Abstract
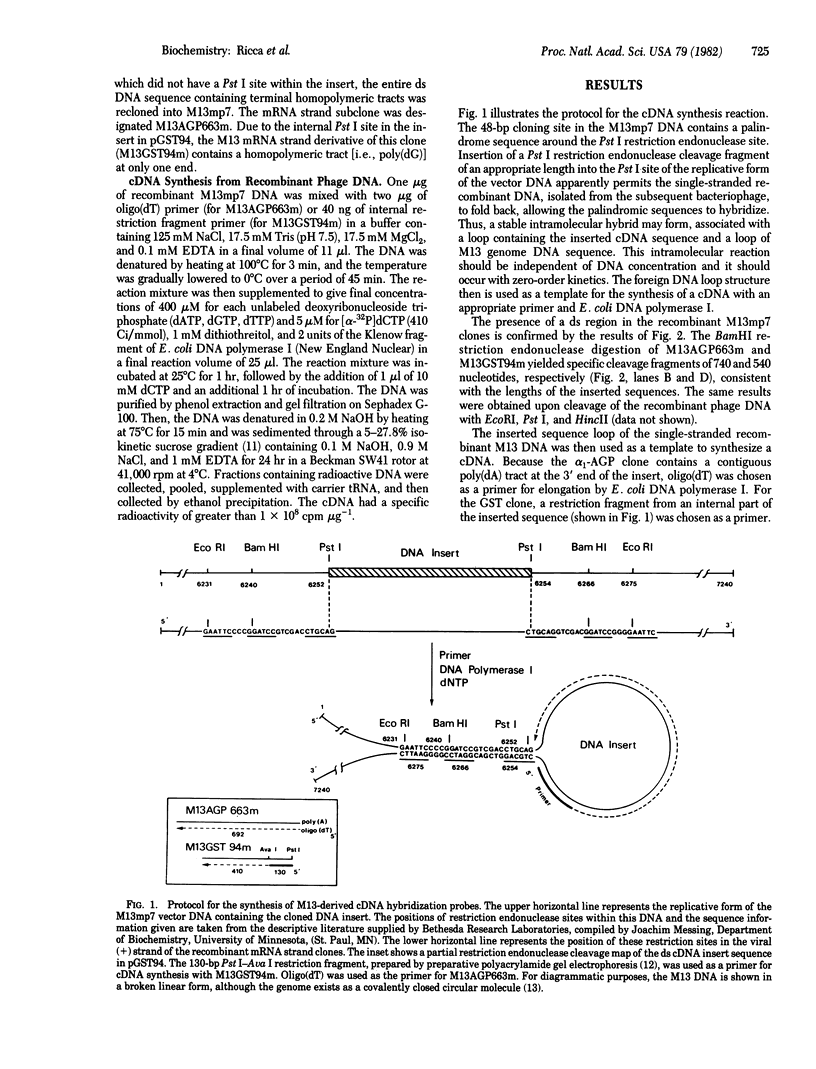
Double-stranded cDNA sequences for rat alpha 1-acid glycoprotein and rat glutathione S-transferase mRNAs were inserted into the Pst I site of bacteriophage M13mp7 and used to develop a new method for preparing specific cDNA hybridization probes directly from cloned template DNA. A palindrome sequence surrounding the Pst I site in the vector DNA permitted single-stranded DNA isolated from the recombinant phage to fold back, thus forming a stable hybrid bounded on the ends by a large loop of M13mp7 single-stranded DNA and a small loop of inserted foreign DNA. A primer corresponding to an internal sequence of the foreign DNA was hybridized, then Escherichia coli DNA polymerase I was used to synthesize a 32P-labeled complementary DNA copy of the cloned inserted DNA. The single-stranded cDNA reaction product was easily isolated by subsequent sedimentation through alkaline sucrose gradients. Gel electrophoresis of the labeled cDNA product, after denaturation with glyoxal, indicated a single discrete band with an electrophoretic mobility corresponding to the length of the inserted DNA sequence. About 95% of the cDNA product formed S1 nuclease-resistant hybrids in hybridization reactions with excess RNA in solution. DNA sequences complementary to the M13mp7 vector DNA were not detected in the cDNA product. Thus, these M13mp7-derived probes are the functional equivalent of cDNA copies to mRNAs and can be employed for quantitative measurements of mRNA concentration. This simple, rapid method probably can be used for most cloned DNA sequences to yield single-stranded radioactively labeled DNA, without contaminating vector DNA sequences, for virtually any hybridization requirement.
