Abstract
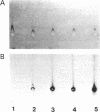
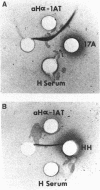
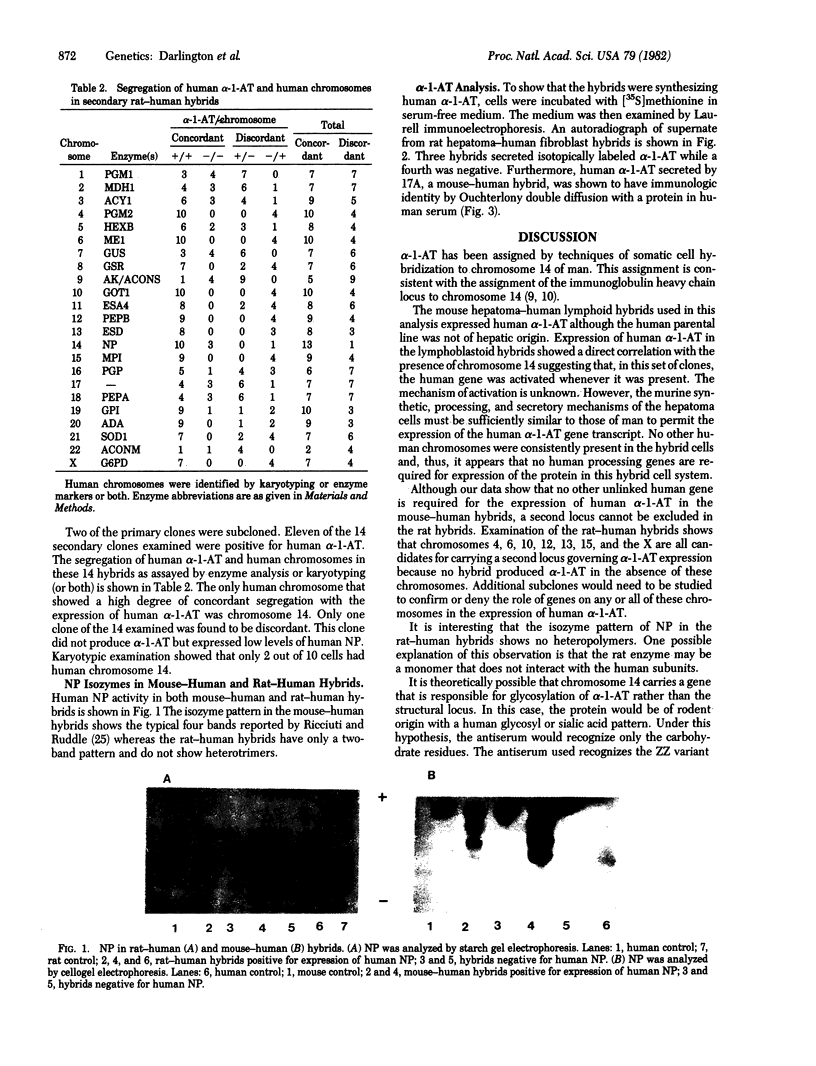
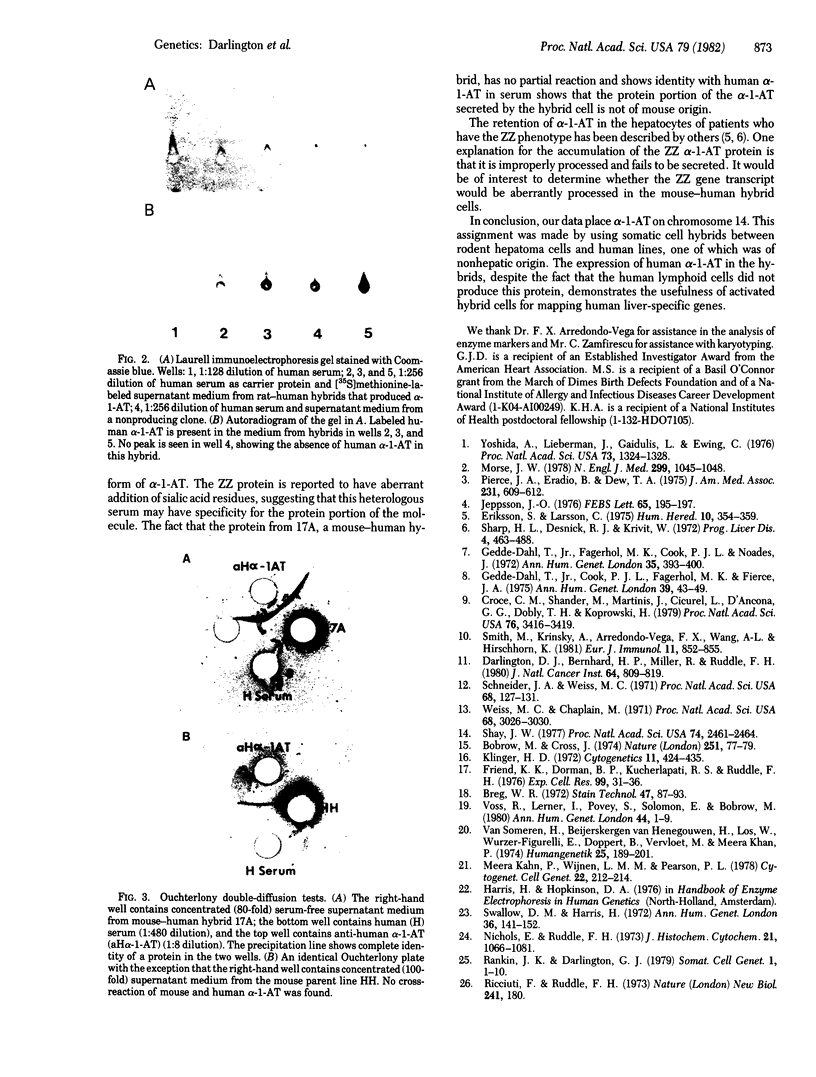
Human alpha 1-antitrypsin ( alpha-1-AT;Pi) production was analyzed in 11 primary mouse hepatoma-human lymphoid cell hybrids and in 14 secondary rat hepatoma-human fetal liver fibroblast hybrids. The presence of human alpha-1-AT was determined by Laurell immunoelectrophoresis of concentrated and isotopically labeled supernatant medium. Human alpha-1-AT production segregated in the mouse-human hybrids concordantly with human purine nucleoside phosphorylase and with chromosome 14. All rat-human hybrids that were alpha-1-AT positive were also positive for human purine nucleoside phosphorylase and chromosome 14. Our study demonstrated the usefulness of rodent hepatoma cell hybrids for mapping human liver-specific genes because differentiated functions are expressed despite the fact that the human parental cells did not express these functions. Our study also showed that human alpha-1-AT gene product can be processed for secretion in the rodent hepatoma cellular environment. The mouse-human hybrids showed that no other human chromosome carries genes necessary for processing or secretion of human alpha-1-AT in the hybrid cell milieu.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bobrow M., Cross J. Differential staining of human and mouse chromosomes in interspecific cell hybrids. Nature. 1974 Sep 6;251(5470):77–79. doi: 10.1038/251077a0. [DOI] [PubMed] [Google Scholar]
- Breg W. R. Quinacrine fluorescence for identifying metaphase chromosomes, with special reference to photomicrography. Stain Technol. 1972 Mar;47(2):87–93. doi: 10.3109/10520297209116456. [DOI] [PubMed] [Google Scholar]
