Abstract
The highly conserved small GTPase Cdc42p is a key regulator of cell polarity and cytoskeletal organization in eukaryotic cells. Multiple effectors of Cdc42p have been identified, although it is unclear how their activities are coordinated to produce particular cell behaviors. One strategy used to address the contributions made by different effector pathways downstream of small GTPases has been the use of “effector-loop” mutants of the GTPase that selectively impair only a subset of effector pathways. We now report the generation and preliminary characterization of a set of effector-loop mutants of Saccharomyces cerevisiae CDC42. These mutants define genetically separable pathways influencing actin or septin organization. We have characterized the phenotypic defects of these mutants and the binding defects of the encoded proteins to known yeast Cdc42p effectors in vitro. The results suggest that these effectors cannot account for the observed phenotypes, and therefore that unknown effectors exist that affect both actin and septin organization. The availability of partial function alleles of CDC42 in a genetically tractable system serves as a useful starting point for genetic approaches to identify such novel effectors.
INTRODUCTION
The establishment and regulation of cell polarity are critical for cell shape, for cell motility and migration, and for directional cell–cell communication. Although the detailed forms and consequences of polarization vary widely depending on the cell type and the biological context, the underlying molecular machinery used to establish cell polarity appears to be remarkably well conserved among eukaryotes. In particular, the small GTPase Cdc42p, originally identified through its role in polarization of yeast cells during bud formation (Adams et al., 1990), plays a widespread role in polarity establishment and cytoskeletal regulation throughout eukaryotes (Hall, 1998; Johnson, 1999). Cdc42p can stimulate actin polymerization and reorganization (Li et al., 1995; Zigmond et al., 1998; Bi and Zigmond, 1999; Rohatgi et al., 1999), affect vesicle trafficking (Brown et al., 1998; Kroschewski et al., 1999; Garrett et al., 2000), and influence transcriptional regulation (Coso et al., 1995; Minden et al., 1995; Simon et al., 1995; Zhao et al., 1995). These functions are thought to be mediated by numerous effectors (proteins that are regulated through their specific interaction with GTP-bound Cdc42p), and intensive efforts are underway to identify effectors and to elucidate how Cdc42p coordinates their actions to promote appropriate effects.
Several potential Cdc42p effectors have been identified through a variety of interaction-cloning strategies (Manser et al., 1993, 1994), and recognition of a conserved Cdc42/Rac-interactive-binding (CRIB) domain (Burbelo et al., 1995) has allowed the discovery of further effectors by virtue of sequence homology (Brown et al., 1997; Chen et al., 1997; Martin et al., 1997; Pirone et al., 2000). However, there is considerable controversy regarding the role of particular effectors in specific responses (Lamarche et al., 1996; Sells et al., 1997), and it is not clear whether the majority of effectors have been identified or whether many yet remain to be discovered. Perhaps surprisingly, genetic approaches in yeast have thus far contributed little to the identification of Cdc42p effectors, possibly because until recently very few mutant cdc42 alleles were available for analysis.
One approach that has enjoyed remarkable success in the analysis of pathways downstream of Ras-related GTPases is the use of mutants that alter residues in the “effector loop” of the protein, which is thought to enable interacting proteins to recognize the “activated” GTP-bound conformation (Wittinghofer and Nassar, 1996). In many instances, effector-loop mutations appear to selectively disrupt the interaction of GTP-bound Ras relatives with only a subset of their effectors (Nobes and Hall, 1995; White et al., 1995; Joneson et al., 1996a,b; Khosravi-Far et al., 1996; Lamarche et al., 1996; White et al., 1996; Joneson and Bar-Sagi, 1997; Sahai et al., 1998; Owen et al., 2000). When effector-loop mutant alleles are introduced into cells, phenotypic deficits in specific biological outputs can be correlated with biochemical deficits in binding to particular effectors. Although this approach has been applied to ask whether known effectors are likely to participate in particular pathways, it has not yet contributed to the search for novel effectors.
We reasoned that introduction of effector-loop mutants of CDC42 into the genetically tractable yeast system should provide strains containing partial function cdc42 alleles that could serve as a productive starting point for genetic approaches to identify novel Cdc42p effectors and to assign roles for known effectors in particular pathways. Here we report the generation and phenotypic and biochemical characterization of 10 effector-loop mutants. The results suggest that as-yet-unknown effectors exist in yeast, and that some “effectors” may operate upstream of Cdc42p as well as downstream of Cdc42p.
MATERIALS AND METHODS
Strains, Plasmids, and PCR Manipulations
Standard media and methods were used for plasmid manipulations (Ausubel et al., 1995) and yeast genetic manipulations (Guthrie and Fink, 1991). The Saccharomyces cerevisiae strains used in this study are listed in Table 1, plasmids are listed in Table 2, and oligonucleotides are listed in Table 3.
Table 1.
Yeast strains used in this studya
| Strain | Genotype |
|---|---|
| DLY1 | a bar1 |
| DLY5 | a/α |
| DLY680 | α cdc42-1 |
| DLY3067 | abar1 cdc42∷LEU2∷GAL1p-CDC42 |
| DLY3496 | abar1 leu2∷GAL1p-CDC42∷LEU2 cdc42N39A |
| DLY3497 | abar1 leu2∷GAL1p-CDC42∷LEU2 cdc42V36A |
| DLY3500 | abar1 leu2∷GAL1p-CDC42∷LEU2 cdc42V36T |
| DLY3509 | abar1 cdc42∷LEU2∷GAL1p-CDC42 his2∷cdc42D381∷HIS2 |
| DLY3511 | abar1 cdc42∷LEU2∷GAL1p-CDC42 his2∷cdc42T35A∷HIS2 |
| DLY3513 | abar1 cdc42∷LEU2∷GAL1p-CDC42 his2∷cdc42Y40K∷HIS2 |
| DLY3515 | abar1 cdc42∷LEU2∷GAL1p-CDC42 his2∷cdc42D38A∷HIS2 |
| DLY3517 | abar1 cdc42∷LEU2∷GAL1p-CDC42 his2∷cdc42F37G∷HIS2 |
| DLY3519 | abar1 cdc42∷LEU2∷GAL1p-CDC42 his2∷cdc42Y40C∷HIS2 |
| DLY3525 | α bar1 cdc42∷LEU2∷GAL1p-CDC42 his2∷cdc42Y40C∷HIS2 |
| DLY3540 | α bar1 his2∷cdc42Y40K∷HIS2 cdc42-6 |
| DLY3541 | α bar1 his2∷cdc42D38A∷HIS2 cdc42-6 |
| DLY3553 | α bar1 leu2∷GAL1p-CDC42∷LEU2 cdc42V36T |
| DLY3554 | α bar1 leu2∷GAL1p-CDC42∷LEU2 cdc42V36A |
| DLY3556 | α bar1 leu2∷GAL1p-CDC42∷LEU2 cdc42N39A |
| DLY3571b | a/α bar1/bar1 cdc42∷LEU2∷GAL1p-CDC42/cdc42∷LEU2∷GAL1p-CDC42 his2∷cdc42F37G∷HIS2/his2∷cdc42F37G∷HIS2 |
| DLY3572b | a/α bar1/bar1 cdc42∷LEU2∷GAL1p-CDC42/cdc42∷LEU2∷GAL1p-CDC42 his2∷cdc42Y40K∷HIS2/his2∷cdc42Y40K∷HIS2 |
| DLY3579 | α bar1 his2∷cdc42F37G∷HIS2 cdc42-6 |
| DLY3804 | α bar1 his2∷cdc42D38I∷HIS2 cdc42-6 |
| DLY3809 | α bar1 cdc42∷LEU2∷GAL1p-CDC42 his2∷cdc42T35A∷HIS2 |
| DLY3880 | α bar1 cdc42∷LEU2∷GAL1p-CDC42 his2∷cdc42F37Y∷HIS2 |
| DLY3881 | α bar1 cdc42∷LEU2∷GAL1p-CDC42 his2∷cdc42F37Y∷HIS2 |
| DLY3891 | a/α bar1/bar1 leu2∷GAL1p-CDC42∷LEU2/leu2 cdc42∷LEU2∷GAL1p-CDC42/cdc42V36This2∷cdc42F37G∷HIS2/his2 |
| DLY3895 | a/α bar1/bar1 leu2∷GAL1p-CDC42∷LEU2/leu2 cdc42∷LEU2∷GAL1p-CDC42/cdc42V36This2∷cdc42Y40K∷HIS2/his2 |
| DLY3923 | a/α bar1/bar1 leu2∷GAL1p-CDC42∷LEU/leu2∷GAL1p-CDC42∷LEU2 cdc42V36A/cdc42V36A |
| DLY3924 | a/α bar1/bar1 leu2∷GAL1p-CDC42∷LEU/leu2∷GAL1p-CDC42∷LEU2 cdc42V36T/cdc42V36T |
| DLY4756 | a/α bar1/bar1 cdc42∷LEU2∷GAL1p-CDC42/cdc42∷LEU2∷GAL1p-CDC42 his2∷cdc42F37Y∷HIS2/his2∷cdc42F37Y∷HIS2 |
| DLY4757 | a/α bar1/bar1 leu2∷GAL1p-CDC42∷LEU2/leu2∷GAL1p-CDC42∷LEU2 cdc42N39A/cdc42N39A |
| MOSY0090 | abar1 leu2∷GAL1p-CDC42∷LEU2 cdc42∷URA3 |
| MOSY0121 | α bar1 cdc42-6 |
All strains are in the BF264-15Du (Richardson et al., 1989) background (ade1 his2 leu2-3, 112 trp1-1 ura3Δns).
DLY3571 and DLY3572 were generated by HO-induced diploidization of DLY3517 and DLY3513, respectively.
Table 2.