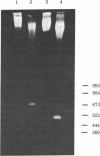
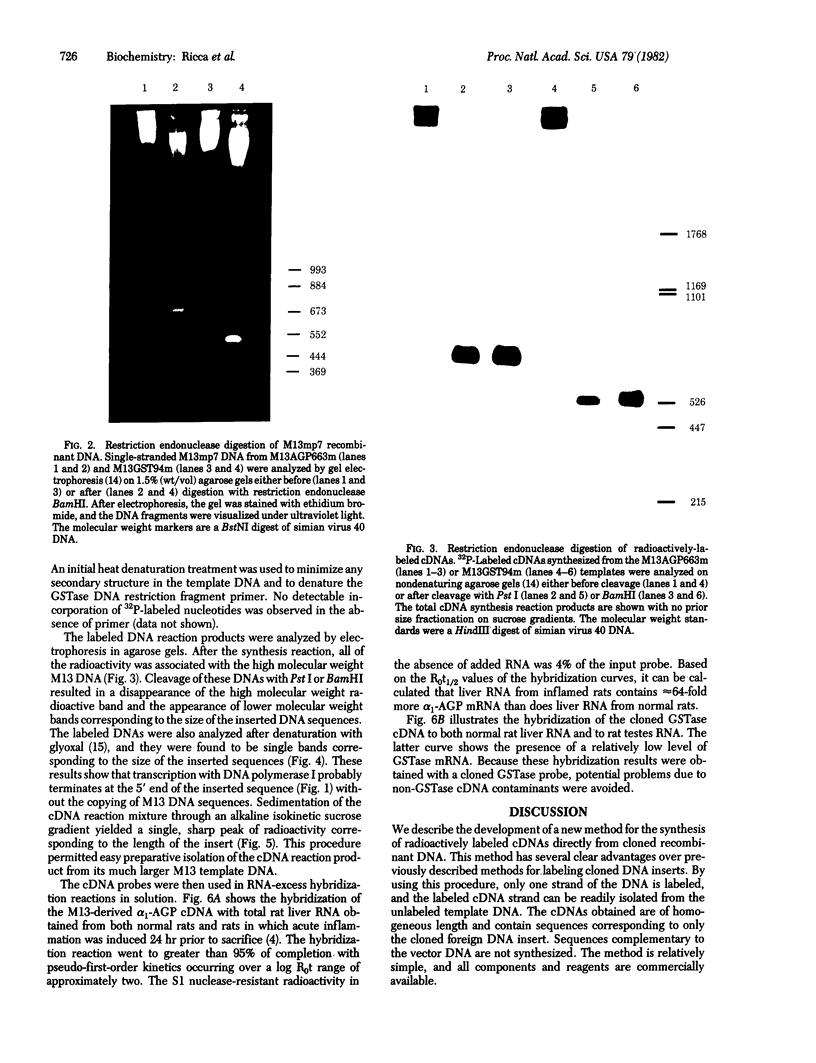
Full text
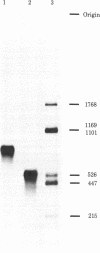
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Parker B. A., Reiser J., Renart J., Stark G. R., Wahl G. M. Detection of specific RNAs or specific fragments of DNA by fractionation in gels and transfer to diazobenzyloxymethyl paper. Methods Enzymol. 1979;68:220–242. doi: 10.1016/0076-6879(79)68017-5. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W. S., Ricca G. A., Taylor J. M. Cloning of rat alpha-fetoprotein 3'-terminal complementary deoxyribonucleic acid sequences and preparation of radioactively labeled hybridization probes from cloned deoxyribonucleic acid inserts. Biochemistry. 1981 Mar 17;20(6):1646–1652. doi: 10.1021/bi00509a037. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Marvin D. A., Hohn B. Filamentous bacterial viruses. Bacteriol Rev. 1969 Jun;33(2):172–209. doi: 10.1128/br.33.2.172-209.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McCarty K. S., Jr, Vollmer R. T., McCarty K. S. Improved computer program data for the resolution and fractionation of macromolecules by isokinetic sucrose density gradient sedimentation. Anal Biochem. 1974 Sep;61(1):165–183. doi: 10.1016/0003-2697(74)90343-1. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgard M. V., Keem K., Monahan J. J. Factors affecting the transformation of Escherichia coli strain chi1776 by pBR322 plasmid DNA. Gene. 1978 Jul;3(4):279–292. doi: 10.1016/0378-1119(78)90038-0. [DOI] [PubMed] [Google Scholar]
- Ricca G. A., Hamilton R. W., McLean J. W., Conn A., Kalinyak J. E., Taylor J. M. Rat alpha 1-acid glycoprotein mRNA. Cloning of double-stranded cDNA and kinetics of induction of mRNA levels following acute inflammation. J Biol Chem. 1981 Oct 25;256(20):10362–10368. [PubMed] [Google Scholar]
- Ricca G. A., Taylor J. M. Nucleotide sequence of rat alpha 1-acid glycoprotein messenger RNA. J Biol Chem. 1981 Nov 10;256(21):11199–11202. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]