Abstract
Endocrine disruption, the guiding theme of the 27th International Neurotoxicology Conference, merged into the neurotoxicology agenda largely because hormones help steer the process of brain development. Although the disruption motif first attracted public health attention because of reproductive anomalies in both wildlife and humans, the neurobehavioral implications had been planted decades earlier. They stemmed from the principle that sex differences in behavior are primarily the outcomes of differences in how the brain is sexually differentiated during early development by gonadal hormones (the Organizational Hypothesis). We also now understand that environmental chemicals are capable of altering these underlying events and processes. Among those chemicals, the group labeled as endocrine disrupting chemicals (EDCs) offers the clearest evidence of such selectivity, a consequence of their actions on the endogenous sex steroids, androgens and estrogens. Two EDCs in particular offer useful and intriguing examples. One is phthalate esters. The other is bisphenol A. Both agents are used extensively in plastics manufacture, and are pervasive in the environment. Both are produced in immense quantities. Both are found in almost all humans. Phthalates are considered to function in essence as anti-androgens, while bisphenol A is labeled as an estrogen. Their associations with brain sexual differentiation are reviewed and further questions noted. Both EDCs produce a wider spectrum of health effects, however, than would be extrapolated simply from their properties as anti-androgens and estrogens. Obesity is one example. Further complicating their assessment as health risks are questions about nonmonotonic dose-response functions and about transgenerational effects incurred via epigenetic mechanisms. All these facets of endocrine disruption are pieces of a puzzle that challenge neurotoxicologists for solutions.
Keywords: Bisphenol A; Development, Fetus; Dimorphism, sexual; Dose-response; Endocrine, disruptors; Hormones; Phthalates
Introduction
Endocrine disruption, the global theme of the 27th International Neurotoxicology conference, can trace its connections to neurotoxicology from the first stirrings of the environmentalist movement. Rachel Carson’s Silent Spring (1962) sounded alarms about the decline of bird populations (Figure 1) but touched peripherally on implications for human health. Her message arrived in the midst of rising concerns about environmental contamination, and the erosion, not only of avian populations, but of aquatic mammals and various fish species from both marine and freshwater habitats. Reproductive anomalies were also observed in terrestrial mammals such as mink fed fish from the Great Lakes. Bald eagle populations were declining at disturbing rates. Around the Great Lakes, herring gulls displayed peculiar developmental anomalies such as twisted bills. Participants in the first Rochester Conference on Environmental Toxicity (Berg and Miller, 1969), trying to explain the evidence that eggshell thinning might explain diminished eagle populations, offered the proposition that pesticides such as DDT could be responsible because of their estrogenic properties, while others saw a connection with dioxins and allied chemicals (reviewed in Schecter, 1994). The growing swell of such information, and the puzzling changes taking place in wildlife populations spurred the 1991 Wingspread Conference (Markey et al, 2002), led by Theo Colborn, to its conclusion that these phenomena could be the result of environmental contamination by chemicals that altered hormone status and function. Endocrine disruption was later featured as a theme for neurotoxicology, especially as a developmental neurotoxicant, at the 1995 International Neurotoxicology Conference (Tilson, 1998; Weiss, 1997).
Figure 1.
Rachel Carson (1907–1964) lifted a nascent environmental movement into public awareness with the publication of Silent Spring (1962). It highlighted the environmental risks of pesticides such as DDT, which earlier had been overlooked.
Endocrine disruptor describes those chemical agents that interfere with the biological actions of hormones by blocking, mimicking, displacing, or acting through a variety of other mechanisms to subvert their natural roles. Like some other neurotoxicants, they present a special challenge to traditional toxicology because, rather than expressing their effects in the form of tissue pathology, clinical disorders, or death, they may simply distort or shift the organism’s normal or characteristic patterns of response to environmental or internal conditions.
Endocrine disruption offered a connection between environmental contaminants and human reproduction with publication of a 1992 article contending that semen quality had undergone a steady decline during the previous 50 years (Carlsen et al., 1992). It aroused a spirited debate about its validity and the underlying mechanisms. Sharpe and Skakkebaek (1993) interpreted these findings as evidence of exposure to estrogenic chemicals in the environment. To Skakkebaek (2003), viewing the landscape of male reproductive disorders ten years later, they appeared to blend into an identifiable syndrome. He wrote, “There is evidence that poor semen quality, testicular cancer, undescended testes and hypospadias are symptoms of one underlying entity, testicular dysgenesis syndrome (TDS)… Experimental and epidemiological studies suggest that TDS is the result of disruption of embryonal programming and gonadal development during fetal life.” The syndrome is now presumed to arise from exposure to environmental endocrine disruptors at a critical stage of development (e.g., Sharpe & Skakkebaek, 2008; Wohlfahrt-Veje et al., 2009).
Neurotoxicology’s main connection with endocrine disruption arose through what might be called the sexual brain. The same gonadal hormones that fashion the reproductive system are also fundamental in molding the brain. A connection between reproductive disorders and neurobehavioral function can be devised in a variety of mechanistic formulations, but a statement by Richard Sharpe (2008) frames the context in an engaging way:
“The difference between becoming a male rather than a female is about as fundamental as you can get, as it will alter that individual’s place in society, transform the shape of his body, reshape his inherent abilities, his thought processes and his behaviors [my italics]. Whilst it is a constant source of debate and amusement as to whether this “transformation” process represents an improvement or not, when compared with the “set-up” program which would have led to a female, it is becomingly increasingly clear that “making a male” is a rather perilous process.”
The publication of Our Stolen Future (Colborn et al., 1996) firmly placed endocrine disruption on the agenda of neurotoxicology. Theo Colborn, honored at the 27th conference, had the insight to foresee this development. Many of the observations that created the book’s thesis, that environmental chemicals had been fomenting turbulence in hormonal function, arose from puzzling instances of animal behavior. When George and Molly Hunt (1977) observed the presence of female–female pairings of western gulls on Santa Barbara Island, California, they invoked the term “lesbian gulls.” Michael Fry (1995) attributed such pairings to both a reduced male population and anomalies in male reproductive structures and behavior. He proposed DDT and other “estrogenic” contaminants in the environment as causes. Because behavior is a reflection of events and processes in the brain, it became necessary to explain the coupling between aberrant behavior and endocrine disruption by determining how such environmental agents alter brain anatomy and function. In particular, to borrow Sharpe’s term, how they proceed to alter the events that “make” a male. A succinct review of this sequence follows to help provide a context for the topics discussed at the conference.
Molding The Sexual Brain
Psychologists are responsible for establishing the principle that now governs how we conceive of sexual differentiation of the brain (Wallen, 2009). What came to be known as the Organizational Hypothesis first appeared in a publication by Phoenix et al. (1959). As recounted by Wallen (2009), “In the 50 years since its publication it has transformed common views of the actions of hormones on the nervous system. The notion that hormones could permanently alter the structure of the nervous system, radical when it was first published, is currently taught in high school and undergraduate classes in psychology and neuroscience. It has become the dominant explanation for the genesis of behavioral sex differences.”
The differentiation process is largely controlled by the sex steroid hormones, that is, androgens and estrogens. Although these chemicals can apparently modify brain organization at other times of life, such as puberty (Mouritsen et al., 2010), the most critical phase occurs during pregnancy, when pronounced changes in the fetal brain are taking place almost hour by hour. Within this period lie specific temporal windows during which sexual differentiation proceeds most forcefully. For the genitals, this process in humans takes place during the first 2–3 months of pregnancy. For the brain, the next few months of pregnancy seem to be the more decisive period according to Bao and Swaab (2011).
A terse description of the sexual differentiation process in mammals begins with the release of testosterone from the fetal testis, a process programmed by the SRY gene and that will sculpt both the reproductive tract and the brain. Although early in infancy males experience another testosterone surge, it seems to play a lesser role in brain organization (Bao and Swaab, 2011, in humans; Brown and Dixon, 1999 in nonhuman primates; Mack et al., 1996 in rodents). For both girls and boys, however, testosterone levels during this period are associated with sex-linked play behaviors at 14 months of age (Lamminmäki et al., 2012). In females, the absence of SRY leads the primitive gonads to differentiate into the ovaries (Figure 2).
