Abstract
Stromal interaction molecule (STIM) proteins function in cells as dynamic coordinators of cellular calcium (Ca2+) signals. Spanning the endoplasmic reticulum (ER) membrane, they sense tiny changes in the levels of Ca2+ stored within the ER lumen. As ER Ca2+ is released to generate primary Ca2+ signals, STIM proteins undergo an intricate activation reaction and rapidly translocate into junctions formed between the ER and the plasma membrane. There, STIM proteins tether and activate the highly Ca2+-selective Orai channels to mediate finely controlled Ca2+ signals and to homeostatically balance cellular Ca2+. Details are emerging on the remarkable organization within these STIM-induced junctional microdomains and the identification of new regulators and alternative target proteins for STIM.
Calcium (Ca2+) signals are crucial for the control a broad range of cellular functions, including secretion, excitation, contraction, motility, metabolism, transcription, growth, cell division and apoptosis. The numerous pumps and channels that comprise the machinery for generating cellular Ca2+ signals are functionally well defined. However, less understood are the mechanisms that coordinate the operation of this machinery to generate the temporally and spatially precise Ca2+ signals that selectively control individual cell functions. Ca2+ signalling involves the concerted action of Ca2+ release channels in Ca2+ storage organelles and Ca2+ entry channels in the plasma membrane (BOX 1). The recently identified stromal interaction molecule (STIM) proteins1,2, STIM1 and STIM2, are crucial in coordinating Ca2+ release and entry signals and in maintaining cellular Ca2+ homeostasis.
Box 1. Principles of Ca2+ signalling.
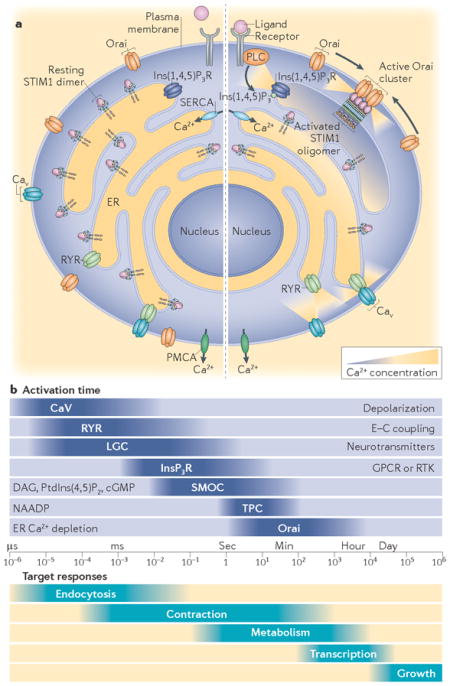
The events that maintain cellular Ca2+ signalling during homeostasis are shown in part a of the figure. Resting cells (left) maintain cytosolic Ca2+ in the nM range through sarcoplasmic reticulum Ca2+ ATPase (SERCA) and plasma membrane Ca2+ ATPase (PMCA) pumps. Following ligand binding to phospholipase C (PLC)-coupled receptors (right), the second messengers inositol-1,4,5-trisphosphate (Ins(1,4,5) P3) and diacylglycerol (DAG) are generated through breakdown of phosphatidylinositol-4,5- bisphosphate (PtdIns(4,5)P2). Ins(1,4,5)P3 diffuses rapidly within the cytosol to interact with endoplasmic reticulum (ER)-located Ins(1,4,5)P3 receptors (Ins(1,4,5)P3Rs), which are channels that release Ca2+ from the ER lumen to generate the initial Ca2+ signal phase149. Following depletion of ER Ca2+ (right), stromal interaction molecule (STIM) proteins are activated and translocate by diffusion into ER–plasma membrane junctions, where they interact with the plasma membrane. Here, STIM proteins tether and gate Orai1 Ca2+ entry channels. ER Ca2+ release leading to STIM activation can also be mediated by ryanodine receptor (RYR) activation. In skeletal muscle, RYRs are permanently coupled to plasma membrane voltage-operated Ca2+ channel (CaV) isoform CaV1.1, which is activated by depolarization. In other cells, RYRs are activated by the entry of Ca2+ into the cytosol through other CaV channel subtypes.
The properties and function of different Ca2+ channels are shown in part b of the figure. Ca2+ signals are generated by several different channels with widely differing timescales of activation (upper panel). As a result, each Ca2+ channel mediates temporally distinct Ca2+-dependent cellular responses (lower panel). Voltage-operated CaV channels are activated by membrane-depolarization in the μS timescale. In skeletal muscle, RYRs are coupled to CaV channels and open rapidly thereafter. In cardiac muscle, smooth muscle and neurons, RYR activation is slightly slower than in skeletal muscle and depends on CaV-mediated Ca2+ entry. Ligand-gated channels (LGCs) are activated by the binding of extracellular ligands (neurotransmitters such as N-methyl-d-aspartate (NMDA), acetyl choline or nucleotides) and display varying activation kinetics depending on the rate of ligand induction or release. The activation rates of Ins(1,4,5) P3Rs, second messenger-operated channels (SMOCs) and two-pore channels (TPCs) depend on the production rates of their respective ligands. Orai channels have the slowest activation kinetics, which depend on the rate of Ca2+ release from the ER and on the diffusion rate of STIM and Orai proteins into ER– plasma membrane junctions. Orai channels can remain active for long time periods under conditions of prolonged store depletion. The cellular responses shown in the lower panel are commensurate with the timescales of channel activation: CaV and RYR channels mediate rapid events including exocytosis and contraction, whereas Orai channels regulate long-term events such as gene transcription. cGMP, cyclic GMP; E–C coupling, exitation–contraction coupling; NAADP, nicotinic acid adenine dinucleotide phosphate; RTK, receptor Tyr kinase.
STIM proteins are finely tuned sensors of Ca2+ levels in the interior of the endoplasmic reticulum (ER). ER Ca2+ levels are needed for generating rapid Ca2+ signals in cells, but they are also vital for maintaining the correct protein folding and trafficking environment within the ER. Thus, decreased ER luminal Ca2+ levels are a major stress condition. Small changes in ER Ca2+ levels trigger STIM proteins to rapidly translocate into specialized junctions where they interact with the plasma membrane. There, STIM proteins directly couple with and gate plasma membrane Orai channels3–5, which mediate an extraordinarily selective entry of Ca2+ ions. The entering Ca2+ ions provide precise local Ca2+ signals that are crucial for controlling long-term cellular responses, including gene expression and growth. The entering Ca2+ is also essential for the homeostatic control of cellular Ca2+ levels.
Emerging studies reveal much about the molecular sensing properties of STIM proteins: how they become activated, how they unfold and form complexes that can translocate into junctions and how they specifically engage and activate their channel targets. New information reveals that the junctions have an astonishing complexity, comprising an array of previously known and newly identified regulatory proteins. Organized around STIM proteins, this complex of proteins finely choreographs the interactions within junctional domains and controls the flow of Ca2+ to generate the exact Ca2+ signals that are required by the cell. Recent studies have also revealed that the junctions include several other crucial Ca2+ channels and Ca2+ pumps that are controlled by STIM proteins. Hence, in addition to generating finely tuned Ca2+ signals, STIM proteins are essential modulators of the Ca2+ homeostatic machinery in cells. Here, we describe the current understanding on STIM proteins as central communicating intermediaries in cellular signalling and highlight some fascinating new areas of work that provide insights into their structure and mechanism of activation.
Store-operated Ca2+ entry: concept and history
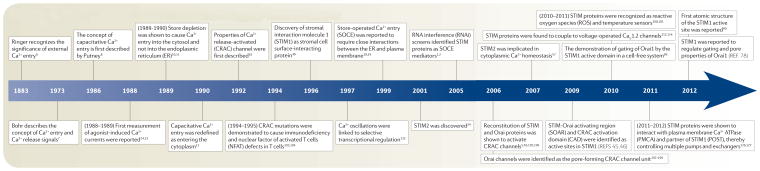
The concept of Ca2+ signalling was first recognized by Ringer who revealed the importance of extracellular Ca2+ in maintaining contraction of isolated hearts6. The regulation of intracellular Ca2+ levels and the generation of Ca2+ signals are much newer concepts7. Ca2+ signals are a combination of both Ca2+ entry across the plasma membrane and Ca2+ release from intracellular stores, predominantly from the ER in higher eukaryotic cells. The causal relationship and coordinated control of these two processes was not appreciated until Putney proposed the model of capacitative Ca2+ entry in 1986 (REF. 8) (TIMELINE). After the discovery that inositol-1,4,5-triphosphate (Ins(1,4,5)P3) activates Ca2+ release from ER Ca2+ stores9, the apparently ‘privileged’ movement of Ca2+ from outside to replenish stores led Putney to propose that “a specialized region exists where ER and plasma membrane are closely apposed and Ca2+ diffusion laterally is therefore geometrically restricted”8. This concept was visionary. However, the idea of a direct capacitative pathway allowing extracellular Ca2+ to directly enter depleted Ca2+ stores needed refinement. Studies showed that Ca2+ store depletion activates Ca2+ entry initially into the cytosol10,11, a process that is triggered solely by decreased Ca2+ store content and does not require Ins(1,4,5)P3 production12. Thus, Putney presented a revised model in 1990 depicting the activation of Ca2+ channels in the plasma membrane as a direct consequence of Ca2+ store depletion13, the process now referred to as store-operated Ca2+ entry (SOCE). Simultaneously, small agonist-induced Ca2+ currents were being defined in haematopoietic cells14,15, and in 1992 Hoth and Penner16 described the Ca2+ release-activated Ca2+ current (CRAC current), which is a small, inwardly rectifying, highly Ca2+-selective current, in mast cells. In keeping with the recognition by Putney that Ca2+ entry is solely related to the Ca2+ content of the ER, the CRAC current is activated regardless of whether Ca2+ is released from stores following Ins(1,4,5)P3 receptor (Ins(1,4,5) P3R) activation, ER Ca2+ pump blockade, Ca2+ ionophore application or luminal Ca2+ chelation. Theories to explain how sensing of ER Ca2+ stores and the activation of specific channels could be coupled ranged from ER-derived diffusible messengers to direct conformational coupling between ER and plasma membrane proteins as envisaged by Berridge17. Close ER–plasma membrane interactions were clearly important for coupling to occur18,19, and, ultimately, with the identification of STIM proteins in 2005 (REFS 1,2) and Orai channels a year later3–5, the original ER–plasma membrane junctional coupling model of Putney8 was proven correct.
Timeline.
Major events in the discovery and characterization of store-operated Ca2+ entry
The discovery of the involvement of STIM proteins in SOCE came from two studies performing RNA interference (RNAi) screens. The first study, which examined Ca2+ responses in Drosophila melanogaster S2 cells, led to the identification of the single D. melanogaster STIM protein1, the other study, which monitored Ca2+ signalling in HeLa cells, identified the pair of human STIM proteins2. STIM proteins are now clearly recognized as the store Ca2+ sensors that trigger SOCE (BOX 1; FIG. 1a). Three genome-wide screens in S2 cells were successful in identifying the SOCE channel3–5 that is now known as Orai (FIG. 1b). In mammals, there are three Orai genes encoding store-operated channels with significantly different properties. The gene encoding Orai1 was identified by linkage analysis as being mutated in individuals with a rare immunodeficiency, in which T cells display defective SOCE3. Subsequently, other mutations in ORAI1 (REF. 20) and STIM1 (REF. 21) have been linked to human immunodeficiencies. Overall, the revelation that STIM proteins could mediate SOCE1,2 provided the mechanistic coupling paradigm predicted within the models of Putney8,13 and Berridge17.
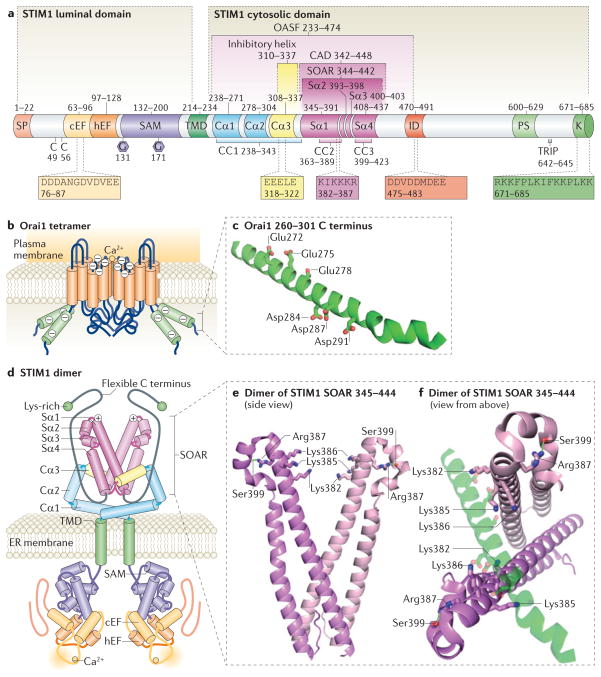
Figure 1. Structure and activation of STIM1.
a| The molecular domains of stromal interaction molecule 1 (STIM1). Endoplasmic reticulum (ER) STIM1 contains a luminal and a cytosolic domain. The amino-terminal signal peptide (SP) is cleaved during translation. The ER luminal N-terminal domain includes a conserved Cys pair, a Ca2+-binding canonical EF-hand domain, (cEF), a non-Ca2+-binding hidden EF-hand (hEF) domain, a sterile α-motif (SAM) with two Asn-linked glycosylation sites (shown as hexagons) and a single transmembrane domain (TMD). The cytosolic carboxy-terminal domain is considered to include three coiled-coil regions74 (called CC1, CC2 and CC3). CC1 is divided into three α-helices (termed Cα1, Cα2 and Cα3) on the basis of sequence analysis predictions by using JPred3 (REF. 188). The structure of Cα3 is also determined on the basis of homology with the recently solved Caenorhabditis elegans STIM structure40. SOAR (STIM–Orai activating region) is the minimal sequence required for the activation of Orai1 (REF. 45). SOAR contains four α-helices, termed Sα1, Sα2, Sα3 and Sα4 (REF. 40). The segments CAD (Ca2+ release-activated Ca2+ (CRAC) activation domain)46 and OASF (Orai1-activating small fragment)47 are larger than SOAR, contain the CC1 region and also activate Orai1. SOAR includes the polybasic region, with the sequence KIKKKR (amino acids 382–387), which is crucial for the interaction with Orai1 (REFS 40,68,71). Cα3 contains an inhibitory helix that inhibits SOAR function40,68,71. The acidic EEELE (residues 318–322) region is required for the action of the inhibitory helix40,68. Downstream of SOAR resides an acidic inhibitory domain (ID) that mediates fast Ca2+-dependent inactivation of Orai1 (REFS 89,92,93). The C-terminal tail contains a Pro/Ser-rich domain (PS), a microtubule interacting domain (TRIP) and a Lys-rich domain responsible for phospholipid interaction at the plasma membrane. b | The tetrameric structure of the Orai1 channel is shown, highlighting the TMDs (shown in orange), extracellular and intracellular sequences (blue) and C-terminal predicted α-helices (shown in green). Negatively charged residues are indicated in the C-terminal helices and in the Ca2+ selectivity filter at the predicted mouth of the pore. c | The predicted α-helical structure of the Orai1 C terminus and potential sites for SOAR binding are illustrated on the basis of secondary structure prediction by using JPred3 (REF. 188). Side chains from acidic residues Glu272, Glu275, Glu278, Asp284, Asp287 and Asp291 are shown as amphipathic helices that may electrostatically interact with basic residues in the SOAR dimer. d | Proposed structure of a resting STIM1 dimer. The predicted α-helices in CC1 are indicated in the figure and are shown in a folded configuration. The inhibitory Cα3 helix (highlighted in yellow) is bound to SOAR (shown in red), which comprises four α-helices as indicated. The SOAR polybasic region is shown (+). The STIM1 dimer is held together predominantly by interactions between the CC1 and SOAR regions37,40. The C-terminal flexible region (shown in grey) together with the C-terminal Lys-rich domain (shown in green) may provide some steric shielding of SOAR77. α-helices are shown as cylinders; flexible regions as lines. e | The side view of the SOAR structure reveals that Lys382, Lys385 and Lys386 are located in the polybasic Orai-interacting region, and that these residues are oriented towards the centre of the cleft. Lys384 (not shown) is oriented in another direction. The Arg387 residue may be involved in hydrogen bonding with Ser399 (indicated by a green line). Coordinates were obtained from Protein data bank entry 3TEQ40. f | SOAR structure shown in (e) rotated 90° to illustrate the potential sites of association with the Orai1 C terminus depicted in (c). The hypothetical electrostatic binding of the Orai1 C terminus within the cleft between the SOAR monomers is shown.
