Abstract
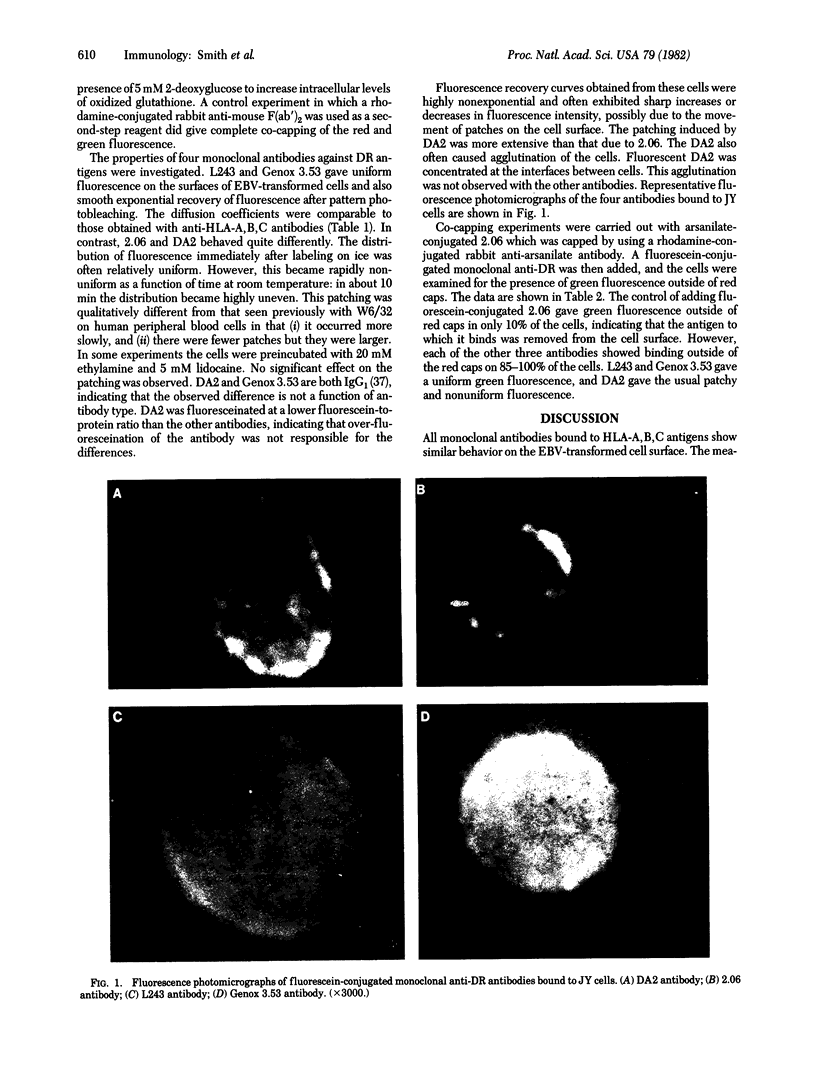
A number of monoclonal antibodies have been used to investigate the distributions and rates of lateral motion of the HLA-A,B, and-DR antigens on several Epstein--Barr virus-transformed B-cell lines. The lateral diffusion coefficients (D) of fluorescein conjugates of the monoclonal antibodies bound to the cell surface were determined by fluorescence recovery after pattern photobleaching. Ds of HLA-A and-B were found to be comparable and of the order of 10(-9) to 10(-10) cm2/sec for each of the seven monoclonal antibodies and four cell lines examined. The HLA antigens appear to be monomeric on the cell surface based on experiments using mixtures of arsanilic acid-conjugated and fluorescein-conjugated antibodies. Four monoclonal antibodies against DR antigens were examined. Two of these, Genox 3.53 and L243, labeled the cell surface uniformly and gave Ds comparable to those obtained for the HLA-A and -B antigens. The other two, DA2 and 2.06, rapidly patched on the cell surface and were immobile. The DA2, L243, and Genox 3.53 antibodies bound outside of the caps formed with the arsanilic acid-conjugated 2.06 antibody and a second-step rhodamine-conjugated rabbit anti-arsanilate antibody. This is consistent with recent biochemical evidence that there are multiple distinct antigens coded for by the HLA-DR region.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison J. P., Walker L. E., Russell W. A., Pellegrino M. A., Ferrone S., Reisfeld R. A., Frelinger J. A., Silver J. Murine Ia and human DR antigens: homology of amino-terminal sequences. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3953–3956. doi: 10.1073/pnas.75.8.3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach F. H., van Rood J. J. The major histocompatibility complex - genetics and biology (third of three parts). N Engl J Med. 1976 Oct 21;295(17):927–936. doi: 10.1056/NEJM197610212951705. [DOI] [PubMed] [Google Scholar]
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Brodsky F. M., Parham P., Barnstable C. J., Crumpton M. J., Bodmer W. F. Monoclonal antibodies for analysis of the HLA system. Immunol Rev. 1979;47:3–61. doi: 10.1111/j.1600-065x.1979.tb00288.x. [DOI] [PubMed] [Google Scholar]
- Brodsky F. M., Parham P., Bodmer W. F. Monoclonal antibodies to HLA--DRw determinants. Tissue Antigens. 1980 Jul;16(1):30–48. doi: 10.1111/j.1399-0039.1980.tb00285.x. [DOI] [PubMed] [Google Scholar]
- Brodsky F. M., Parham P., Bodmer W. F. Monoclonal antibodies to HLA--DRw determinants. Tissue Antigens. 1980 Jul;16(1):30–48. doi: 10.1111/j.1399-0039.1980.tb00285.x. [DOI] [PubMed] [Google Scholar]
- Charron D. J., McDevitt H. O. Analysis of HLA-D region-associated molecules with monoclonal antibody. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6567–6571. doi: 10.1073/pnas.76.12.6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charron D. J., McDevitt H. O. Characterization of HLA-D-region antigens by two-dimensional gel electrophoresis. Molecular-genotyping. J Exp Med. 1980 Aug 1;152(2 Pt 2):18s–36s. [PubMed] [Google Scholar]