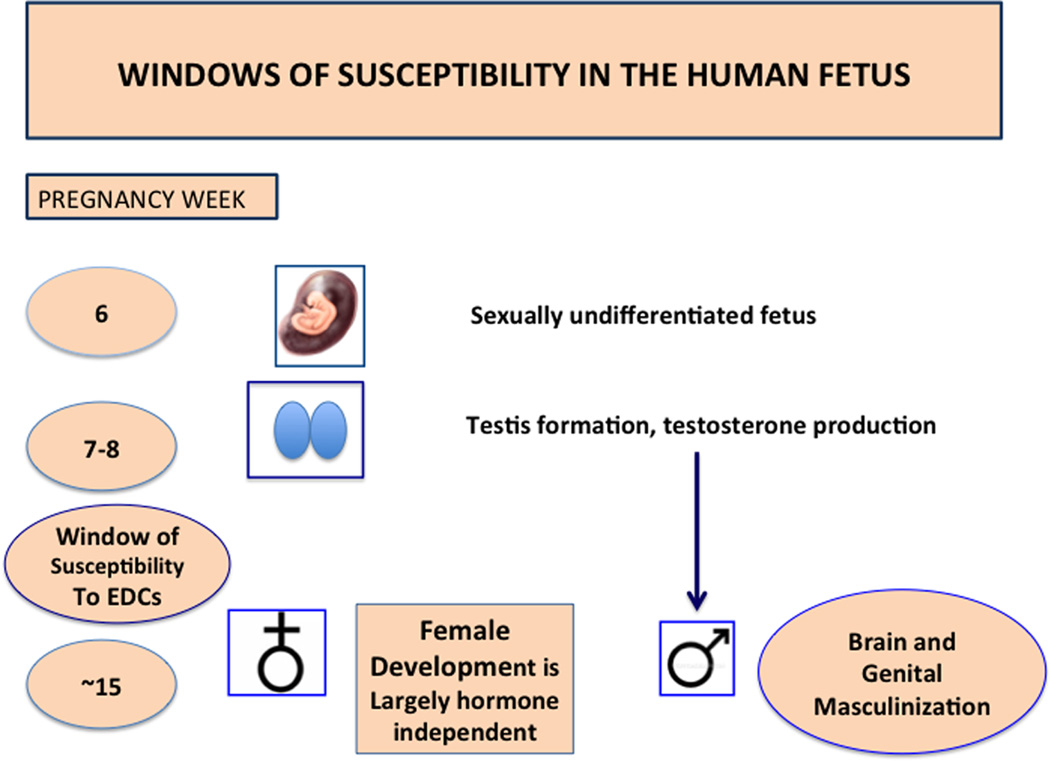
Figure 2.
Basic sketch of human sexual differentiation, depicting windows of susceptibility to endocrine-disrupting chemicals. Until about gestational week 6, the fetus in essence is sexually undifferentiated. Production of testosterone by the fetal testis, beginning at about week 7, as well as programmed gene expression, transforms the sexually undifferentiated fetus into the male form. At this sensitive juncture, anti-androgens such as phthalates can begin to exert their effects. In the rat, testosterone secretion peaks at about embryonic days 16–18.
In the rodent brain, fetal testosterone is converted by aromatase (CYP19) to estradiol, which then acts as the masculinizing blueprint. In the rhesus monkey brain (Thornton et al., 2009), and presumably the human brain, both testosterone and dihydrotestosterone (DHT), which is not aromatized, are able to shape its masculine properties. Estrogen may still play a role, however. Azcoitia et al., (2011) reported that human brains also synthesize estradiol via aromatase, supporting the earlier findings of MacLusky et al. (1987). Further, DHT can itself be metabolized to 5α-androstane-3α,17β-diol (3α-diol) or 5α-androstane-3β,17β-diol (3β-diol), which seem preferentially to bind ERβ (Handa et al., 2009). Moreover, it is also now clear that the fetal rodent brain is itself capable of synthesizing androgens independently of testicular generation (e.g., Konkle and McCarthy, 2011), findings that are altering our understanding of the differentiation process.
In essence, male and female brains develop under contrasting hormonal circumstances. Exposure to testosterone and its metabolites (estradiol via aromatase, DHT via 5α-reductase) spells the difference; without it, brain development would follow the basic female pattern. It is predominantly the hormonal environment that dictates the biological basis for sex differences in the brain (Figure 3). Such processes and outcomes are not confined to mammals. Nottebohm et al (1987) described how gonadal hormones forge changes in the structure of songbird brains and song learning patterns. While not directly reprising their roles in mammalian brains, they demonstrate how endocrine mechanisms are conserved from phylum to phylum.
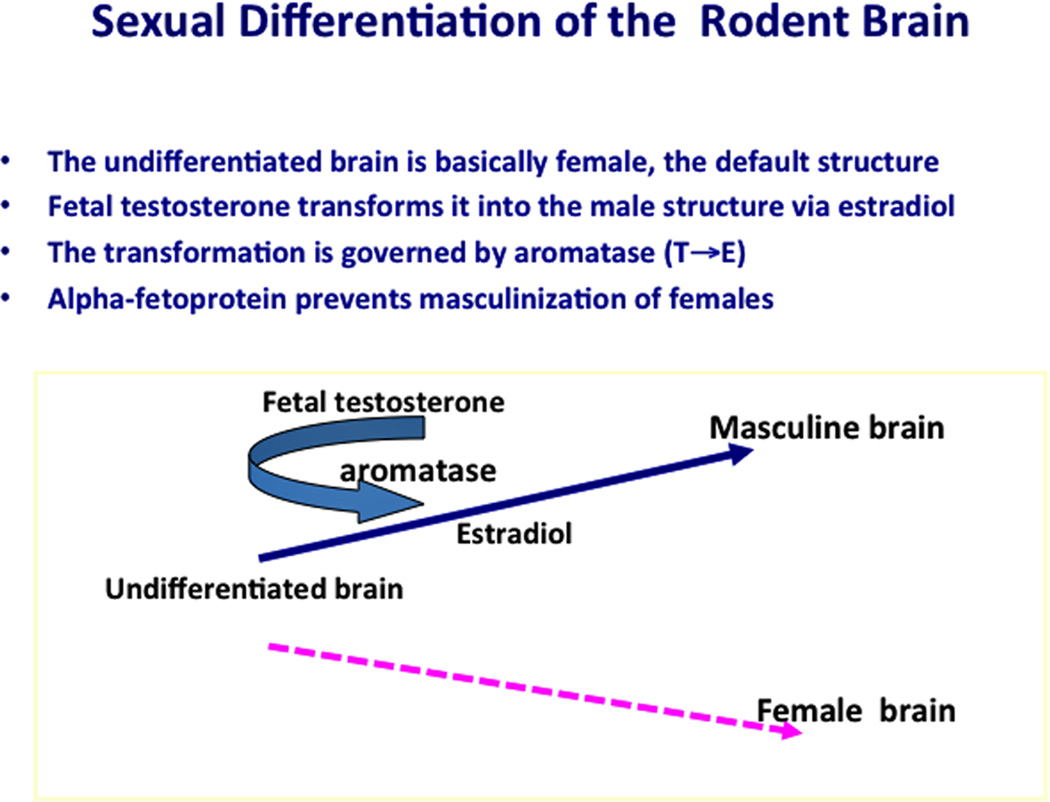
Figure 3.
The process of brain sexual differentiation as seen in rodents, the primary experimental model for studies of brain sexual differentiation. Here, masculinization is governed by estradiol, converted from testosterone by the enzyme aromatase (CYP19). In humans and other primates, dihydrotestosterone performs this function.
The anatomical outcomes of this process of differentiation are visible in numerous ways: total brain volume, relative sizes of different structures such as the hippocampus and corpus callosum, cortical thickness and symmetry, lipid content versus gray matter, and distribution of androgen and estrogen receptors (Weiss, 2007). One outcome of nervous system sexual differentiation is how, in sexually mature animals, it responds to what are termed the “activational” (also reversible) effects of sex hormones, those that produce transient changes in behavior such as those accompanying estrus, and only emerge following puberty. The nervous system, that is, has to be “primed” during early development to respond to sex hormones later in life.
The basic Organizational Hypotheses has been modified to acknowledge the genetic component in brain sexual differentiation that is independent of hormonal action. As put by Davies and Wilkinson (2006), “It is not all hormones,” a point made by others as well (Dewing et al, 2006; Reinius et al, 2008; Arnold, 2004). Recent years have also witnessed a spate of advances, not reviewed here, in grasping the cellular and molecular mechanisms by which testosterone and estradiol generate a sexually differentiated brain and in how we measure the behavioral outcomes.
The Translation Step
These advances parallel our increased comprehension of the risks posed to brain development by environmental chemicals. None exceed in subtlety the ways in which one class of chemicals, EDCs, engage provocative societal issues arising from the role of biological determinism in explaining behavior. Among some segments of the public, intense disputes about sex differences in, say, cognitive performance and children’s play preferences still provoke arguments about the roles of nature (the genome, in essence) and nurture, defined as the social environment. For scientists, the argument for biological determinism as the foundation for sex differences in behavior was settled some time ago. But the argument is now complicated by a different kind of environmental question, chemical exposure, that joins it to neurotoxicology.
