Abstract
Background
Proponents of flexible annuloplasty rings have hypothesized that such devices maintain annular dynamics. This hypothesis is based on the supposition that annular motion is relatively normal in patients undergoing mitral valve repair. We hypothesized that mitral annular dynamics are impaired in ischemic mitral regurgitation (IMR) and myxomatous mitral regurgitation (MMR).
Methods and Results
Philips iE33 echocardiographic module and X7-2t probe were used to acquire full volume rt-3DTEE loops in 11 normal subjects, 11 patients with IMR and 11 patients with MMR. Image analysis was performed using Tomtec Image Arena -4D-MV Assessment© - 2.1 (Munich, Germany). A midsystolic frame was selected for the initiation of annular tracking using the semi-automated program. Continuous parameters were normalized in time to provide for uniform systolic and diastolic periods. Both IMR (9.98±155 cm2) and MMR annuli (13.29±3.05 cm2) were larger in area than normal annuli (7.95±1.40 cm2) at midsystole. In general, IMR annuli were less dynamic than controls. In MMR, annular dynamics were also markedly abnormal with the mitral annulus dilating rapidly in early systole in response to rising ventricular pressure.
Conclusions
In both IMR and MMR, annular dynamics and anatomy are abnormal. Flexible annuloplasty devices used in mitral valve repair are, therefore, unlikely to result in either normal annular dynamics or normal anatomy.
Keywords: mitral valve, dynamics, valvuloplasty, echocardiography
It has been well reported in the literature that the mitral valve annulus assumes a complex, saddle shaped 3D geometry which alters configuration throughout the cardiac cycle. [1–4] Annular dynamic motion has been thought to contribute to leaflet coaptation, valve competence, adequate diastolic filling of the left ventricle (LV) and effective LV systolic ejection. [5–6] Previous experimental work in theoretical studies, animal models and clinical echocardiographic studies, have shown that the normal annulus contracts during early systole and deepens in saddle shape thereby improving leaflet coaptation. [4,7–8] This physiology has been shown to be altered in animal models of ischemic mitral regurgitation (IMR) with the annulus dilating, flattening, and losing its ability to alter its annular height and saddle geometry throughout the cardiac cycle. [9] More recently, with advances in real-time three-dimensional echocardiography (rt-3DE), investigators have begun studying the dynamic effects of the mitral annulus in clinical scenarios including IMR, nonischemic dilated cardiomyopathy and myxomatous mitral regurgitation (MMR). [7, 10–11]
As the clinical interest in accurately describing annular physiology has increased, so has the interest in developing a better understanding of how modern mitral valve repair techniques affect this physiology. Annuloplasty rings are routinely used in MV repair surgery to restore annular size and geometry in both IMR and MMR, and the design of 3D saddle shaped rings has been shown to restore annular height. [12] The use of flexible annuloplasty devices had been advocated by some, based on the belief that they preserve annular dynamics. We hypothesized that mitral annular dynamics are impaired in both IMR and MMR. In this study, we describe the dynamic motion of normal, IMR and MMR annuli for a variety of measured parameters throughout the cardiac cycle using rt-3DE and high resolution, semi-automated annular tracking.
Methods
Patients
A total of 33 patients were enrolled in this study, which was approved by the University of Pennsylvania School of Medicine Institutional Review Board, prior to undergoing cardiac surgery for all causes. Patients were divided into three groups based on baseline physiology: normal physiology (N=11), IMR (N=11) or MMR (N=11). Patients were excluded from the normal group if they had any evidence on prior echocardiography of mitral valve disease (including more than mild regurgitation or stenosis), depressed ejection fraction (defined as less than 40%), prior history of mitral valve surgery, rheumatic valve disease or endocarditis. Inclusion criteria for the ischemic group included history of coronary artery disease documented by angiography or prior documented history of myocardial infarction with or without previous intervention, and moderate or greater functional mitral regurgitation on echocardiography. Inclusion criteria for the myxomatous group included documentation of P2 prolapse or flail, and moderate or greater mitral regurgitation on echocardiography. Patients were excluded from these cohorts if they had prior history of mitral valve surgery, mitral stenosis, rheumatic valve disease, endocarditis or were in atrial fibrillation at the time of image acquisition. Written informed consent was obtained from each subject.