Plasmids used in this study
| Plasmid | Vector | Insert | Source |
|---|---|---|---|
| pDLB678 | 2 μm URA3 | BEM1 | Bender and Pringle, 1991 |
| pDLB722 | 2 μm URA3 | CLA4 | Erfei Bi |
| pDLB723 | 2 μm URA3 | STE20 | Erfei Bi |
| pDLB935 | YEp24 | CDC24 | John Moskowa |
| pDLB1025 | pUNI10b | CLA4 CRIB | This study |
| pDLB1026 | pUNI10b | GIC1 CRIB | This study |
| pDLB1027 | pUNI10b | GIC2 CRIB | This study |
| pDLB1034 | pUNI10b | cdc42Q61L | This study |
| pDLB1035 | pUNI10b | cdc42D57Y | This study |
| pDLB1126 | pHB2-GSTb | CLA4 CRIB | This study |
| pDLB1127 | pHB2-GSTb | GIC1 CRIB | This study |
| pDLB1128 | pHB2-GSTb | GIC2 CRIB | This study |
| pDLB1159 | pUNI10b | cdc42V36T,Q61L | This study |
| pDLB1160 | pUNI10b | cdc42V36A,Q61L | This study |
| pDLB1161 | pUNI10b | cdc42F37G,Q61L | This study |
| pDLB1234 | pHB1-myc3b | cdc42D57Y | This study |
| pDLB1238 | pHB1-myc3b | cdc42Q61L | This study |
| pDLB1239 | pHB1-myc3b | cdc42T35A,Q61L | This study |
| pDLB1240 | pHB1-myc3b | cdc42V36A,Q61L | This study |
| pDLB1241 | pHB1-myc3b | cdc42V36T,Q61L | This study |
| pDLB1242 | pHB1-myc3b | cdc42Y40C,Q61L | This study |
| pDLB1243 | pHB1-myc3b | cdc42Y40K,Q61L | This study |
| pDLB1274 | pUNI10b | BEM1 | This study |
| pDLB1275 | pUNI10b | cdc42T35A,Q61L | This study |
| pDLB1277 | pUNI10b | cdc42Y40C,Q61L | This study |
| pDLB1278 | pUNI10b | cdc42F37Y,Q61L | This study |
| pDLB1279 | pUNI10b | cdc42D38A,Q61L | This study |
| pDLB1280 | pUNI10b | cdc42N39A,Q61L | This study |
| pDLB1282 | pHB1-myc3b | cdc42F37G,Q61L | This study |
| pDLB1283 | pHB1-myc3b | cdc42F37Y,Q61L | This study |
| pDLB1284 | pHB1-myc3b | cdc42D38A,Q61L | This study |
| pDLB1285 | pHB1-myc3b | cdc42D38I,Q61L | This study |
| pDLB1286 | pHB1-myc3b | cdc42N39A,Q61L | This study |
| pDLB1305 | pHB2-GSTb | BEM1 | This study |
| pDLB1307 | pUNI10b | cdc42Y40K,Q61L | This study |
| pDLB1308 | 2 μm URA3 | HA-BNI1 | Mark Longtine |
| pDLB1309 | pHB2-GSTb | cdc42Q61L | This study |
| pDLB1311 | pHB2-GSTb | cdc42D57Y | This study |
| pDLB1313 | pHB2-GSTb | cdc42T35A,Q61L | This study |
| pDLB1314 | pHB2-GSTb | cdc42V36A,Q61L | This study |
| pDLB1315 | pHB2-GSTb | cdc42V36T,Q61L | This study |
| pDLB1316 | pHB2-GSTb | cdc42F37G,Q61L | This study |
| pDLB1317 | pHB2-GSTb | cdc42F37Y,Q61L | This study |
| pDLB1318 | pHB2-GSTb | cdc42D38A,Q61L | This study |
| pDLB1319 | pHB2-GSTb | cdc42D38I,Q61L | This study |
| pDLB1320 | pHB2-GSTb | cdc42N39A,Q61L | This study |
| pDLB1321 | pHB2-GSTb | cdc42Y40C,Q61L | This study |
| pDLB1322 | pHB2-GSTb | cdc42Y40K,Q61L | This study |
| pDLB1323 | CEN URA3 | cdc42D38I | This study |
| pDLB1656 | 2 μm URA3 | cdc42T35A | This study |
| pDLB1657 | 2 μm URA3 | cdc42V36A | This study |
| pDLB1658 | 2 μm URA3 | cdc42V36T | This study |
| pDLB1659 | 2 μm URA3 | cdc42F37G | This study |
| pDLB1660 | 2 μm URA3 | cdc42F37Y | This study |
| pDLB1661 | 2 μm URA3 | cdc42D38A | This study |
| pDLB1662 | 2 μm URA3 | cdc42D381 | This study |
| pDLB1663 | 2 μm URA3 | cdc42N39A | This study |
| pDLB1664 | 2 μm URA3 | cdc42Y40C | This study |
| pDLB1665 | 2 μm URA3 | cdc42Y40K | This study |
| pDLB1666 | 2 μm URA3 | CDC42 | This study |
| pDLB1723 | 2 μm TRP1 | cdc42T35A | This study |
| pDLB1724 | 2 μm TRP1 | cdc42V36A | This study |
| pDLB1726 | 2 μm TRP1 | cdc42F37G | This study |
| pDLB1727 | 2 μm TRP1 | cdc42F37Y | This study |
| pDLB1728 | 2 μm TRP1 | cdc42D38A | This study |
| pDLB1729 | 2 μm TRP1 | cdc42D38I | This study |
| pDLB1730 | 2 μm TRP1 | cdc42N39A | This study |
| pDLB1731 | 2 μm TRP1 | cdc42Y40C | This study |
| pDLB1732 | 2 μm TRP1 | cdc42Y40K | This study |
| pDLB1733 | 2 μm TRP1 | CDC42 | This study |
| pDLB1861 | pHB1-myc3b | CLA4 CRIB | This study |
| pMOSB14 | pCR2.1c | cdc42V36T | This study |
| pMOSB15 | pCR2.1c | cdc42T35A | This study |
| pMOSB16 | pCR2.1c | cdc42V36A | This study |
| pMOSB17 | pCR2.1c | cdc42D38A | This study |
| pMOSB18 | pCR2.1c | cdc42F37Y | This study |
| pMOSB19 | pCR2.1c | cdc42F37G | This study |
| pMOSB23 | pCR2.1c | cdc42Y40K | This study |
| pMOSB26 | pCR2.1c | cdc42D38I | This study |
| pMOSB27 | pCR2.1c | cdc42N39A | This study |
| pMOSB53 | pCR2.1c | cdc42Y40C | This study |
| pMOSB40 | CEN URA3 | cdc42F37G | This study |
| pMOSB41 | CEN URA3 | cdc42Y40K | This study |
| pMOSB45 | CEN TRP1 | CDC42 | This study |
| pMOSB46 | CEN TRP1 | cdc42T35A | This study |
| pMOSB47 | CEN TRP1 | cdc42V36A | This study |
| pMOSB48 | CEN TRP1 | cdc42F37G | This study |
| pMOSB49 | CEN TRP1 | cdc42D38I | This study |
| pMOSB50 | CEN TRP1 | cdc42D38A | This study |
| pMOSB51 | CEN TRP1 | cdc42Y40K | This study |
| pMOSB55 | CEN URA3 | CDC42 | This study |
| pMOSB57 | CEN URA3 | cdc42V36T | This study |
| pMOSB58 | CEN URA3 | cdc42F37Y | This study |
| pMOSB59 | CEN URA3 | cdc42N39A | This study |
| pMOSB175 | CEN TRP1 | cdc42Y40C | This study |
| pMOSB176 | CEN URA3 | cdc42Y40C | This study |
| pMOSB177 | CEN URA3 | cdc42V36A | This study |
| pMOSB186 | CEN URA3 | cdc42T35A | This study |
| pMOSB229 | 2 μm URA3 | GIC1 | Matthias Peter |
| pMOSB230 | 2 μm URA3 | GIC2 | Jaquenoud et al. (1998) |
| pMOSB240 | pGEX | STE20 CRIB | Moskow et al. (2000) |
| pRS314 | CEN TRP1 | Sikorski and Hieter (1989) | |
| pRS316 | CEN URA3 | Sikorski and Hieter (1989) | |
| pRS426 | 2 μm URA3 | Christianson et al. (1992) | |
| pRS424 | 2 μm TRP1 | Christianson et al. (1992) | |
| YEp24 | 2 μm URA3 | New England Biolabs (Beverly, MA) |
Isolated from Yep24 genomic library. Insert containing full-length CDC24 was confirmed by sequencing.
Univector and host plasmids as previously described (Liu et al., 1998).
pCR2.1 from Invitrogen (San Diego, CA).
Table 3.
Oligonucleotides used in this study
| Oligo | Description | Oligonucleotide sequence |
|---|---|---|
| cdc-1a | cdc42-T35A | GCAGTGTTCGATAACTATGC |
| cdc-3a | cdc42-V36T | ACAACGTTCGATAACTATGCGG |
| cdc-4a | cdc42-V36A | ACAGCGTTCGATAACTATGCGG |
| cdc-5a | cdc42-F37Y | ACAGTGTACGATAACTATGCGGTG |
| cdc-6a | cdc42-F37G | ACAGTGGGCGATAACTATGCGGTG |
| cdc-7a | cdc42-D38I | ACAGTGTTCATTAACTATGCGGTGACTG |
| cdc-8a | cdc42-D38A | ACAGTGTTCGCTAACTATGCGGTGACTG |
| cdc-9a | cdc42-N39A | ACAGTGTTCGATGCCTATGCGGTGACTGTG |
| cdc-10a | cdc42-Y40K | ACAGTGTTCGATAACAAGGCGGTGACTGTGATG |
| cdc-11b | reverse primer | TGGAACATAGTCAGCTGGAAATTGATTCG |
| cdc-22a | cdc42-Y40C | ACAGTGTTCGATAACTGTGCGGTGACTGTGATG |
| DJL42-3 | CDC42 promoter | CCACCGTCGATTCAAGGGTC |
| DJL42-6 | TDH3 terminator | CTACTACAGATATTACATGTGGCG |
| DJL42-4 | CDC42 stop | GCGAAACGAGTCTCTAGA |
| CLA4-UNI1c | CLA4-CRIB/NdeI | GGAATTCCATATGGATTTGCATAGTTGGTTAGACGCC |
| CLA4-UNI2c | CLA4-CRIB/SacI | CCGACTCTCAATCTTCTCCTGTAATTCTGGAGTG |
| GIC1-UNI1c | GIC1-CRIB/NdeI | GGAATTCCATATGCTGTTGTCGAGGAGACATGGGTCTGCC |
| GIC1-UNI2c | GIC1-CRIB/SacI | CCGAGCTCTCATGGTCGGGGTTGCGGTGCCAAAGACG |
| GIC2-UNI1c | GIC2-CRIB/NdeI | GGAATTCCATATGGGTGCCCAACCGGACATAAGAGGT |
| GIC2-UNI2c | GIC2-CRIB/SacI | CCGAGCTCTCAGGTATAGTCGTCCTTAATCTCTGTGG |
| BEM1-2 | BEM1 | GATCCATATGCTGAAAAACTTCAAACTC |
| BEM1-3 | BEM1 | GCTTCGTCTTCTAACACTAG |
Sequence changes are indicated by bases in boldface.
cdc-11 has a silent mutation that introduces a PvuII site (underlined).
Underlined sequences indicate NdeI and SacI restriction sites used for cloning.
The CDC42 effector-loop mutants were constructed using the ExSite polymerase chain reaction (PCR)-based site-directed mutagenesis kit (Stratagene, La Jolla, CA). For each mutation, PCR was performed using the mutagenic and cdc-11 oligonucleotides (Table 3) with pDLB643 as a template. This plasmid contains the CDC42 promoter and coding region (from 366 bp upstream of the start codon to 30 bp downstream of the stop codon) fused to TDH3 transcription terminator sequences in a pCR2.1 (Invitrogen, Carlsbad, CA) backbone (Moskow et al., 2000).
Using the above-mentioned mutants as template, the CDC42 and TDH3 sequences were amplified by PCR with the oligonucleotides DJL42-3 and DJL42-6 (Table 3) and introduced into pDLB644 (Moskow et al., 2000) by gap repair, generating plasmids for expression of cdc42 alleles in yeast. Mutants were sequenced to confirm the presence of the desired mutation and the absence of any other mutations. These plasmids contain a pRS316 (Sikorski and Hieter, 1989) backbone (low-copy URA3), and parallel sets of plasmids was generated by subcloning the 2.1-kb BamHI/XhoI fragments containing cdc42 alleles into the corresponding sites in pRS314 (low-copy TRP1), pRS424 (high-copy TRP1), and pRS426 (high-copy URA3) vectors (Sikorski and Hieter, 1989).
Two strategies were taken to integrate the cdc42 alleles into the genome. First, a linear 1-kb EcoRI fragment containing the promoter and open reading frame of the mutant was transformed together with an uncut pRS314 “carrier” plasmid into strain MOSY0090, which contains a cdc42::URA3 disruption (missing all but the last 78 bp of the CDC42 open reading frame) at the CDC42 locus and a copy of CDC42 under control of the GAL1 promoter at the LEU2 locus (Moskow et al., 2000). Trp+ transformants containing the carrier plasmid were first selected on galactose-containing plates lacking tryptophan, and then those transformants in which the cdc42 allele had replaced the cdc42::URA3 deletion were selected by replica-plating onto dextrose-containing plates with 5-fluoroorotic acid, which kills URA3 cells. Gene replacement was confirmed by PCR with the oligonucleotides DJL42-3 and DJL42-4, which amplify a 1-kb product from the effector-loop alleles but not from cdc42::URA3 (lacking the region complementary to DJL42-4) or GAL1p-CDC42 (lacking the promoter region complementary to DJL42-3). Haploid mutants were backcrossed to a wild-type strain, and the cdc42 phenotypes (see text) were observed to segregate 2:2 in at least 10 tetrads, indicating that the phenotypes are caused by the cdc42 allele alone (and not due to second site mutations).
This strategy was successful for the cdc42V36A, cdc42V36T, and cdc42N39A alleles, but we were unable to apply the 5-fluoroorotic selection on galactose-containing plates (Ura+ cells grew on such plates), so the above-described strategy was not workable for cdc42 alleles that were unable to support growth on dextrose (i.e., as the sole copy of CDC42). In an alternative strategy, the 1-kb EcoRI fragments containing the effector-loop alleles were cloned into the EcoRI site of the integrating vector YIpGAP2 (Sia et al., 1996), which contains the HIS2 marker in a pUC18 backbone. The resulting plasmids were digested with XbaI, which cuts at a unique site within HIS2, and transformed into strain DLY3067 (Moskow et al., 2000), in which the genomic CDC42 is placed under control of the GAL1 promoter. Integration of the mutant alleles at HIS2 in His+ transformants (selected on galactose-containing plates) was confirmed by PCR as described above. The same strategy was used to introduce the alleles into strain MOSY0121 (cdc42-6). The phenotype of each mutant was identical whether it was analyzed by glucose shift (depleting wild-type Cdc42p in the GAL1p-CDC42 strains) or by temperature shift (inactivating Cdc42-6p in cdc42-6 strains).
The cdc42-6 allele was generated by the same PCR mutagenesis and gap repair strategy described for isolation of pheromone-resistant cdc42-md alleles (Moskow et al., 2000) except that transformants were screened for temperature-sensitive growth rather than pheromone resistance. cdc42-6 was then integrated at the genomic CDC42 locus by the cdc42::URA3 replacement strategy described above. The resulting cdc42-6 strain grows well at 23°C but arrests with a uniform large round unbudded cell phenotype at 37°C. The minimal restrictive temperature for this strain is 33°C, but we shifted the cells to 37°C to eliminate as much residual function as possible. We first characterized the tightness of this mutant by staining cells grown at the permissive temperature with fluorescein isothiocyanate-concanvalin A (a vital stain for cell wall polysaccharide (Adams and Pringle, 1984) and then shifting them to 37°C in medium lacking fluorescein isothiocyanate-concanvalin A. Any buds formed after the temperature shift would then appear as dark buds attached to bright green mother cells, allowing an accurate estimate of how rapidly bud formation ceased after temperature shift. By this criterion, <5.0% of cdc42-6 cells initiated bud formation after the shift to 37°C (in comparison >20% of cdc42-1 cells did so), indicating a tight and fast-acting phenotype for this mutant.