STIM1 and STIM2
Dynamic intermembrane communicators
STIM proteins are type 1A single-span membrane proteins that are largely conserved across species from D. melanogaster1 to Caenorhabditis elegans22. STIM proteins are likely to have evolved earlier than Ins(1,4,5)P3Rs23 and may have originally had a greater role in Ca2+ homeostasis than in Ca2+ signalling. In vertebrates, STIM1 and STIM2 are expressed ubiquitously throughout cell types24. In most tissues STIM1 levels are higher than STIM2 levels24,25, however, STIM2 expression predominates in the brain26 and dendritic cells27. STIM1 and STIM2 have high homology through most of their length, with variations in their amino-terminal and carboxy-terminal segments28 (see Supplementary information S1 (figure)). The functions of STIM1 and STIM2 are subtly distinct with important physiological implications.
Triggering and translocation of STIM1
STIM proteins are located predominantly in the ER29–31, and they undergo rapid and reversible translocation into close ER– plasma membrane junctions to couple with and activate Orai channels following store depletion28,32 (BOX 1). Under resting conditions, STIM1 is distributed throughout the ER2,33,34 probably in a dimeric form35–40 (BOX 1). The dimers undergo rapid oligomerization and move into plasma membrane junctions within a few seconds following store depletion2,34 (BOX 1). The luminal N-terminal domain of STIM1 contains a tightly clustered assembly of short α-helices comprising two EF-hand domains and a sterile α-motif (SAM) domain41,42 (FIG. 1a,d). This region enables STIM1 to sense small changes in luminal Ca2+ levels and triggers intermolecular interactions. The cytoplasmic C terminus of STIM1 contains extensive coiledcoil regions that can span the ER– plasma membrane junctional gap, which is estimated to be ~15 nm2,33,34,43,44. The C-terminal region includes an ~100 amino acid segment named the STIM–Orai activating region (SOAR), which mediates direct coupling with Orai channels45 (FIG. 1a,d–f). This is similar to the CRAC activation domain (CAD)46 or Orai1-activating small fragment47 (OASF) (FIG. 1a). Crystallization approaches now provide the first evidence for the α-helical structure of these domains40.
Although the role of STIM1 is now mostly defined in the ER, STIM1 was originally identified48 on the surface of stromal cells mediating interactions with pre-B cells. Orai channel activation does not require plasma membrane STIM1, and thus tagged STIM1 that cannot insert into the plasma membrane49 still fully activates Orai channels31,35. Interestingly, replacement of the Ca2+-sensing luminal N-terminal domain of STIM1 with protein domains that oligomerize in response to a Ca2+-independent signal revealed that simple STIM1 crosslinking is sufficient for its activation44. Moreover, the ER membrane-attached STIM1 C terminus alone is capable of clustering in junctions and activating Orai50. However, these observations belie the complex conformational transition that the resting STIM protein must undergo to present itself within junctions.
STIM1 is a finely tuned ER Ca2+ sensor
The two EF-hand domains within STIM1 operate together in a tightly associated complex with the SAM domain, which comprises five α-helices, to finely sense luminal Ca2+ levels41,42,51–53 (FIG. 1d) At resting luminal Ca2+ levels (that is, ~400 μM) the Ca2+-binding canonical EF-hand (cEF) domain of STIM1 forms a tight, stable EF-hand–SAM configuration. Decreased luminal Ca2+ levels cause Ca2+ to dissociate from the cEF-hand domain. This leads to unfolding and destabilization of the EF-hand–SAM complex as hydrophobic residues are exposed within both the EF-hand domain and the SAM domain42, which triggers activation and oligomerization of STIM1 (REFS 35,37,46,50,54) (FIG. 2). The Ca2+ dissociation-induced EF-hand–SAM domain interactions are reversed when luminal Ca2+ returns to resting levels42. STIM1 rapidly retreats from ER–plasma membrane junctions and Orai channels become deactivated2,33,55,56. The luminal EF-hand–SAM domain of STIM1 binds Ca2+ with a dissociation constant (Kd) of ~200 μM41,42,57,58. The dependence of STIM1 oligomerization on Ca2+ has a Hill coefficient of almost 4 which is similar to that for Orai channel activation44. Thus, STIM-induced Orai channel activation is triggered in a narrow range of luminal Ca2+ depletion. Mutation of acidic residues in the cEF-hand domain to lower its Ca2+ affinity causes STIM1 oligomerization just as if luminal Ca2+ levels had been lowered42. Expression of STIM1 with such cEF-hand domain mutations results in activated STIM1 localized almost entirely within junctions and constitutively active CRAC channels2,30,43,59. Mutation of equivalent EF-hand residues in the non-Ca2+-binding hidden EF-hand (hEF) loop causes similar oligomerization and constitutive Ca2+ entry42. This tandem functioning of the two EF-hand domains contributes to the narrow Ca2+-dependency of STIM1 activation.
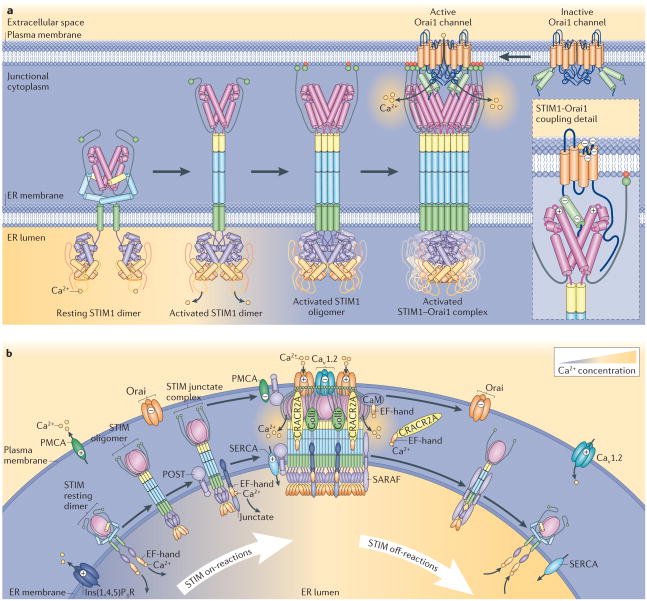
Figure 2. STIM activation and organization of the Ca2+ signalling junction.
a | Hypothetical model of stromal interaction molecule 1 (STIM1) activation and coupling to Orai1. The resting STIM1 dimer in the Ca2+-replete endoplasmic reticulum (ER) is shown on the left. The activation of the STIM1 dimer is initiated by Ca2+ dissociation from the STIM1 dimer. This causes EF-hand–SAM domains within the STIM1 dimer to interact, which induces an extended configuration of the cytoplasmic coiled-coil domains74, dissociation of the Cα3 inhibitory helix from SOAR (STIM–Orai activating region)40,68,74, and the carboxy-terminal flexible domains recede and expose SOAR. STIM1 continues to oligomerize and migrates into ER–plasma membrane junctions, and the polybasic C termini bind and anchor STIM1 to negatively charged phospholipids in the plasma membrane46 (shown in red) and active SOAR is fully exposed. Large aggregates of anchored STIM1 within ER–plasma membrane junctions are able to tether and activate Orai1 proteins. Each SOAR dimer interacts with one Orai1 protein, therefore eight STIM1 molecules form an active complex with one tetrameric Orai1 channel. b | Role of regulatory and target proteins in the STIM-activated Ca2+ signalling junction. In response to inositol-1,4,5-trisphosphate (Ins(1,4,5)P3) receptor (Ins(1,4,5)P3R)-mediated ER Ca2+ depletion, Ca2+ dissociates from STIM1, and STIM1 aggregates and translocates into ER–plasma membrane junctions. During activation, STIM1 initially interacts with plasma membrane lipids through its Lys-rich domain assisted by interaction with junctate in or near ER–plasma membrane junctions81,82. This interaction is stabilized by the Ca2+-free form of Ca2+ release-activated (CRAC) regulatory protein 2A (CRACR2A)87 and golli96,98. During targeting, STIM1 interacts with and activates Orai1 channels and inhibits CaV1.2 channels112,114. STIM1 recruits partner of STIM1 (POST) to the junction, and this adaptor protein recruits both plasma membrane Ca2+ ATPase (PMCA) and sarcoendoplasmic reticulum Ca2+ ATPase (SERCA) to the Ca2+ signalling junction127. POST127 and STIM1 (REF. 126) inhibit PMCA-mediated Ca2+ efflux from the cell and thereby increase Ca2+ availability for signalling. SERCA recruited into junctions by POST may also assist ER Ca2+ loading. During STIM deactivation, increased cytosolic Ca2+-dependent loss of both CRACR2A87 and golli98 destabilizes the STIM complex. Ca2+-binding to calmodulin (CaM) promotes Orai channel inactivation. The STIM-binding protein store-operated Ca2+ entry (SOCE)-associated regulatory factor (SARAF)88 also mediates dissociation of STIM proteins, resulting in their configuration into a resting state.
Distinct roles for STIM2
STIM2 is a significantly weaker activator of Orai channels than STIM1 (REF. 60) but is a more sensitive sensor of ER luminal Ca2+ (REF. 57). STIM2 has a strong C-terminal ER-retention sequence and is exclusively localized in the ER, whereas STIM1 lacks this sequence, and therefore ~10% of STIM1 localizes to the plasma membrane30,61,62. Unlike STIM1 (REFS 30,59), overexpressed STIM2 has a strong negative effect on endogenous SOCE30,57 and mediates slower Orai1 activation than STIM1 (REFS 63,64). The Kd of STIM2 for Ca2+ (that is ~400 μM) is 2-fold higher than that of STIM1, which possibly reflects the differences in EF-hand domain structures. Hence, STIM2 is more sensitive to small changes in luminal Ca2+ levels57, and its overexpression results in higher constitutive Ca2+ entry and CRAC channel activity65,66. The STIM2 EF-hand–SAM domain undergoes slower unfolding and aggregation following Ca2+ withdrawal52,53. This delay in STIM2 aggregation may reflect increased stability conferred by the short flexible N-terminal sequences52,64. The slower rate of STIM2 unfolding and aggregation is likely to account for the slower kinetics of store-operated channel activation by STIM2 (REFS 63,64) and the negative dominance of overexpressed STIM2 on SOCE30. Thus, whereas STIM2 is sensitized to small changes in ER Ca2+ levels, the slow activation and poor Orai-coupling efficacy may be important in preventing uncontrolled activation of storeoperated channels. The differences in the sensitivity of STIM1 and STIM2 to ER luminal Ca2+ levels are important for the maintenance of Ca2+ oscillations that are generated in response to distinct Ins(1,4,5) P3-activating agonists67, as described below.
A molecular model for STIM1 activation
STIM proteins undergo a remarkable series of molecular rearrangements triggered by and intimately linked to their ER Ca2+-sensing ability. STIM and Orai protein coupling seems sufficient for SOCE induction (FIGS 1b–f,2a). Thus, Orai channels are activated by cytoplasmically expressed C-terminal fragments of STIM1 (REFS 36,45–47,56,66) and even by STIM1 domains targeted to mitochondria68. Moreover, SOAR added to vesicles extracted from yeast expressing human Orai1 can directly activate the channel69. Here we describe recent progress in understanding the molecular role of STIM1.
Transition from resting to activated state
Within the Ca2+-replete ER, the resting dimeric state of STIM1 (REFS 36,37,40,47) is maintained through interactions mediated by the coiled-coil 1 (CC1) and SOAR domains37 (FIG. 2a). These coiled-coil sequences, which comprise a large segment of the STIM1 C-terminal region (that is, amino acids 238–423), mediate STIM1–STIM1 interactions both before and after depletion of Ca2+ stores37,40. Earlier studies suggested that oligomerization of STIM1 is mediated mainly by EF-hand–SAM domain interactions42. However, when a truncated form of STIM1 lacking the STIM1 cytoplasmic domain (that is, the EF-hand–SAM domain-containing N terminus and transmembrane region) is expressed, the oligomerization of this STIM1 fragment in response to store depletion is unstable37. Thus, although EF-hand–SAM domains are necessary for sensing Ca2+ levels and triggering STIM1 oligomerization, they may not be the major crosslinking domains between STIM1 proteins. The STIM1 cytoplasmic coiled-coil regions are important for oligomerization in the resting state54, and deletion of the amino acids 249–390 of the coiled-coil segment prevents constitutive STIM1 interactions35. The resting STIM1 dimers are held together primarily by interactions between both the CC1 and SOAR regions37 (FIG. 1d). In this state, the EF-hand–SAM domains are likely to be monomeric and contribute little to STIM1–STIM1 interactions. Following store depletion, the EF-hand–SAM domains undergo interactions to trigger the oligomerization of STIM1 (REFS 41,42), and these relatively unstable N-terminal interactions are stabilized by C-terminal interactions. Although the CC1 region alone may not support store depletion-induced STIM1 oligomerization, the coiledcoil domains within SOAR allow STIM1 to undergo robust multimerization following store depletion forming stable oligomers, which translocate into ER–plasma membrane junctions to activate Orai channels37. Thus, the SOAR region of STIM1 has a dual role within the STIM molecule: it mediates the transition of STIM1 into an oligomeric active conformation37, and it binds directly to and activates Orai channels45,46.
STIM–Orai interaction sites
The short coiled-coil C-terminal cytoplasmic domain of Orai1 is an important STIM1-interacting site (FIG. 1b,c), and its disruption prevents STIM1-mediated activation of Orai channels56. A cluster of acidic residues within this Orai1 segment (that is, amino acids 272–291; ELNELAEFARLQDQLDHRGD) (FIG. 1d) was identified as necessary for store-dependent binding of STIM1 and channel activation70. The amphipathically coiled, acidic Orai1 segments may interact electrostatically with the highly conserved, short polybasic region within the SOAR domain (that is, amino acids 382–387; KIKKKR)68,71 (FIG. 1f). Mutations to neutralize this basic region in either intact STIM1, C-terminal STIM1 fragments (STIM1CT) or SOAR, prevent coupling to and activation of Orai1 channels68,71. Disruption of the adjacent CC2 region in STIM1 (FIG. 1a) also prevents coupling to Orai1 channels72. However, despite mutation of the polybasic STIM1 residues and Orai1 acidic residues, STIM1–Orai1 interactions can still be measured by fluorescence resonance energy transfer (FRET)71, which supports the evidence that STIM1 has a second site of interaction with Orai1 in its N-terminal segment46. The second site is likely to be within the region spanning amino acids 73–91 of Orai1 close to its first transmembrane spanning segment46, a region that is crucial for channel gating and STIM1 interaction73,74.