Image Acquisition
Rt-3DE images were acquired through a mid-esophageal view with a Philips ie33 (Andover, MA, USA) ultrasound system equipped with a 2 to 7 MHz X7-2t TEE matrix transducer. Electrocardiographically gated full-volume loops were acquired over 4 cardiac cycles at a frame rate of 17–30 frames/s. Color flow Doppler images of the mitral valve were acquired after adjusting the Nyquist limit to approximately 60 cm/sec.
Image Analysis
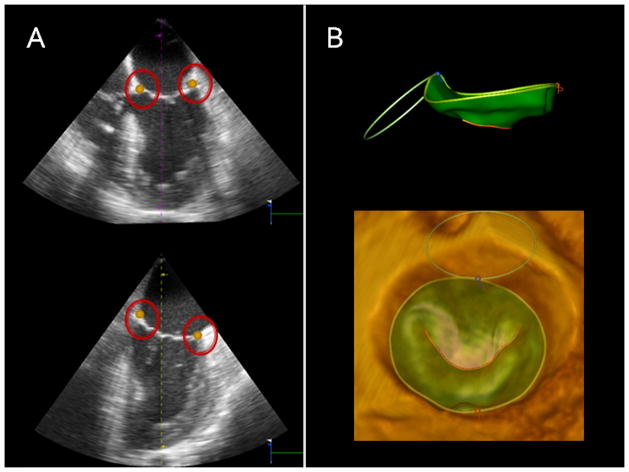
Each full-volume data set was exported offline to an Image Arena - 4D-MV Assessment© - 2.1 (Tomtec Imaging Systems, Munich, Germany) software workstation for image analysis. The highest-quality data set was selected for each subject. A mid-systolic frame was selected for the initiation of annular tracking using the semiautomated program which marks 80 points around the circumference of the annulus in order to create a static 3D mitral model. Manual edits of the 3D model were performed to ensure adequate automation. Dynamic models were then created from the 80 circumferential points using 3D speckle tracking algorithms which tracks the annulus throughout each frame of the cardiac cycle. Manual edits of the dynamic models were performed in cases where gross discontinuity of the tracking was observed, however, given the superiority of the tracking algorithms, this adjustment was rarely needed. Figure 1. Measured parameters were automatically generated from the resultant models including annular area, annular circumference, septolateral diameter (anterior-posterior (AP) diameter) and mitral valve transverse diameter (anterolateral-posteromedial (AL-PM) diameter). Annular height was determined from the actual Cartesian coordinates of the 80 circumferential points. For each patient, the dynamic datasets were time shifted such that end systole became the first timepoint. The data was then normalized in time to provide for uniform systolic and diastolic periods such that the end diastolic datapoint move to the midpoint of the cycle. Continuous parameters were computed at interval timepoints after having rotated the annulus such that its least squares plane lay on the XY axis. A smoothing spline was used to approximate the data. This spline was weighted at the endpoints to ensure that the curve began and ended at the end systolic value. The slopes of the curves were computed using a gradient function and recorded for determining rate of annular change.
Figure 1.
(Panel A) Identification of 4 annular points for semi-automated circumferential annular tracking. (Panel B, Top) Side view of completed 3D dynamic mitral model used for annular analysis and (Bottom) surgeon’s view of 3D mitral valve reconstruction superimposed on 3D echocardiography image.
Statistical Analysis
All statistical analysis was performed using Matlab 7.11, (The MathWorks Inc., Natick, Massachusetts). Continuous variables are reported as mean ± standard deviation. Categorical variables are reported as percentages and were compared using a Fisher’s exact test. A MANOVA was performed to determine significance between groups for both static and dynamic measurements. If significance was determined by MANOVA, individual one-way ANOVAs were performed followed by post-hoc testing using a Bonferroni correction where appropriate. A P value less than 0.05 was considered statistically significant.