- Cherry R. J. Rotational and lateral diffusion of membrane proteins. Biochim Biophys Acta. 1979 Dec 20;559(4):289–327. doi: 10.1016/0304-4157(79)90009-1. [DOI] [PubMed] [Google Scholar]
- Corte G., Calabi F., Damiani G., Bargellesi A., Tosi R., Sorrentino R. Human Ia molecules carrying DC1 determinants differ in both alpha- and beta-subunits from Ia molecules carrying DR determinants. Nature. 1981 Jul 23;292(5821):357–360. doi: 10.1038/292357a0. [DOI] [PubMed] [Google Scholar]
- Cresswell P., Dawson J. R. Dimeric and monomeric forms of HL-A antigens solubilized by detergent. J Immunol. 1975 Jan;114(1 Pt 2):523–525. [PubMed] [Google Scholar]
- Cresswell P., Geler S. S. Antisera to human B-lymphocyte membrane glycoproteins block stimulation in mixed lymphocyte culture. Nature. 1975 Sep 11;257(5522):147–149. doi: 10.1038/257147a0. [DOI] [PubMed] [Google Scholar]
- Cullen S. E., David C. S., Shreffler D. C., Nathenson S. G. Membrane molecules determined by the H-2 associated immune response region: isolation and some properties. Proc Natl Acad Sci U S A. 1974 Mar;71(3):648–652. doi: 10.1073/pnas.71.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edidin M., Wei T. Y. Diffusion rates of cell surface antigens of mouse-human heterokaryons. I. Analysis of the population. J Cell Biol. 1977 Nov;75(2 Pt 1):475–482. doi: 10.1083/jcb.75.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser D. L., Winters B. A., Haas J. B., McKearn T. J., Kennett R. H. Monoclonal antibody directed to a B-cell antigen present in rats, mice, and humans. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4636–4640. doi: 10.1073/pnas.76.9.4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J. The major histocompatibility complex of the mouse. Science. 1979 Feb 9;203(4380):516–521. doi: 10.1126/science.104386. [DOI] [PubMed] [Google Scholar]
- Koppel D. E., Sheetz M. P., Schindler M. Matrix control of protein diffusion in biological membranes. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3576–3580. doi: 10.1073/pnas.78.6.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampson L. A., Levy R. Two populations of Ia-like molecules on a human B cell line. J Immunol. 1980 Jul;125(1):293–299. [PubMed] [Google Scholar]
- Letarte M., Falk J. Analysis of serologic cross-reactions between human and mouse Ia antigens. J Immunol. 1980 Sep;125(3):1210–1215. [PubMed] [Google Scholar]
- Markert M. L., Cresswell P. Polymorphism of human B-cell alloantigens: evidence for three loci within the HLA system. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6101–6104. doi: 10.1073/pnas.77.10.6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster W. R., Winearls B. C., Parham P. A monoclonal mouse anti-rat Ia antibody which cross reacts with a human HLA-DRw determinant. Tissue Antigens. 1979 Nov;14(5):453–458. doi: 10.1111/j.1399-0039.1979.tb00875.x. [DOI] [PubMed] [Google Scholar]