Intramolecular control of SOAR
Whereas the short SOAR45,46 fragments of STIM1 constitutively bind to and activate Orai channels, these sequences are much less active within longer, cytosolically expressed C-terminal constructs46,66,68,74,75. This suggests that a conformational transition may occur to either expose SOAR or relieve it from functional constraint. The nature of this important priming reaction is intriguing. Mutation of a series of acidic residues (in particular amino acids 318–322; EEELE) upstream of SOAR leads to constitutive STIM1 activation68. Moreover, co-expression of the STIM1 fragment (amino acids 283–343) that contains these acidic residues interferes with Orai1 activation by STIM1. The concept of a possible electrostatic ‘intramolecular clamp’ that prevents STIM1 activation at rest was supported by FRET studies of a larger segment of STIM1CT (amino acids 233–474; the region termed OASF)74 (FIG. 1a). These studies revealed that OASF undergoes significant unfolding into an extended state as it interacts with the Orai1 channel. This unfolding does not depend on Ca2+ passage through Orai channels. Intriguingly, mutations to neutralize the cluster encompassing the acidic residues 318–322 in the CC1 domain caused some unfolding of OASF. In addition, considerable unfolding was induced by mutations that replace hydrophobic residues in several of the coiled-coil segments of OASF, which suggests that hydrophobic interactions also contribute to its folded inactive conformation74,76.
New insights from STIM1 atomic structure
Recent crystallographic analyses provide valuable insights into the structure and intramolecular control of SOAR activation40. SOAR was observed as a dimeric structure by ultracentrifugation, in agreement with earlier reports showing that active C-terminal STIM1 fragments are dimers45,74, although a tetrameric configuration had also been reported46. The crystallized structure revealed four α-helices within the SOAR peptide (that is, amino acids 345–444), two long helices (Sα1, Sα4) and two very short helices (Sα2, Sα3) (FIG. 1d,e). The atomic structure identified residues through which cross-peptide interactions mediate dimerization of SOAR: specific residues in the N-terminal region of Sα1 (within the 347–354 amino acid segment) from one SOAR molecule interact through either hydrophobic or hydrogen bonds with residues in the C-terminal region of Sα4 (within the 429–436 amino acid segment) in the other SOAR molecule. Importantly, mutating these dimerization regions within SOAR or full-length STIM1 prevented interaction with and activation of Orai1 (REF. 40), suggesting that the active functional unit within STIM1 is a SOAR dimer. Furthermore, the atomic structure revealed that the long rigid Sα1 helix within SOAR (which comprises residues 345–391) exposes the polybasic Orai-interacting residues (which are amino acids 382–387) at its apex, with Lys382, Lys385 and Lys386 amphipathically oriented towards the centre of the dimer (FIG. 1f). Hypothetically, this structure could provide a cleft within which the two amphipathic acidic groups of the Orai C-terminal helix might electrostatically interact (FIG. 1b,c,f). A further important conclusion is that the acidic residues (which are residues 318–322) within the inhibitory domain that are located close to the C-terminal end of the Sα1 helix would be unable to directly interact with the polybasic active sites (that is, amino acids 382–387) within the dimer by virtue of the intervening long (47 residues) Sα1 helix, militating against the electrostatic clamp model. The C. elegans STIM sequence encompassing residues 214–410 (REF. 40), including the complete CC1 and SOAR regions, was also successfully crystallized. The structural data indicate that the above described inhibitory region in CC1 is α-helical and undergoes tight interactions through several hydrogen bonding and hydrophobic interactions with the N-terminal start of SOAR and the C-terminal end of SOAR — the two regions in SOAR furthest away from the middle ‘apical’ polybasic active site. The corresponding inhibitory sequence identified in human STIM1 (which are residues 310–337), when eliminated from full-length STIM1, leads to constitutive interaction with and activation of Orai1, which is consistent with the previous findings68. The unfolding of the STIM1 coiledcoil region that is required for Orai channel activation74 may thus correspond to the detachment of the inhibitory helix from SOAR. The acidic residues in this sequence known to prevent STIM1 activation68 may in fact mediate its interaction with SOAR40.
A consolidated activation model
The new structural data provide strong evidence for the central role of the SOAR dimer in both presentation of the active Orai-interacting site of STIM1 and in the unfolding and activation of the entire STIM1 C-terminal domain (FIG. 2a). The model of one STIM dimer interacting with each Orai1 subunit of the active Orai1 channel is in good agreement with recent stoichiometric data showing that eight STIM1 molecules are coupled to activate each tetrameric Orai1 channel38.
The schematic in FIG. 2a attempts to bring together what is currently known about the STIM protein activation and Orai coupling processes. The SOAR dimer is shown as a central core structure throughout STIM1 activation. In the STIM1 resting state, the predicted three α-helices of CC1 are shown in a folded configuration with the Cα1 N terminus close to the C terminus of SOAR as suggested by FRET analysis of OASF74. The inhibitory Cα3 helix is shown to interact with SOAR at its far C- and N-terminal regions as suggested by the recent structural data for C. elegans STIM-1 (REF. 40). During activation, Ca2+ dissociation-induced interactions between the EF-hand and SAM domains may trigger unfolding and elongation of the STIM1 C-terminal coiled-coil domains, separating the Cα3 inhibitory helix of CC1 from SOAR to provide an activated SOAR domain40,68,74. The unfolding of the STIM protein may also allow interactions of its Lys-rich C-terminus with plasma membrane lipids46 and exposure of SOAR, which enables the activation of Orai channels40,77. Although convincing evidence exists for the interactions between the polybasic SOAR region and the acidic regions of Orai1 (REFS 40,68,71), a direct electrostatic interaction has not yet been shown. SOAR also interacts with the N-terminal region of Orai1 (that is, amino acids 73–91)46. This segment of Orai1 is crucial for channel gating, and the Orai1-K85E mutation alters STIM1-mediated gating without altering SOAR–Orai1 interactions73. Indeed, recent studies revealed that the pore properties and gating of Orai1 channels are intimately associated78.
Thus, by binding to Orai1, STIM1 not only induces channel opening but also increases the Ca2+ selectivity of the channel78, which suggests that STIM1 functions in a similar fashion to a regulatory subunit of the Orai1 channel. This is remarkable considering that it exists in a separate membrane. Theoretically, the SOAR–Orai interactions may involve tethering at the Orai1 C terminus and ‘regulatory’ interactions at the N-terminus of Orai1, leading to channel gating and control of ion selectivity. In addition, the Lys-rich far C-terminal end of STIM1 strongly interacts with acidic phospholipids in the plasma membrane33,46,79, which stabilizes its attachment and allows it to trap and activate Orai channels.
The function of the remainder of the large (~200 residues) C-terminal tail of STIM proteins remains unclear. The regions are dissimilar in STIM1 and STIM2 (see Supplementary information S1 (figure)) and absent in D. melanogaster STIM and may function sterically as intramolecular shields occluding the SOAR region77 to maintain STIM proteins in an inactive state. The attachment of STIM1 to the plasma membrane through its Lys-rich C terminus may assist in relieving this occlusion.
Control of the Ca2+ signalling junction
STIM proteins are central components in ER–plasma membrane junctions as they mediate crucial Ca2+ communication among the ER lumen, the cytoplasm and the extracellular space. Under resting conditions, these junctions account for approximately 5% of the plasma membrane surface34,80 and can become considerably larger upon STIM protein activation34. The junctions are complex structures and include several recently identified STIM-interacting proteins that control junctional assembly, disassembly and function (FIG. 2b).
Junctional assembly: STIM turn-on events
The interaction between oligomerized STIM and Orai proteins can itself drive the formation of junctions68,69. However, the emerging picture is that junctions are highly organized domains that contain an array of protein modulators (FIG. 2b). Although STIM–Orai interactions are important, the interaction between the Lys-rich C-termini of STIM proteins and plasma membrane acidic phospholipids provides another major driving force in junctional assembly33,46,79 (FIG. 2a). Although the seven C-terminal Lys residues of a single STIM1 protein (and eight C-terminal Lys residues in STIM2) provide only weak lipid interactions58, oligomerized STIM proteins provide a robust multivalent interaction with plasma membrane surface lipids. The D. melanogaster STIM protein lacks the Lysrich C-terminus, and therefore Orai activation does not require lipid-interactions, although lipid association may assist junctional localization46.
The Ca2+-binding integral ER membrane protein junctate, known to assist in ER–plasma membrane interactions, was shown to be present in STIM–Orai containing junctions and to be important in mediating SOCE81,82. Indeed, junctate is an interacting partner within the STIM1–Orai1 complex and can recruit STIM1 into the ER–plasma membrane junctions83. Interestingly, junctate contains a Ca2+-binding EF-hand domain, which, when mutated to prevent Ca2+ binding, causes CRAC channel activation even without store depletion83 by promoting the formation of ER–plasma membrane junctions (FIG. 2b). Junctate does not seem to be widely distributed throughout the ER but remains close to the junctions and may represent an important additional factor contributing to STIM protein recruitment and Orai channel activation (REF. 83). Its role may be to mediate CRAC activation in response to local Ca2+ depletion events and/or to amplify the STIM-mediated activation of Orai channels.
Although in most cells STIM–Orai junctional assembly occurs slowly (within 10–30 seconds), in skeletal muscle the kinetics of activation of Orai channels by Ca2+ depletion can be much faster, and it seems that STIM and Orai proteins remain junctionally coupled within cisternae84,85. Interestingly, the unique actin-binding domain within the STIM1 splice variant STIM1L may mediate this junctional pre-coupling86. Thus, local control of assembly and the degree of ‘pre-assembly’ of junctions are crucial factors in controlling SOCE kinetics.
Turning off STIM–Orai coupling
The activation and deactivation of STIM–Orai coupling is a steady-state event that is controlled by the local junctional environment. The entry of Ca2+ into the junctional space through Orai channels provides powerful feedback control on STIM-induced Orai channel activation. STIM and Orai proteins function together as a coupled channel assembly78, and turn-off events reflect properties of both proteins. In addition, several regulatory proteins within this complex were recently identified87–89. Turning off the activated STIM–Orai complex involves two major events: rapid Ca2+-dependent inactivation (CDI) of the Orai channel, and the subsequent slow dissociation of the STIM–Orai junctional complex. CDI of CRAC channels has long been recognized90,91, and several recent studies revealed that a short segment of acidic residues in the STIM1 cytoplasmic inhibitory domain region (ID region; which comprises residues 475–483) (FIG. 1a) is necessary for CDI89,92,93. Orai1 activation by SOAR fragments that lack this region does not exhibit CDI46,93, and replacement of a few or all of these acidic residues in STIM1 prevents CDI89,92,93. Ca2+ interacts with the ID region, however, it is not clear whether Ca2+ binding to the ID region is required for CDI89. Calmodulin is a powerful mediator of CDI94 and seems to induce this effect through direct interaction with the Orai1 channel. This interaction site was shown to reside at a region within the N-terminal cytoplasmic domain (that is, residues 69–91), close to the second STIM1-interaction site89 (FIG. 2b). The slow dissociation of the STIM–Orai complex and the disaggregation of STIM proteins is mainly mediated by increased ER luminal Ca2+ levels, which lead to STIM protein disaggregation28. However, careful manipulation of cytosolic and ER Ca2+ levels reveals that localized cytoplasmic Ca2+ in ER–plasma membrane junctions also has a role in STIM1 de-oligomerization95.
Several other proteins seem to be important in controlling the turn-off of STIM–Orai coupling. The cytoplasmic protein CRAC regulatory protein 2A (CRACR2A) was recently identified as being tightly associated with the STIM–Orai1 complex87. CRACR2A is a soluble, cytoplasmic protein with two predicted EF-hand motifs (FIG. 2b). In vitro binding reveals strong interactions with the same Orai1 N-terminal sequence (that is, residues 64–93) that binds calmodulin. The Orai1–CRACR2A interaction is inhibited by Ca2+ binding to CRACR2A, and a mutation in the CRACR2A EF-hand domain that prevents Ca2+ binding eliminates the Ca2+-mediated inhibition of this interactions87. Furthermore, CRACR2A also binds directly to STIM1. CRACR2A may function as a stabilizer of STIM1–Orai interactions, but only when junctional Ca2+ levels are low. As Ca2+ levels increase at ER–plasma membrane junctions, CRACR2A dissociates from and destabilizes the STIM1–Orai1 complex, thereby terminating the interaction (FIG. 2b). Similarly to CRACR2A, calmodulin also binds Ca2+ via its high affinity EF-hand domains, and both CRACR2A and calmodulin may function synergistically when junctional Ca2+ levels increase, with calmodulin inactivating Orai and CRACR2A enhancing STIM1–Orai1 dissociation.
The cytoplasmic protein golli, which is an alternatively spliced isoform of myelin basic protein (MBP), negatively controls SOCE96 and also enhances CaV1.2 channel activity97. Golli directly interacts with the C-terminal domain of STIM1, an interaction that may reflect cytoplasmic Ca2+ levels. As golli colocalizes with STIM1–Orai1 complexes after store depletion98, it may also promote Ca2+-dependent turning off of STIM1–Orai1 coupling (FIG. 2b).
SOCE-associated regulatory factor (SARAF) has also recently been implicated in the deactivation of the STIM1–Orai1 complex88. SARAF, which was identified by high-throughput functional screening, is a singlepass ER membrane protein that is closely associated with STIM1 and that moves with STIM1 into junctions following store depletion (FIG. 2b). Although SARAF does not contain Ca2+-sensing EF-hand domains, it seems to be important in mediating the effects of cytosolic Ca2+ on Orai uncoupling and inhibition of SOCE. A cluster of basic residues in the cytoplasmic tail of SARAF might interact with plasma membrane lipids in a similar manner to STIM1. Perhaps as a key player in deactivating SOCE, SARAF may be crucial in controlling store refilling and protecting cells from ER Ca2+ overload. Clearly, the inactivation of SOCE and disassembly of STIM–Orai junctions are under the tight control of Ca2+ and are mediated by several ancillary proteins.
New STIM protein triggers, targets and partners
Recent information revealed that in addition to sensing decreased ER Ca2+ levels, STIM proteins are triggered by several other distinct stress conditions99, including oxidative stress100, temperature change101,102, hypoxia and acidification103. Details of these additional stress-sensing roles of STIM proteins are shown in BOX 2. Recent studies also revealed various new targets and partners for STIM proteins that operate within ER–plasma membrane junctions. Thus, STIM proteins interact with and control a range of other channels, pumps and scaffolding proteins suggesting that STIM proteins have many other Ca2+ signalling targets besides Orai channels (FIG. 2b).
Box 2. Multiple sensing roles of STIM1.