To express GTP-locked versions of the effector-loop Cdc42p variants in bacteria, double mutant alleles containing an additional Q61L substitution were generated by a “gap-repair” strategy and then cloned into the “univector” pUNI-10 (Liu et al., 1998) as described previously for pheromone-resistant cdc42 alleles (Moskow et al., 2000). These plasmids were then recombined with the “host” vector pHB1-MYC3 (Liu et al., 1998) by using Cre recombinase in vitro, generating bacterial expression plasmids directing production of double-mutant alleles fused to three c-myc epitopes at the N terminus. To express the effector CRIB domains, the relevant regions of CLA4 (amino acids 164–225), GIC1 (amino acids 109–171), and GIC2 (amino acids 124–172) were amplified by PCR with yeast genomic DNA as template and the oligonucleotides listed in Table 3. PCR products were digested with NdeI and SacI and cloned into the corresponding sites of pUNI-10. To express full-length Bem1p, the entire BEM1 gene was amplified from yeast genomic DNA by using the BEM1-2 and BEM1-3 oligonucleotides listed in Table 3. The resulting PCR fragment was cloned into pCR2.1 (Invitrogen), and a NdeI/XhoI BEM1 fragment was then cloned into the NdeI/SalI sites of pUNI-10. All pUNI constructs were sequenced to confirm that no additional mutations occurred as a result of PCR manipulations. pUNIGIC1 CRIB, pUNIGIC2 CRIB, and pUNIBEM1 were then recombined with the host vector pHB2-GST (Liu et al., 1998), generating a bacterial expression plasmid directing production of Bem1p fused to GST at the N terminus. pUNICLA4 CRIB was recombined with pHB1-MYC3 (Liu et al., 1998) to express a fusion protein. The plasmid pGEX-Ste20CRIB (Moskow et al., 2000) was used to express the region encoding Ste20p amino acids 328–428 fused to glutathione S-transferase (GST) at the N terminus.
Media, Growth Conditions, and Depletion of Wild-Type Cdc42p
Strains were grown in YEPD (1% yeast extract, 2% bacto-peptone, 2% dextrose, and 0.01% adenine), YEPG (as YEPD but with 2% galactose instead of dextrose), or (for strains containing plasmids) drop-out medium (Guthrie and Fink, 1991) lacking uracil, tryptophan, or histidine, as appropriate. For strains containing both an effector-loop allele of CDC42 under control of the CDC42 promoter and a wild-type copy of CDC42 under control of the GAL1 promoter, the wild-type Cdc42p was depleted by growth of the cells in dextrose-containing medium for at least 24 h. Control experiments confirmed that this was sufficient to deplete the protein to undetectable levels and to produce a uniform unbudded arrest in cells lacking an additional copy of CDC42.
Intragenic Complementation Analysis
The crosses performed to generate the intragenic complementation strains are listed in Table 4. Starting haploid strains contained the relevant effector-loop allele of CDC42 and an additional copy of either GAL1p-CDC42 or the temperature-sensitive cdc42-6 allele. Intragenic complementation was evaluated on dextrose medium (to deplete GAL1p-CDC42 where relevant) at 37°C (to inactivate cdc42-6). For the GAL1p-CDC42 strains the analysis was also performed at 37°C, with identical results. To evaluate intragenic complementation at higher copy-number, DLY3067 was transformed with pairwise combinations of alleles on TRP1 and URA3 marked plasmids. Wild-type Cdc42p was depleted by growth for 30 h at 30°C on dextrose medium before evaluating complementation.
Table 4.
Crosses used for complementation analysisa
| T35A | V36T | V36A | F37G | F37Y | D38A | D38I | N39A | Y40C | Y40K | |
|---|---|---|---|---|---|---|---|---|---|---|
| T35A | 3511 × 3809 | 3500 × 3809 | 3497 × 3809 | 3517 × 3809 | 3881 × 3809 | 3515 × 3809 | 3509 × 3809 | 3496 × 3809 | 3519 × 3809 | 3513 × 3809 |
| V36A | 3511 × 3554 | 3500 × 3554 | 3497 × 3554 | 3517 × 3554 | 3881 × 3554 | 3515 × 3554 | 3509 × 3554 | 3496 × 3554 | 3519 × 3554 | 3513 × 3554 |
| V36T | 3511 × 3553 | 3500 × 3553 | 3497 × 3553 | 3517 × 3553 | 3881 × 3553 | 3515 × 3553 | 3509 × 3553 | 3496 × 3553 | 3519 × 3553 | 3513 × 3553 |
| F37G | 3809 × 3517 | 3553 × 3517 | 3554 × 3517 | 3517 × 3579 | 3880 × 3517 | 3515 × 3579 | 3509 × 3579 | 3556 × 3517 | 3540 × 3517 | 3513 × 3579 |
| F37Y | 3511 × 3880 | 3500 × 3880 | 3497 × 3880 | 3517 × 3880 | 3881 × 3880 | 3515 × 3880 | 3509 × 3880 | 3496 × 3880 | 3519 × 3880 | 3513 × 3880 |
| D38A | 3515 × 3809 | 3553 × 3515 | 3554 × 3515 | 3517 × 3541 | 3880 × 3515 | 3515 × 3541 | 3509 × 3541 | 3556 × 3515 | 3540 × 3515 | 3513 × 3541 |
| D38I | 3509 × 3809 | 3553 × 3509 | 3554 × 3509 | 3517 × 3804 | 3880 × 3509 | 3515 × 3804 | 3509 × 3804 | 3556 × 3509 | 3540 × 3509 | 3513 × 3804 |
| N39A | 3511 × 3556 | 3500 × 3556 | 3497 × 3556 | 3517 × 3556 | 3881 × 3556 | 3515 × 3556 | 3509 × 3556 | 3496 × 3556 | 3519 × 3556 | 3513 × 3556 |
| Y40C | 3511 × 3525 | 3500 × 3525 | 3497 × 3525 | 3517 × 3525 | 3881 × 3525 | 3515 × 3525 | 3509 × 3525 | 3496 × 3525 | 3519 × 3525 | 3513 × 3525 |
| Y40K | 3513 × 3809 | 3553 × 3513 | 3554 × 3513 | 3517 × 3540 | 3880 × 3513 | 3515 × 3540 | 3509 × 3540 | 3556 × 3513 | 3525 × 3513 | 3513 × 3540 |
All numbers are DLY strains listed in Table 1.
Quantitation of Cdc42p Expression in Yeast
Centromeric plasmids directing expression of CDC42 alleles were transformed into the cdc42-1 strain DLY680. Cdc42-1p is expressed at very low levels (Ziman et al., 1991; Kozminski et al., 2000), so that detectable signals by Western blot represent the abundance of Cdc42p expressed from the plasmid. Strains were grown in YEPD at 24°C, so that Cdc42-1p was able to provide the functions necessary for viability and the blot reflects the abundance of Cdc42p effector-loop variants in proliferating cells. Yeast cells were then harvested by centrifugation and protein extracts were prepared by resuspending the pellets in NP-40 lysis buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 5 mM EDTA, 1% NP-40, 1 mM sodium pyrophosphate, 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate, and 2 μg/ml each of pepstatin A and leupeptin [Sigma, St. Louis, MO]) and vortexing with acid-washed glass beads. Lysates were clarified by centrifugation for 10 min at 14,000 rpm in an Eppendorf microfuge at 4°C. Protein concentration was determined by the Bradford method (Bio-Rad, Hercules, CA), and equal amounts of total protein were resolved by SDS-PAGE and immunoblotted using polyclonal rabbit anti-Cdc42p antibody (diluted 1/500) kindly provided by Patrick Brennwald, and horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (diluted 1/2000). As a loading control, filters were subsequently incubated with monoclonal anti-PSTAIRE antibody (Yamashita et al., 1991) (ascites preparation diluted 1/20000), which recognizes both Cdc28p and Pho85p in yeast, and horseradish peroxidase-conjugated goat anti-mouse secondary antibody (diluted 1/2000). Blots were developed using the Renaissance Chemiluminescence Reagent Plus (PerkinElmer Life Science Products, Boston, MA).
Immunofluorescence and Other Microscopic Analysis
Overall cell morphologies were examined by differential-interference-contrast microscopy, and cells were stained with 4,6-diamidino-2-phenylindole (Sigma) to visualize DNA (Pringle, 1991), with 10 μg/ml Calcofluor (Sigma) to visualize chitin (Pringle, 1991), or with rhodamine-phalloidin (Molecular Probes, Eugene, OR) to visualize F-actin (Bi et al., 1998). To visualize septins, cells were fixed by addition of formaldehyde (3.7% final concentration) to the medium and incubated for 75 min at 30°C before processing for immunofluorescence as described previously (Pringle, 1991). Rabbit anti-Cdc11p antibody (Santa Cruz Biotechnology, Santa Cruz, CA) was used at 1/10 dilution and Cy2-conjugated goat anti-rabbit secondary antibody was used at a 1/100 dilution (Jackson Immunoresearch Laboratories, West Grove, PA). To localize Cdc42p, cells were fixed for 2.5 h in as described (Lehman et al., 1999). Fixed cells were incubated with 0.5% SDS and processed for immunofluorescence as described (Redding et al., 1991). Anti-Cdc42p antibody (used at 1/100 dilution) was generously provided by Patrick Brennwald. Cells were examined using a Zeiss Axioscop. Images were captured using a Pentamax cooled charge-coupled device camera (Princeton Instruments, Princeton, NJ), interfaced with MetaMorph software (Universal Imaging, Silver Spring, MD).
Production of Recombinant Proteins and Binding Assays
Plasmids directing expression of myc-tagged cdc42 alleles were transformed into Escherichia coli BL21(DE3) (Stratagene) and GST-tagged effectors were transformed into E. coli BL21. Extracts were prepared as described previously (Moskow et al., 2000). To ensure that binding reactions contained equal amounts of each effector-loop variant, titration series of each bacterial extract containing Cdc42p-myc were resolved by SDS-PAGE, transferred to Immobilon-P nylon membrane (Millipore, Bedford, MA), and immunoblotted with monoclonal anti-myc antibodies (9E10; Santa Cruz Biotechnology) by using standard procedures (Ausubel et al., 1995). Lysate concentrations were then adjusted so that equal amounts of each Cdc42p-myc variant were added to the binding reactions. GST-effectors were purified using glutathione Sepharose 4B (Amersham Pharmacia Biotech, Piscataway, NJ), and the beads were incubated together with the normalized bacterial extracts containing Cdc42p-myc, washed, and analyzed to detect bound Cdc42p as described previously (Moskow et al., 2000), except that 125 mM NaCl was added to the wash buffer. A modification of this strategy was used for Cla4p because the GST-tagged Cla4p CRIB domain did not display specific binding to myc-tagged Cdc42p. In this case the procedure was reversed and GST-tagged Cdc42p variants were immobilized on beads and incubated with bacterial lysates expressing myc-tagged Cla4p CRIB domain. GST-tagged protein concentrations were normalized using the Bradford assay (Bio-Rad). In all cases, india ink staining of the blots confirmed that equal amounts of GST-tagged proteins were present in each set of binding assays.