Results
3D dynamic mitral models were created for all 33 patients. Preoperative patient characteristics are presented in Table 1. There were no differences in gender between cohorts. Patients in the MMR cohort were slightly younger than patients in the IMR cohort. Ejection fraction was decreased in the IMR cohort relative to the normal and MMR cohorts.
Table 1.
Preoperative Patient Characteristics
| Normal | IMR | MMR | |
|---|---|---|---|
|
| |||
| Age (years) | 63.7±16.8 | 72.8±8.7 | 57.7±14.5* |
|
| |||
| Women (%) | 45.5% | 36.4% | 27.3% |
|
| |||
| Ejection Fraction (%) | 65.7±15.0 | 34.5±14.7† | 60.9±9.6 |
|
| |||
| MR grade | |||
| None | 100%‡ | - | - |
| Mild | - | - | - |
| Moderate | - | 54.5% | 27.3 |
| Severe | - | 45.5% | 72.7% |
P=0.03by ANOVA; post hoc analysisshowed P=0.03for IMR vs MMR
P<0.001 by ANOVA; post hoc analysis showed P<0.05 for Normal vs IMR and IMR vs MMR
P<0.05 by Fisher exact probability test for all groups
Static Annular Geometry Comparison
The static annular measurements in the three study groups differed significantly by MANOVA, P< 0.001. These results are summarized in Table 2. Both IMR and MMR annuli were larger than normal annuli for a variety of measured parameters based on the static midsystolic frame. 2D annular area tended to be larger in the ischemic group 9.98 ± 1.55 cm2 vs. 7.95 ± 1.40 cm2 in the normal group. Septolateral diameter was 3.46 ± 0.29 cmin the ischemic group vs 2.84 ± 0.25 cm in the normal group (P=0.001). Annular height showed a decreasing trend from 6.84 ± 1.55 mm in normals to 5.92 ± 1.15 mm in ischemics. Annular height to septolateral diameter ratio was significantly reduced from 0.24 ± 0.05 to 0.17 ± 0.03 (P<0.001). Interestingly, there was no significant difference in mitral valve transverse diameter. These findings confirm that IMR annuli lose 3D geometry and remain relatively narrow by dilating in an anterior to posterior direction. Similar trends were noted in the MMR annuli. 2D annular area for the myxomatous cohort was 13.29 ± 3.05 cm2 vs. 7.95 ± 1.40 cm2 (P<0.001) when compared to normal annuli. Annular circumference, septolateral diameter and mitral valve transverse diameter were also increased in a statistically significant fashion. At the midsystolic frame, annular height was similar between the myxomatous group and the normal group however, annular height to septolateral diameter ratio was significantly different. MMR annuli were similar to IMR in terms of dilatation in the septolateral dimension (3.67 ± 0.51 cm vs 3.46 ± 0.29 cm, P=0.57), however, they demonstrated greater widening in the anterolateral-posteromedial direction than IMR patients (4.46 ± 0.50 cm vs. 3.65 ± 0.29 cm, P<0.001)
Table 2.