- Neauport-Sautes C., Lilly F., Silvestre D., Kourilsky F. M. Independence of H-2K and H-2D antigenic determinants on the surface of mouse lymphocytes. J Exp Med. 1973 Feb 1;137(2):511–526. doi: 10.1084/jem.137.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr H. T., Lancet D., Robb R. J., Lopez de Castro J. A., Strominger J. L. The heavy chain of human histocompatibility antigen HLA-B7 contains an immunoglobulin-like region. Nature. 1979 Nov 15;282(5736):266–270. doi: 10.1038/282266a0. [DOI] [PubMed] [Google Scholar]
- Parham P., Barnstable C. J., Bodmer W. F. Use of a monoclonal antibody (W6/32) in structural studies of HLA-A,B,C, antigens. J Immunol. 1979 Jul;123(1):342–349. [PubMed] [Google Scholar]
- Parham P., Bodmer W. F. Monoclonal antibody to a human histocompatibility alloantigen, HLA-A2. Nature. 1978 Nov 23;276(5686):397–399. doi: 10.1038/276397a0. [DOI] [PubMed] [Google Scholar]
- Parham P., McLean J. Characterization, evolution, and molecular basis of a polymorphic antigenic determinant shared by HLA-A and B products. Hum Immunol. 1980 Sep;1(2):131–139. doi: 10.1016/0198-8859(80)90100-7. [DOI] [PubMed] [Google Scholar]
- Parham P. Monoclonal antibodies against two separate alloantigenic sites of HLA-B40. Immunogenetics. 1981;13(6):509–527. doi: 10.1007/BF00343719. [DOI] [PubMed] [Google Scholar]
- Peterson P. A., Cunningham B. A., Berggård I., Edelman G. M. 2 -Microglobulin--a free immunoglobulin domain. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1697–1701. doi: 10.1073/pnas.69.7.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petty H. R., Smith L. M., Fearon D. T., McConnell H. M. Lateral distribution and diffusion of the C3b receptor of complement, HLA antigens, and lipid probes in peripheral blood leukocytes. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6587–6591. doi: 10.1073/pnas.77.11.6587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierres M., Rebouah J. P., Kourilsky F. M., Dosseto M., Mercier P., Mawas C., Malissen B. Cross-reactions between mouse Ia and human HLA-D/DR antigens analyzed with monoclonal alloantibodies. J Immunol. 1981 Jun;126(6):2424–2429. [PubMed] [Google Scholar]
- Pober J. S., Guild B. C., Strominger J. L. Phosphorylation in vivo and in vitro of human histocompatibility antigens (HLA-A and HLA-B) in the carboxy-terminal intracellular domain. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6002–6006. doi: 10.1073/pnas.75.12.6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pober J. S., Strominger J. L. Transglutaminase modifies the carboxy-terminal intracellular region of HLA-A and -B antigens. Nature. 1981 Feb 26;289(5800):819–821. doi: 10.1038/289819a0. [DOI] [PubMed] [Google Scholar]
- Quaranta V., Walker L. E., Pellegrino M. A., Ferrone S. Purification of immunologically functional subsets of human Ia-like antigens on a monoclonal antibody (Q5/13) immunoadsorbent. J Immunol. 1980 Oct;125(4):1421–1425. [PubMed] [Google Scholar]