This conjunction brings neurotoxicology into an arena fraught with vocal disagreements aired in public statements by proponents from different cultural milieus. These disagreements arise partly from arguments about the roots of homosexuality, and about questions of gender identity and others that at one time would have seemed quite remote from neurotoxicology. For example, issues such as the increasing dominance of females in college completion rates are arousing speculations in some quarters about whether environmental factors such as male vulnerability to environmental chemicals may be responsible (e.g., Sax, 2007). It will be difficult for neurotoxicologists to detach themselves from such discussions because their science is driving much of the debate.
And the implications of the science are hardly abstractions. It is worth noting that the nurture argument found favor in the past even with some professionals, who maintained that children are born as sexually neutral beings who then are shaped by the social environment to behave as males or females (e.g., Money, 1975). It was a view that supported radical surgery in children with ambiguous genitals (intersex) designed to have them conform anatomically to whichever was selected as the appropriate sex. Such arbitrary decisions generated tragic consequences. Later, many of these children, responding as adults to the behavioral signals from their brains, so to speak, rebelled against the sex assigned to them and underwent corrective surgery to restore them to the sex with which they identified (Diamond, 2011). When insights from basic research were finally accepted, they forestalled ruinous “corrective” surgery in many children, a triumph of translation.
Despite how far biological sex has penetrated current public discourse, some scientists are not yet comfortable with the message. For certain types of queries, toxicology readily accepts assessments of sex differences in response to potentially toxic agents. The standard cancer bioassay, for example, requires two species (usually mice and rats) and both sexes. Sex is a reasonable parameter in this instance; mammary tumor potential cannot be assessed in males. Clinical medicine and pharmaceuticals, partly in response to federal directives, have moved toward a more balanced assessment of sex-based outcomes and risks (Nicolson et al., 2010). In fact, gender medicine has now attained the status of an independent area of research (Oertelt-Prigione et al., 2010).
Perhaps because they are not forced to defend clinical accountability, laboratory researchers in the life sciences, including neurotoxicology, are still prone to overlook sex as a variable, mostly choosing males as subjects (Beery and Zucker, 2011; Wald and Wu, 2010) or, especially with in vitro experiments, ignoring sex altogether (Weiss, 2010). Given the scope of sex differences in virtually every biological function, confining the study of environmental chemicals to a single sex or neglecting it can deprive the outcomes of relevance. We now are aware, as the 27th Conference told us, that to understand neurobehavioral disorders such as autism, ADHD, and schizophrenia, including their associations with gene expression, we have to acknowledge their “strong gender bias.” The program wove that theme into the fabric of the entire conference.
The other part of the translation process is how the investigator measures and interprets sex differences. When an outcome such as fertility, for example, is the aim of a study, evaluating the toxicity of an agent in both sexes entails different measures for males and females. Unusual behaviors, such as lordosis in male rodents, or mounting in females, would signal disruptions in sex-specific actions of the chemical under study. When the outcome is cognitive function, mood, fine motor performance, social responsiveness (“social cognition”), or most other forms of non-reproductive behavior, the investigator is dealing with differences that take the form of distributions exhibiting significant overlap between the sexes. Intelligence test scores show such a blending, and are the source of endless and passionate debates about the validity of sex differences. The charts in Weiss (2010) show how small differences in means and variances between the sexes in measures such as mathematical aptitude can elicit a variety of interpretations about their significance. Anatomical differences between male and female brains revealed by imaging techniques require complex algorithms for their depiction (e.g., Goldstein 2001). Simplicity is not one of the virtues of sex differences research. Blackless et al (2000), in their review of intersex variations and prevalence, estimated that the prevalence of individuals whose bodies differ from standard male or female approximated only two percent. The absence of easily visible external guideposts to identify the brain’s sexuality creates challenges for neurotoxicology research that are heightened by endocrine disruptors.
One of the serious and perplexing features of the human literature is how often sex or gender is treated as a “confounder” in the statistical analysis and modeling of research results so that it obliterates sex differences. This practice can be just as pernicious as confining the investigation to a single sex. The 27th Conference presentations should convince researchers that such a practice is scientifically untenable.
Chemical Beacons
Sex differences in response to chemical exposures are so embedded in biology, and so ubiquitous, that only an encyclopedic survey of the literature would encompass most of the pertinent agents. Metals such as lead, mercury, and manganese, pesticides from the organophosphate and organochlorine classes, persistent organic pollutants (POPS) such as PCBs, and other agents often investigated in neurotoxicology were again explored at the 27th Conference. These introductory comments are centered, instead, on two case studies designed to illuminate the current landscape and to find novel, unexpected, puzzling, and instructive findings pertinent to neurotoxicology and discussed at the Conference. Unlike heavy metals, POPS, pesticides, and other recognized neurotoxicants, endocrine disruptors still face skepticism in some quarters of the toxicology community about their neurotoxic potential at common environmental exposure levels. Harris (2001) framed the issue from the standpoint of effects in wildlife. She noted that environmental chemicals such as nonylphenols, that can induce intersex in fish, also had the potential to alter brain development.
Two chemicals, phthalate esters and bisphenol A, exemplify the kinds of questions and enigmas aroused by the endocrine disruptor literature and are the target of intense public scrutiny. Their history demonstrates how easily fundamental toxic outcomes may elude scientists.
Phthalates
Phthalate esters pervade our environment. They are produced in quantities of billions of kilograms per year (Koch and Calafat, 2009). They are ubiquitous in cosmetics. Large quantities are used to treat polyvinyl chloride (PVC) plastic to make it soft or flexible. Phthalates were added to children’s toys for that reason, a practice now banned in several countries and states; chewing on toys was a major source of exposure. They are found in medications. They are “inert” ingredients in pesticide formulations. We can be exposed orally (food), dermally (cosmetics), through inhalation (dust particles from vinyl flooring), and even subdermally (medical tubing).
As early as the 1950s, the widespread adoption of phthalates for many products provoked questions about their adverse effects (Bushnell et al., 2010). By 1980, they had been identified as testicular poisons that could directly alter testicular function in rats. Sharpe et al (1995), after finding that gestational and lactational exposure of rats to environmental estrogens could reduce testicular size and sperm production, suggested that, because phthalates also produced such effects, their estrogenic properties warranted investigation. Not until the late 1990s (Mylchreest et al., 1999, 2002) was it asked whether these early developmental effects might be a consequence of anti-androgenic mechanisms.
Now their anti-androgenic properties are known in greater detail (e.g., Howdeshell et al., 2008; Fisher, 2003, 2004), and their congruency with the testicular dysgenesis syndrome appears quite striking (Bay et al., 2006; Toppari et al., 2010). Moreover, we now have data from humans about their anti-androgenic properties. Until 2005, the possibility of such effects in humans exposed to low environmental levels of phthalates had been discounted because most of the animal studies had been based on high doses. Swan et al. (2005) then reported an association between levels of certain phthalate metabolites in maternal urine during pregnancy and reduced anogenital distance (AGD) in male offspring. In both rodents and humans, AGD is 1.5–2 times as long in males as in females (cf., Swan, 2008), so it serves as an index of masculine development.
From the standpoint of brain development, the primary effects of phthalates would presumably reflect the quenching of testosterone production by the fetal testis. They do not act at the receptor level, as do other anti-androgens such as flutamide. The consequence would be that the two main processes that govern masculinization of the brain, transformation of testosterone by aromatiztion to estradiol (rodents), or via 5α-reductase conversion to dihydrotestosterone (primates), lack sufficient substrate. We would correspondingly expect phthalates to partially strip the brain of its masculine potential or even to feminize it. It is important, however, to recognize that, because we are exposed to a variety of phthalate esters, each with their own distinct metabolites, epidemiologic studies correspondingly measure a variety of metabolites. As would be expected, their associations with the selected endpoints vary. Similarly, in laboratory studies, effects on development vary with the particular phthalate chosen for assessment. One complication is that combinations of phthalates are additive in their effects (Howdeshell et al., 2008) so that correlations of single metabolites with endpoints can be misleading.
Human studies show more complicated outcomes than would be predicted from such assumptions. One clear outcome, consistent with that assumption, comes from Swan et al. (2010). They measured play behavior in children 4–9 years of age by means of a questionnaire. Higher concentrations of several phthalate metabolites during pregnancy significantly reduced masculine play behavior in boys, with minor effects in girls. Other reports also indicate greater adverse effects on males, based on concurrent diethylhexyl phthalate (DEHP) metabolite concentrations, for measures such as IQ and vocabulary (Cho et al., 2010) in children 8–11 years of age. In a study based on metabolite concentrations during pregnancy, Engel et al. (2010) found elevated phthalate concentrations associated with poorer scores on the aggression, attention problems, conduct problems, depression, and externalizing problems scales, and poorer executive functioning in children 4–9 years of age. These effects were seen among the boys, but not the girls. Overall, then, males seem to be at greater risk for functional impairments, but the endpoints are not sharply differentiated by sex. Studies with infants (Engel et al., 2009; Yolton et al., 2011) are difficult to interpret, but indicate some potential behavioral problems although possible positive effects. Consequences that appear later in life are both easier to measure and generally more crucial for the individual.