Midsystolic Annular Dimensions
| Annular Parameter | Normal | IMR | MMR | P value |
|---|---|---|---|---|
| Annular Height (mm) | 6.84±1.55 | 5.92±1.15 | 6.45±1.42 | P = 0.32 |
| Septolateral Diameter (cm) | 2.84±0.25 | 3.46±0.29* | 3.67±0.51† | P <0.001 |
| Annular Height to Septolateral Ratio | 0.24±0.05 | 0.17±0.03* | 0.18±0.04† | P <0.001 |
| Mitral Valve Transverse Diameter | 3.49±0.39 | 3.65±0.29 | 4.46±0.50† | P <0.001 |
| 2D Mitral Annular Area (cm2) | 7.95±1.40 | 9.98±1.55 | 13.29±3.05† | P <0.001 |
| 3D Mitral Annular Area (cm2) | 8.24±1.47 | 10.22±1.55 | 13.56±3.09† | P <0.001 |
| Annular Circumference (cm) | 10.64±1.00 | 11.66±0.90 | 13.48±1.50† | P <0.001 |
P < 0.001 by MANOVA for the entire table; P values presented are those of one-way ANOVA
denotes significance (P< 0.05) between normal and IMR by post-hoc testing
denotes significance (P< 0.05) between normal and MMR by post-hoc testing
Normal Annular Dynamic Motion
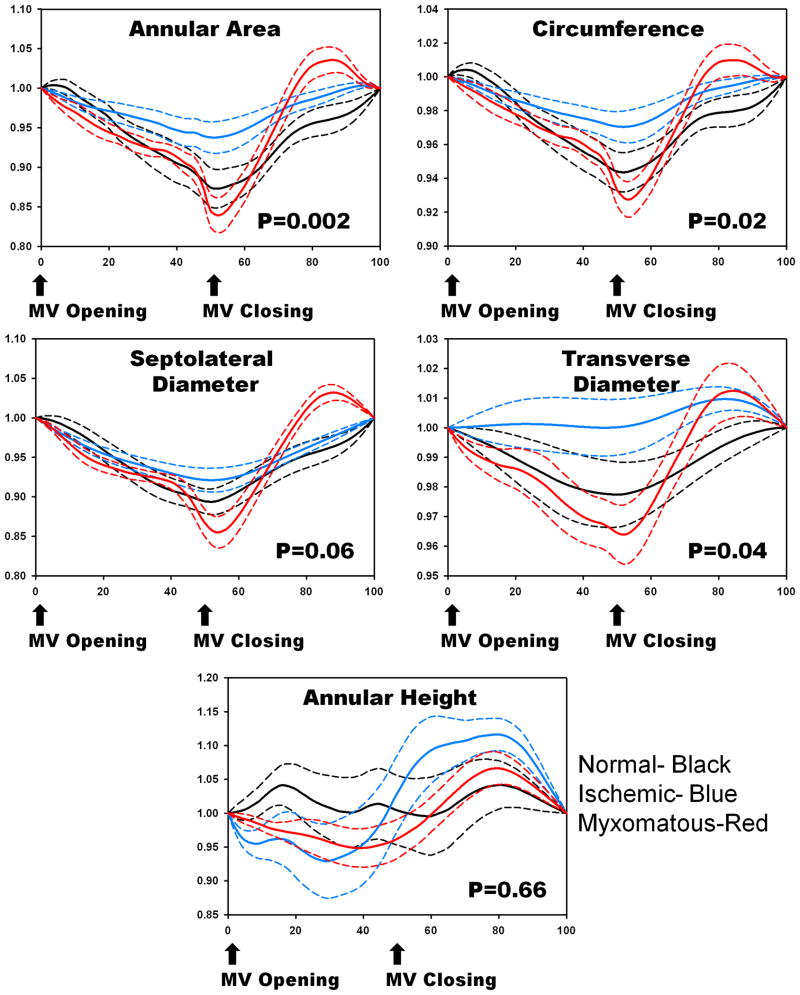
Normal mitral annuli demonstrated early systolic area contraction when the cardiac cycle was normalized to align MV closing for all patients. Early systolic contraction was followed by gradual annular area increase until end systole. The 2D annular area fraction change defined as (Area max-Area min)/(Area max) was 19.02 ± 4.94%. Additionally, as demonstrated in Figure 2, normal annuli demonstrated early systolic narrowing in the septolateral direction followed by a gradual increase in diameter. This followed along with progressive systolic increases in annular height. In contrast, the dimensions of the mitral valve transverse diameter changed little over time corroborating the findings that as annular height increases with a relatively fixed annular width, the relative saddle shape becomes more accentuated throughout systole. By MANOVA (P=.02), there was significant difference in annular parameters amongst the normal, IMR, and MMR groups.