RESULTS
Generation of Effector-Loop Mutants of CDC42
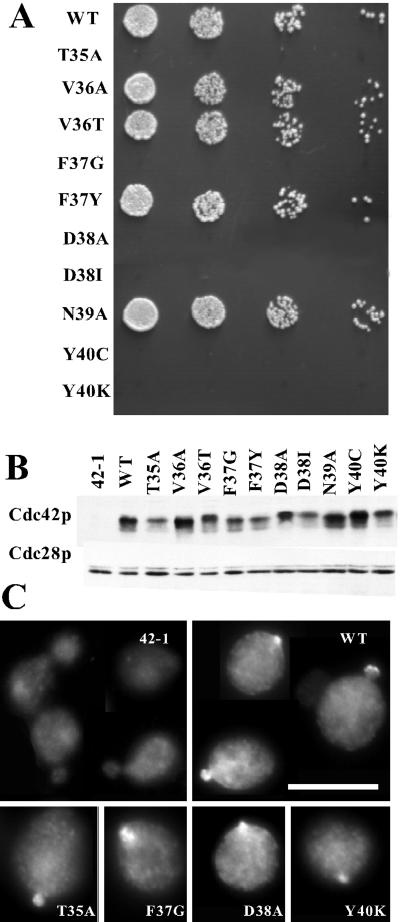
The effector loop comprises the “Switch I” region (residues 30–40) of Cdc42p, one of two regions that assume different conformations depending on whether the protein is GDP- or GTP-bound (Wittinghofer and Nassar, 1996). The most commonly used “effector” mutation, altering Thr35 to Ala, is thought to block binding of all effectors to small G proteins and affects coordination of the Mg2+ ion in the complex (Pai et al., 1990). Other mutations in this region of Ras were shown to partially attenuate the transforming potential of oncogenic Ras by crippling specific downstream pathways (Joneson and Bar-Sagi, 1997). Subsequent studies on Rho, Rac, and Cdc42p confirmed that similar selective effects could be obtained by mutating this region in members of the Rho subfamily (Diekmann et al., 1995; Nobes and Hall, 1995; Lamarche et al., 1996). Based on the available literature we generated the T35A mutant and nine more mutants (Figure 1) by site-directed mutagenesis. Initially, we introduced a single copy of each mutant under control of the CDC42 promoter into the genome of a strain containing a second copy of (wild-type) CDC42 under control of the regulatable GAL1 promoter (see MATERIALS AND METHODS for details). Growth of this strain in dextrose-containing medium promotes repression of the GAL1 promoter, permitting analysis of the phenotypes of strains expressing only the mutant forms of Cdc42p. Control experiments showed that wild-type Cdc42p was depleted within 10 generations of a shift from galactose-to dextrose-containing medium, and cells lacking a second copy of CDC42 arrested uniformly as large, round, unbudded cells with depolarized actin and no assembled septin structures (similar to the cdc42-1 arrest phenotype at the restrictive temperature [Adams et al., 1990]). Of the 10 effector-loop alleles, four were able to sustain cell proliferation when present as the only expressed copy of CDC42, whereas six others were not (Figure 1A). The inviability of these six mutants was recessive to wild type (our unpublished results), consistent with impairment of the function of at least one essential effector pathway. In addition, the inviability of the mutants was not suppressed by incubation at low temperature (14°C) or in media of elevated osmolarity (supplemented with 1 M sorbitol or 1 M NaCl) (our unpublished results). None of the other four mutants were temperature-sensitive (37°C) or cold-sensitive (14°C) for viability and only cdc42V36T grew somewhat slower than wild type at 37°C. The phenotypes of these mutants were not significantly enhanced or suppressed on media with elevated osmolarity (our unpublished results).
Figure 1.
Characterization of cdc42 effector-loop alleles. (A) Wild-type (DLY1) and cdc42 effector-loop mutant strains DLY3511 (T35A), DLY3497 (V36A), DLY3500 (V36T), DLY3517(F37G), DLY3881 (F37Y), DLY3515 (D38A), DLY3509 (D38I), DLY3496(N39A), DLY3519 (Y40C), and DLY3513 (Y40K) were grown in YEPD at 30°C for 24 h to deplete wild-type Cdc42p expressed from the GAL1 promoter. Cells were counted with a hemacytometer and fivefold serial dilutions were spotted onto a YEPD plate, which was incubated for 36 h at 30°C. (B) Strain DLY680 (cdc42-1) was transformed with plasmids expressing the indicated cdc42 alleles pMOSB55 (WT), pMOSB186 (T35A), MOSB177(V36A), pMOSB57(V36T), pMOSB40 (F37G), pMOSB58 (F37Y), pMOSB50(D38A), pDLB1323 (D38I), pMOSB59(N39A), pMOSB176 (Y40C), and pMOSB41 (Y40K). Cells were grown to exponential phase in YEPD at 24°C. Lysates were prepared and analyzed by immunoblotting with anti-Cdc42p or anti-PSTAIRE antibodies (to control for loading). (C) The same strains used in B were grown to exponential phase in YEPD at 24°C and processed to visualize localization of Cdc42p. Bar, 10 μm.
To determine the level of expression of the Cdc42p variants encoded by the effector-loop alleles, we expressed each variant in a cdc42-1 strain. It has been shown that despite its ability to sustain cell proliferation at 24°C, Cdc42-1p is expressed at very low levels (Ziman et al., 1991; Kozminski et al., 2000), providing a very low background signal (Figure 1B). Most of the effector-loop variants were expressed at approximately similar levels to the wild type (Figure 1B), indicating that phenotypic differences are unlikely to stem simply from differences in expression level.
Wild-type Cdc42p is concentrated at the bud site and at the bud tips of cells with small buds (Ziman et al., 1993). To determine whether the Cdc42p variants encoded by the effector-loop alleles were able to localize to these sites we again expressed the variants in a cdc42-1 strain, providing a very low background in which localization of other Cdc42p variants could be detected (Figure 1C). Perhaps surprisingly, all of the Cdc42p variants could be found localized at the bud site and at the bud tips at 24°C (Figure 1C). This suggests that effector interactions are not critical for Cdc42p localization. However, polarity functions provided by Cdc42-1p may contribute to localization of the other Cdc42p variants in these strains. In addition, we only detected Cdc42p staining in a minority of cells even for wild-type Cdc42p, and it appeared that signals from the variants might be qualitatively less frequent and/or intense.
Phenotypic Characterization of Effector-Loop Mutants
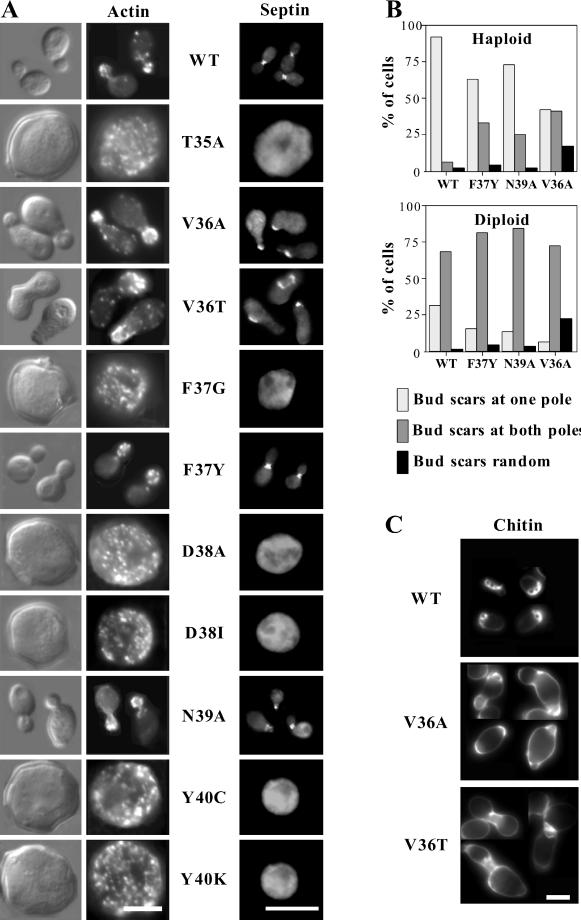
Polarization of yeast cells before budding is triggered by activation of the cyclin-dependent kinase Cdc28p by G1 cyclins (Lew and Reed, 1993). Upon Cdc28p activation, Cdc42p becomes concentrated beneath a patch of plasma membrane at the presumptive bud site (Ziman et al., 1993). At about the same time, the actin cables become oriented, cortical actin patches are clustered, septins (filament-forming proteins that remain at the mother-bud neck during subsequent bud growth) assemble into a ring, and several other proteins including the “polarisome” components Bni1p and Spa2p congregate in a patch at the prebud site (Pringle et al., 1995; Johnson, 1999). All of these polarized distributions require Cdc42p activity; conversely, Cdc42p polarization does not require F-actin, assembled septins, or polarisome components. In addition, the F-actin, septin, and polarisome reorganizations are mutually independent: elimination of one does not affect polarization of the others (Pringle et al., 1995; Ayscough et al., 1997). These findings suggest that Cdc42p promotes the independent polarization of several cytoskeletal structures, perhaps through separate effector pathways. At least two of these polarization targets (F-actin and septins) are essential for yeast viability. We examined the phenotypes of the effector-loop cdc42 mutants with respect to cell morphology, F-actin organization, and septin organization after depletion of the wild-type Cdc42p on dextrose medium (Figure 2). These are described below, going from least to most severe.
Figure 2.
Phenotype of cdc42 effector-loop alleles. (A) Cell morphology, F-actin and septin distribution in cdc42 effector-loop mutants. DLY1 (WT), DLY3511 (T35A), DLY3497 (V36A), DLY3500 (V36T), DLY3517 (F37G), DLY3881 (F37Y), DLY3515 (D38A), DLY3509 (D38I), DLY3496 (N39A), DLY3519 (Y40C), and DLY3513 (Y40K) strains were grown in YEPD at 30°C for 36 h, fixed, and stained with rhodamine-phalloidin (actin) or anti-Cdc11p (septin) as described in MATERIALS AND METHODS. Bar, 5 μm. (B) Bud-site selection in cdc42 effector-loop mutants. Haploid DLY1 (WT), DLY3881 (F37Y), DLY3496 (N39A), and DLY3497 (V36A) strains and diploid DLY5 (WT), DLY4756 (F37Y), DLY4757 (N39A), and DLY3923 (V36A) strains were grown to exponential phase in YEPD at 30°C, and stained with calcofluor to visualize bud scars. Cells with two or more bud scars were scored as having scars at only one pole (expected for axial or bipolar budding), at both poles (expectedfor bipolar budding), or distributed randomly. (C) Chitin deposition in V36A and V36T mutants. Haploid DLY1 (WT), DLY3497 (V36A), and DLY3553 (V36T) strains were grown to exponential phase in YEPD at 30°C, and stained with calcofluor to visualize chitin deposition. Bar, 5 μm. Apparent size differences between cells stained for chitin (unfixed), actin (fixed with ethanol), or septins (fixed with formaldehyde) are due to cell shrinkage during fixation.
Cells containing the cdc42F37Y or cdc42N39A alleles were generally wild type with respect to cell morphology, actin organization, and septin organization. At low penetrance (<10% of cells), cdc42N39A cells displayed lumps protruding from the base of the bud or aberrant septin staining, but these defects were subtle. Previous studies indicated that perturbation of actin organization could cause defects in the bipolar pattern of bud site selection observed in diploid cells (Yang et al., 1997), whereas perturbation of septin organization could cause defects in the axial pattern of bud site selection observed in haploid cells (Flescher et al., 1993). We found that cdc42F37Y and cdc42N39A mutant cells displayed normal bipolar bud site selection in diploids but were mildly defective in axial bud site selection in haploids (Figure 2B). Thus, any effector pathways compromised in these mutants are not critical for cytoskeletal organization.
Cells containing the cdc42V36T or cdc42V36A alleles displayed apparently normal actin organization but had wide and misshapen mother-bud necks and poorly organized septins (Figure 2A). These defects were qualitatively similar for both mutants but were more penetrant and more severe in cdc42V36T cells. The most frequent defect was a wider neck (67% of cdc42V36T cells, n = 200), and at lower frequencies knobby, kinked, or stretched necks were observed (often in the same cells that had wider necks). In cells with wide necks, septin staining was generally fainter, patchy, and occasionally even undetectable at the neck, and in some cases septin staining was observed at the tip of the bud (36% of cells, n = 200). Aberrant septin staining was observed for three different septins: Cdc11p (Figure 2A), Cdc3p (detected using anti-Cdc3p antibody or Cdc3p-GFP; data not shown), and Cdc12p (detected using Cdc12p-GFP; data not shown). In addition, calcofluor staining revealed a severe defect in the localization of chitin deposition, which is guided by the underlying septin ring, in cdc42V36T mutants (Figure 2C). These defects included increased staining all over the cell wall, broader and irregular zones of bright staining at the neck, and bright uneven staining in the vicinity of bud scars. Similar but less severe defects were observed in cdc42V36A mutants (Figure 2C), which also displayed a severe axial bud site selection defect (Figure 2B). All of the septin defects were corrected in cdc42V36A/CDC42 and cdc42V36T/CDC42 heterozygotes (our unpublished results), indicating that these are loss-of-function alleles that are defective in pathways important for neck morphology and septin organization.
For each of the six effector-loop alleles that could not sustain cell proliferation, the populations arrested uniformly as large, round, unbudded cells with depolarized actin patches and no detectable assembled septins, similar to the arrest observed in the absence of Cdc42p (Figure 2A). This is thought to be the cdc42 null phenotype, and suggests that each of these alleles has crippled interactions with crucial effectors of Cdc42p.