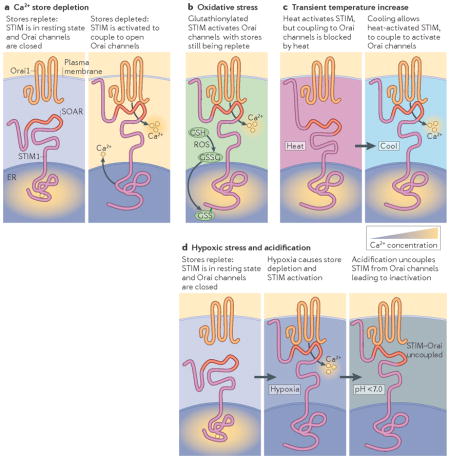
Stromal interaction molecule (STIM) proteins are activated in response to several different cellular stress conditions. ER Ca2+ store depletion is itself a major cellular stress condition leading to protein misfolding and STIM activation (see the figure, part a). Reactive oxygen species (ROS) were shown to induce STIM1 aggregation, STIM translocation to ER–plasma membrane junctions and activation of Orai channels without Ca2+ store depletion100 (see the figure, part b). ROS-induced S-glutathionylation of Cys56 in the amino terminus of STIM1 decreases Ca2+ binding by the EF-hand domain and triggers STIM1 activation. Increased temperature from 37°C to 41°C also triggers STIM1 activation independently of Ca2+ store depletion101 (see the figure, part c). However, the higher temperature (left panel) prevents coupling to and activation of Orai channels, which can occur only after cooling (right panel). The role of STIM1 in temperature sensing may be important in priming haematopoietic cells during fever101. It is also possible that high temperature controls STIM–Orai uncoupling to protect cells from temperature-induced Ca2+ overload99,102. Hypoxic stress and ensuing decreased ATP levels cause Ca2+ store depletion and activation of STIM proteins (see the figure, part d). However, coupling to Orai channels is prevented in this case through hypoxia-induced cytoplasmic acidification which may prevent the electrostatic STIM-Orai interactions that are crucial for Orai channel activation (FIG. 1). Thus, STIM–Orai uncoupling in response to decreased pH may be a protective mechanism against hypoxia-induced Ca2+ overload103. GSH, reduced glutathione; GSSG, oxidized glutathione disulphide; SOAR, STIM-Orai activating region.
Control of other channel targets
Although Orai channels are the most established STIM protein targets, other channels are either directly controlled by or closely associated with STIM proteins. The family of transient receptor potential channels (TRPCs) has long been linked to SOCE28. The fly TRP homologues were reported to be Ca2+ store-dependent104,105, and mammalian TRPCs seem to couple with Ins(1,4,5)P3Rs and mediate SOCE106. The involvement of STIM proteins in activating TRPC has been controversial28. As non-selective cation channels, TRPCs mediate cation currents with properties distinct from those of the CRAC current. Whereas some prominent studies have described direct STIM-induced activation of TRPCs75,107–109, other studies have presented strong evidence against this hypothesis110–112. TRPC activation by phospholipase C (PLC)-coupled receptors may result from direct actions of changes in diacylglycerol (DAG) or phosphatidylinositol-4,5-bisphosphate (PtdIns(4,5) P2) levels rather than Ins(1,4,5)P3-mediated Ca2+ store depletion. However, close functional connections between TRPCs and the STIM–Orai machinery have been suggested from studies revealing that Ca2+ entering through Orai channels induces plasma membrane insertion of TRPCs113.
STIM proteins also interact with and control the function and turnover of CaV1.2 voltage-operated Ca2+ channels40,112,114. In contrast to its effects on Orai channels, STIM1 inhibits CaV1.2 channel function. Store depletion causes STIM1, CaV1.2 and Orai1 channels to accumulate within the same junctional areas112, but STIM1-mediated CaV1.2 channel inhibition does not require Orai channel activation. The reciprocal actions of STIM1 on Orai1 and CaV1.2 channels are both mediated by the cytoplasmic SOAR region of STIM1 (REFS 112,114), which interacts with the C-terminal tail of the CaV1.2 channel α1C subunit114. In addition to inhibiting channel function, the interaction of STIM1 causes a dramatic increase in CaV1.2 channel internalization, which results in its decreased surface expression114. STIM1 also influences Orai channel turnover by increasing the insertion and retention of Orai channels in the oocyte plasma membrane115. During meiosis, STIM protein activation is turned off, which leads to prominent internalization of Orai channels and the prevention of SOCE116. Thus, STIM protein activation has reciprocal effects on turnover of Orai and CaV1.2 channels. As CaV1.2 channels and Orai channels are widely expressed in excitable and non-excitable cells, their reciprocal control by STIM proteins is likely to have important functional consequences.
The STIM-dependent ARC channel has been reported to comprise a unique configuration of Orai1 and Orai3 subunits117–119. The ARC channel is not activated by Ca2+ store depletion but by receptor-induced arachidonic acid generation. ARC channels were found to depend exclusively on cis-located STIM1 within the plasma membrane. However, it is unclear how STIM1, the structure of which has evolved to enable interaction with and activation of Orai channels in a junctional trans-membrane configuration, can also activate Orai channels within a cis-membrane configuration.
Control of other major regulatory proteins
The ER– plasma membrane junctional domain, as defined by the presence of STIM proteins, is becoming recognized as a Ca2+ signalling microdomain in which not only Ca2+ channels but also Ca2+ pumps are recruited. Evidence indicates that sarcoendoplasmic reticulum Ca2+ ATPase (SERCA) pumps in the ER are functionally close to STIM–Orai junctions120. Furthermore, FRET imaging studies revealed that SERCA2A is recruited into junctions together with STIM1 (FIG. 2b), whereas after store refilling STIM1, Orai1 and SERCA2A dissociate121. Studies combining targeted aequorins to measure local Ca2+ with imaging of labelled STIM1, Orai1 and SERCA2B revealed local Ca2+ pumping and the formation of a triple complex of STIM1, Orai1 and SERCA2B122,123.
The plasma membrane Ca2+ ATPase (PMCA) pump also seems to be closely controlled within the STIM–Orai junction (FIG. 2b). PMCA is modulated by CRAC channel activation, independent of increases in local cytosolic Ca2+ levels124, and it was recently shown to be inhibited in the immunological synapse in T cells125,126. PMCA inhibition was proposed to be mediated by mitochondrial Ca2+ loading at a location remote from the STIM–Orai interacting site where PMCA and mitochondria closely interact125. However, more recently it was revealed that activated STIM1 proteins within STIM–Orai junctions interact directly with PMCA and induce an inhibitory effect on Ca2+ pumping independent of mitochondrial function126.
The identification of partner of STIM1 (POST) provides clues about the role of STIM1 in organizing crucial Ca2+ regulatory proteins and other functionally important proteins127. POST is a 10-transmembrane spanning ‘adaptor’ protein existing, like STIM1, predominantly in the ER, although ~5–10% of POST is present in the plasma membrane. Following Ca2+ store depletion, POST binds strongly to STIM1 and moves with STIM1 into precisely the same ER–plasma membrane junctions (FIG. 2b). POST is not required for STIM–Orai interactions and altered expression does not change CRAC channel activation. Store depletion leads to POST-dependent binding of STIM1 to SERCA and PMCA pumps. This provides a mechanistic basis for the above described functioning of SERCA pumps within STIM–Orai junctions (FIG. 2b). Moreover, POST was shown to inhibit the Ca2+ pumping activity of PMCA independently of mitochondria127, and this is consistent with the inhibitory effect of STIM1 on PMCA126. Thus, POST-mediated organization and control of PMCA and SERCA pumps within STIM–Orai junctions is a powerful means to control and direct the entering Ca2+ (FIG. 2b). Intriguingly, POST also interacts with the Na+/ Ca2+ exchanger (NCX), consistent with reports that STIM1 promotes reverse-mode NCX activity in smooth muscle128. In addition, POST was found to bind the Na+/ K+ ATPase and the nuclear transporters importin-β and exportin127, and may explain why STIM1 can immunoprecipitate with importin-β and exportin62.
STIM1 also interacts with the ER chaperones calnexin 62 and ERp57 (REF. 129). Moreover, STIM1 may exert control over plasma membrane adenylyl cyclases130. Recent work revealed direct binding between Orai1 and the adenylyl cyclase isoform AC8, which indicates that SOCE exerts local control of cyclic AMP (cAMP) production. Overall, STIM proteins seem to interact with and control several distinct classes of Ca2+-regulatory proteins and other signalling proteins.
STIM proteins, Ca2+ oscillations and gene expression
Transient oscillations in cytosolic Ca2+ provide long-term signals while avoiding the cytotoxic effects of prolonged Ca2+ increases131 and are crucial in transcriptional control132. The coordinated function of ER Ca2+ release and SOCE is essential in generating Ca2+ oscillations133,134 and controlling transcription (FIG. 3). During oscillations, SOCE replenishes ER Ca2+ stores and oscillations cannot be sustained without SOCE134. During individual oscillations, STIM1 migrates into junctions and activates Orai channels60. Although more sensitive to store depletion57, STIM2 was not found to enter junctions during individual oscillations60. However, both STIM1 and STIM2 are important in long-term transcriptional responses mediated by Ca2+ oscillations (FIG. 3). Nuclear factor of activated T cells (NFAT)-driven interleukin 2 (IL-2) production is decreased in STIM2-deficient T cells, and STIM2 is necessary for long-term SOCEdriven gene expression25. In B cells, STIM2 is required for NFAT-driven transcription, although STIM2 is only modestly involved in mediating Ca2+ signals135. A recent study revealed that Ca2+ release spikes in response to G protein-coupled receptor activation induce extensive store depletion and drive STIM1 (the activation of which has a high Ca2+ release threshold) to mediate Ca2+ entry67. By contrast, more subtle Tyr kinase-induced oscillations involve moderate store depletion and drive both STIM1 and STIM2 (the activation of which has a lower Ca2+ release threshold) to activate Ca2+ entry67.
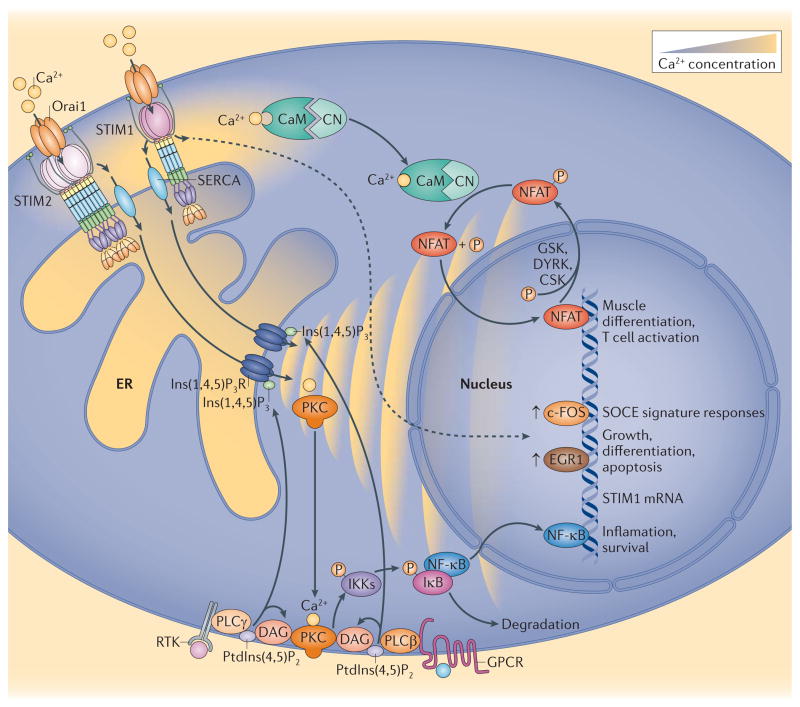
Figure 3. STIM proteins control gene expression by generating spatiotemporally controlled Ca2+ signals.
Activation of either receptor Tyr kinase-coupled or G protein-coupled receptors (RTK or GPCR, respectively) result in phospholipase C (PLC)-mediated production of inositol-1,4,5-trisphosphate (Ins(1,4,5)P3) and diacylglycerol (DAG). RTK-mediated PLCγ activation can be slower and more gradual than GPCR-mediated PLCβ responses67. The rapid ER Ca2+ depletion mediated by GPCR leads to stromal interaction protein 1 (STIM1)-mediated but not STIM2-mediated Orai channel activation. By contrast, ‘shallow’ Ca2+ release through RTK may cause both STIM1- and STIM2-mediated Orai channel activation. Close apposition of sarcoplasmic reticulum Ca2+ ATPase (SERCA) with junctions allows the entry of Ca2+ to refill stores and maintain oscillations while contributing only minimally to global changes in cytosolic Ca2+ concentration60. However, the local increase in Ca2+ near the STIM1–Orai junction is crucial for the activation of both c-FOS136 and nuclear factor of activated T cells (NFAT)139,140. Hence, Ca2+-Calmodulin (CaM) activates calcineurin (CN), which dephosphorylates NFAT, resulting in nuclear translocation. Casein kinase (CK), dual specificity Tyr phosphorylationregulated kinase 2 (DYRK) and glycogen synthase kinase (GSK) phosphorylate NFAT, which results in a cycling of NFAT across the nuclear membrane as long as the Ca2+ signal continues. Store-operated Ca2+ entry (SOCE) can also lead to both nuclear factor-κB (NF-κB) activation189,179 and early growth response protein 1 (EGR1) upregulation190, both of which directly stimulate STIM1 transcription (not shown)191,192. Ca2+-mediated NF-κB activation may be mediated by protein kinase C (PKC), which phosphorylates (P) inhibitor of NF-κB (IκB) kinases (IKKs). IKKs phosphorylate IκB, resulting in its dissociation from NF-κB.
Although SOCE contributes only a small fraction of each global Ca2+ spike, local contributions of SOCE to Ca2+ oscillations seem to be crucial in mediating transcriptional control136,137. Blocking both Orai-mediated entry and PMCA-mediated exit of Ca2+ across the plasma membrane with lanthanides allows oscillations to continue in a ‘closed system’ purely through Ca2+ release138. In mast cells, leukotriene C4-induced Ca2+ oscillations seem to be identical in the presence or absence of SOCE blockers. However, when the SOCE component is eliminated, leukotriene C4-induced Ca2+ oscillations fail to induce c-fos expression and, thus, a c-FOS-mediated transcriptional response in activated mast cells136. This implicates the local Ca2+ environment (which is also known as the ‘Ca2+ signature’) created by SOCE as being crucial for c-FOS activation. Similar requirements for SOCE were demonstrated for NFAT activation139,140. Nuclear translocation of NFAT requires dephosphorylation by calcineurin141,142, and continued NFAT activation requires extended cytosolic Ca2+ level increases to maintain calcineurin activation. Calcineurin localized within near-plasma membrane scaffolds143,144 may be selectively activated in the immediate environment of SOCE (FIG. 3).
Conclusions and implications
The pace of discovery in understanding the function of STIM proteins continues to accelerate. New information reveals the remarkable molecular coordination between STIM proteins and their growing number of interacting partners and targets. Also emerging is an understanding of the broader sensing and coupling properties of STIM proteins. As ‘stress-transducers’, STIM proteins can respond to cellular stressors, such as Ca2+, oxidative and hypoxic conditions, temperature fluctuation and changes in pH (BOX 2), and this extends their potential as homeostatic regulators.