Figure 2.
Comparison of 2D annular area (Panel A), annular circumference (Panel B), septolateral diameter (Panel C), mitral valve transverse diameter (Panel D) and annular height (Panel E) for normal (black), ischemic (green) and myxomatous (red) annuli after being normalized to annulus size at the time of valve opening. Systolic changes in these measures were significantly different among groups by MANOVA, P=0.02. Ischemic annuli appear to have less contraction and motion compared to normal annuli. In contrast, mxyomatous annuli appear to demonstrate rapid systolic dilatation compared to normal and ischemic annuli. Dotted lines=standard error of the mean
Ischemic Annular Dynamic Motion
As demonstrated in Figure 2, IMR annuli appeared less dynamic than normal annuli, with decreased motion in a variety of directions. The annular area contracted less during early systole when compared to controls in terms of area. Average 2D annular area fraction change was 10.26 ± 6.58%. The IMR annulus also displayed a decreased shortening in the septolateral direction and reduced ability to increase annular height.
Myxomatous Annular Dynamic Motion
Annuli in the MMR group demonstrated a different pattern of behavior from either the normal patients or ischemic patients, as also demonstrated in Figure 2. Even though MMR appeared larger than both the normals and ischemics, they maintained the ability to contract and modify shape. MMR expand rapidly during systole. The maximum rate of increase (max Δy/Δx) for septolateral diameter, mitral valve transverse diameter, annular circumference, and annular area in the MMR group was 4.53 ± 1.76 cm/s, 2.68 ± 1.55 cm/s, 8.09 ±3.37 cm/s and 15.61 ±6.95 cm2/s respectively as compared to 3.17 ±1.46 cm/s, 1.44 ±0.63 cm/s, 5.25 ±1.97 cm/s, and 7.81 ±3.83 cm2/s in the normal group. This shows that that there is a rapid rise in annular dilatation in multiple directions as the left ventricular pressure increases. Figure 2. 2D average annular area fractional change was 20.87 ±8.05%.
Discussion
In the current study, we analyzed the dynamic motion of the mitral valve annulus in a comprehensive manner in both normal and pathologic patient cohorts. Our results demonstrate that mitral annular dynamics is abnormal in patients with both IMR and MMR. The ischemic annulus is adynamic relative to the normal annulus and therefore, loses its ability to modify shape throughout the cardiac cycle as it dilates and flattens. The myxomatous annulus is also severely dilated and exhibits rapid expansion during systole, including in the AL-PM direction which is not part of normal physiology. These patterns of abnormal behavior may be important factors in contributing to the degree of mitral regurgitation witnessed in these pathologies.
There have been a number of previous studies in the literature which have sought to accurately describe annular dynamic motion in both normal and pathologic valves. One of the limitations of previous work has included the fact that some authors only report data on a few measured annular parameters which only modestly describes annular physiology. [7, 13] Other studies have been limited in terms of annular dynamics by using a minimum number of circumferential reference points, or in terms of reporting only a limited number of static timepoints (ED, middiastole, ES, midsystole, etc.) throughout the cardiac cycle. [11] One of the advantages to this study was that we used 3DE with innovative tracking features to accurately follow the annulus throughout time, and reported all clinically relevant parameters in a continuous fashion throughout the cardiac cycle.
The current work is limited by a relatively small clinical population; furthermore, the control population did not consist of disease-free individuals. In order to obtain transesophageal 3DE studies on patients with “normal mitral valves,” patients undergoing cardiac surgery for causes unrelated to mitral valve pathology were deemed “normal.”