Varying interpretations of such findings might have been predicted from the earlier literature. Building on Sharpe and Skakkebeak (1993), who voiced the possibility that exposure to environmental estrogens might explain falling sperm counts, Sharpe et al. (1995) undertook a study of phthalates (benzyl butyl phthalate, BBP) and other presumed xenoestrogens. They identified phthalates as among a number of …” estrogenic chemicals [that] are ubiquitous in the environment, and [that] humans are exposed to … daily by a number of routes.” Their findings, based on perinatal exposures and a variety of markers of testicular toxicity, led them to note that, “…the present findings suggest that further studies of the estrogenicity of phthalates should be a priority.”
But Sharpe et al. (1995), despite the subsequent findings, may not have missed the target completely. The estrogenicity of phthalates has been observed under various conditions. One example is that of Parveen et al. (2008). These investigators applied DNA microarray technology to screen for these properties by examining the gene expression profiles of various phthalates and of estradiol. They observed significant correlations, in MCF-7 cells, between the two profiles, especially for genes related to enzymes and transcription. And Ma et al. (2006) exposed female prepubertal rats to DEHP via inhalation. Both the age of vaginal opening and first estrus cycle were advanced in the exposed subjects. DEHP also raised the expression of aromatase mRNA, the enzyme that catalyzes the conversion of testosterone to estradiol, somewhat similar to the findings of Andrade et al. (2006) on brain aromatase in neonatal females. More recent data from a human population indicate that delayed pubarche in girls was associated with the higher levels of phthalate metabolites in urine samples (Frederiksen et al. 2012)
Finally, among the molecular mechanisms underlying phthalate toxicity, peroxisome proliferator-activated receptors PPAR-α and PPAR-γ have been advanced as possibilities (Hurst and Waxman, 2004) and PPAR-β(δ) may play a role in protection against brain damage (Hall et al. 2008). Because PPARs, when activated, can induce the expression of genes related to lipid and glucose metabolism, which imply consequences for obesity (Desvergne et al., 2009) they may explain the effects reported by Stahlhut et al. (2007) and Hatch et al. (2008) on the tendency of phthalate exposures to increase waist circumference, insulin resistance, and body mass index, BMI. Here is a domain that, superficially at least, carries us beyond the scope of gonadal hormones.
Bisphenol A
Like phthalates, bisphenol A (BPA) is manufactured in prodigious quantities: 3.0 million kg/yr. It was evaluated as a synthetic estrogen in the 1930s, but never used for that purpose once diethylstilbestrol (DES) became available. It is now mainly used as a synthetic monomer in the manufacturing of polycarbonate plastic, polystyrene resins, and dental sealants, in printing store receipts, and as an epoxy resin for can linings. BPA can be found in a multitude of products. Polycarbonate baby bottles had been identified as one notorious source, leading to its removal from such products. Leaching of BPA from various products is common, and is accelerated by high temperatures and acidic or basic environments. Experiments have been undermined as a result of laboratory animal exposure to BPA released from used polycarbonate cages (Howdeshell et al., 2003; Hunt, 2008). And one recent study (Carwile et al, 2011) reported that daily consumption of one can of vegetable soup for five consecutive days raised urinary levels of BPA by 1,200%. In one experiment, families eliminated canned foods and those wrapped in plastic for a three-day period (Rudel et al. 2011). Metabolites of both BPA and phthalates fell sharply during this brief period.
Given the numerous sources of BPA in the environment, it is hardly unexpected that 93% of the population over six years old shows evidence of exposure in urine samples analyzed by CDC (Calafat et al. 2008). Because it was first developed as a synthetic estrogen, its structural similarity to diethylstilbestrol (DES) drew notice. (Doherty et al. 2010). DES has become a model for creating synthetic estrogenic havoc, so that the similarities were bound to arouse questions about its potential adverse effects. DES was given to pregnant women to prevent miscarriage (which it failed to do), but its biological legacy appeared years later in the form of a high lifetime risk of many kinds of adverse health outcomes such as infertility, cancer, and others (Hoover et al., 2011).
Intense interest in BPA’s health effects has stimulated a still expanding literature, much of which addresses brain function and behavior, with an emphasis on early development. For researchers engaged in neurotoxicology research, the message it conveys is unambiguous: BPA influences neurobehavioral development, and this influence tends to be expressed differently in males and females. Where ambiguity resides is in dose and timing. Dosing regimens are rather variable, ranging from nanograms to grams.
Although early development is usually adopted as the most critical window of vulnerability, even here we find considerable variability. Some experimenters, to simulate human exposure patterns, begin dosing before mating. Some choose a period during gestation. Some continue dosing after birth, usually until weaning. Earlier reviews by Vandenberg et al., (2009) and vom Saal et al., (2007), surveyed the breadth of BPA toxicity. More recent reviews by Rubin (2011), Kundakovic and Champagne (2011) and Wolstenholme et al., (2011). have expanded that territory into domains such as epigenetics and obesity. A comprehensive review of BPA’s action on many of these issues and others is available from WHO (Castoldi et al, 2011).
Findings in animal models include masculinization of brain anatomy in females, impaired maternal behavior, reduced aromatase activity, altered dopaminergic function, impaired learning, increased aggressive behavior, altered infant behavior in male monkeys toward mother, altered attractiveness of males by females. In two human studies, Braun et al., (2009, 2011) reported a correlation between prenatal exposure levels and externalizing behavior (increased hyperactivity and aggression) in girls two years of age and poor emotional control and anxiety and depression at three years of age. In contrast, Yolton et al., (2011) found no correlations between maternal BPA metabolite concentrations and neurobehavioral measures in infants.
Bisphenol A is a risk factor for other kinds of health endpoints as well as neurotoxicity. They include immune system function, distorted mammary development, sperm abnormalities, prostate cancer, cardiovascular disease, and others. Its obesogenic properties, for example, are now a growing arena of research (Schug et al., 2011). As noted by Ben-Jonathan et al., (2009), discussing its implications for the metabolic syndrome, “The mechanism by which BPA exerts its actions is enigmatic.” The recent report by the French Agency for Food, Environmental and Occupational Health and Safety (ANSES, 2011) came to the same conclusion while also accepting the scientific literature as forceful enough to warrant restriction of exposure.
Endocrine Disruption and Aging
As we know from research on other classes of environmental chemicals, the full panoply of deficits they impose can only be appreciated from a lifetime perspective (e.g., Weiss, 2000). Sometimes they unfold late in life, having lain dormant for decades, much like the herpes zoster virus. Sometimes the waning compensatory capacities that accompany aging magnify vulnerability to exposure, a problem with pharmaceuticals discussed at length in the medical literature.
Endocrine-disrupting chemicals are surely no exception (Weiss, 2007). Leranth et al., (2008a, 2008b) have now demonstrated that bisphenol A interferes with hormonally-induced synaptogenesis, triggered by estradiol (female) and testosterone (males), in adult nonhuman primates. We now know, in contrast to the doctrine the prevailed until about fifteen years ago, that the adult brain is capable of neurogenesis. This recognition has prompted many authors to speculate about how this process could be directed toward repairing or impeding the deficits of Alzheimer’s disease. Mu and Gage (2012), for example, describe the mechanisms of plasticity that could be enlisted as therapeutic strategies. Environmental chemicals that blunt or eliminate this process undermine societies, like ours, now enduring an inexorable shift to an aging demographic and its burden of neurodegenerative diseases such as dementia. The fact that endogenous estrogen, androgen, and thyroid function all decline with aging, that all play a role in brain plasticity, and that all three endocrine systems are vulnerable to EDCs should generate more recognition of how the latter may threaten the aging brain (Weiss, 2007, 2011).
Non-Monotonic Functions
Traditional toxicology, grounded in measures of lethality and pathology, applied the mantra, “The dose makes the poison,” to all forms of toxicity. It has governed risk assessment since its inception. It guided the traditional approach to cancer bioassay in which high doses provide the foundation for extrapolation to risks, by a variety of statistical models, at low doses. The 27th International Neurotoxicology Conference evoked further doubts about this archaic dogma. It contained numerous examples of departures from the traditional toxicologic assumptions of linear, monotonic dose-response functions. On this occasion, though, unlike attitudes in the past, the researchers did not present nonmonotonic dose-response functions as a perplexing incongruity. Such functions are now accepted as routine outcomes.