Perhaps the most important goal of future studies will be the discovery of target and regulatory proteins that closely coexist with STIM proteins within junctions. The organization of these junctional domains with the participation of major Ca2+ channel and pump proteins suggests that the local Ca2+ signalling environment has a profound impact on cell function. The application of super-resolution imaging and FRET technology may provide enhanced understanding of the structure, operation and role of these domains.
STIM proteins have novel and unexpected physiological and pathophysiological roles in several tissues (BOX 3). Animal studies revealed that the phenotypes of Stim1 and Stim2 knockout mice are surprisingly different. The use of conditional tissue-specific knockout mouse models will provide important information on the sometimes subtle tissue-specific functions of STIM proteins. For example, STIM proteins have novel and surprising roles in growth and development of skeletal muscle145,146, cardiac muscle147 and smooth muscle148. With new structural details emerging on STIM proteins, the generation of transgenic mouse models carrying Stim genes mutationally modified at key regulatory and interactive sites will help understand the role and pathophysiology of STIM proteins and provide new information on functional differences between STIM1 and STIM2. The Ca2+ sensing properties, activation kinetics and target coupling efficiency of the two STIM proteins are quite different. The exquisite sensitivity, ubiquitous expression and breadth of control over Ca2+ signals mediated by STIM proteins predict that we are only beginning to understand the implications and importance of their cellular function.
Box 3. Physiological and pathophysiological roles of STIM and Orai proteins.
Lung
Stromal interaction molecule 1 (STIM1)–Orai1 complexes mediate store-operated Ca2+ entry (SOCE) in airway smooth muscle cells150. STIM1-dependent Ca2+ release-activated Ca2+ (CRAC) channel activation participates in hypoxia-induced AMP-activated protein kinase (AMPK) activation151. STIM1–Orai1 upregulation correlates with pulmonary smooth muscle cell proliferation. Increased Orai1 insertion contributes to the inflammatory response in cystic fibrosis152. Orai1 knockdown attenuates endothelial cell migration and angiogenesis153.
Heart
STIM1-dependent SOCE contributes to cardiac hypertrophy147,154. STIM1 knockdown diminishes diastolic Ca2+ levels and Orai1 knockdown abrogates hypertrophic signalling155.
Liver
Hormone-induced cytosolic Ca2+ oscillations require Ca2+ entry through SOCE156. STIM1 contributes to hepatocyte injury during cholestasis157.
Kidney
In glomerular mesangial cells, SOCE requires STIM1 and regulates glomerular haemodynamics158.
Reproductive tract
STIM1 is required for sheath cell and spermatheca contractile activity, which contributes to fertility in Caenorhabditis elegans159. STIM1–Orai1-dependent SOCE contributes to oocyte maturation and fertilization in mammals160.
Bone
Orai1 is required for the formation of multinuclear osteoclasts161,162. Orai1 knockout animals exhibit low bone density, implicating additional roles in osteoblast function163.
Skeletal muscle
STIM1 and Orai1 are expressed in skeletal muscle164. STIM1 ablation reduces SOCE, which causes muscle weakness and neonatal lethality21,165. STIM1 and STIM2 are necessary for exitation–contraction coupling and myoblast differentiation166. The fast STIM–Orai1 activation in skeletal muscle depends on the actin-binding STIM1L splice variant86.
Brain
STIM proteins and Orai channels are implicated in neuronal development and memory167. Loss of STIM2 affects cognitive function but protects from neuronal damage after ischaemic stroke26.
Breast
During lactation, the expression of Orai1 and STIM2 is increased, whereas STIM1 is downregulated168. The STIM1– Orai1 complex is required for breast tumour cell migration and metastasis169. Orai1–SPCA2 (secretory pathway Ca2+ ATPase 2)-mediated store-independent Ca2+ entry influences mammary tumorigenesis170. STIM1-, STIM2- and Orai3-induced SOCE contributes to breast cancer progression171,172.
Pancreas
STIM1 is localized exclusively to the lateral and basal regions in pancreatic acinar cells173, whereas Orai1 is restricted to the apical region174. SOCE is implicated in insulin secretion from pancreatic β-cells175.
Intestine
In colonic epithelial cells, STIM1 is required for cAMP production triggered by polyunsaturated fatty acids176. CRAC channels do not contribute to oscillatory Ca2+ signalling in worm intestines159.
T cells
Mutations in either Orai1 or STIM1 results in defective SOCE that leads to severe combined immunodeficiency3,21,25. Mice lacking either STIM1 or STIM2 are protected from developing multiple sclerosis177.
B cells
SOCE-induced Ca2+ signalling contributes to the generation of interleukin 6 (IL-6), IL-10 and immunoglobulin M (IgM)135. Loss of STIM1 and STIM2 exacerbates autoimmune encephalomyelitis in mice. STIM1 overexpression sensitizes developing B cells to negative selection178.
Mast cells
Mast cells lacking either STIM1 (REF. 179) or Orai1 (REF. 180) exhibit defective cytokine production and release, which prevents allergic responses. No defects in proliferation or differentiation were observed in these cells.
Platelets
Constitutive STIM1 activation causes premature platelet activation181. STIM1 is required for physiological platelet activation in arterial thrombus formation182,183. The STIM1–Orai1 complex is essential for occlusive thrombus formation during stroke182,184.
Granulocytes
STIM1–Orai1 mediated increases in Ca2+ levels are key for the modulation of NADPH oxidase activity and the rise in Ca2+ levels are crucial for phagocytic activity185. In murine eosinophils, Orai1 contributes to allergic rhinitis186.
Myeloid cells
STIM1 ablation causes phagocytosis defects without affecting cytokine induction. Mice that lack STIM1 are resistant to experimental immune thrombocytophenia, autoimmune haemolytic anaemia and acute pneumonitis187. Dendritic cells predominantly express STIM2, but not STIM1, and STIM2 moves to the immunological synapse27.
Supplementary Material
Acknowledgments
This work was supported by US National Institutes of Health (NIH) grants AI058173 (to D.L.G.), GM097335 (to J.S.), HL109920 (to M.M. and J.S.), HL086699 (to M.M.) and GM068523(to B.S.R.).
Glossary
- Ca2+ signalling
The process through which spatially and temporally controlled changes in Ca2+ levels are induced in response to external and/or internal cellular activation events
- Stromal interaction molecule (STIM)
The Ca2+ sensing endoplasmic reticulum (ER) transmembrane protein that becomes activated upon decrease of ER luminal Ca2+. Higher eukaryotes contain two similar proteins, STIM1 and STIM2. STIM2 is exclusively expressed in the ER, whereas ~5–10% of STIM1 is located in the plasma membrane. Activated STIM proteins translocate into ER–plasma membrane junctions where they activate plasma membrane Orai Ca2+ channels
- Ca2+ homeostasis
The process of global Ca2+ maintenance within the entire cell and/or sub-compartments therein. The term refers to the regulation of Ca2+ within cells through the operation of all Ca2+ pumps, channels, binding proteins and other regulatory proteins within every cellular compartment, including the cytosol, endoplasmic reticulum, mitochondria, Golgi apparatus and endolysosomal system
- Endoplasmic reticulum (ER)
The extensive subcellular tubular network that has crucial roles in lipid and protein synthesis and serves also as the major Ca2+ storage organelle
- Orai channels
A family of plasma membrane Ca2+ entry channels comprising three mammalian homologues termed Orai1, Orai2 and Orai3. Orai proteins have four transmembrane domains and form tetrameric channels. Orai1 tetramers are the pore-forming units of Ca2+ release-activated Ca2+ (CRAC) channels, gated by stromal interaction molecule (STIM) proteins. Orai2 and Orai3 can also form STIM-responsive channels, although the physiological roles of these proteins are less clear
- Capacitative Ca2+ entry
This term was introduced to describe the activation of Ca2+ entry across the plasma membrane in response to the depletion of Ca2+ levels in the endoplasmic reticulum. Currently known as storeoperated Ca2+ entry (SOCE)
- Inositol-1,4,5-trisphosphate (Ins(1,4,5)P3)
A product derived from breakdown of phosphatidylinositol-4,5- bisphosphate by phospholipase C. It functions as a cytosolic second messenger by binding to Ins(1,4,5)P3 receptors, which are located on the endoplasmic reticulum membrane and operate as ER Ca2+ release channels
- Store-operated Ca2+ entry (SOCE)
The activation of a Ca2+ channel in the plasma membrane in response to the depletion of Ca2+ levels in the endoplasmic reticulum. Formerly known as capacitative Ca2+ entry
- Ca2+ release-activated Ca2+ current (CRAC current or ICRAC)
The electrophysiologically defined current mediated by the Orai family of channels
- Mast cells
Leukocyte cells with large secretory granules that contain histamine and various protein mediators. Mast cells contain an unusually high density of Ca2+ release-activated Ca2+ (CRAC) channels, which function to mediate mast cell activation
- EF-hand domains
Highly conserved Ca2+-binding domains comprising two helices (E and F after the 5th and 6th helices of parvalbumin) that are linked by a short acidic Ca2+-binding loop
- STIM–Orai activating region (SOAR)
A ~100 amino acid segment within the cytosolic domain of stromal interaction molecule 1 (STIM1) that is the minimal sequence for mediating interaction with and activation of Orai channels
- Pre-B cells
Cells in a stage of B cell development in the bone marrow that are characterized by complete immunoglobulin heavy-chain rearrangement in the absence of immunoglobulin light-chain rearrangement. They express the pre-B cell receptor, which comprises a pseudo light chain and a heavy chain. Cells are phenotypically CD19+ cytoplasmic immunoglulin M+ (IgM+) or are sometimes defined as B220+ CD43−cell surface IgM− (which is known as the Hardy classification)
- Hill coefficient
Quantifies the cooperativity of ligand binding by an allosteric protein and indicates the minimal number of interacting binding sites. A Hill coefficient of 1 indicates independent binding even when ligands are bound to different binding sites, and a coefficient of >1 reflects positive cooperativity
- Junctate
A Ca2+-binding, integral endoplasmic reticulum (ER) membrane protein that induces and/or stabilizes peripheral coupling between the ER and the plasma membrane
- Ca2+-dependent inactivation (CDI)
The process whereby an increase in cytosolic Ca2+ levels leads to inactivation of the Ca2+ release-activated Ca2+ (CRAC) current. CDI is mediated by residues located in stromal interaction molecule 1 (STIM1) (in the cytosolic domain) and Orai1 (within the amino terminus)
- Inhibitory domain region (ID region)
A short sequence in the carboxy-terminal region of stromal interaction molecule 1 (STIM1) containing acidic residues that is important for mediating Ca2+-dependent inactivation of Orai channels
- Calmodulin
A highly conserved cytosolic Ca2+-binding protein that mediates several Ca2+-dependent responses in cells by interaction with and modification of calmodulin-binding target proteins. Calmodulin is expressed in all eukaryotic cell types
- CRAC regulatory protein 2A (CRACR2A)
A recently described stromal interaction molecule (STIM)–Orai-binding protein shown to regulate the stability of the Ca2+ signalling junction
- Golli
A member of the myelin basic protein (MBP) family that interacts with stromal interaction molecule 1 (STIM1) and regulates store operated Ca2+ entry (SOCE)
- SOCE-associated regulatory factor (SARAF)
A recently defined stromal interaction molecule (STIM)-binding protein that mediates the dissociation of STIM proteins from Ca2+ signalling junctions
- Transient receptor potential channels (TRPCs)
A family of seven non-selective cation channels defined on the basis of homology with the Drosophila melanogaster TRP channel. TRPCs have been extensively investigated as potential mediators of store-operated Ca2+ entry (SOCE). However, this notion is highly controversial mainly because they are activated downstream of phospholipase C and mediate non-selective cation currents easily distinguishable from Ca2+ release-activated Ca2+(CRAC)
- Phospholipase C (PLC)
A signalling enzyme that breaks down phosphatidylinositol-4,5-bisphosphate into inositol-1,4,5-trisphosphate and diacylglycerol in response to the activation of both G protein-coupled receptor and Tyr kinase-coupled receptor
- Diacylglycerol (DAG)
Lipid product of phospholipase C that activates and modulates the function of several proteins including various ion channels
- Phosphatidylinositol-4 5-bisphosphate, (PtdIns(4,5)P2)
A negatively charged phospholipid found primarily in the inner leaflet of the plasma membrane. As the substrate for phospholipase C, PtdIns(4,5)P2 breakdown leads to the release of Ins(1,4,5)P3 and diacylglycerol
- ARC channel
An arachidonic acid-responsive channel activity comprising a unique combination of Orai1 and Orai3 subunits
- Sarcoendoplasmic reticulum Ca2+ATPase (SERCA)
A family of Ca2+ pumps comprising three members, all of which pump Ca2+ from the cytosol into the endoplasmic reticulum (ER) or the sacroeplamic reticulum lumen. Each family member has several splice variants. Due to the leakiness of the ER to Ca2+, SERCA is constitutively active
- Aequorins
Ca2+-sensitive photoproteins isolated from luminescent jellyfish that are used to detect the Ca2+ content in different subcellular compartments
- Plasma membrane Ca2+ ATPase (PMCA)
A family of Ca2+ pumps comprising four members that pump Ca2+ from the cytosol to the extracellular milieu. Each family member has at least two splice variants. PMCA pumps primarily function to maintain cytosolic Ca2+ levels
- Immunological synapse
A region that can form between two cells of the immune system that are in close proximity. The name derives from similarities to the synapse that is found in the nervous system. The immunological synapse refers to the interaction between a T cell or natural killer cell and an antigen-presenting cell
- Partner of STIM1 (POST)
A 12-transmembrane spanning protein found mostly in the endoplasmic reticulum membrane, but also in the plasma membrane. POST binds activated stromal interaction molecule 1 (STIM1) and modulates the function of multiple target proteins
- Calnexin
Ca2+-binding transmembrane endoplasmic reticulum chaperone protein that functions to support correct folding of new proteins
Footnotes
Competing interests statement
The authors declare competing financial interests; see Web version for details.