Effect of Increased Gene Dosage on Phenotype of Effector-Loop Mutants
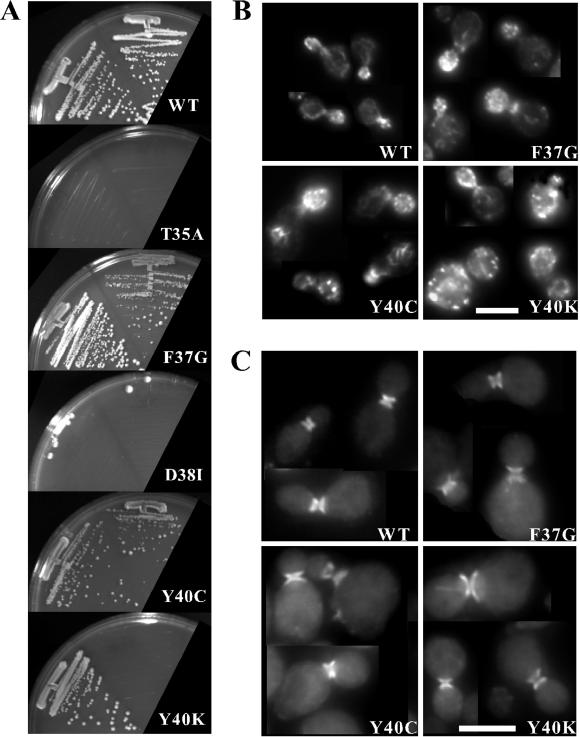
The findings described above seemed rather surprising, in that several mutant alleles that had been described as specific partial function mutants in other G proteins (including in some cases similar alleles of human CDC42 [Lamarche et al., 1996]) appeared to behave as null alleles (Figure 2). However, a significant difference with prior studies was that in this study the alleles were expressed at single copy from the endogenous promoter. In contrast, most previous reports either overexpressed or injected large amounts of proteins encoded by alleles that also contained a second mutation locking the protein in the GTP-bound form. These considerations suggested at least two possible reasons for the apparent discrepancies. First, it seemed possible that the more severe alleles had (in addition to some specific defects in effector interaction) a general defect in GTP-loading, which would render them inactive in our study but not when GTP hydrolysis was inhibited. Second, it seemed possible that the proteins encoded by these alleles might have global, general defects in effector interactions superimposed upon a more specific defect. In either case, assaying mutant phenotypes when the alleles are expressed at single copy would reveal the general defect, whereas overexpression might overcome the global defect but now reveal more specific phenotypic defects. To address this possibility, we subcloned the effector-loop alleles (still expressed from the CDC42 promoter) into low- and high-copy vectors (CEN ARS and 2 μm, respectively).
We found that cdc42Y40C and cdc42F37G were able to sustain some proliferation (albeit poorly) when expressed from a low-copy plasmid, and that proliferation was significantly improved when the alleles were expressed from a high-copy plasmid (Figure 3A). In addition, cdc42Y40K was able to sustain proliferation when expressed from a high-copy plasmid, though not from a low-copy plasmid (Figure 3A). However, cdc42T35A, cdc42D38I, and cdc42D38A were unable to sustain proliferation even when expressed from high-copy plasmids (Figure 3A; data not shown).
Figure 3.
Effect of increased gene dosage on cdc42 effector-loop mutant phenotype. (A) Strain DLY3067 (GAL1p-CDC42) was transformed with low-copy plasmids pMOSB55 (WT), pMOSB186 (T35A), pMOSB40 (F37G), pDLB1323 (D38I), pMOSB176 (Y40C), and pMOSB41 (Y40K), or with high-copy plasmids pDLB1666 (WT), pDLB1656 (T35A), pDLB1659 (F37G), pDLB1662 (D38I), pDLB1664 (Y40C), and pDLB1665 (Y40K). Cells were depleted of GAL1-regulated wild-type Cdc42p by growth on dextrose-containing medium for 30 h, streaked out on dextrose plates lacking uracil and incubated for 72 h at 30°C. Left streaks have high-copy plasmid and right streaks have low-copy plasmid. Actin (B) and septin (C) staining of cells containing the indicated high-copy plasmids grown as described above.
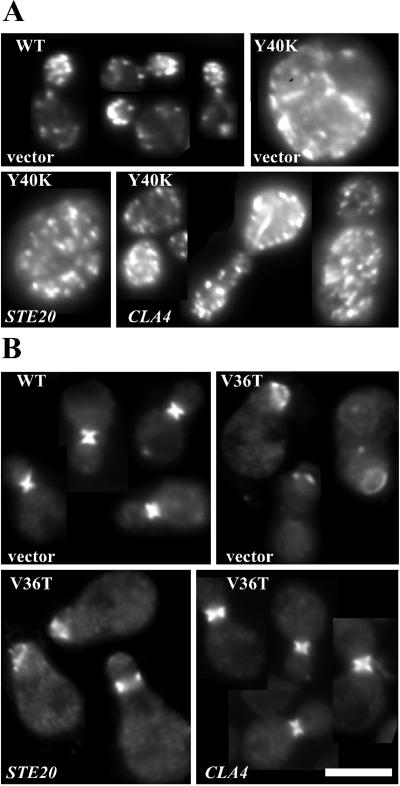
Even when overexpressed, cdc42Y40C, cdc42Y40K, and cdc42F37G mutants displayed severe defects in cell morphology. In all three cases, the mutants were generally larger and rounder than wild-type cells (Figure 3, B and C). About half of the cells containing the high-copy cdc42Y40C plasmid exhibited depolarized actin patches and slightly fainter actin cables, although when visible most actin cables were polarized (Figure 3B). Additionally, knots of actin “ropes” that appeared brighter and thicker than normal actin cables were observed in about half of the budded cells (though these were not correlated with depolarized patches; Figure 3B). Cells containing the high-copy cdc42Y40K displayed a more severe depolarization of actin patches (73% of cells, n = 200), but ropes were not detected (Figure 3B). Both the high-copy cdc42Y40C (40% of unbudded cells, n = 131) and the high-copy cdc42Y40K (29% of unbudded cells, n = 148) cell populations contained binucleate or multinucleate cells as judged by 4,6-diamidino-2-phenylindole staining. Septin organization in cells containing high-copy cdc42Y40C or high-copy cdc42Y40K plasmids was relatively normal: the septin rings were always localized to the neck, but they sometimes appeared broader than in wild-type cells (Figure 3C). Cells expressing high-copy cdc42F37G generally contained polarized actin patches (80% of cells, n = 200) and cables, though the cables appeared fainter than in wild-type cells (Figure 3B). These cells also had fewer multinucleate cells (15% of unbudded cells, n = 125). Septin rings were localized to the neck, but were frequently faint (49% of the cells, n = 200) and the septin ring often had diffuse tendrils of septin staining extending out from the ring perpendicular to the neck (30% of the cells, n = 200; Figure 3C). Such tendrils were not observed in the cdc42Y40C and cdc42Y40K strains. Thus, at high gene dosage cdc42Y40C and cdc42Y40K show relatively specific defects in actin organization, whereas cdc42F37G shows more subtle defects in both actin and septin organization.
Intragenic Complementation among Effector-Loop Alleles
We generated heterozygous diploid strains containing all possible pairs of cdc42 effector-loop alleles (see MATERIALS AND METHODS) to ask whether they had defects in separate or overlapping functions of Cdc42p. Heterozygotes containing the pseudo wild-type alleles cdc42F37Y and cdc42N39A together with any other allele appeared wild type, as expected (our unpublished results). Heterozygotes containing the two alleles displaying neck organization defects (cdc42V36A and cdc42V36T) were phenotypically similar to homozygotes for the milder allele (cdc42V36A), indicating that these alleles affect similar pathways (our unpublished results).
All of the heterozygotes between severe effector-loop alleles (cdc42T35A, cdc42F37G, cdc42D38I, cdc42D38A, cdc42Y40C, or cdc42Y40K) arrested with a similar phenotype to the starting haploids (large, round, unbudded cells lacking polarized actin or assembled septins; our unpublished results). This result suggests that all of these alleles share at least one common defect. Even when expressed at high copy, we failed to detect a significant improvement in the phenotype of cells containing pairs of these alleles compared with cells containing the milder allele on its own (our unpublished results). Thus, even the apparently more specific phenotypes exhibited by cells containing high-copy cdc42F37G, cdc42Y40C, or cdc42Y40K plasmids do not seem to arise from defects in cleanly separable pathways.
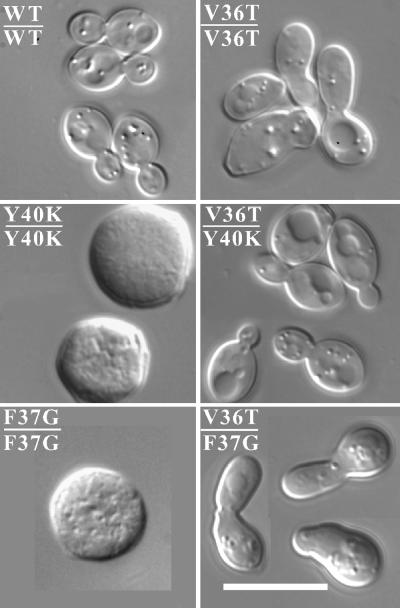
Strikingly, we found that cdc42V36A/cdc42Y40C, cdc42V36T/cdc42Y40C, cdc42V36A/cdc42Y40K, and cdc42V36T/cdc42Y40K heterozygotes appeared fully wild type with respect to growth, cell and mother-bud neck morphology, actin organization, and septin organization (Figure 4; our unpublished results). Thus, even though cdc42Y40C and cdc42Y40K were unable to promote septin organization on their own at single copy (Figure 2A), they apparently retained the ability to activate the effector pathway(s) impaired in the cdc42V36A and cdc42V36T mutants. In contrast, cdc42F37G did not complement these defects when present at single copy, and only mildly ameliorated the cdc42V36T neck defect when present at high copy, promoting slightly more narrow but still abnormal necks. cdc42T35A, cdc42D38I, and cdc42D38A were unable to rescue the neck defect of cdc42V36A and cdc42V36T mutants even when present at high copy (our unpublished results).
Figure 4.
Intragenic complementation between cdc42Y40K and cdc42V36T. Cell morphology of diploid strains DLY5 (WT/WT), DLY3924 (V36T/V36T), DLY3572 (Y40K/Y40K), DLY3895 (V36T/Y40K), DLY3571 (F37G/F37G), and DLY3891 (V36T/F37G) grown in YEPD at 30°C for at least 36 h. Bar, 5 μm.
Binding of Cdc42p Variants Encoded by Effector-Loop Alleles to Putative Effectors
There are five genes in the yeast genome encoding proteins that contain a CRIB domain and are presumed to be Cdc42p effectors. CRIB domains also bind to Rac-family proteins (Burbelo et al., 1995) but yeast does not contain a Rac representative, so it seems likely that in yeast Cdc42p is the sole small GTPase that binds to this domain. Three of the effectors are p21-activated kinases (Cla4p, Ste20p, and Skm1p) (Leberer et al., 1992; Cvrckova et al., 1995; Martin et al., 1997) and the other two (Gic1p and Gic2p) are homologous proteins that do not possess obvious enzymatic activity (Brown et al., 1997; Chen et al., 1997). Cla4p is required for normal septin organization (Longtine et al., 2000) and shares an essential function in cell polarization with Ste20p (Cvrckova et al., 1995; Holly and Blumer, 1999). Ste20p also plays a specific role in the pheromone response and pseudohyphal differentiation pathways (Leberer et al., 1992, 1997; Peter et al., 1996), whereas Skm1p does not appear to play a major role in any of the pathways yet examined (Martin et al., 1997). Gic1p and Gic2p share an important (though not essential) role in cell polarization, particularly at elevated temperatures (Brown et al., 1997; Chen et al., 1997; Bi et al., 2000). The scaffold protein Bem1p is also important for polarization (Bender and Pringle, 1991), and binds directly to GTP-Cdc42p (but not GDP-Cdc42p [Bose et al., 2001]), raising the possibility that it also acts as an effector. Although several other proteins have been implicated as possible Cdc42p effectors in yeast (Evangelista et al., 1997; Bi et al., 2000), direct binding to Cdc42p has yet to be demonstrated.
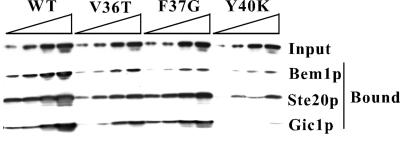
To ask whether the Cdc42p effector-loop variants were defective in binding to the known Cdc42p effectors, we assayed the ability of bacterially expressed Cdc42p variants to interact with bacterially expressed effector CRIB domains or to full-length Bem1p (see MATERIALS AND METHODS). With one exception, the variants showed binding defects whose severity was correlated with the severity of the phenotypic defect (Figure 5 and Table 5). The pseudo wild-type Cdc42pF37Y and Cdc42pN39A bound as well as the wild type to all of the effectors tested. All of the other variants displayed binding deficits that were not limited to a single effector. The variants displaying neck organization defects, Cdc42pV36A and Cdc42pV36T, were severely impaired in binding to Cla4p and mildly impaired in binding to Bem1p, Gic1p, Gic2p, and Ste20p (in all cases the binding defect, like the phenotypic defect, was a little more severe for Cdc42pV36T than Cdc42pV36A). Surprisingly, Cdc42pF37G, which at single copy was unable to promote either actin polarization or septin organization, displayed a similar profile of binding defects, being slightly more impaired in binding to Cla4p but less defective in binding to Ste20p and Gic2p. The fact that Cdc42pF37G displayed generally milder binding defects but much stronger phenotypic defects than Cdc42pV36T suggests that other important effectors exist whose binding is more affected by Cdc42pF37G than by Cdc42pV36T.