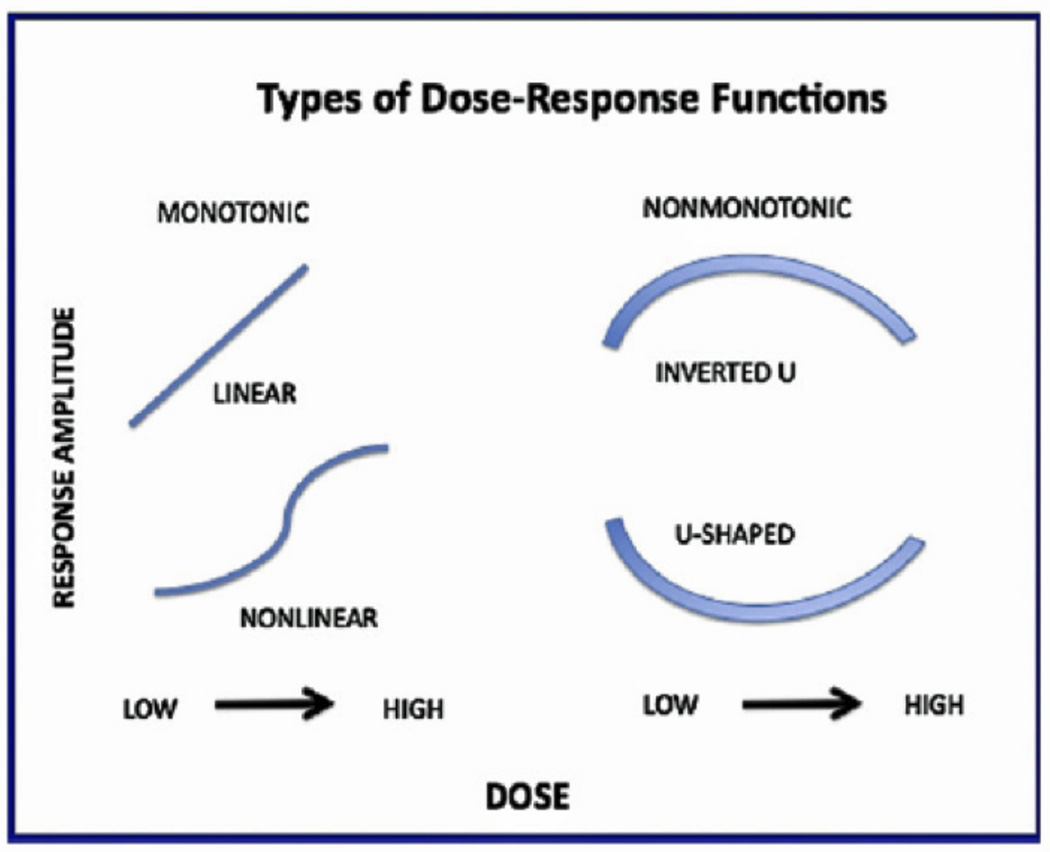
BPA has become the model chemical for examples of nonmonotonic dose-response functions, as depicted in Figure 4. Traditional versions of dose-response relationships assume a monotonic function. This version is depicted in two common forms on the left of Figure 4. Two non-monotonic versions are depicted on the right side of the figure, and have appeared in the BPA literature. Welshons et al (2006) discussed such phenomena in this way:
Figure 4.
Different types of dose-response functions. Traditional toxicology assumes monotonic relationships. Endocrine disruptors often display nonmonotonic relationships. Cf., Welshons et al, 2006: Vandenberg et al 2012.
“In experiments with hormones, drugs, and other chemicals that act via hormonal, receptor-mediated mechanisms, it is very common for the dose-response curve to be nonmonotonic and form an inverted U, which endocrinologists typically refer to as a biphasic dose-response curve. In contrast, there appears to be a lack of awareness of this phenomenon in toxicology…”
A comprehensive review of low dose effects and nonmonotonic doseresponse relationships appeared recently in Vandenberg et al (2012). It sets forth in detail, and with mechanistic explanations, the science underlying rejection of linearity and monotonicity as the guiding principle of dose-response relationships.
It is critical to recognize this departure from traditional toxicological dogma because it lies behind some of the arguments about the health risks of endocrine disruptors. The conventional position assumes that the lack of an adverse effect at dose, D say, implies the absence of such an effect at doses lower than D. Experimental data, such as those from BPA studies, contradict the conventional position and generate understandable confusion on the part of regulators and risk assessors. How does a regulatory agency prescribe an exposure standard confronted with such a function? How should it move to adapt its rules to such new scientific developments? Vandenberg et al (2012) describe the core of the problem in this way: “When nonmonotonic dose-response curves occur, the effects of low doses cannot be predicted by the effects observed at high doses.”
For example, in two neurobehavioral experiments with dioxin (TCDD), we showed U-shaped dose-response functions, as in Figure 5 (Markowski et al, 2001; Hojo et al, 2002). In discussions with USEPA risk assessors about our data, they detailed the problem posed by the lack of policies to deal with such functions. They typically rely on indices such as the No Observed Adverse Effect Level (NOAEL) as the value to which they apply uncertainty (safety) factors such as a 100-fold denominator. U-shaped functions pose a conundrum for assessors because the calculations of uncertainty factors assume monotonicity.
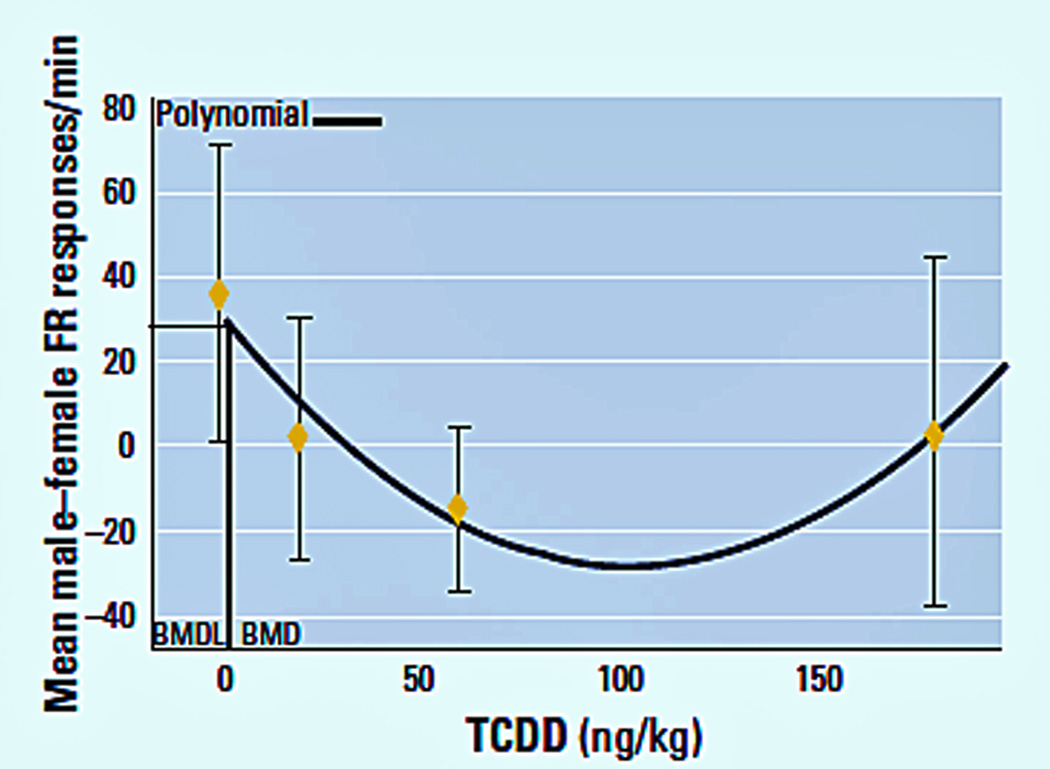
Figure 5.
Polynomial model for benchmark dose ED10 value and 95% lower confidence limit for the male–female littermate differences in Fixed Ratio response rate during the fixed-ratio component of a multiple schedule (Hojo et al, 2002). Pregnant Long-Evans rats were administered TCDD doses of 0, 20, 60, or 180 ng/kg on GD 8. Male and female offspring were trained on a multiple Fixed Ratio-DRL schedule for food pellet rewards. The polynomial was calculated from a quadratic fit to the dose–effect data. A similar plot was calculated for Markowski et al. (2001) for wheel running.
This question has not been pursued in depth with phthalates, but there are hints that it deserves exploration. One example comes from Lin et al (2008), who found that a dose of 10 mg/kg DEHP to pregnant mice raised testosterone levels in male offspring while a dose of 100 mg/kg lowered it, which would be the expected outcome. The plots by Hatch et al (2008) of relationships between phthalate exposure and indices of obesity in groups of different ages show many examples of convoluted dose-response functions.