FURTHER INFORMATION
Temple University School of Medicine Department of Biochemistry: http://www.temple.edu/medicine/departments_centers/basic_science/biochemistry.htm
SUPPLEMENTARY INFORMATION
See online article: S1 (table)
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
References
- 1.Roos J, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liou J, et al. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feske S, et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 4.Vig M, et al. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang SL, et al. Genome-wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release-activated Ca2+ channel activity. Proc Natl Acad Sci USA. 2006;103:9357–9362. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ringer S. A further contribution regarding the influence of the different constituents of the blood on the contraction of the heart. J Physiol. 1883;4:29–42. doi: 10.1113/jphysiol.1883.sp000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bohr DF. Vascular smooth muscle updated. Circ Res. 1973;32:665–672. doi: 10.1161/01.res.32.6.665. [DOI] [PubMed] [Google Scholar]
- 8.Putney JW. A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 9.Streb H, Irvine RF, Berridge MJ, Schulz I. Release of Ca2+from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5- trisphosphate. Nature. 1983;306:67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- 10.Takemura H, Putney JW., Jr Capacitative calcium entry in parotid acinar cells. Biochem J. 1989;258:409–412. doi: 10.1042/bj2580409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muallem S, Khademazad M, Sachs G. The route of Ca2+ entry during reloading of the intracellular Ca2+ pool in pancreatic acini. J Biol Chem. 1990;265:2011–2016. [PubMed] [Google Scholar]
- 12.Takemura H, Hughes AR, Thastrup O, Putney JW. Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J Biol Chem. 1989;264:12266–12271. [PubMed] [Google Scholar]
- 13.Putney JW. Capacitative calcium entry revisited. Cell Calcium. 1990;11:611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- 14.Lewis RS, Cahalan MD. Mitogen-induced oscillations of cytosolic Ca2+ and transmembrane Ca2+ current in human leukemic T cells. Cell Regul. 1989;1:99–112. doi: 10.1091/mbc.1.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Penner R, Matthews G, Neher E. Regulation of calcium influx by second messengers in rat mast cells. Nature. 1988;334:499–504. doi: 10.1038/334499a0. [DOI] [PubMed] [Google Scholar]
- 16.Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- 17.Berridge MJ. Capacitative calcium entry. Biochem J. 1995;312:1–11. doi: 10.1042/bj3120001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patterson RL, van Rossum DB, Gill DL. Store operated Ca2+ entry: evidence for a secretion-like coupling model. Cell. 1999;98:487–499. doi: 10.1016/s0092-8674(00)81977-7. [DOI] [PubMed] [Google Scholar]
- 19.Yao Y, Ferrer-Montiel AV, Montal M, Tsien RY. Activation of store-operated Ca2+ current in Xenopus oocytes requires SNAP-25 but not a diffusible messenger. Cell. 1999;98:475–485. doi: 10.1016/s0092-8674(00)81976-5. [DOI] [PubMed] [Google Scholar]
- 20.McCarl CA, et al. ORAI1 deficiency and lack of store-operated Ca2+ entry cause immunodeficiency, myopathy, and ectodermal dysplasia. J Allergy Clin Immunol. 2009;124:1311–1318. doi: 10.1016/j.jaci.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Picard C, et al. STIM1 mutation associated with a syndrome of immunodeficiency and autoimmunity. N Engl J Med. 2009;360:1971–1980. doi: 10.1056/NEJMoa0900082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strange K, Yan X, Lorin-Nebel C, Xing J. Physiological roles of STIM1 and Orai1 homologs and CRAC channels in the genetic model organism Caenorhabditis elegans. Cell Calcium. 2007;42:193–203. doi: 10.1016/j.ceca.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collins SR, Meyer T. Evolutionary origins of STIM1 and STIM2 within ancient Ca2+ signaling systems. Trends Cell Biol. 2011;21:202–211. doi: 10.1016/j.tcb.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams RT, et al. Identification and characterization of the STIM (stromal interaction molecule) gene family: coding for a novel class of transmembrane proteins. Biochem J. 2001;357:673–685. doi: 10.1042/0264-6021:3570673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oh-Hora M, et al. Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nature Immunol. 2008;9:432–443. doi: 10.1038/ni1574. An important study examining the roles of both STIM1 and STIM2 in mediating Ca2+entry in T3cells and fibroblasts and revealing the roles of STIM proteins in the development and function of regulatory T cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berna-Erro A, et al. STIM2 regulates capacitive Ca2+ entry in neurons and plays a key role in hypoxic neuronal cell death. Sci Signal. 2009;2:ra67. doi: 10.1126/scisignal.2000522. [DOI] [PubMed] [Google Scholar]
- 27.Bandyopadhyay BC, Pingle SC, Ahern GP. Store-operated Ca2+ signaling in dendritic cells occurs independently of STIM1. J Leukoc Biol. 2011;89:57–62. doi: 10.1189/jlb.0610381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deng X, Wang Y, Zhou Y, Soboloff J, Gill DL. STIM and Orai-dynamic intermembrane coupling to control cellular calcium signals. J Biol Chem. 2009;284:22501–22505. doi: 10.1074/jbc.R109.018655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manji SS, et al. STIM1: a novel phosphoprotein located at the cell surface. Biochim Biophys Acta. 2000;1481:147–155. doi: 10.1016/s0167-4838(00)00105-9. [DOI] [PubMed] [Google Scholar]
- 30.Soboloff J, et al. STIM2 is an inhibitor of STIM1-mediated store-operated Ca2+ entry. Curr Biol. 2006;16:1465–1470. doi: 10.1016/j.cub.2006.05.051. [DOI] [PubMed] [Google Scholar]
- 31.Hewavitharana T, et al. Location and function of STIM1 in the activation of Ca2+ entry signals. J Biol Chem. 2008;283:26252–26262. doi: 10.1074/jbc.M802239200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewis RS. The molecular choreography of a store-operated calcium channel. Nature. 2007;446:284–287. doi: 10.1038/nature05637. [DOI] [PubMed] [Google Scholar]
- 33.Liou J, Fivaz M, Inoue T, Meyer T. Live-cell imaging reveals sequential oligomerization and local plasma membrane targeting of stromal interaction molecule 1 after Ca2+ store depletion. Proc Natl Acad Sci USA. 2007;104:9301–9306. doi: 10.1073/pnas.0702866104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol. 2006;174:803–813. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baba Y, et al. Coupling of STIM1 to store-operated Ca2+entry through its constitutive and inducible movement in the endoplasmic reticulum. Proc Natl Acad Sci USA. 2006;103:16704–16709. doi: 10.1073/pnas.0608358103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Penna A, et al. The CRAC channel consists of a tetramer formed by Stim-induced dimerization of Orai dimers. Nature. 2008;456:116–120. doi: 10.1038/nature07338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Covington ED, Wu MM, Lewis RS. Essential role for the CRAC activation domain in store-dependent oligomerization of STIM1. Mol Biol Cell. 2010;21:1897–1907. doi: 10.1091/mbc.E10-02-0145. Reveals that the active domain within STIM1 that interacts with and triggers Orai channel opening is also involved in STIM1 oligomerization, which is the initial event promoted by store-depletion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Z, et al. Graded activation of CRAC channel by binding of different numbers of STIM1 to Orai1 subunits. Cell Res. 2011;21:305–315. doi: 10.1038/cr.2010.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoover PJ, Lewis RS. Stoichiometric requirements for trapping and gating of Ca2+ release-activated Ca2+ (CRAC) channels by stromal interaction molecule 1 (STIM1) Proc Natl Acad Sci USA. 2011;108:13299–13304. doi: 10.1073/pnas.1101664108. Examines the stoichiometry of interaction between STIM and Orai proteins, indicating that eight STIM1 molecules interact with a single Orai channel comprising four Orai subunits. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang X, Jin H, Cai X, Li S, Shen Y. Structural and mechanistic insights into the activation of stromal interaction molecule 1 (STIM1) Proc Natl Acad Sci USA. 2012;109:5657–5662. doi: 10.1073/pnas.1118947109. The first atomic structure of SOAR, which is the active site within the C-terminal domain of STIM1, derived from X-ray analysis of a crystallized form of the STIM1 fragment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stathopulos PB, Li GY, Plevin MJ, Ames JB, Ikura M. Stored Ca2+ depletion-induced oligomerization of STIM1 via the EF-SAM region: an initiation mechanism for capacitive Ca2+ entry. J Biol Chem. 2006;281:35855–35862. doi: 10.1074/jbc.M608247200. [DOI] [PubMed] [Google Scholar]
- 42.Stathopulos PB, Zheng L, Li GY, Plevin MJ, Ikura M. Structural and mechanistic insights into STIM1-mediated initiation of store-operated calcium entry. Cell. 2008;135:110–122. doi: 10.1016/j.cell.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 43.Zhang SL, et al. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luik RM, Wang B, Prakriya M, Wu MM, Lewis RS. Oligomerization of STIM1 couples ER calcium depletion to CRAC channel activation. Nature. 2008;454:538–542. doi: 10.1038/nature07065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yuan JP, et al. SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nature Cell Biol. 2009;11:337–343. doi: 10.1038/ncb1842. Identifies SOAR as the active site of STIM1 that interacts with and triggers the opening of Orai channels. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park CY, et al. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell. 2009;136:876–890. doi: 10.1016/j.cell.2009.02.014. A detailed study revealing that the STIM1 segment CAD is the Orai coupling site and providing a detailed characterization of the molecular nature of the STIM–Orai interaction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muik M, et al. A cytosolic homomerization and a modulatory domain within STIM1 C-terminus determine coupling to ORAI1 channels. J Biol Chem. 2009;284:8421–8426. doi: 10.1074/jbc.C800229200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oritani K, Kincade PW. Identification of stromal cell products that interact with pre-B cells. J Cell Biol. 1996;134:771–782. doi: 10.1083/jcb.134.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hauser CT, Tsien RY. A hexahistidine-Zn2+-dye label reveals STIM1 surface exposure. Proc Natl Acad Sci USA. 2007;104:3693–3697. doi: 10.1073/pnas.0611713104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Z, et al. Mapping the interacting domains of STIM1 and Orai1 in CRAC channel activation. J Biol Chem. 2007;282:29448–29456. doi: 10.1074/jbc.M703573200. [DOI] [PubMed] [Google Scholar]
- 51.Zheng L, Stathopulos PB, Li GY, Ikura M. Biophysical characterization of the EF-hand and SAM domain containing Ca2+ sensory region of STIM1 and STIM2. Biochem Biophys Res Commun. 2008;369:240–246. doi: 10.1016/j.bbrc.2007.12.129. [DOI] [PubMed] [Google Scholar]
- 52.Stathopulos PB, Zheng L, Ikura M. Stromal interaction molecule (STIM)1 and STIM2 EF-SAM regions exhibit distinct unfolding and oligomerization kinetics. J Biol Chem. 2009;284:728–732. doi: 10.1074/jbc.C800178200. [DOI] [PubMed] [Google Scholar]
- 53.Zheng L, et al. Auto-inhibitory role of the EF-SAM domain of STIM proteins in store-operated calcium entry. Proc Natl Acad Sci USA. 2011;108:1337–1342. doi: 10.1073/pnas.1015125108. Nuclear magnetic resonance (NMR)-based structural approaches revealing that the EF-hand–SAM domains of STIM1 and STIM2 may have distinct stabilization properties that could explain important differences in SOCE mediated by each protein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Williams RT, et al. Stromal interaction molecule 1 (STIM1), a transmembrane protein with growth suppressor activity, contains an extracellular SAM domain modified by N-linked glycosylation. Biochim Biophys Acta. 2002;1596:131–137. doi: 10.1016/s0167-4838(02)00211-x. [DOI] [PubMed] [Google Scholar]
- 55.Smyth JT, Dehaven WI, Bird GS, Putney JW. Jr Ca2+-store-dependent and -independent reversal of Stim1 localization and function. J Cell Sci. 2008;121:762–772. doi: 10.1242/jcs.023903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Muik M, et al. Dynamic coupling of the putative coiled-coil domain of ORAI1 with STIM1 mediates ORAI1 channel activation. J Biol Chem. 2008;283:8014–8022. doi: 10.1074/jbc.M708898200. [DOI] [PubMed] [Google Scholar]
- 57.Brandman O, Liou J, Park WS, Meyer T. STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell. 2007;131:1327–1339. doi: 10.1016/j.cell.2007.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carrasco S, Meyer T. STIM proteins and the endoplasmic reticulum-plasma membrane junctions. Annu Rev Biochem. 2011;80:973–1000. doi: 10.1146/annurev-biochem-061609-165311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spassova MA, et al. STIM1 has a plasma membrane role in the activation of store-operated Ca2+ channels. Proc Natl Acad Sci USA. 2006;103:4040–4045. doi: 10.1073/pnas.0510050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bird GS, et al. STIM1 is a calcium sensor specialized for digital signaling. Curr Biol. 2009;19:1724–1729. doi: 10.1016/j.cub.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Soboloff J, Spassova MA, Dziadek MA, Gill DL. Calcium signals mediated by STIM and Orai proteins — a new paradigm in inter-organelle communication. Biochim Biophys Acta. 2006;1763:1161–1168. doi: 10.1016/j.bbamcr.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 62.Saitoh N, et al. Identification of functional domains and novel binding partners of STIM proteins. J Cell Biochem. 2011;112:147–156. doi: 10.1002/jcb.22910. [DOI] [PubMed] [Google Scholar]
- 63.Parvez S, et al. STIM2 protein mediates distinct store-dependent and store-independent modes of CRAC channel activation. FASEB J. 2008;22:752–761. doi: 10.1096/fj.07-9449com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou Y, et al. The short N-terminal domains of STIM1 and STIM2 control the activation kinetics of Orai1 channels. J Biol Chem. 2009;284:19164–19168. doi: 10.1074/jbc.C109.010900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Soboloff J, et al. Orai1 and STIM reconstitute store-operated calcium channel function. J Biol Chem. 2006;281:20661–20665. doi: 10.1074/jbc.C600126200. [DOI] [PubMed] [Google Scholar]