Despite these limitations, the normal patient group demonstrated early systolic annular contraction and anterior-posterior shortening similar to what has been described by Grewal et al (2010). In their study, they reference the fact that early annular contraction aids in approximating the leaflets when ventricular pressures are low, thereby serving to prevent early systolic valve incompetence. [11] Moreover, Grewal described myxomatous annular dynamics. Our study expands upon those results in demonstrating inherent dynamic annular differences between normal and myxomatous valves. Additionally, rather than extract annular measurements at single timepoints from 3DE, measurements were performed throughout every frame of the cardiac cycle (regardless of number of frames) and normalized across patient populations for a continuous rather than discrete analysis. These data are also consistent with reports demonstrating reduced geometric deformation in ischemic mitral regurgitation. [7,14]
There is an ongoing debate in the literature regarding the failure rates and long term clinical outcomes of mitral valve repair procedures performed with either flexible or rigid annuloplasty rings. Yamaura et al reported that mitral annular dynamics are preserved in patients who receive a flexible ring as compared to those who receive a rigid ring. [15] Additionally, early reports suggested that the use of a flexible ring in degenerative valve disease may improve LV systolic function defined strictly by echocardigraphic criteria. [16] As such, the past 20 years has seen a number of partial and complete flexible ring designs aimed at maintaining physiologic annular motion. More recently, follow-up clinical studies analyzing the long term data have been described. For example, Silberman et al compared flexible vs rigid annuloplasty in repair of ischemic mitral regurgitation. [17] They found that clinical and hemodynamic results are better with rigid annuloplasty. In their study, both ring types reduced MR post repair, however a more pronounced reduction was noted with the rigid group as well as a decreased incidence of recurrence in the rigid group. Chang et al performed a prospective, randomized study which looked at the long term results of both ring types for all causes of mitral valve repair. [18] They found no differences between annuloplasty types, however, they did not perform subanalyses on patients with ischemic or degenerative disease. Moreover, their study demonstrated a nonsignificant trend favoring the rigid annuloplasty group. As such, flexible annuloplasty devices have not been shown to improve clinical outcomes. [18–20]
The findings of this study suggest that reestablishing normal systolic annular saddle shape in patients requiring mitral valve repair is probably the best approach for improving valve function. Such a strategy has been shown to reduce annular strain [21,22] optimize leaflet stress profile [23] and increase leaflet coaptation area [24]. Maintenance of annular function as a goal of ring annuloplasty may not benefit patients with already abnormal dynamics.
In conclusion, both IMR and MMR annular dynamics and anatomy are abnormal. Flexible annuloplasty devices used in mitral valve repair are, therefore, unlikely to result in either normal annular dynamics or normal anatomy.
Acknowledgments
Funding
This work was supported by grants from the National Heart, Lung and Blood Institute of the National Institutes of Health, Bethesda, MD (HL63954, HL73021 and HL103723). R. Gorman and J. Gorman are supported by individual Established Investigator Awards from the American Heart Association, Dallas, TX. A. Jassar was supported by a postdoctoral fellowship from the American Heart Association.