Figure 5.
Binding of Cdc42p variants encoded by effector-loop mutants to known Cdc42p effectors. Plasmids pDLB1305 (Bem1p), pMOSB240 (Ste20p), pDLB1127 (Gic1p), pDLB1238 (WT), pDLB1241 (V36T), pDLB1282 (F37G), or pDLB1243 (Y40K) were used to express recombinant GST-tagged effector domains or myc-tagged Cdc42p variants in bacteria. Increasing amounts (twofold increases going from left to right) of lysates containing the myc-tagged Cdc42p variants (“input”, top) were incubated with constant (excess) amounts of the indicated GST-effectors immobilized on beads, and the amount of myc-Cdc42p remaining bound after washing was determined by immunoblotting.
Table 5.
Binding of Cdc42p variants to recombinant effector domainsa
| Ste20p | Cla4p | Gic1p | Gic2p | Bem1p | |
|---|---|---|---|---|---|
| WTb | ++ | ++ | ++ | ++ | ++ |
| D57Y | − | − | − | − | − |
| T35Ab | − | − | − | − | − |
| V36Ab | + | +/− | + | + | + |
| V36Tb | + | +/− | + | + | + |
| F37Gb | ++ | − | + | ++ | + |
| F37Yb | ++ | ++ | ++ | ++ | ++ |
| D38Ab | + | − | +/− | +/− | +/− |
| D38Ib | − | − | − | − | − |
| N39Ab | ++ | ++ | ++ | ++ | ++ |
| Y40Cb | +/− | +/− | + | +/− | + |
| Y40Kb | +/− | − | − | − | +/− |
Binding was evaluated as >70% of wild-type (++), 30–70% of wild-type (+), <30% of wild-type (+/−), or undetectable (−).
All recombinant Cdc42p proteins, except D57Y, had Q61L substitutions to prevent GTP hydrolysis.
Cdc42pY40C was severely impaired in binding to Cla4p, Ste20p, and Gic2p, but only mildly impaired in binding to Gic1p and Bem1p, whereas Cdc42pY40K, which displayed similar but more severe phenotypic defects, was severely impaired in binding to all effectors. Cdc42pD38A was severely impaired for binding to all effectors except Ste20p. Cdc42pT35A and Cdc42pD38I did not exhibit detectable binding to any of the tested effectors, consistent with their behavior as null alleles in all of the genetic analyses. The finding that Cdc42pY40K displayed across-the-board binding defects (Figure 5 and Table 1) was unexpected because the intragenic complementation results (Figure 4) had suggested that Cdc42pY40K could activate effector pathways that were not engaged by Cdc42pV36T.
Suppression of Effector-Loop cdc42 Mutants by Overexpression of Effectors
If the phenotypic defect of a cdc42 mutant results from impaired binding to a particular effector, then increasing the abundance of that effector might suppress the phenotype. To determine whether this was the case for any of the effector-loop mutants, we transformed high-copy plasmids expressing CLA4, STE20, GIC1, GIC2, BEM1, or BNI1 (a formin-homology protein that has been implicated as a possible Cdc42p effector for cell polarization during mating [Evangelista et al., 1997]) into mutant strains expressing the severe cdc42 mutants on low-copy plasmids (Table 6 and Figure 6A), or the mild cdc42 mutants at single copy (Figure 6B; data not shown). Strikingly, we found that overexpression of Cla4p was able to partially suppress the growth defect of all of the alleles except the apparently null cdc42T35A. Similarly, overexpression of Bem1p could partially suppress the growth defect of all of the alleles except cdc42T35A and cdc42D38I (Table 6). Suppression was not complete, because even the cells from strains exhibiting robust growth still displayed significant actin patch depolarization and morphological abnormalities (Figure 6A; data not shown). In addition, overexpression of either Cla4p or Bem1p effectively suppressed the neck morphology and septin organization defects of the cdc42V36A and cdc42V36T mutants (Figure 6B; data not shown).
Table 6.
Suppression of cdc42 mutant growth defect by overexpressed effectorsa
| STE20 | CLA4 | GIC1 | GIC2 | BEM1 | BNI1 | CDC24 | vector | |
|---|---|---|---|---|---|---|---|---|
| T35A | − | − | − | − | − | − | − | − |
| F37G | + | ++ | +/− | +/− | ++ | +/− | +/− | +/− |
| D38A | − | + | − | − | + | − | − | − |
| D38I | − | +/− | − | − | − | − | − | − |
| Y40C | + | ++ | + | ++ | ++ | +/− | +/− | +/− |
| Y40K | − | + | − | − | + | − | − | − |
aDLY3067 was transformed with the low-copy pMOSB46 (T35A), pMOSB48 (F37G), pMOSB49 D38I), pMOSB50 (D38A), pMOSB51 (Y40K), and pMOSB175 (Y40C) plasmids, and then with the high-copy pDLB678 (BEM1), pDLB722 (CLA4), pDLB723 (STE20), pDLB935 (CDC24), pDLB1308 BNI1), pMOSB229 (GIC1), pMOSB230 (GIC2), or YEp 24 (vector) plasmids. Growth on dextrose medium was classified as robust (++), slow (+), very poor (+/−), or none (−).
Figure 6.
Suppression of cdc42 effector-loop mutants by overexpression of CLA4. DLY1 (WT), DLY3513 (Y40K), and DLY3500 (V36T) strains were transformed with high-copy plasmids YEp24 (vector), pDLB722 (CLA4), or pDLB723 (STE20) as indicated and grown in dextrose medium lacking uracil at 30°C. (A) Cells were stained with rhodamine-phalloidin to visualize F-actin. (B) Cells were stained with anti-Cdc11p antibody to visualize septins. Bar, 5 μm.
In contrast to the general suppression conferred by Cla4p and Bem1p, other effectors were more restricted and less effective in their actions. Overexpression of Gic1p, Gic2p, or Ste20p weakly suppressed the growth defect of cdc42Y40C mutants, whereas overexpression of Ste20p weakly suppressed the growth defect of cdc42F37G mutants (Table 6). We did not observe suppression of any other cdc42 mutant by these effectors, and none of the mutants was suppressed by overexpressed Bni1p or Cdc24p (Table 6).
DISCUSSION
Comparison with Other Studies of Effector-Loop Mutants of Small GTPases
Previous studies conducted using effector-loop mutants in mammalian GTPases have examined the effects of the mutations on the dramatic and sometimes lethal phenotypes (e.g., oncogenic transformation or cytoskeletal derangement) induced upon overexpression of GTP-locked forms of the GTPases. Phenotypic differences in the mutants were then correlated with biochemical defects in binding to recombinant effectors in vitro, helping to elucidate the signaling pathways involved. Our strategy for characterizing the cdc42 effector-loop mutants in yeast sought to combine the advantages of this approach with the genetic tractability of the yeast system. In particular, we characterized mutant phenotypes in the context of non-GTP-locked variants, and examined recessive defects by replacing the endogenous wild-type CDC42 with the mutant alleles. One surprising result of this analysis was that several of the mutants displayed an essentially null phenotype rather than the expected specific defect in one or two pathways. In at least one case (cdc42Y40C), the very same allele of mammalian CDC42 had been reported to display specific defects in p21-activated kinase activation while retaining the ability to alter cytoskeletal behavior and promote cell cycle progression (Lamarche et al., 1996). It is possible that different effectors mediate cytoskeletal control pathways in yeast and mammalian cells, because many of the putative Cdc42p effectors identified in mammals (Van Aelst and D'Souza-Schorey, 1997) have no clear homologues in yeast, where only CRIB-domain containing proteins have been clearly established as Cdc42p effectors. However, another factor likely to contribute to the difference is the level of expression of the mutant forms of Cdc42p. We found that for several alleles, elevated expression suppressed the phenotypic defect; for instance, cdc42Y40C was able to sustain proliferation, to polarize actin (albeit imperfectly), and to organize septins when expressed at higher levels. This finding suggests that the defects due to effector-loop mutants may be less specific than previously appreciated, and that overexpression studies may reveal only the most critically affected pathways.
While this work was in progress, an overlapping set of cdc42 mutants was generated by Kozminski et al. (2000). That study is complementary to ours in that we report more in-depth analysis of mutant phenotypes and effector interactions, whereas they report a considerably broader spectrum of mutants, the majority of which lie outside of the effector loop. The two studies are in agreement on the lethality of several mutants when expressed at single copy, but report some differences in the phenotype of the milder cdc42V36T allele (discussed in more detail below), which are likely due to strain background differences. Very recently, Richman and Johnson (2000) also reported the generation of effector-loop mutants of CDC42 in yeast, focused on characterization of a novel mutant, cdc42D38E, that produced a phenotype distinct from any of the mutants reported here. Specifically, that mutant had an apparent defect in maintaining polarization during bud growth, leading to the frequent abandonment of small buds followed by repolarization toward new sites (Richman and Johnson, 2000). The availability of a much wider range of cdc42 mutants should accelerate progress in understanding the pathways whereby Cdc42p controls cell polarity in yeast. Finally, using a random mutagenesis approach to investigate the role of Cdc42p in the pheromone-stimulated signal transduction pathway, we recently identified effector-loop mutations implicating Ste20p and Bem1p as the relevant effectors of Cdc42p in that pathway (Moskow et al., 2000).
Phenotypes of cdc42 Effector-Loop Mutants
When expressed at elevated levels, cdc42Y40C and cdc42Y40K were able to sustain proliferation, but displayed considerably reduced polarization of actin patches (more severe for cdc42Y40K than cdc42Y40C) and frequent generation of multinucleate cells (more severe for cdc42Y40C than cdc42Y40K). These phenotypes are reminiscent of those in null mutants lacking the polarity establishment proteins Bem1p or Bem2p (Bender and Pringle, 1991; Pringle et al., 1995), and may indicate a severe defect in promoting proper actin polarization. Interpretation of a “depolarized actin patch” phenotype is complicated by the recent finding that actin patch depolarization is induced by plasma membrane or cell wall stress-signaling pathways involving Pkc1p (Delley and Hall, 1999). However, the proportion of cells with depolarized patches was only reduced by 20–30% when these strains were grown in osmotically stabilized media (containing 1 M sorbitol, which suppresses cell wall defects and Pkc1p pathway activation [Kamada et al., 1995]), suggesting that most of the patch delocalization is due to a primary defect in actin organization (our unpublished results).
In addition to actin patch depolarization, cdc42Y40C mutant cells frequently contained actin “ropes,” thicker than normal cables and generally forming knotted clumps within the mother portion of budded cells. Because these structures were observed using phalloidin, we assume that they consist of polymerized actin and most likely represent aberrant cables. Because actin cables play a role in spindle orientation and nuclear segregation (Theesfeld et al., 1999), these aberrant cables may contribute to the increased frequency of binucleate and multinucleate cells in the cdc42Y40C population. This is a novel cdc42 phenotype that may indicate a role for Cdc42p in regulating the cross-linking or assembly dynamics of actin cables.
Two other alleles, cdc42V36A and cdc42V36T, were able to sustain proliferation even when expressed at single copy, but displayed aberrant morphology of the mother-bud neck accompanied by defects in septin localization and associated defects in chitin deposition and bud site selection (more severe for cdc42V36T than cdc42V36A). These phenotypes suggest a primary defect in septin organization, although they do not exclude the possibility (previously suggested for ste20Δ cla4-Ts mutants [Cvrckova et al., 1995]) that a primary defect in some other aspect of neck organization produces secondary effects on septin localization and function.
The cdc42V36T mutant was also analyzed by Kozminski et al. (2000), who did not examine septin localization or mention neck morphology but did report that the mutants exhibited elongated buds and hyperpolarized actin patches. They concluded that this mutant had a primary defect in the switch from apical-to-isotropic growth, which is associated with a depolarization of actin patches within the bud (Kozminski et al., 2000). However, examination of the cdc42V36T cells in that report suggests that they also have defects in neck morphology similar to those observed in our strain background. Furthermore, recent studies (Barral et al., 1999; Longtine et al., 2000) indicate that defects in septin organization frequently trigger a Swe1p-dependent G2 delay that delays the apical-isotropic switch and leads to bud elongation, raising the possibility that the bud elongation observed by Kozminski et al. (2000) was a secondary consequence of altered septin organization. In this context, it is noteworthy that another mutant, cdc42V44A, was recently reported to show both septin organization defects and elongated buds, and in that case the bud elongation was shown to be Swe1p-dependent (Richman et al., 1999). We did not observe significant bud elongation in cdc42V36T cells in our strain background, but previous observations suggest that mutants causing increased Swe1p activity (e.g., hsl1Δ or hsl7Δ mutants [McMillan et al., 1999]) display much weaker elongated-bud phenotypes in this strain background. Thus, we suggest that the primary defect in cdc42V36T mutants lies in a pathway important for septin or neck organization, which in some strain backgrounds gives rise to a secondary Swe1p-dependent bud elongation.