EPILOGUE
Tracing a direct connection between indices of neurobehavioral function and environmental exposures sets us on a path marked by an abundance of diversions. The purity of a definitive criterion, the Platonic ideal of risk assessment, can rarely be met in the labyrinthine world of endocrine disruption. What the 27th Conference, and the terse summary here of findings based on bisphenol A and phthalate exposures most notably reveals, is how misleading it is to expect EDCs to produce profiles of effects, such as sexually dimorphic behaviors, as literal copies of those produced by native hormones. Such agents are not hormones. They should not be expected to act precisely as hormones. The term disruptor is a far more accurate depiction of our current depth of understanding. Whoever first applied the disruptor label to this class of chemicals turned out to be a gifted visionary. My thesaurus offers the following synonyms for “disrupt”:
throw into confusion, throw into disorder, throw into disarray, cause confusion/turmoil in, play havoc with; disturb, interfere with, upset, unsettle; obstruct, impede, hold up, delay, interrupt, suspend.
This article’s attempt to create a context for neurotoxicology and endocrine disruption, drawing on two model chemicals, exemplifies the accuracy of these entries. The list of synonyms also can be interpreted as a guide for neurotoxicologists. We mostly know about the relationship between EDC exposure and neurobehavioral function through an examination of outcomes within a limited sphere of questions. We fundamentally lack insights about how EDCs act—the mechanisms aptly described by the synonym list. So far, we have seen few serious collaborations between neurotoxicology and neuroendocrinology, for example. Or projects attempting to integrate mechanisms and outcomes. Perhaps the current climate for funding assigns such research to wishful thinking, but scientists in the past have often succeeded in overcoming such obstacles.
ACKNOWLEDGMENTS
Preparation was supported in part by research grant RC2 ES018736 and Center grant ES01247 from the National Institute of Environmental Health Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrade AJ, Grande SW, Talsness CE, Grote K, Chahoud I. A dose-response study following in utero and lactational exposure to di-(2-ethylhexyl)-phthalate (DEHP): non-monotonic dose-response and low dose effects on rat brain aromatase activity. Toxicology. 2006;227:185–192. doi: 10.1016/j.tox.2006.07.022. [DOI] [PubMed] [Google Scholar]
- ANSES. French Agency for Food, Environmental and Occupational Health & Safety. [Accessed 21 May 2012];Effets sanitaires du bisphénol A. 2011 2009-SA-0331, at http://www.anses.fr/Documents/CHIM-Ra-BisphenolA.pdf.
- Azcoitia I, Yague JG, Garcia-Segura LM. Estradiol synthesis within the human brain. Neuroscience. 2011;191:139–147. doi: 10.1016/j.neuroscience.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Bao AM, Swaab DF. Sexual differentiation of the human brain: relation to gender identity, sexual orientation and neuropsychiatric disorders. Front Neuroendocrinol. 2011;32:214–226. doi: 10.1016/j.yfrne.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Bay K, Asklund C, Skakkebaek NE, Andersson AM. Testicular dysgenesis syndrome: possible role of endocrine disrupters. Best Pract Res Clin Endocrinol Metab. 2006;20:77–90. doi: 10.1016/j.beem.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Beery AK, Zucker I. Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev. 2010 doi: 10.1016/j.neubiorev.2010.07.002. doi: 10.1016/j.neubiorev.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Jonathan N, Hugo ER, Brandebourg TD. Effects of bisphenol A on adipokine release from human adipose tissue: Implications for the metabolic syndrome. Mol Cell Endocrinol. 2009;304:49–54. doi: 10.1016/j.mce.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg GC, Miller MW. Chemical Fallout. Springfield, IL: Charles C. Thomas; 1969. [Google Scholar]
- Blackless M, Charuvastra A, Derryck A, Fausto-Sterling A, Lauzanne K, Lee E. How sexually dimorphic are we? Review and synthesis. Am J Hum Biol. 2000;12:151–166. doi: 10.1002/(SICI)1520-6300(200003/04)12:2<151::AID-AJHB1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Braun JM, Kalkbrenner AE, Calafat AM, Yolton K, Ye X, Dietrich KN, Lanphear BP. Impact of early-life bisphenol A exposure on behavior and executive function in children. Pediatrics. 2011;128:873–882. doi: 10.1542/peds.2011-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Yolton K, Dietrich KN, Hornung R, Ye X, Calafat AM, Lanphear BP. Prenatal bisphenol A exposure and early childhood behavior. Environ Health Perspect. 2009;117:1945–1952. doi: 10.1289/ehp.0900979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GR, Dixson AF. Investigation of the role of postnatal testosterone in the expression of sex differences in behavior in infant rhesus macaques (Macaca mulatta) Horm Behav. 1999;35:186–194. doi: 10.1006/hbeh.1999.1512. [DOI] [PubMed] [Google Scholar]
- Bushnell PJ, Kavlock RJ, Crofton KM, Weiss B, Rice DC. Behavioral toxicology in the 21st century: challenges and opportunities for behavioral scientists. Neurotoxicol Teratol; Summary of a symposium presented at the annual meeting of the neurobehavioral teratology society; June, 2009; 2010. pp. 313–328. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ Health Perspect. 2008;116:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992;305:609–613. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson R. Silent Spring. New York: Houghton Mifflin; 1962. [Google Scholar]
- Carwile JL, Ye X, Zhou X, Calafat AM, Michels KB. Canned soup consumption and urinary bisphenol A: a randomized crossover trial. JAMA. 2011;306:2218–2220. doi: 10.1001/jama.2011.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castoldi AF, Patisaul HB, Aungst J. Geneva: WHO; 2011. Neurotoxic, Neuroendocrine and Neurobehavioural Effects of Bisphenol A. [Google Scholar]
- Cho SC, Bhang SY, Hong YC, Shin MS, Kim BN, Kim JW, Yoo HJ, Cho IH, Kim HW. Relationship between environmental phthalate exposure and the intelligence of school-age children. Environ Health Perspect. 2010;118:1027–1032. doi: 10.1289/ehp.0901376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colborn T, Dumanoski D, Myers JM. Our Stolen Future. New York: Dutton; 1996. [Google Scholar]
- Davies W, Wilkinson LS. It is not all hormones: Alternative explanations for sexual differentiation of the brain. Brain Res. 2006;1126:36–45. doi: 10.1016/j.brainres.2006.09.105. [DOI] [PubMed] [Google Scholar]
- Desvergne B, Feige JN, Casals-Casas C. PPAR-mediated activity of phthalates: A link to the obesity epidemic? Mol Cell Endocrinol. 2009;304:43–48. doi: 10.1016/j.mce.2009.02.017. [DOI] [PubMed] [Google Scholar]
- Dewing P, Shi T, Horvath S, Vilain E. Sexually dimorphic gene expression in mouse brain precedes gonadal differentiation. Brain Res Mol Brain Res. 2003;118:82–90. doi: 10.1016/s0169-328x(03)00339-5. [DOI] [PubMed] [Google Scholar]
- Diamond M. Developmental, sexual and reproductive neuroendocrinology: historical, clinical and ethical considerations. Front Neuroendocrinol. 2011;32:255–263. doi: 10.1016/j.yfrne.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Doherty LF, Bromer JG, Zhou Y, Aldad TS, Taylor HS. In utero exposure to diethylstilbestrol (DES) or bisphenol-A (BPA) increases EZH2 expression in the mammary gland: an epigenetic mechanism linking endocrine disruptors to breast cancer. Horm Cancer. 2010;1:146–155. doi: 10.1007/s12672-010-0015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SM, Miodovnik A, Canfield RL, Zhu C, Silva MJ, Calafat AM, Wolff MS. Prenatal phthalate exposure is associated with childhood behavior and executive functioning. Environ Health Perspect. 2010;118:565–571. doi: 10.1289/ehp.0901470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SM, Zhu C, Berkowitz GS, Calafat AM, Silva MJ, Miodovnik A, Wolff MS. Prenatal phthalate exposure and performance on the Neonatal Behavioral Assessment Scale in a multiethnic birth cohort. Neurotoxicology. 2009 doi: 10.1016/j.neuro.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JS. Environmental anti-androgens and male reproductive health: focus on phthalates and testicular dysgenesis syndrome. Reproduction. 2004;127:305–315. doi: 10.1530/rep.1.00025. [DOI] [PubMed] [Google Scholar]