- Croce C. M., Shander M., Martinis J., Cicurel L., D'Ancona G. G., Dolby T. W., Koprowski H. Chromosomal location of the genes for human immunoglobulin heavy chains. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3416–3419. doi: 10.1073/pnas.76.7.3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington G. J., Bernhard H. P., Miller R. A., Ruddle F. H. Expression of liver phenotypes in cultured mouse hepatoma cells. J Natl Cancer Inst. 1980 Apr;64(4):809–819. [PubMed] [Google Scholar]
- Friend K. K., Dorman B. P., Kucherlapati R. S., Ruddle F. H. Detection of interspecific translocations in mouse-human hybrids by alkaline Giemsa staining. Exp Cell Res. 1976 Apr;99(1):31–36. doi: 10.1016/0014-4827(76)90676-5. [DOI] [PubMed] [Google Scholar]
- Gedde-Dahl T., Jr, Cook P. J., Fagerhol M. K., Pierce J. A. Improved estimate of the Gm-Pi linkage. Ann Hum Genet. 1975 Jul;39(1):43–50. doi: 10.1111/j.1469-1809.1975.tb00106.x. [DOI] [PubMed] [Google Scholar]
- Gedde-Dahl T., Jr, Fagerhol M. K., Cook P. J., Noades J. Autosomal linkage between the Gm and Pi loci in man. Ann Hum Genet. 1972 Apr;35(4):393–399. [PubMed] [Google Scholar]
- Jeppsson J. O. Amino acid substitution Glu leads to Lys alpha1-antitrypsin PiZ. FEBS Lett. 1976 Jun 1;65(2):195–197. doi: 10.1016/0014-5793(76)80478-4. [DOI] [PubMed] [Google Scholar]
- Klinger H. P. Rapid processing of primary embryonic tissues for chromosome banding pattern analysis. Cytogenetics. 1972;11(5):424–435. doi: 10.1159/000130208. [DOI] [PubMed] [Google Scholar]
- Meera Khan P., Wijnen L. M., Pearson P. L. Assignment of the mitochondrial aconitase gene (ACONM) to human chromosome 22. Cytogenet Cell Genet. 1978;22(1-6):212–214. doi: 10.1159/000130938. [DOI] [PubMed] [Google Scholar]
- Morse J. O. alpha1-antitrypsin deficiency (first of two parts). N Engl J Med. 1978 Nov 9;299(19):1045–1048. doi: 10.1056/NEJM197811092991905. [DOI] [PubMed] [Google Scholar]