Footnotes
Poster presentation at the American Heart Association Scientific Sessions 2011, Orlando, FL, November 2011
Disclosures
None
References
- 1.Levine RA, Handschumacher MD, Sanfilippo AJ, Hagege AA, Harrigan P, Marshall JE, Weyman AE. Three-dimensional echocardiographic reconstruction of the mitral valve, with implications for the diagnosis of mitral valve prolapse. Circulation. 1989;80:589–598. doi: 10.1161/01.cir.80.3.589. [DOI] [PubMed] [Google Scholar]
- 2.Gorman JH, III, Gupta KB, Streicher JT, Gorman RC, Jackson BM, Ratcliffe RB, Bogen DK, Edmunds H. Dynamic Three-Dimensional Imaging of the Mitral Valve and Left Ventricle by Rapid Sonomicrometry Array Localization. J Thorac Cardiovasc Surg. 1996;112:712–726. doi: 10.1016/S0022-5223(96)70056-9. [DOI] [PubMed] [Google Scholar]
- 3.Ryan LP, Jackson BM, Enomoto Y, Parish L, Plappert TJ, St John-Sutton MG, Gorman RC, Gorman JH., 3rd Description of regional mitral annular nonplanarity in healthy human subjects: a novel methodology. J Thorac Cardiovasc Surg. 2007;134:644–648. doi: 10.1016/j.jtcvs.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Rausch MK, Bothe W, Kvitting JP, Swanson JC, Ingels NB, Jr, Miller DC, Kuhl E. Characterization of mitral valve annular dynamics in the beating heart. Ann Biomed Eng. 2011;39:1690–1702. doi: 10.1007/s10439-011-0272-y. [DOI] [PubMed] [Google Scholar]
- 5.Enriquez-Sarano M, Frye RL. Mitral valve disease. In: Willerson JT, Cohn JN, Wellens HJJ, Holmes DR, editors. Cardiovascular Medicine. 3. London, UK: Springer-Verlag; 2007. pp. 397–430. [Google Scholar]
- 6.Carlhäll C, Wigström L, Heiberg E, Karlsson M, Bolger AF, Nylander E. Contribution of mitral annular excursion and shape dynamics to total left ventricular volume change. Am J Physiol Heart Circ Physiol. 2004;287:H1836–1841. doi: 10.1152/ajpheart.00103.2004. [DOI] [PubMed] [Google Scholar]
- 7.Daimon M, Saracino G, Fukuda S, Koyama Y, Kwan J, Song JM, Agler DA, Gillinov AM, Thomas JD, Shiota T. Dynamic change of mitral annular geometry and motion in ischemic mitral regurgitation assessed by a computerized 3D echo method. Echocardiography. 2010;27:1069–1077. doi: 10.1111/j.1540-8175.2010.01204.x. [DOI] [PubMed] [Google Scholar]
- 8.Eckert CE, Zubiate B, Vergnat M, Gorman JH, 3rd, Gorman RC, Sacks MS. In vivo dynamic deformation of the mitral valve annulus. Ann Biomed Eng. 2009;37:1757–1771. doi: 10.1007/s10439-009-9749-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorman JH, 3rd, Jackson BM, Enomoto Y, Gorman RC. The effect of regional ischemia on mitral valve annular saddle shape. Ann Thorac Surg. 2004;77:544–548. doi: 10.1016/S0003-4975(03)01354-7. [DOI] [PubMed] [Google Scholar]
- 10.Veronesi F, Corsi C, Sugeng L, Caiani EG, Weinert L, Mor-Avi V, Cerutti S, Lamberti C, Lang RM. Quantification of mitral apparatus dynamics in functional and ischemic mitral regurgitation using real-time 3-dimensional echocardiography. J Am Soc Echocardiogr. 2008;21:347–354. doi: 10.1016/j.echo.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 11.Grewal J, Suri R, Mankad S, Tanaka A, Mahoney DW, Schaff HV, Miller FA, Enriquez-Sarano M. Mitral annular dynamics in myxomatous valve disease: new insights with real-time 3-dimensional echocardiography. Circulation. 2010;121:1423–1431. doi: 10.1161/CIRCULATIONAHA.109.901181. [DOI] [PubMed] [Google Scholar]