- 66.Wang Y, et al. STIM protein coupling in the activation of Orai channels. Proc Natl Acad Sci USA. 2009;106:7391–7396. doi: 10.1073/pnas.0900293106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kar P, Bakowski D, Di Capite J, Nelson C, Parekh AB. Different agonists recruit different stromal interaction molecule proteins to support cytoplasmic Ca2+ oscillations and gene expression. Proc Natl Acad Sci USA. 2012;109:6969–6974. doi: 10.1073/pnas.1201204109. Reveals interesting differences in the ability of STIM1 and STIM2 to mediate Ca2+ entry in response to different PLC-coupled receptors and Ca2+oscillations that lead to the activation of gene expression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Korzeniowski MK, Manjarres IM, Varnai P, Balla T. Activation of STIM1-Orai1 involves an intramolecular switching mechanism. Sci Signal. 2010;3:ra82. doi: 10.1126/scisignal.2001122. Provides information on the interactive sites in STIM1 and Orai1 that are involved in an electrostatic interaction and proposes an intramolecular switch to maintain STIM1 in an inactive state. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou Y, et al. STIM1 gates the store-operated calcium channel ORAI1 in vitro. Nature Struct Mol Biol. 2010;17:112–116. doi: 10.1038/nsmb.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Calloway N, Vig M, Kinet JP, Holowka D, Baird B. Molecular clustering of STIM1 with Orai1/CRACM1 at the plasma membrane depends dynamically on depletion of Ca2+ stores and on electrostatic interactions. Mol Biol Cell. 2009;20:389–399. doi: 10.1091/mbc.E07-11-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Calloway N, Holowka D, Baird B. A basic sequence in STIM1 promotes Ca2+ influx by interacting with the C-terminal acidic coiled coil of Orai1. Biochemistry. 2010;49:1067–1071. doi: 10.1021/bi901936q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Frischauf I, et al. Molecular determinants of the coupling between STIM1 and Orai channels: differential activation of Orai1–3 channels by a STIM1 coiled-coil mutant. J Biol Chem. 2009;284:21696–21706. doi: 10.1074/jbc.M109.018408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lis A, Zierler S, Peinelt C, Fleig A, Penner R. A single lysine in the N-terminal region of store-operated channels is critical for STIM1-mediated gating. J Gen Physiol. 2010;136:673–686. doi: 10.1085/jgp.201010484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Muik M, et al. STIM1 couples to ORAI1 via an intramolecular transition into an extended conformation. EMBO J. 2011;30:1678–1689. doi: 10.1038/emboj.2011.79. Reveals by using FRET technology that a segment of the STIM1 C-terminal domain containing the active site undergoes an important intramolecular transition into an extended conformation upon interaction with Orai. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang GN, et al. STIM1 carboxyl-terminus activates native SOC, I(crac) and TRPC1 channels. Nature Cell Biol. 2006;8:1003–1010. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- 76.Kim JY, Muallem S. Unlocking SOAR releases STIM. EMBO J. 2011;30:1673–1675. doi: 10.1038/emboj.2011.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yu F, Sun L, Courjaret R, Machaca K. Role of the STIM1 C-terminal domain in STIM1 clustering. J Biol Chem. 2011;286:8375–8384. doi: 10.1074/jbc.M110.188789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McNally BA, Somasundaram A, Yamashita M, Prakriya M. Gated regulation of CRAC channel ion selectivity by STIM1. Nature. 2012;482:241–245. doi: 10.1038/nature10752. An important study revealing the intimate control mediated by STIM1on Orai1channels and revealing that STIM1 functions similar to a channel subunit controlling both gating and the pore properties of the Orai1 channel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Korzeniowski MK, et al. Dependence of STIM1/Orai1-mediated calcium entry on plasma membrane phosphoinositides. J Biol Chem. 2009;284:21027–21035. doi: 10.1074/jbc.M109.012252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Luik RM, Wu MM, Buchanan J, Lewis RS. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J Cell Biol. 2006;174:815–825. doi: 10.1083/jcb.200604015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Treves S, et al. Junctate is a key element in calcium receptors and/or entry induced by activation of InsP3 calcium store depletion. J Cell Biol. 2004;166:537–548. doi: 10.1083/jcb.200404079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Treves S, et al. Agonist-activated Ca2+ influx occurs at stable plasma membrane and endoplasmic reticulum junctions. J Cell Sci. 2010;123:4170–4181. doi: 10.1242/jcs.068387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Srikanth S, et al. Junctate is a Ca2+-sensing structural component of Orai1 and stromal interaction molecule31 (STIM1) Proc Natl Acad Sci USA. 2012;109:8682–8687. doi: 10.1073/pnas.1200667109. Identifies the ER protein junctate as a luminal Ca2+-sensing component within ER–plasma membrane junctions and shows that junctate mediates and controls recruitment of STIM1 into junctions to activate Orai channels. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Edwards JN, Blackmore DG, Gilbert DF, Murphy RM, Launikonis BS. Store-operated calcium entry remains fully functional in aged mouse skeletal muscle despite a decline in STIM1 protein expression. Aging Cell. 2011;10:675–685. doi: 10.1111/j.1474-9726.2011.00706.x. [DOI] [PubMed] [Google Scholar]
- 85.Stiber JA, Rosenberg PB. The role of store-operated calcium influx in skeletal muscle signaling. Cell Calcium. 2011;49:341–349. doi: 10.1016/j.ceca.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Darbellay B, Arnaudeau S, Bader CR, Konig S, Bernheim L. STIM1L is a new actin-binding splice variant involved in fast repetitive Ca2+ release. J Cell Biol. 2011;194:335–346. doi: 10.1083/jcb.201012157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Srikanth S, et al. A novel EF-hand protein, CRACR2A, is a cytosolic Ca2+ sensor that stabilizes CRAC channels in T cells. Nature Cell Biol. 2010;12:436–446. doi: 10.1038/ncb2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Palty R, Raveh A, Kamisky I, Meller R, Reuveny E. SARAF inactivates the store operated calcium entry machinery to prevent excess calcium refilling. Cell. 2012;149:425–438. doi: 10.1016/j.cell.2012.01.055. Screening approaches that reveal the ER protein SARAF as a potentially important negative regulator of STIM protein function and indicate that SARAF may have an important function in limiting Ca2+ signals in cells. [DOI] [PubMed] [Google Scholar]
- 89.Mullins FM, Park CY, Dolmetsch RE, Lewis RS. STIM1 and calmodulin interact with Orai1 to induce Ca2+-dependent inactivation of CRAC channels. Proc Natl Acad Sci USA. 2009;106:15495–15500. doi: 10.1073/pnas.0906781106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hoth M, Penner R. Calcium release-activated calcium current in rat mast cells. J Physiol. 1993;465:359–386. doi: 10.1113/jphysiol.1993.sp019681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zweifach A, Lewis RS. Rapid inactivation of depletion-activated calcium current (ICRAC) due to local calcium feedback. J Gen Physiol. 1995;105:209–226. doi: 10.1085/jgp.105.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Derler I, et al. A Ca2+ release-activated Ca2+ (CRAC) modulatory domain (CMD) within STIM1 mediates fast Ca2+-dependent inactivation of ORAI1 channels. J Biol Chem. 2009;284:24933–24938. doi: 10.1074/jbc.C109.024083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee KP, et al. Molecular determinants of fast Ca2+-dependent inactivation and gating of the Orai channels. Proc Natl Acad Sci USA. 2009;106:14687–14692. doi: 10.1073/pnas.0904664106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Litjens T, Harland ML, Roberts ML, Barritt GJ, Rychkov GY. Fast Ca2+-dependent inactivation of the store-operated Ca2+current (ISOC) in liver cells: a role for calmodulin. J Physiol. 2004;558:85–97. doi: 10.1113/jphysiol.2004.065870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shen WW, Frieden M, Demaurex N. Local cytosolic Ca2+ elevations are required for stromal interaction molecule 1 (STIM1) de-oligomerization and termination of store-operated Ca2+ entry. J Biol Chem. 2011;286:36448–36459. doi: 10.1074/jbc.M111.269415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Feng JM, et al. Golli protein negatively regulates store depletion-induced calcium influx in T cells. Immunity. 2006;24:717–727. doi: 10.1016/j.immuni.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 97.Fulton D, et al. Regulation of L-type Ca++ currents and process morphology in white matter oligodendrocyte precursor cells by gollimyelin proteins. Glia. 2010;58:1292–1303. doi: 10.1002/glia.21008. [DOI] [PubMed] [Google Scholar]
- 98.Walsh CM, Doherty MK, Tepikin AV, Burgoyne RD. Evidence for an interaction between Golli and STIM1 in store-operated calcium entry. Biochem J. 2010;430:453–460. doi: 10.1042/BJ20100650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Soboloff J, Madesh M, Gill DL. Sensing cellular stress through STIM proteins. Nature Chem Biol. 2011;7:488–492. doi: 10.1038/nchembio.619. Provides current information on the role of STIM proteins in sensing and mediating responses to oxidative-, temperature- and hypoxic stress conditions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hawkins BJ, et al. S-glutathionylation activates STIM1 and alters mitochondrial homeostasis. J Cell Biol. 2010;190:391–405. doi: 10.1083/jcb.201004152. Describes that STIM1 can sense ROS, couple to and activate Orai channels within ER–plasma membrane junctions without store deletion through glutathionylation of a specific N-terminal Cys residue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xiao B, Coste B, Mathur J, Patapoutian A. Temperature-dependent STIM1 activation induces Ca2+ influx and modulates gene expression. Nature Chem Biol. 2011;7:351–358. doi: 10.1038/nchembio.558. Interesting study indicating that STIM1 becomes activated by modest increases in temperature, but that coupling to Orai channels requires cooling. These findings suggest that STIM1 is an important temperature sensor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mancarella S, Wang Y, Gill DL. Signal transduction: STIM1 senses both Ca2+ and heat. Nature Chem Biol. 2011;7:344–345. doi: 10.1038/nchembio.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mancarella S, et al. Hypoxia-induced acidosis uncouples the STIM–Orai calcium signaling complex. J Biol Chem. 2011;286:44788–44798. doi: 10.1074/jbc.M111.303081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gillo B, et al. Coexpression of Drosophila TRP and TRP-like proteins in Xenopus oocytes reconstitutes capacitative Ca2+ entry. Proc Natl Acad Sci USA. 1996;93:14146–14151. doi: 10.1073/pnas.93.24.14146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xu XZ, Li HS, Guggino WB, Montell C. Coassembly of TRP and TRPL produces a distinct store-operated conductance. Cell. 1997;89:1155–1164. doi: 10.1016/s0092-8674(00)80302-5. [DOI] [PubMed] [Google Scholar]
- 106.Kiselyov KI, et al. Functional interaction between InsP3 receptors and store-operated Htrp3 channels. Nature. 1998;396:478–482. doi: 10.1038/24890. [DOI] [PubMed] [Google Scholar]
- 107.Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S. STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nature Cell Biol. 2007;9:636–645. doi: 10.1038/ncb1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zeng W, et al. STIM1 gates TRPC channels but not Orai1 by electrostatic interaction. Mol Cell. 2008;32:439–448. doi: 10.1016/j.molcel.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee KP, Yuan JP, So I, Worley PF, Muallem S. STIM1-dependent and STIM1-independent function of transient receptor potential canonical (TRPC) channels tunes their store-operated mode. J Biol Chem. 2010;285:38666–38673. doi: 10.1074/jbc.M110.155036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Varga-Szabo D, et al. Store-operated Ca2+ entry in platelets occurs independently of transient receptor potential (TRP) C1. Pflügers Arch. 2008;457:377–387. doi: 10.1007/s00424-008-0531-4. [DOI] [PubMed] [Google Scholar]
- 111.Dehaven W, et al. TRPC channels function independently of STIM1 and Orai1. J Physiol. 2009;587:2275–2298. doi: 10.1113/jphysiol.2009.170431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang Y, et al. The calcium store-sensor, STIM1, reciprocally controls Orai and CaV1.2 channels. Science. 2010;330:105–109. doi: 10.1126/science.1191086. Describes how STIM proteins interact with and mediate control over the function of voltage-operated CaV1.2 channels within the same ER–plasma membrane junctional regions in which STIM proteins interact with Orai channels. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cheng KT, Liu X, Ong HL, Swaim W, Ambudkar IS. Local Ca2+ entry via Orai1 regulates plasma membrane recruitment of TRPC1 and controls cytosolic Ca2+ signals required for specific cell functions. PLoS Biol. 2011;9:e1001025. doi: 10.1371/journal.pbio.1001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Park CY, Shcheglovitov A, Dolmetsch R. The CRAC channel activator STIM1 binds and inhibits L-type voltage-gated calcium channels. Science. 2010;330:101–105. doi: 10.1126/science.1191027. Shows the molecular nature of the interactions between STIM1 and CaV1.2 channels and describes how these interactions lead to both inhibition of function and increased turnover and downregulation of CaV1.2 channels. [DOI] [PubMed] [Google Scholar]
- 115.Woodard GE, Salido GM, Rosado JA. Enhanced exocytotic-like insertion of Orai1 into the plasma membrane upon intracellular Ca2+ store depletion. Am J Physiol Cell Physiol. 2008;294:C1323–C1331. doi: 10.1152/ajpcell.00071.2008. [DOI] [PubMed] [Google Scholar]
- 116.Yu F, Sun L, Machaca K. Orai1 internalization and STIM1 clustering inhibition modulate SOCE inactivation during meiosis. Proc Natl Acad Sci USA. 2009;106:17401–17406. doi: 10.1073/pnas.0904651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mignen O, Thompson JL, Shuttleworth TJ. STIM1 regulates Ca2+ entry via arachidonate-regulated Ca2+-selective (ARC) channels without store depletion or translocation to the plasma membrane. J Physiol. 2007;579:703–715. doi: 10.1113/jphysiol.2006.122432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mignen O, Thompson JL, Shuttleworth TJ. Both Orai1 and Orai3 are essential components of the arachidonate-regulated Ca2+-selective (ARC) channels. J Physiol. 2008;586:185–195. doi: 10.1113/jphysiol.2007.146258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mignen O, Thompson JL, Shuttleworth TJ. The molecular architecture of the arachidonate-regulated Ca2+-selective ARC channel is a pentameric assembly of Orai1 and Orai3 subunits. J Physiol. 2009;587:4181–4197. doi: 10.1113/jphysiol.2009.174193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jousset H, Frieden M, Demaurex N. STIM1 knockdown reveals that store-operated Ca2+ channels located close to SERCA pumps silently refill the endoplasmic reticulum. J Biol Chem. 2007;282:11456–11464. doi: 10.1074/jbc.M609551200. [DOI] [PubMed] [Google Scholar]
- 121.Sampieri A, Zepeda A, Asanov A, Vaca L. Visualizing the store-operated channel complex assembly in real time: dentification of SERCA2 as a new member. Cell Calcium. 2009;45:439–146. doi: 10.1016/j.ceca.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 122.Manjarres IM, Rodriguez-Garcia A, Alonso MT, Garcia-Sancho J. The sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) is the third element in capacitative calcium entry. Cell Calcium. 2010;47:412–418. doi: 10.1016/j.ceca.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 123.Manjarres IM, Alonso MT, Garcia-Sancho J. Calcium entry-calcium refilling (CECR) coupling between store-operated Ca2+ entry and sarco/ endoplasmic reticulum Ca2+-ATPase. Cell Calcium. 2011;49:153–161. doi: 10.1016/j.ceca.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 124.Bautista DM, Lewis RS. Modulation of plasma membrane calcium-ATPase activity by local calcium microdomains near CRAC channels in human T cells. J Physiol. 2004;556:805–817. doi: 10.1113/jphysiol.2003.060004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Quintana A, et al. Calcium microdomains at the immunological synapse: how ORAI channels, mitochondria and calcium pumps generate local calcium signals for efficient T-cell activation. EMBO J. 2011;30:3895–3912. doi: 10.1038/emboj.2011.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ritchie MF, Samakai E, Soboloff J. STIM1 is required for attenuation of PMCA-mediated Ca2+ clearance during T-cell activation. EMBO J. 2012;31:1123–1133. doi: 10.1038/emboj.2011.495. Shows both functional and physical interactions between STIM1 and PMCA with important implications on the generation of Ca2+ signals in many cell types. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Krapivinsky G, Krapivinsky L, Stotz SC, Manasian Y, Clapham DE. POST, partner of stromal interaction molecule 1 (STIM1), targets STIM1 to multiple transporters. Proc Natl Acad Sci USA. 2011;108:19234–19239. doi: 10.1073/pnas.1117231108. The work identifies POST as a membrane protein present in both the ER and plasma membrane that moves with STIM1 into junctions upon store depletion and mediates interactions with important proteins including Ca2+ pumps. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liu B, Peel SE, Fox J, Hall IP. Reverse mode Na+/Ca2+ exchange mediated by STIM1 contributes to Ca2+ influx in airway smooth muscle following agonist stimulation. Respir Res. 2010;11:168. doi: 10.1186/1465-9921-11-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Prins D, Groenendyk J, Touret N, Michalak M. Modulation of STIM1 and capacitative Ca2+ entry by the endoplasmic reticulum luminal oxidoreductase ERp57. EMBO Rep. 2011;12:1182–1188. doi: 10.1038/embor.2011.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lefkimmiatis K, et al. Store-operated cyclic AMP signalling mediated by STIM1. Nature Cell Biol. 2009;11:433–442. doi: 10.1038/ncb1850. [DOI] [PubMed] [Google Scholar]