- Fisher JS, Macpherson S, Marchetti N, Sharpe RM. Human 'testicular dysgenesis syndrome': a possible model using in-utero exposure of the rat to dibutyl phthalate. Hum Reprod. 2003;18:1383–1394. doi: 10.1093/humrep/deg273. [DOI] [PubMed] [Google Scholar]
- Frederiksen H, Sorensen K, Mouritsen A, Aksglaede L, Hagen CP, Petersen JH, Skakkebaek NE, Andersson AM, Juul A. High urinary phthalate concentration associated with delayed pubarche in girls. Int J Androl. 2012 doi: 10.1111/j.1365-2605.2012.01260.x. [DOI] [PubMed] [Google Scholar]
- Fry DM. Reproductive effects in birds exposed to pesticides and industrial chemicals. Environ Health Perspect. 1995;103(Suppl 7):165–171. doi: 10.1289/ehp.95103s7165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS, Jr, Faraone SV, Tsuang MT. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb Cortex. 2001;11:490–497. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- Hall MG, Quignodon L, Desvergne B. Peroxisome Proliferator-Activated Receptor beta/delta in the Brain: Facts and Hypothesis. PPAR Res. 2008;2008:780452. doi: 10.1155/2008/780452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa RJ, Weiser MJ, Zuloaga DG. A role for the androgen metabolite, 5alpha-androstane-3beta, 17beta-diol, in modulating oestrogen receptor beta-mediated regulation of hormonal stress reactivity. J Neuroendocrinol. 2009;21:351–358. doi: 10.1111/j.1365-2826.2009.01840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris CA. Sex-change chemicals and their influence on the brain. ScientificWorldJournal. 2001;1:681–683. doi: 10.1100/tsw.2001.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch EE, Nelson JW, Qureshi MM, Weinberg J, Moore LL, Singer M, Webster TF. Association of urinary phthalate metabolite concentrations with body mass index and waist circumference: a cross-sectional study of NHANES data, 1999–2002. Environ Health. 2008;7:27. doi: 10.1186/1476-069X-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojo R, Stern S, Zareba G, Markowski VP, Cox C, Kost JT, Weiss B. Sexually dimorphic behavioral responses to prenatal dioxin exposure. Environ Health Perspect. 2002;110:247–254. doi: 10.1289/ehp.02110247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover RN, Hyer M, Pfeiffer RM, Adam E, Bond B, Cheville AL, Colton T, Hartge P, Hatch EE, Herbst AL, Karlan BY, Kaufman R, Noller KL, Palmer JR, Robboy SJ, Saal RC, Strohsnitter W, Titus-Ernstoff L, Troisi R. Adverse health outcomes in women exposed in utero to diethylstilbestrol. N Engl J Med. 2011;365:1304–1314. doi: 10.1056/NEJMoa1013961. [DOI] [PubMed] [Google Scholar]
- Howdeshell KL, Peterman PH, Judy BM, Taylor JA, Orazio CE, Ruhlen RL, Vom Saal FS, Welshons WV. Bisphenol A is released from used polycarbonate animal cages into water at room temperature. Environ Health Perspect. 2003;111:1180–1187. doi: 10.1289/ehp.5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howdeshell KL, Rider CV, Wilson VS, Gray LE., Jr Mechanisms of action of phthalate esters, individually and in combination, to induce abnormal reproductive development in male laboratory rats. Environ Res. 2008;108:168–176. doi: 10.1016/j.envres.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Hunt GL, Jr, Hunt MW. Female-Female Pairing in Western Gulls (Larus occidentalis) in Southern California. Science. 1977;196:1466–1467. doi: 10.1126/science.196.4297.1466. [DOI] [PubMed] [Google Scholar]
- Hunt P. Lab disinfectant harms mouse fertility. Patricia Hunt interviewed by Brendan Maher. Nature. 2008;453:964. doi: 10.1038/453964a. [DOI] [PubMed] [Google Scholar]
- Hurst CH, Waxman DJ. Environmental phthalate monoesters activate pregnane X receptor-mediated transcription. Toxicol Appl Pharmacol. 2004;199:266–274. doi: 10.1016/j.taap.2003.11.028. [DOI] [PubMed] [Google Scholar]
- Koch HM, Calafat AM. Human body burdens of chemicals used in plastic manufacture. Philos Trans R Soc Lond B Biol Sci. 2009;364:2063–2078. doi: 10.1098/rstb.2008.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkle AT, McCarthy MM. Developmental time course of estradiol, testosterone, and dihydrotestosterone levels in discrete regions of male and female rat brain. Endocrinology. 2011;152:223–235. doi: 10.1210/en.2010-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundakovic M, Champagne FA. Epigenetic perspective on the developmental effects of bisphenol A. Brain Behav Immun. 2011;25:1084–1093. doi: 10.1016/j.bbi.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamminmaki A, Hines M, Kuiri-Hanninen T, Kilpelainen L, Dunkel L, Sankilampi U. Testosterone measured in infancy predicts subsequent sex-typed behavior in boys and in girls. Horm Behav. 2012;61:611–616. doi: 10.1016/j.yhbeh.2012.02.013. [DOI] [PubMed] [Google Scholar]
- Leranth C, Hajszan T, Szigeti-Buck K, Bober J, MacLusky NJ. Bisphenol A prevents the synaptogenic response to estradiol in hippocampus and prefrontal cortex of ovariectomized nonhuman primates. Proc Natl Acad Sci U S A. 2008;105:14187–14191. doi: 10.1073/pnas.0806139105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leranth C, Szigeti-Buck K, Maclusky NJ, Hajszan T. Bisphenol A prevents the synaptogenic response to testosterone in the brain of adult male rats. Endocrinology. 2008;149:988–994. doi: 10.1210/en.2007-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Ge RS, Chen GR, Hu GX, Dong L, Lian QQ, Hardy DO, Sottas CM, Li XK, Hardy MP. Involvement of testicular growth factors in fetal Leydig cell aggregation after exposure to phthalate in utero. Proc Natl Acad Sci U S A. 2008;105:7218–7222. doi: 10.1073/pnas.0709260105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M, Kondo T, Ban S, Umemura T, Kurahashi N, Takeda M, Kishi R. Exposure of prepubertal female rats to inhaled di(2-ethylhexyl)phthalate affects the onset of puberty and postpubertal reproductive functions. Toxicol Sci. 2006;93:164–171. doi: 10.1093/toxsci/kfl036. [DOI] [PubMed] [Google Scholar]
- Mack CM, McGivern RF, Hyde LA, Denenberg VH. Absence of postnatal testosterone fails to demasculinize the male rat's corpus callosum. Brain Res Dev Brain Res. 1996;95:252–255. doi: 10.1016/0165-3806(96)00093-4. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Clark AS, Naftolin F, Goldman-Rakic PS. Estrogen formation in the mammalian brain: possible role of aromatase in sexual differentiation of the hippocampus and neocortex. Steroids. 1987;50:459–474. doi: 10.1016/0039-128x(87)90032-8. [DOI] [PubMed] [Google Scholar]
- Markey CM, Rubin BS, Soto AM, Sonnenschein C. Endocrine disruptors: from Wingspread to environmental developmental biology. J Steroid Biochem Mol Biol. 2002;83:235–244. doi: 10.1016/s0960-0760(02)00272-8. [DOI] [PubMed] [Google Scholar]
- Markowski VP, Zareba G, Stern S, Cox C, Weiss B. Altered operant responding for motor reinforcement and the determination of benchmark doses following perinatal exposure to low-level 2,3,7,8-tetrachlorodibenzo-p-dioxin. Environ Health Perspect. 2001;109:621–627. doi: 10.1289/ehp.01109621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Money J, Clarke F, Mazur T. Families of seven male-to-female transexuals after 5–7 years: Sociological sexology. Arch Sex Behav. 1975;4:187–197. doi: 10.1007/BF01541082. [DOI] [PubMed] [Google Scholar]
- Mouritsen A, Aksglaede L, Sorensen K, Mogensen SS, Leffers H, Main KM, Frederiksen H, Andersson AM, Skakkebaek NE, Juul A. Hypothesis: exposure to endocrine-disrupting chemicals may interfere with timing of puberty. Int J Androl. 2010;33:346–359. doi: 10.1111/j.1365-2605.2010.01051.x. [DOI] [PubMed] [Google Scholar]
- Mu Y, Gage FH. Adult hippocampal neurogenesis and its role in Alzheimer's disease. Mol Neurodegener. 2011;6:85. doi: 10.1186/1750-1326-6-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylchreest E, Sar M, Cattley RC, Foster PM. Disruption of androgen-regulated male reproductive development by di(n-butyl) phthalate during late gestation in rats is different from flutamide. Toxicol Appl Pharmacol. 1999;156:81–95. doi: 10.1006/taap.1999.8643. [DOI] [PubMed] [Google Scholar]
- Mylchreest E, Sar M, Wallace DG, Foster PM. Fetal testosterone insufficiency and abnormal proliferation of Leydig cells and gonocytes in rats exposed to di(n-butyl) phthalate. Reprod Toxicol. 2002;16:19–28. doi: 10.1016/s0890-6238(01)00201-5. [DOI] [PubMed] [Google Scholar]