Biochemical analysis indicated that cdc42V36T mutants were defective in binding to the Cla4p CRIB domain, and cla4Δ mutants display defects in septin organization (Cvrckova et al., 1995; Longtine et al., 2000), raising the possibility that the neck defects of this mutant are due to impairment of Cla4p function. However, other mutants (cdc42Y40C and cdc42Y40K) that also failed to bind to the Cla4p CRIB domain were nevertheless effective in complementing the cdc42V36T defect. It remains unclear whether the Cdc42p–Cla4p interaction is important for the role of Cla4p in septin organization, and if so whether defects in a Cla4p-mediated pathway are important for the cdc42V36T phenotype.
Evidence for Existence of Further Cdc42p Effectors
By the criterion of functional complementation, the cdc42V36A and cdc42V36T mutants are defective in separate pathways from those that are defective in cdc42Y40C and cdc42Y40K mutants. It was particularly surprising to find that cdc42V36T/cdc42Y40K diploids appeared fully wild type, for two reasons. First, homozygous diploid cells containing two copies of cdc42Y40K were unable to polarize actin or assemble septins and arrested with a characteristic cdc42 null phenotype, yet even at single copy (i.e. one of the two copies in the heterozygote) cdc42Y40K was able to correct the neck defect of cdc42V36T mutants. Second, whereas Cdc42pV36T retained the ability to interact with most of the effectors we tested (Ste20p, Gic1p, Gic2p, Bem1p) at a level only mildly reduced from the wild type, interaction between the effectors and Cdc42pY40K was significantly more impaired in every case. This suggests that none of these effectors is responsible for the complementing activity of Cdc42pY40K, and therefore that a novel effector(s) important for septin or neck organization exists.
The cdc42F37G mutant, at single copy, was also unable to polarize actin or assemble septins and arrested with a characteristic cdc42 null phenotype. However, with the possible exception of Cla4p, Cdc42pF37G bound at least as well to all tested effectors as did Cdc42pV36T. This suggests that none of these effectors accounts for the lethal phenotypic defect of the cdc42F37G mutant, and therefore that a novel effector(s) important for actin polarization and bud formation exists. However, it is also possible that in vitro binding does not accurately reflect productive in vivo interaction. For instance, it may be that interaction of Cdc42pF37G with Ste20p fails to activate Ste20p kinase activity, whereas interaction of Cdc42pV36T with Ste20p, although reduced, can activate Ste20p kinase activity. In that case, the more severe phenotype of cdc42F37G mutants might reflect the reduced productivity of its interactions with known effectors rather than its inability to interact with other (hypothetical) effectors.
Role of Cla4p and Bem1p in Cell Polarity
One remarkable result to emerge from this work is that overexpression of Cla4p or Bem1p was able to partially suppress the phenotypic defects of almost every single effector-loop mutant (with the notable exception of cdc42T35A, which is thought to prevent all effector binding). This is particularly striking because the different alleles affected genetically separable pathways (as discussed above) and displayed distinct phenotypes and effector-binding profiles. In the Kozminski et al. (2000) study Cla4p overexpression did not globally suppress temperature-sensitive growth defects of several mutants, and Bem1p overexpression was not tested. Conceivably, our mutants encompass a special set of alleles that is particularly susceptible to suppression by Cla4p, whereas those investigated by Kozminski et al. (2000) are not. However, the effectiveness of suppression may well depend upon the level of Cla4p expression, and both we (our unpublished results) and Kozminski et al. (2000) have found that excessive Cla4p expression is highly deleterious even in otherwise wild-type cells. This raises the possibility that the stronger expression (driven by the GAL1/10 promoter) used by Kozminski et al. (2000) may have masked suppression of their mutants.
It seems very unlikely that overexpression of Cla4p or Bem1p is simply restoring adequate levels of Cla4p- or Bem1p interaction to our panel of mutants, because the differences between the mutants appear to preclude interpretation of their defects as being due to the same downstream pathways in every case. It seems more likely that these are instances of “bypass suppression,” i.e., that excess Cla4p or Bem1p can cover for loss of particular Cdc42p-directed pathways by stimulating parallel pathways. However, these parallel pathways would still have to correct several separate defects in the various mutants.
An alternative interpretation of the suppression results is that rather than acting solely downstream of Cdc42p or in parallel with Cdc42p, Cla4p and Bem1p can also act effectively “upstream” of Cdc42p (or at the same level, improving overall Cdc42p function). In this scenario, Cla4p and Bem1p may help to increase the activity of the Cdc42p effector-loop variants, yielding a phenotypic improvement similar to (but more potent than) that observed when the alleles were expressed from high-copy plasmids. Support for this hypothesis comes from recent observations that the scaffold protein Bem1p can bind directly to Cla4p, as well as to GTP-Cdc42p and to the exchange factor Cdc24p, in a complex (Bose et al., 2001). Such a complex may assist Cdc24p function and/or localization, thereby increasing GTP-loading and/or localization of Cdc42p in vivo.
In conclusion, our analysis of effector-loop mutants of CDC42 in yeast has identified partial function alleles displaying some novel cdc42 phenotypes, and has revealed a surprisingly broad role for Bem1p and Cla4p in promoting Cdc42p function. Furthermore, the data suggest that unknown effectors involved in actin and septin organization exist, and the mutants provide a starting point for genetic approaches to identify those effectors.
ACKNOWLEDGMENTS
We thank Alan Bender, Erfei Bi, Mark Longtine, and Matthias Peter for plasmids, and Keith Kozminsky, David Drubin, and Pat Brennwald for kindly providing anti-Cdc42p antibodies. Thanks also to John Pringle and members of the Lew lab for stimulating interactions. J.J.M. was supported by American Cancer Society fellowship PF-98-008-01-CSM. This work was supported by National Institutes of Health Grant GM-53050 and American Cancer Society Grant RPG-98-046-CCG to D.J.L.
REFERENCES
- Adams AEM, Johnson DI, Longnecker RM, Sloat BF, Pringle JR. CDC42 and CDC43, two additional genes involved in budding and the establishment of cell polarity in the yeast Saccharomyces cerevisiae. J Cell Biol. 1990;111:131–142. doi: 10.1083/jcb.111.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams AEM, Pringle JR. Relationship of actin and tubulin distribution to bud growth in wild-type and morphogenetic-mutant Saccharomyces cerevisiae. J Cell Biol. 1984;98:934–945. doi: 10.1083/jcb.98.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current Protocols in Molecular Biology. New York: John Wiley & Sons; 1995. [Google Scholar]
- Ayscough KR, Stryker J, Pokala N, Sanders M, Crews P, Drubin DG. High rates of actin filament turnover in budding yeast and roles for actin in establishment and maintenance of cell polarity revealed using the actin inhibitor latrunculin-A. J Cell Biol. 1997;137:399–416. doi: 10.1083/jcb.137.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral Y, Parra M, Bidlingmaier S, Snyder M. Nim1-related kinases coordinate cell cycle progression with the organization of the peripheral cytoskeleton in yeast. Genes Dev. 1999;13:176–187. doi: 10.1101/gad.13.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender A, Pringle JR. Use of a screen for synthetic lethal and multicopy suppressee mutants to identify two new genes involved in morphogenesis in Saccharomyces cerevisiae. Mol Cell Biol. 1991;11:1295–1305. doi: 10.1128/mcb.11.3.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi E, Chiavetta JB, Chen H, Chen GC, Chan CS, Pringle JR. Identification of novel, evolutionarily conserved Cdc42p-interacting proteins and of redundant pathways linking Cdc24p and Cdc42p to actin polarization in yeast. Mol Biol Cell. 2000;11:773–793. doi: 10.1091/mbc.11.2.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi E, Maddox P, Lew DJ, Salmon ED, McMillan JN, Yeh E, Pringle JR. Involvement of an actomyosin contractile ring in Saccharomyces cerevisiae cytokinesis. J Cell Biol. 1998;142:1301–1312. doi: 10.1083/jcb.142.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi E, Zigmond SH. Actin polymerization: where the WASP stings. Curr Biol. 1999;9:R160–R163. doi: 10.1016/s0960-9822(99)80102-x. [DOI] [PubMed] [Google Scholar]
- Bose, I., Irazoqui, J., Moskow, J.J., Bardes, E.S.G., Zyla, T.R., and Lew, D.J. (2001). Assembly of scaffold-mediated complexes containing Cdc42p, the exchange factor Cdc24p, and the effector Cla4p required for cell cycle regulated phosphorylation of Cdc24p. J. Biol. Chem. (in press). [DOI] [PubMed]
- Brown JL, Jaquenoud M, Gulli MP, Chant J, Peter M. Novel Cdc42-binding proteins Gic1 and Gic2 control cell polarity in yeast. Genes Dev. 1997;11:2972–2982. doi: 10.1101/gad.11.22.2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AM, O'Sullivan AJ, Gomperts BD. Induction of exocytosis from permeabilized mast cells by the guanosine triphosphatases Rac and Cdc42. Mol Biol Cell. 1998;9:1053–1063. doi: 10.1091/mbc.9.5.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbelo PD, Drechsel D, Hall A. A conserved binding motif defines numerous candidate target proteins for both Cdc42 and Rac GTPases. J Biol Chem. 1995;270:29071–29074. doi: 10.1074/jbc.270.49.29071. [DOI] [PubMed] [Google Scholar]
- Chen GC, Kim YJ, Chan CS. The Cdc42 GTPase-associated proteins Gic1 and Gic2 are required for polarized cell growth in Saccharomyces cerevisiae. Genes Dev. 1997;11:2958–2971. doi: 10.1101/gad.11.22.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson TW, Sikorski RS, Dante M, Shero JH, Hieter P. Multifunctional yeast high-copy-number shuttle vectors. Gene. 1992;110:119–122. doi: 10.1016/0378-1119(92)90454-w. [DOI] [PubMed] [Google Scholar]
- Coso OA, Chiariello M, Yu J-C, Teramoto H, Crespo P, Xu N, Miki T, Gutkind JS. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell. 1995;81:1137–1146. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- Cvrckova F, De Virgilio C, Manser E, Pringle JR, Nasmyth K. Ste20-like protein kinases are required for normal localization of cell growth and for cytokinesis in budding yeast. Genes Dev. 1995;9:1817–1830. doi: 10.1101/gad.9.15.1817. [DOI] [PubMed] [Google Scholar]
- Delley PA, Hall MN. Cell wall stress depolarizes cell growth via hyperactivation of RHO1. J Cell Biol. 1999;147:163–174. doi: 10.1083/jcb.147.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekmann D, Nobes CD, Burbelo PD, Abo A, Hall A. Rac GTPase interacts with GAPs and target proteins through multiple effector sites. EMBO J. 1995;14:5297–5305. doi: 10.1002/j.1460-2075.1995.tb00214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelista M, Blundell K, Longtine MS, Chow CJ, Adames N, Pringle JR, Peter M, Boone C. Bni1p, a yeast formin linking Cdc42p and the actin cytoskeleton during polarized morphogenesis. Science. 1997;276:118–122. doi: 10.1126/science.276.5309.118. [DOI] [PubMed] [Google Scholar]
- Flescher EG, Madden K, Snyder M. Components required for cytokinesis are important for bud site selection in yeast. J Cell Biol. 1993;122:373–386. doi: 10.1083/jcb.122.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett WS, Chen LM, Kroschewski R, Ebersold M, Turley S, Trombetta S, Galan JE, Mellman I. Developmental control of endocytosis in dendritic cells by Cdc42. Cell. 2000;102:325–334. doi: 10.1016/s0092-8674(00)00038-6. [DOI] [PubMed] [Google Scholar]