- 12.Ryan LP, Jackson BM, Hamamoto H, Eperjesi TJ, Plappert TJ, St John-Sutton M, Gorman RC, Gorman JH., 3rd The influence of annuloplasty ring geometry on mitral leaflet curvature. Ann Thorac Surg. 2008;86:749–60. doi: 10.1016/j.athoracsur.2008.03.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Little SH, Ben Zekry S, Lawrie GM, Zoghbi WA. Dynamic annular geometry and function in patients with mitral regurgitation: insight from three-dimensional annular tracking. J Am Soc Echocardiogr. 2010;23:872–9. doi: 10.1016/j.echo.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe N, Ogasawara Y, Yamaura Y, Wada N, Kawamoto T, Toyota E, Akasaka T, Yoshida K. Mitral annulus flattens in ischemic mitral regurgitation: geometric differences between inferior and anterior myocardial infarction: a real-time 3-dimensional echocardiographic study. Circulation. 2005;112:I458–462. doi: 10.1161/CIRCULATIONAHA.104.524595. [DOI] [PubMed] [Google Scholar]
- 15.Yamaura Y, Yoshikawa J, Yoshida K, Hozumi T, Akasaka T, Okada Y. Three-dimensional analysis of configuration and dynamics in patients with an annuloplasty ring by multiplane transesophageal echocardiography: comparison between flexible and rigid annuloplasty rings. J Heart Valve Dis. 1995;4:618–22. [PubMed] [Google Scholar]
- 16.David TE, Komeda M, Pollick C, Burns RJ. Mitral valve annuloplasty: the effect of the type on left ventricular function. Ann Thorac Surg. 1989;47:524–527. doi: 10.1016/0003-4975(89)90426-8. [DOI] [PubMed] [Google Scholar]
- 17.Silberman S, Klutstein MW, Sabag T, Oren A, Fink D, Merin O, Bitran D. Repair of ischemic mitral regurgitation: comparison between flexible and rigid annuloplasty rings. Ann Thorac Surg. 2009;87:1721–1726. doi: 10.1016/j.athoracsur.2009.03.066. [DOI] [PubMed] [Google Scholar]
- 18.Chang BC, Youn YN, Ha JW, Lim SH, Hong YS, Chung N. Long-term clinical results of mitral valvuloplasty using flexible and rigid rings: a prospective and randomized study. J Thorac Cardiovasc Surg. 2007;133:995–1003. doi: 10.1016/j.jtcvs.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 19.Shahin GM, van der Heijden GJ, Bots ML, Cramer MJ, Jaarsma W, Gadellaa JC, de la Riviere AB, van Swieten HA. The Carpentier-Edwards Classic and Physio mitral annuloplasty rings: a randomized trial. Heart Surg Forum. 2005;8:E389–394. doi: 10.1532/HSF98.20051114. [DOI] [PubMed] [Google Scholar]
- 20.Chung CH, Kim JB, Choo SJ, Kim KS, Song H, Song MG, Song JK, Kang DH, Lee JW. Long-term outcomes after mitral ring annuloplasty for degenerative mitral regurgitation: Duran ring versus Carpentier-Edwards ring. J Heart Valve Dis. 2007;16:536–544. [PubMed] [Google Scholar]
- 21.Jensen MØ, Jensen H, Nielsen SL, Smerup M, Johansen P, Yoganathan AP, Nygaard H, Hasenkam JM. What forces act on a flat rigid mitral annuloplasty ring? J Heart Valve Dis. 2008;17:267–75. [PubMed] [Google Scholar]
- 22.Jensen MO, Jensen H, Smerup M, Levine RA, Yoganathan AP, Nygaard H, Hasenkam JM, Nielsen SL. Saddle-shaped mitral valve annuloplasty rings experience lower forces compared with flat rings. Circulation. 2008;118:S250–255. doi: 10.1161/CIRCULATIONAHA.107.746776. [DOI] [PubMed] [Google Scholar]
- 23.Salgo IS, Gorman JH, 3rd, Gorman RC, Jackson BM, Bowen FW, Plappert T, St John Sutton MG, Edmunds LH., Jr Effect of annular shape on leaflet curvature in reducing mitral leaflet stress. Circulation. 2002;106:711–717. doi: 10.1161/01.cir.0000025426.39426.83. [DOI] [PubMed] [Google Scholar]
- 24.Vergnat M, Jackson BM, Cheung AT, Weiss SJ, Ratcliffe SJ, Gillespie MJ, Woo YJ, Bavaria JE, Acker MA, Gorman RC, Gorman JH., III Saddle-Shape Annuloplasty Increases Mitral Leaflet Coaptation after Repair for Flail Posterior Leaflet. Annals of Thoracic Surgery. 2011;92:797–804. doi: 10.1016/j.athoracsur.2011.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]