- Nicolson TJ, Mellor HR, Roberts RR. Gender differences in drug toxicity. Trends Pharmacol Sci. 2010;31:108–114. doi: 10.1016/j.tips.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Oertelt-Prigione S, Parol R, Krohn S, Preissner R, Regitz-Zagrosek V. Analysis of sex and gender-specific research reveals a common increase in publications and marked differences between disciplines. BMC Med. 2010;8:70. doi: 10.1186/1741-7015-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parveen M, Inoue A, Ise R, Tanji M, Kiyama R. Evaluation of estrogenic activity of phthalate esters by gene expression profiling using a focused microarray (EstrArray) Environ Toxicol Chem. 2008;27:1416–1425. doi: 10.1897/07-399. [DOI] [PubMed] [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Reinius B, Jazin E. Prenatal sex differences in the human brain. Mol Psychiatry. 2009;14:987, 988–989. doi: 10.1038/mp.2009.79. [DOI] [PubMed] [Google Scholar]
- Rubin BS. Bisphenol A: An endocrine disruptor with widespread exposure and multiple effects. J Steroid Biochem Mol Biol. 2011 doi: 10.1016/j.jsbmb.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Rudel RA, Gray JM, Engel CL, Rawsthorne TW, Dodson RE, Ackerman JM, Rizzo J, Nudelman JL, Brody JG. Food packaging and bisphenol A and bis(2-ethyhexyl) phthalate exposure: findings from a dietary intervention. Environ Health Perspect. 2011;119:914–920. doi: 10.1289/ehp.1003170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sax L. Boys Adrift: The Five Factors Driving the Growing Epidemic of Unmotivated Boys and Underachieving Young Men. New York: Basic Books; 2007. [Google Scholar]
- Schecter A, Startin J, Wright C, Kelly M, Papke O, Lis A, Ball M, Olson JR. Congener-specific levels of dioxins and dibenzofurans in U.S. food and estimated daily dioxin toxic equivalent intake. Environ Health Perspect. 1994;102:962–966. doi: 10.1289/ehp.94102962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schug TT, Janesick A, Blumberg B, Heindel JJ. Endocrine disrupting chemicals and disease susceptibility. J Steroid Biochem Mol Biol. 2011;127:204–215. doi: 10.1016/j.jsbmb.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe RM. "Additional" effects of phthalate mixtures on fetal testosterone production. Toxicol Sci. 2008;105:1–4. doi: 10.1093/toxsci/kfn123. [DOI] [PubMed] [Google Scholar]
- Sharpe RM, Fisher JS, Millar MM, Jobling S, Sumpter JP. Gestational and lactational exposure of rats to xenoestrogens results in reduced testicular size and sperm production. Environ Health Perspect. 1995;103:1136–1143. doi: 10.1289/ehp.951031136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe RM, Skakkebaek NE. Testicular dysgenesis syndrome: mechanistic insights and potential new downstream effects. Fertil Steril. 2008;89:e33–e38. doi: 10.1016/j.fertnstert.2007.12.026. [DOI] [PubMed] [Google Scholar]
- Sharpe RM, Skakkebaek NE. Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? Lancet. 1993;341:1392–1395. doi: 10.1016/0140-6736(93)90953-e. [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE. Testicular dysgenesis syndrome. Horm Res. 2003;60(Suppl 3):49. doi: 10.1159/000074499. [DOI] [PubMed] [Google Scholar]
- Stahlhut RW, van Wijngaarden E, Dye TD, Cook S, Swan SH. Concentrations of urinary phthalate metabolites are associated with increased waist circumference and insulin resistance in adult U.S. males. Environ Health Perspect. 2007;115:876–882. doi: 10.1289/ehp.9882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH. Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environ Res. 2008;108:177–184. doi: 10.1016/j.envres.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH, Liu F, Hines M, Kruse RL, Wang C, Redmon JB, Sparks A, Weiss B. Prenatal phthalate exposure and reduced masculine play in boys. Int J Androl. 2010;33:259–269. doi: 10.1111/j.1365-2605.2009.01019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH, Main KM, Liu F, Stewart SL, Kruse RL, Calafat AM, Mao CS, Redmon JB, Ternand CL, Sullivan S, Teague JL. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect. 2005;113:1056–1061. doi: 10.1289/ehp.8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton J, Zehr JL, Loose MD. Effects of prenatal androgens on rhesus monkeys: a model system to explore the organizational hypothesis in primates. Horm Behav. 2009;55:633–645. doi: 10.1016/j.yhbeh.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilson HA. Developmental neurotoxicology of endocrine disruptors and pesticides: identification of information gaps and research needs. Environ Health Perspect. 1998;106(Suppl 3):807–811. doi: 10.1289/ehp.98106807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toppari J, Larsen JC, Christiansen P, Giwercman A, Grandjean P, Guillette LJ, Jr, Jegou B, Jensen TK, Jouannet P, Keiding N, Leffers H, McLachlan JA, Meyer O, Muller J, Rajpert-De Meyts E, Scheike T, Sharpe R, Sumpter J, Skakkebaek NE. Male reproductive health and environmental xenoestrogens. Environ Health Perspect. 1996;104(Suppl 4):741–803. doi: 10.1289/ehp.96104s4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Jr, Lee DH, Shioda T, Soto AM, Vom Saal FS, Welshons WV, Zoeller RT, Myers JP. Hormones and Endocrine-Disrupting Chemicals: Low-Dose Effects and Nonmonotonic Dose Responses. Endocr Rev. 2012 doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr Rev. 2009;30:75–95. doi: 10.1210/er.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vom Saal FS, Akingbemi BT, Belcher SM, Birnbaum LS, Crain DA, Eriksen M, Farabollini F, Guillette LJ, Jr, Hauser R, Heindel JJ, Ho SM, Hunt PA, Iguchi T, Jobling S, Kanno J, Keri RA, Knudsen KE, Laufer H, LeBlanc GA, Marcus M, McLachlan JA, Myers JP, Nadal A, Newbold RR, Olea N, Prins GS, Richter CA, Rubin BS, Sonnenschein C, Soto AM, Talsness CE, Vandenbergh JG, Vandenberg LN, Walser-Kuntz DR, Watson CS, Welshons WV, Wetherill Y, Zoeller RT. Chapel Hill bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod Toxicol. 2007;24:131–138. doi: 10.1016/j.reprotox.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vom Saal FS, Myers JP. Bisphenol A and risk of metabolic disorders. JAMA. 2008;300:1353–1355. doi: 10.1001/jama.300.11.1353. [DOI] [PubMed] [Google Scholar]
- Wald C, Wu C. Biomedical research. Of mice and women: the bias in animal models. Science. 2010;327:1571–1572. doi: 10.1126/science.327.5973.1571. [DOI] [PubMed] [Google Scholar]
- Wallen K. The Organizational Hypothesis: Reflections on the 50th anniversary of the publication of Phoenix, Goy, Gerall, and Young (1959) Horm Behav. 2009;55:561–565. doi: 10.1016/j.yhbeh.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Weiss B. Endocrine disruptors as a threat to neurological function. J Neurol Sci. 2011;305:11–21. doi: 10.1016/j.jns.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B. Same sex, no, sex, unaware sex in neurotoxicology. Neurotoxicology. 2010 doi: 10.1016/j.neuro.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B. Same sex, no sex, unaware sex in neurotoxicology. Neurotoxicology. 2010 doi: 10.1016/j.neuro.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B. Can endocrine disruptors influence neuroplasticity in the aging brain? Neurotoxicology. 2007;28:938–950. doi: 10.1016/j.neuro.2007.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B. Sexually dimorphic nonreproductive behaviors as indicators of endocrine disruption. Environ Health Perspect. 2002;110(Suppl 3):387–391. doi: 10.1289/ehp.02110s3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B. Vulnerability to pesticide neurotoxicity is a lifetime issue. Neurotoxicology. 2000;21:67–73. [PubMed] [Google Scholar]
- Weiss B. Endocrine disruptors and sexually dimorphic behaviors: a question of heads and tails. Neurotoxicology. 1997;18:581–586. [PubMed] [Google Scholar]
- Weiss B. Endocrine disruptors and sexually dimorphic behaviors: a question of heads and tails. Neurotoxicology. 1997;18:581–586. [PubMed] [Google Scholar]
- Welshons WV, Nagel SC, vom Saal FS. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology. 2006;147:S56–S69. doi: 10.1210/en.2005-1159. [DOI] [PubMed] [Google Scholar]
- Wohlfahrt-Veje C, Main KM, Skakkebaek NE. Testicular dysgenesis syndrome: foetal origin of adult reproductive problems. Clin Endocrinol (Oxf) 2009;71:459–465. doi: 10.1111/j.1365-2265.2009.03545.x. [DOI] [PubMed] [Google Scholar]
- Wolstenholme JT, Rissman EF, Connelly JJ. The role of Bisphenol A in shaping the brain, epigenome and behavior. Horm Behav. 2011;59:296–305. doi: 10.1016/j.yhbeh.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolton K, Xu Y, Strauss D, Altaye M, Calafat AM, Khoury J. Prenatal exposure to bisphenol A and phthalates and infant neurobehavior. Neurotoxicol Teratol. 2011;33:558–566. doi: 10.1016/j.ntt.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]