- Guthrie, C., and Fink, G.R. (1991). Guide to Yeast Genetics and Molecular Biology. [PubMed]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Holly SP, Blumer KJ. PAK-family kinases regulate cell and actin polarization throughout the cell cycle of Saccharomyces cerevisiae. J Cell Biol. 1999;147:845–856. doi: 10.1083/jcb.147.4.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaquenoud M, Gulli MP, Peter K, Peter M. The Cdc42p effector Gic2p is targeted for ubiquitin-dependent degradation by the SCFGrr1 complex. EMBO J. 1998;17:5360–5373. doi: 10.1093/emboj/17.18.5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DI. Cdc42: an essential Rho-type GTPase controlling eukaryotic cell polarity. Microbiol Mol Biol Rev. 1999;63:54–105. doi: 10.1128/mmbr.63.1.54-105.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joneson T, Bar-Sagi D. Ras effectors and their role in mitogenesis and oncogenesis. J Mol Med. 1997;75:587–593. doi: 10.1007/s001090050143. [DOI] [PubMed] [Google Scholar]
- Joneson T, McDonough M, Bar-Sagi D, Van Aelst L. RAC regulation of actin polymerization and proliferation by a pathway distinct from Jun kinase. Science. 1996a;274:1374–1376. doi: 10.1126/science.274.5291.1374. [DOI] [PubMed] [Google Scholar]
- Joneson T, White MA, Wigler MH, Bar-Sagi D. Stimulation of membrane ruffling and MAP kinase activation by distinct effectors of RAS. Science. 1996b;271:810–812. doi: 10.1126/science.271.5250.810. [DOI] [PubMed] [Google Scholar]
- Kamada Y, Jung US, Piotrowski J, Levin DE. The protein kinase C-activated MAP kinase pathway of Saccharomyces cerevisiae mediates a novel aspect of the heat shock response. Genes Dev. 1995;9:1559–1571. doi: 10.1101/gad.9.13.1559. [DOI] [PubMed] [Google Scholar]
- Khosravi-Far R, White MA, Westwick JK, Solski PA, Chrzanowska-Wodnicka M, Van Aelst L, Wigler MH, Der CJ. Oncogenic Ras activation of Raf/mitogen-activated protein kinase-independent pathways is sufficient to cause tumorigenic transformation. Mol Cell Biol. 1996;16:3923–3933. doi: 10.1128/mcb.16.7.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozminski KG, Chen AJ, Rodal AA, Drubin DG. Functions and functional domains of the GTPase Cdc42p. Mol Biol Cell. 2000;11:339–354. doi: 10.1091/mbc.11.1.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroschewski R, Hall A, Mellman I. Cdc42 controls secretory and endocytic transport to the basolateral plasma membrane of MDCK cells. Nat Cell Biol. 1999;1:8–13. doi: 10.1038/8977. [DOI] [PubMed] [Google Scholar]
- Lamarche N, Tapon N, Stowers L, Burbelo PD, Aspenstrom P, Bridges T, Chant J, Hall A. Rac and Cdc42 induce actin polymerization and G1 cell cycle progression independently of p65PAK and the JNK/SAPK MAP kinase cascade. Cell. 1996;87:519–529. doi: 10.1016/s0092-8674(00)81371-9. [DOI] [PubMed] [Google Scholar]
- Leberer E, Dignard D, Harcus D, Thomas DY, Whiteway M. The protein kinase homologue Ste20p is required to link the yeast pheromone response G-protein bg subunits to downstream signaling components. EMBO J. 1992;11:4815–4824. doi: 10.1002/j.1460-2075.1992.tb05587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leberer E, Wu C, Leeuw T, Fourest-Lieuvin A, Segall JE, Thomas DY. Functional characterization of the Cdc42p binding domain of yeast Ste20p protein kinase. EMBO J. 1997;16:83–97. doi: 10.1093/emboj/16.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman K, Rossi G, Adamo JE, Brennwald P. Yeast homologues of tomosyn and lethal giant larvae function in exocytosis and are associated with the plasma membrane SNARE, Sec9. J Cell Biol. 1999;146:125–140. doi: 10.1083/jcb.146.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew DJ, Reed SI. Morphogenesis in the yeast cell cycle: regulation by Cdc28 and cyclins. J Cell Biol. 1993;120:1305–1320. doi: 10.1083/jcb.120.6.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Zheng Y, Drubin DG. Regulation of cortical actin cytoskeleton assembly during polarized cell growth in budding yeast. J Cell Biol. 1995;128:599–615. doi: 10.1083/jcb.128.4.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Li MZ, Leibham D, Cortez D, Elledge SJ. The univector plasmid-fusion system, a method for rapid construction of recombinant DNA without restriction enzymes. Curr Biol. 1998;8:1300–1309. doi: 10.1016/s0960-9822(07)00560-x. [DOI] [PubMed] [Google Scholar]
- Longtine MS, Theesfeld CL, McMillan JN, Weaver E, Pringle JR, Lew DJ. Septin-dependent assembly of a cell-cycle-regulatory module in Saccharomyces cerevisiae. Mol Cell Biol. 2000;20:4049–4061. doi: 10.1128/mcb.20.11.4049-4061.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manser E, Leung T, Salihuddin H, Tan L, Lim L. A non-receptor tyrosine kinase that inhibits the GTPase activity of p21 Cdc42. Nature. 1993;363:364–367. doi: 10.1038/363364a0. [DOI] [PubMed] [Google Scholar]
- Manser E, Leung T, Salihuddin H, Zhao Z, Lim L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature. 1994;367:40–46. doi: 10.1038/367040a0. [DOI] [PubMed] [Google Scholar]
- Martin H, Mendoza A, Rodriguez-Pachon JM, Molina M, Nombela C. Characterization of SKM1, a Saccharomyces cerevisiae gene encoding a novel Ste20/PAK-like protein kinase. Mol Microbiol. 1997;23:431–444. doi: 10.1046/j.1365-2958.1997.d01-1870.x. [DOI] [PubMed] [Google Scholar]
- McMillan JN, Longtine MS, Sia RA, Theesfeld CL, Bardes ES, Pringle JR, Lew DJ. The morphogenesis checkpoint in Saccharomyces cerevisiae: cell cycle control of Swe1p degradation by Hsl1p and Hsl7p. Mol Cell Biol. 1999;19:6929–6939. doi: 10.1128/mcb.19.10.6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minden A, Lin A, Claret FX, Abo A, Karin M. Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell. 1995;81:1147–1157. doi: 10.1016/s0092-8674(05)80019-4. [DOI] [PubMed] [Google Scholar]
- Moskow JJ, Gladfelter AS, Lamson RE, Pryciak PM, Lew DJ. Role of Cdc42p in pheromone-stimulated signal transduction in Saccharomyces cerevisiae. Mol Cell Biol. 2000;20:7559–7571. doi: 10.1128/mcb.20.20.7559-7571.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Owen D, Mott HR, Laue ED, Lowe PN. Residues in Cdc42 that specify binding to individual CRIB effector proteins. Biochemistry. 2000;39:1243–1250. doi: 10.1021/bi991567z. [DOI] [PubMed] [Google Scholar]
- Pai EF, Krengel U, Petsko GA, Goody RS, Kabsch W, Wittinghofer A. Refined crystal structure of the triphosphate conformation of H-ras p21 at 1.35 A resolution: implications for the mechanism of GTP hydrolysis. EMBO J. 1990;9:2351–2359. doi: 10.1002/j.1460-2075.1990.tb07409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter M, Neiman AM, Park H-O, van Lohuizen M, Herskowitz I. Functional analysis of the interaction between the small GTP binding protein Cdc42 and the Ste20 protein kinase in yeast. EMBO J. 1996;15:7046–7059. [PMC free article] [PubMed] [Google Scholar]
- Pirone DM, Fukuhara S, Gutkind JS, Burbelo PD. SPECs, small binding proteins for Cdc42. J Biol Chem. 2000;275:22650–22656. doi: 10.1074/jbc.M002832200. [DOI] [PubMed] [Google Scholar]
- Pringle JR. Staining of bud scars and other cell wall chitin with Calcofluor. Methods Enzymol. 1991;194:732–735. doi: 10.1016/0076-6879(91)94055-h. [DOI] [PubMed] [Google Scholar]
- Pringle JR, Bi E, Harkins HA, Zahner JE, De Virgilio C, Chant J, Corrado K, Fares H. Establishment of cell polarity in yeast. Cold Spring Harbor Symp Quant Biol. 1995;60:729–744. doi: 10.1101/sqb.1995.060.01.079. [DOI] [PubMed] [Google Scholar]
- Redding K, Holcomb C, Fuller RS. Immunolocalization of Kex2 protease identifies a putative late Golgi compartment in the yeast Saccharomyces cerevisiae. J Cell Biol. 1991;113:527–538. doi: 10.1083/jcb.113.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson HE, Wittenberg C, Cross F, Reed SI. An essential G1 function for cyclin-like proteins in yeast. Cell. 1989;59:1127–1133. doi: 10.1016/0092-8674(89)90768-x. [DOI] [PubMed] [Google Scholar]
- Richman TJ, Johnson DI. Saccharomyces cerevisiae Cdc42p GTPase is involved in preventing the recurrence of bud emergence during the cell cycle. Mol Cell Biol. 2000;20:8548–8559. doi: 10.1128/mcb.20.22.8548-8559.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman TJ, Sawyer MM, Johnson DI. The Cdc42p GTPase is involved in a G2/M morphogenetic checkpoint regulating the apical-isotropic switch and nuclear division in yeast. J Biol Chem. 1999;274:16861–16870. doi: 10.1074/jbc.274.24.16861. [DOI] [PubMed] [Google Scholar]
- Rohatgi R, Ma L, Miki H, Lopez M, Kirchhausen T, Takenawa T, Kirschner MW. The interaction between N-WASP and the Arp2/3 complex links Cdc42- dependent signals to actin assembly. Cell. 1999;97:221–231. doi: 10.1016/s0092-8674(00)80732-1. [DOI] [PubMed] [Google Scholar]
- Sahai E, Alberts AS, Treisman R. RhoA effector mutants reveal distinct effector pathways for cytoskeletal reorganization, SRF activation and transformation. EMBO J. 1998;17:1350–1361. doi: 10.1093/emboj/17.5.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sells MA, Knaus UG, Bagrodia S, Ambrose DM, Bokoch GM, Chernoff J. Human p21-activated kinase (Pak1) regulates actin organization in mammalian cells. Curr Biol. 1997;7:202–210. doi: 10.1016/s0960-9822(97)70091-5. [DOI] [PubMed] [Google Scholar]
- Sia RAL, Herald HA, Lew DJ. Cdc28 tyrosine phosphorylation and the morphogenesis checkpoint in budding yeast. Mol Biol Cell. 1996;7:1657–1666. doi: 10.1091/mbc.7.11.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon MN, De Virgilio C, Souza B, Pringle JR, Abo A, Reed SI. Role for the Rho-family GTPase Cdc42 in yeast mating-pheromone signal pathway. Nature. 1995;376:702–705. doi: 10.1038/376702a0. [DOI] [PubMed] [Google Scholar]
- Theesfeld CL, Irazoqui JE, Bloom K, Lew DJ. The role of actin in spindle orientation changes during the Saccharomyces cerevisiae cell cycle. J Cell Biol. 1999;146:1019–10132. doi: 10.1083/jcb.146.5.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Aelst L, D'Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- White MA, Nicolette C, Minden A, Polverino A, Van Aelst L, Karin M, Wigler MH. Multiple Ras functions can contribute to mammalian cell transformation. Cell. 1995;80:533–541. doi: 10.1016/0092-8674(95)90507-3. [DOI] [PubMed] [Google Scholar]
- White MA, Vale T, Camonis JH, Schaefer E, Wigler MH. A role for the Ral guanine nucleotide dissociation stimulator in mediating Ras-induced transformation. J Biol Chem. 1996;271:16439–16442. doi: 10.1074/jbc.271.28.16439. [DOI] [PubMed] [Google Scholar]
- Wittinghofer A, Nassar N. How Ras-related proteins talk to their effectors. Trends Biochem Sci. 1996;21:488–491. doi: 10.1016/s0968-0004(96)10064-5. [DOI] [PubMed] [Google Scholar]
- Yamashita M, Yoshikuni M, Hirai T, Fukada S, Nagahama Y. A monoclonal antibody against the PSTAIR sequence of p34cdc2, catalytic subunit of maturation-promoting factor and key regulator of the cell cycle. Develop Growth Differ. 1991;33:617–624. doi: 10.1111/j.1440-169X.1991.00617.x. [DOI] [PubMed] [Google Scholar]
- Yang S, Ayscough KR, Drubin DG. A role for the actin cytoskeleton of Saccharomyces cerevisiae in bipolar bud-site selection. J Cell Biol. 1997;136:111–123. doi: 10.1083/jcb.136.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZS, Leung T, Manser E, Lim L. Pheromone signaling in Saccharomyces cerevisiae requires the small GTP-binding protein Cdc42p and its activator Cdc24p. Mol Cell Biol. 1995;15:5246–5257. doi: 10.1128/mcb.15.10.5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond SH, Joyce M, Yang C, Brown K, Huang M, Pring M. Mechanism of Cdc42-induced actin polymerization in neutrophil extracts. J Cell Biol. 1998;142:1001–1012. doi: 10.1083/jcb.142.4.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziman M, O'Brien JM, Ouellette LA, Church WR, Johnson DI. Mutational analysis of CDC42Sc, a Saccharomyces cerevisiae gene that encodes a putative GTP-binding protein involved in the control of cell polarity. Mol Cell Biol. 1991;11:3537–3544. doi: 10.1128/mcb.11.7.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziman M, Preuss D, Mulholland J, O'Brien JM, Botstein D, Johnson DI. Subcellular localization of Cdc42p, a Saccharomyces cerevisiae GTP-binding protein involved in the control of cell polarity. Mol Biol Cell. 1993;4:1307–1316. doi: 10.1091/mbc.4.12.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]