- Schlessinger J., Webb W. W., Elson E. L., Metzger H. Lateral motion and valence of Fc receptors on rat peritoneal mast cells. Nature. 1976 Dec 9;264(5586):550–552. doi: 10.1038/264550a0. [DOI] [PubMed] [Google Scholar]
- Schreiner G. F., Unanue E. R. Membrane and cytoplasmic changes in B lymphocytes induced by ligand-surface immunoglobulin interaction. Adv Immunol. 1976;24:37–165. doi: 10.1016/s0065-2776(08)60329-6. [DOI] [PubMed] [Google Scholar]
- Schwartz B. D., Kato K., Cullen S. E., Nathenson S. G. H-2 histocompatibility alloantigens. Some biochemical properties of the molecules solubilized by NP-40 detergent. Biochemistry. 1973 May 22;12(11):2157–2164. doi: 10.1021/bi00735a023. [DOI] [PubMed] [Google Scholar]
- Shackelford D. A., Mann D. L., van Rood J. J., Ferrara G. B., Strominger J. L. Human B-cell alloantigens DC1, MT1, and LB12 are identical to each other but distinct from the HLA-DR antigen. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4566–4570. doi: 10.1073/pnas.78.7.4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheetz M. P., Schindler M., Koppel D. E. Lateral mobility of integral membrane proteins is increased in spherocytic erythrocytes. Nature. 1980 Jun 12;285(5765):510–511. doi: 10.1038/285510a0. [DOI] [PubMed] [Google Scholar]
- Smith L. M., Rubenstein J. L., Parce J. W., McConnell H. M. Lateral diffusion of M-13 coat protein in mixtures of phosphatidylcholine and cholesterol. Biochemistry. 1980 Dec 9;19(25):5907–5911. doi: 10.1021/bi00566a037. [DOI] [PubMed] [Google Scholar]
- Snary D., Goodfellow P., Hayman M. J., Bodmer W. F., Crumpton M. J. Subcellular separation and molecular nature of human histocompatibility antigens (HL-A). Nature. 1974 Feb 15;247(5441):457–461. doi: 10.1038/247457a0. [DOI] [PubMed] [Google Scholar]
- Springer T. A., Mann D. L., DeFranco A. L., Strominger J. L. Detergent solubilization, purification, and separation of specificities of HLA antigens from a cultured human lymphoblastoid line, RPMI 4265. J Biol Chem. 1977 Jul 10;252(13):4682–4693. [PubMed] [Google Scholar]
- Trägärdh L., Rask L., Wiman K., Fohlman J., Peterson P. A. Complete amino acid sequence of pooled papain-solubilized HLA-A, -B, and -C antigens: relatedness to immunoglobulins and internal homologies. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1129–1133. doi: 10.1073/pnas.77.2.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhr J. W., Capra J. D., Vitetta E. S., Cook R. G. Organization of the immune response genes. Science. 1979 Oct 19;206(4416):292–297. doi: 10.1126/science.113876. [DOI] [PubMed] [Google Scholar]
- Vaz W. L., Jacobson K., Wu E. S., Derzko Z. Lateral mobility of an amphipathic apolipoprotein, ApoC-III, bound to phosphatidylcholine bilayers with and without cholesterol. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5645–5649. doi: 10.1073/pnas.76.11.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]