- 131.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nature Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 132.Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386:855–858. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- 133.Berridge MJ. Inositol trisphosphate and calcium oscillations. Biochem Soc Symp. 2007;74:1–7. doi: 10.1042/BSS0740001. [DOI] [PubMed] [Google Scholar]
- 134.Putney JW, Bird GS. Cytoplasmic calcium oscillations and store-operated calcium influx. J Physiol. 2008;586:3055–3059. doi: 10.1113/jphysiol.2008.153221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Matsumoto M, et al. The calcium sensors STIM1 and STIM2 control B cell regulatory function through interleukin-10 production. Immunity. 2011;34:703–714. doi: 10.1016/j.immuni.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 136.Di Capite J, Ng SW, Parekh AB. Decoding of cytoplasmic Ca2+ oscillations through the spatial signature drives gene expression. Curr Biol. 2009;19:853–858. doi: 10.1016/j.cub.2009.03.063. [DOI] [PubMed] [Google Scholar]
- 137.Mancarella S, Wang Y, Gill DL. Calcium signals: STIM dynamics mediate spatially unique oscillations. Curr Biol. 2009;19:R950–R952. doi: 10.1016/j.cub.2009.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bird GS, Putney JW., Jr Capacitative calcium entry supports calcium oscillations in human embryonic kidney cells. J Physiol. 2005;562:697–706. doi: 10.1113/jphysiol.2004.077289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kar P, Nelson C, Parekh AB. Selective activation of the transcription factor NFAT1 by calcium microdomains near Ca2+ release-activated Ca2+ (CRAC) channels. J Biol Chem. 2011;286:14795–14803. doi: 10.1074/jbc.M111.220582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kar P, Nelson C, Parekh ABCRAC. Channels drive digital activation and provide analog control and synergy to Ca2+-dependent gene regulation. Curr Biol. 2012;22:242–247. doi: 10.1016/j.cub.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 141.Loh C, et al. Calcineurin binds the transcription factor NFAT1 and reversibly regulates its activity. J Biol Chem. 1996;271:10884–10891. doi: 10.1074/jbc.271.18.10884. [DOI] [PubMed] [Google Scholar]
- 142.Gwack Y, Feske S, Srikanth S, Hogan PG, Rao A. Signalling to transcription: store-operated Ca2+ entry and NFAT activation in lymphocytes. Cell Calcium. 2007;42:145–156. doi: 10.1016/j.ceca.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 143.Li H, et al. Balanced interactions of calcineurin with AKAP79 regulate Ca2+–calcineurin–NFAT signaling. Nature Struct Mol Biol. 2012;19:337–345. doi: 10.1038/nsmb.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wu X, et al. Plasma membrane Ca2+-ATPase isoform 4 antagonizes cardiac hypertrophy in association with calcineurin inhibition in rodents. J Clin Invest. 2009;119:976–985. doi: 10.1172/JCI36693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kiviluoto S, et al. STIM1 as a key regulator for Ca2+ homeostasis in skeletal-muscle development and function. Skelet Muscle. 2011;1:16. doi: 10.1186/2044-5040-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Seth M, et al. Dynamic regulation of sarcoplasmic reticulum Ca(2+) stores by stromal interaction molecule 1 and sarcolipin during muscle differentiation. Dev Dyn. 2012;241:639–647. doi: 10.1002/dvdy.23760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hulot JS, et al. Critical role for stromal interaction molecule 1 in cardiac hypertrophy. Circulation. 2011;124:796–805. doi: 10.1161/CIRCULATIONAHA.111.031229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhang W, et al. Orai1-mediated I (CRAC) is essential for neointima formation after vascular injury. Circ Res. 2011;109:534–542. doi: 10.1161/CIRCRESAHA.111.246777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nature Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 150.Zou JJ, Gao YD, Geng S, Yang J. Role of STIM1/Orai1-mediated store-operated Ca2+ entry in airway smooth muscle cell proliferation. J Appl Physiol. 2011;110:1256–1263. doi: 10.1152/japplphysiol.01124.2010. [DOI] [PubMed] [Google Scholar]
- 151.Mungai PT, et al. Hypoxia triggers AMPK activation through reactive oxygen species-mediated activation of calcium release-activated calcium channels. Mol Cell Biol. 2011;31:3531–3545. doi: 10.1128/MCB.05124-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Balghi H, et al. Enhanced Ca2+ entry due to Orai1 plasma membrane insertion increases IL-8 secretion by cystic fibrosis airways. FASEB J. 2011;25:4274–4291. doi: 10.1096/fj.11-187682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Li J, et al. Orai1 and CRAC channel dependence of VEGF-activated Ca2+ entry and endothelial tube formation. Circ Res. 2011;108:1190–1198. doi: 10.1161/CIRCRESAHA.111.243352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ohba T, et al. Essential role of STIM1 in the development of cardiomyocyte hypertrophy. Biochem Biophys Res Commun. 2009;389:172–176. doi: 10.1016/j.bbrc.2009.08.117. [DOI] [PubMed] [Google Scholar]
- 155.Voelkers M, et al. Orai1 and Stim1 regulate normal and hypertrophic growth in cardiomyocytes. J Mol Cell Cardiol. 2010;48:1329–1334. doi: 10.1016/j.yjmcc.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jones BF, Boyles RR, Hwang SY, Bird GS, Putney JW. Calcium influx mechanisms underlying calcium oscillations in rat hepatocytes. Hepatology. 2008;48:1273–1281. doi: 10.1002/hep.22461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Aromataris EC, Castro J, Rychkov GY, Barritt GJ. Store-operated Ca2+ channels and stromal interaction molecule 1 (STIM1) are targets for the actions of bile acids on liver cells. Biochim Biophys Acta. 2008;1783:874–885. doi: 10.1016/j.bbamcr.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 158.Sours-Brothers S, Ding M, Graham S, Ma R. Interaction between TRPC1/TRPC4 assembly and STIM1 contributes to store-operated Ca2+ entry in mesangial cells. Exp Biol Med. 2009;234:673–682. doi: 10.3181/0809-RM-279. [DOI] [PubMed] [Google Scholar]
- 159.Yan X, et al. Function of a STIM1 homologue in C. elegans: evidence that store-operated Ca2+ entry is not essential for oscillatory Ca2+ signaling and ER Ca2+ homeostasis. J Gen Physiol. 2006;128:443–459. doi: 10.1085/jgp.200609611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gomez-Fernandez C, Lopez-Guerrero AM, Pozo-Guisado E, Alvarez IS, Martin-Romero FJ. Calcium signalling in mouse oocyte maturation: the roles of STIM1, ORAI1 and SOCE. Mol Hum Reprod. 2012;18:194–203. doi: 10.1093/molehr/gar071. [DOI] [PubMed] [Google Scholar]
- 161.Hwang SY, Putney JW. Orai1-mediated calcium entry plays a critical role in osteoclast differentiation and function by regulating activation of the transcription factor NFATc1. FASEB J. 2011;26:1484–1492. doi: 10.1096/fj.11-194399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhou Y, et al. The role of calcium release activated calcium channels in osteoclast differentiation. J Cell Physiol. 2011;226:1082–1089. doi: 10.1002/jcp.22423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Robinson LJ, et al. Gene disruption of the calcium channel Orai1 results in inhibition of osteoclast and osteoblast differentiation and impairs skeletal development. Lab Invest. 2012;92:1071–1083. doi: 10.1038/labinvest.2012.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lyfenko AD, Dirksen RT. Differential dependence of store-operated and excitation-coupled Ca2+ entry in skeletal muscle on STIM1 and Orai1. J Physiol. 2008;586:4815–4824. doi: 10.1113/jphysiol.2008.160481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Stiber J, et al. STIM1 signalling controls store-operated calcium entry required for development and contractile function in skeletal muscle. Nature Cell Biol. 2008;10:688–697. doi: 10.1038/ncb1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Darbellay B, et al. Human muscle economy myoblast differentiation and excitation-contraction coupling use the same molecular partners, STIM1 and STIM2. J Biol Chem. 2010;285:22437–22447. doi: 10.1074/jbc.M110.118984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Venkiteswaran G, Hasan G. Intracellular Ca2+ signaling and store-operated Ca2+ entry are required in Drosophila neurons for flight. Proc Natl Acad Sci USA. 2009;106:10326–10331. doi: 10.1073/pnas.0902982106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.McAndrew D, et al. ORAI1-mediated calcium influx in lactation and in breast cancer. Mol Cancer Ther. 2011;10:448–460. doi: 10.1158/1535-7163.MCT-10-0923. [DOI] [PubMed] [Google Scholar]
- 169.Yang S, Zhang JJ, Huang XY. Orai1 and STIM1 are critical for breast tumor cell migration and metastasis. Cancer Cell. 2009;15:124–134. doi: 10.1016/j.ccr.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 170.Feng M, et al. Store-independent activation of Orai1 by SPCA2 in mammary tumors. Cell. 2010;143:84–98. doi: 10.1016/j.cell.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Motiani RK, Abdullaev IF, Trebak M. A novel native store-operated calcium channel encoded by Orai3: selective requirement of Orai3 versus Orai1 in estrogen receptor-positive versus estrogen receptor-negative breast cancer cells. J Biol Chem. 2010;285:19173–19183. doi: 10.1074/jbc.M110.102582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Faouzi M, et al. Down-regulation of Orai3 arrests cell-cycle progression and induces apoptosis in breast cancer cells but not in normal breast epithelial cells. J Cell Physiol. 2011;226:542–551. doi: 10.1002/jcp.22363. [DOI] [PubMed] [Google Scholar]
- 173.Lur G, et al. Ribosome-free terminals of rough ER allow formation of STIM1 puncta and segregation of STIM1 from IP3 receptors. Curr Biol. 2009;19:1648–1653. doi: 10.1016/j.cub.2009.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lur G, et al. InsP3 receptors and Orai channels in pancreatic acinar cells: co-localization and its consequences. Biochem J. 2011;436:231–239. doi: 10.1042/BJ20110083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Tamarina NA, Kuznetsov A, Philipson LH. Reversible translocation of EYFP-tagged STIM1 is coupled to calcium influx in insulin secreting β-cells. Cell Calcium. 2008;44:533–544. doi: 10.1016/j.ceca.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 176.Roy J, Lefkimmiatis K, Moyer MP, Curci S, Hofer AM. The-ω3 fatty acid eicosapentaenoic acid elicits cAMP generation in colonic epithelial cells via a ‘store-operated’ mechanism. Am J Physiol Gastrointest Liver Physiol. 2010;299:G715–G722. doi: 10.1152/ajpgi.00028.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ma J, McCarl CA, Khalil S, Luthy K, Feske S. T-cell-specific deletion of STIM1 and STIM2 protects mice from EAE by impairing the effector functions of Th1 and Th17 cells. Eur J Immunol. 2010;40:3028–3042. doi: 10.1002/eji.201040614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Limnander A, et al. STIM1, PKC-δ and RasGRP set a threshold for proapoptotic Erk signaling during B cell development. Nature Immunol. 2011;12:425–433. doi: 10.1038/ni.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Baba Y, et al. Essential function for the calcium sensor STIM1 in mast cell activation and anaphylactic responses. Nature Immunol. 2008;9:81–88. doi: 10.1038/ni1546. [DOI] [PubMed] [Google Scholar]
- 180.Vig M, et al. Defective mast cell effector functions in mice lacking the CRACM1 pore subunit of store-operated calcium release-activated calcium channels. Nature Immunol. 2007;9:89–96. doi: 10.1038/ni1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Grosse J, et al. An EF hand mutation in Stim1 causes premature platelet activation and bleeding in mice. J Clin Invest. 2007;117:3540–3550. doi: 10.1172/JCI32312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Varga-Szabo D, et al. The calcium sensor STIM1 is an essential mediator of arterial thrombosis and ischemic brain infarction. J Exp Med. 2008;205:1583–1591. doi: 10.1084/jem.20080302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Gilio K, et al. Roles of platelet STIM1 and Orai1 in glycoprotein VI- and thrombin-dependent procoagulant activity and thrombus formation. J Biol Chem. 2010;285:23629–23638. doi: 10.1074/jbc.M110.108696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Braun A, et al. Orai1 (CRACM1) is the platelet SOC channel and essential for pathological thrombus formation. Blood. 2008;26:2056–2063. doi: 10.1182/blood-2008-07-171611. [DOI] [PubMed] [Google Scholar]
- 185.Steinckwich N, Schenten V, Melchior C, Brechard S, Tschirhart EJ. An essential role of STIM1, Orai1, and S100A8-A9 proteins for Ca2+ signaling and FcγR-mediated phagosomal oxidative activity. J Immunol. 2011;186:2182–2191. doi: 10.4049/jimmunol.1001338. [DOI] [PubMed] [Google Scholar]
- 186.Wang Y, Lin L, Zheng C. Downregulation of the expression of Orai1 in the airway alleviates murine allergic rhinitis. Exp Mol Med. 2011;31:177–190. doi: 10.3858/emm.2012.44.3.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Braun A, et al. STIM1 is essential for Fcγ receptor activation and autoimmune inflammation. Blood. 2008 Oct 21; doi: 10.1182/blood-2008-05-158477. [DOI] [PubMed] [Google Scholar]
- 188.Cole C, Barber JD, Barton GJ. The Jpred 3 secondary structure prediction server. Nucleic Acids Res. 2008;36:W197–W201. doi: 10.1093/nar/gkn238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Yue C, Soboloff J, Gamero AM. Control of type I interferon-induced cell seath by Orai1-mediated calcium entry in T cells. J Biol Chem. 2012;287:3207–3216. doi: 10.1074/jbc.M111.269068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ritchie MF, Zhou Y, Soboloff J. Transcriptional mechanisms regulating Ca2+ homeostasis. Cell Calcium. 2011;49:314–321. doi: 10.1016/j.ceca.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ritchie MF, Yue C, Zhou Y, Houghton PJ, Soboloff J. Wilms tumor suppressor 1 (WT1) and early growth response 1 (EGR1) are regulators of STIM1 expression. J Biol Chem. 2010;285:10591–10596. doi: 10.1074/jbc.M109.083493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Eylenstein A, et al. Transcription factor NF-κB regulates expression of pore-forming Ca2+ channel unit, Orai1, and its activator, STIM1, to control Ca2+ entry and affect cellular functions. J Biol Chem. 2012;287:2719–2730. doi: 10.1074/jbc.M111.275925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Partiseti M, et al. The calcium current activated by T3cell receptor and store depletion in human lymphocytes is absent in a primary immunodeficiency. J Biol Chem. 1994;269:32327–32335. [PubMed] [Google Scholar]
- 194.Fanger CM, Hoth M, Crabtree GR, Lewis RS. Characterization of T cell mutants with defects in capacitative calcium entry: genetic evidence for the physiological roles of CRAC channels. J Cell Biol. 1995;131:655–667. doi: 10.1083/jcb.131.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Peinelt C, et al. Amplification of CRAC current by STIM1 and CRACM1 (Orai1) Nature Cell Biol. 2006;8:771–773. doi: 10.1038/ncb1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mercer JC, et al. Large store-operated calcium selective currents due to co-expression of Orai1 or Orai2 with the intracellular calcium sensor, Stim1. J Biol Chem. 2006;281:24979–24990. doi: 10.1074/jbc.M604589200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Prakriya M, et al. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 198.Yeromin AV, et al. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–229. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Vig M, et al. CRACM1 Multimers Form the Ion-Selective Pore of the CRAC Channel. Curr Biol. 2006;16:2073–2079. doi: 10.1016/j.cub.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.