- Nichols E. A., Ruddle F. H. A review of enzyme polymorphism, linkage and electrophoretic conditions for mouse and somatic cell hybrids in starch gels. J Histochem Cytochem. 1973 Dec;21(12):1066–1081. doi: 10.1177/21.12.1066. [DOI] [PubMed] [Google Scholar]
- Pierce J. A., Eradio B., Dew T. A. Antitrypsin phenotypes in St. Louis. JAMA. 1975 Feb 10;231(6):609–612. [PubMed] [Google Scholar]
- Rankin J. K., Darlington G. J. Expression of human hepatic genes in mouse hepatoma--human amniocyte hybrids. Somatic Cell Genet. 1979 Jan;5(1):1–10. doi: 10.1007/BF01538781. [DOI] [PubMed] [Google Scholar]
- Ricciuti F., Ruddle F. H. Assignment of nucleoside phosphorylase to D-14 and localization of X-linked loci in man by somatic cell genetics. Nat New Biol. 1973 Feb 7;241(110):180–182. doi: 10.1038/newbio241180a0. [DOI] [PubMed] [Google Scholar]
- Schneider J. A., Weiss M. C. Expression of differentiated functions in hepatoma cell hybrids. I. Tyrosine aminotransferase in hepatoma-fibroblast hybrids. Proc Natl Acad Sci U S A. 1971 Jan;68(1):127–131. doi: 10.1073/pnas.68.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp H. L., Desnick R. J., Krivit W. The liver in inherited metabolic diseases of childhood. Prog Liver Dis. 1972;4:463–488. [PubMed] [Google Scholar]
- Shay J. W. Selection of reconstituted cells from karyoplasts fused to chloramphenicol-resistant cytoplasts. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2461–2464. doi: 10.1073/pnas.74.6.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M., Krinsky A., Arredondo-Vega F., Wang A. L., Hirschhorn K. Confirmation of the assignment of genes of human immunoglobulin heavy chains to chromosome 14 by analysis of Ig synthesis by man-mouse hybridomas. Eur J Immunol. 1981 Oct;11(10):852–855. doi: 10.1002/eji.1830111021. [DOI] [PubMed] [Google Scholar]
- Swallow D. M., Harris H. A new variant of the placental acid phosphatases: its implications regarding their subunit structures and genetical determination. Ann Hum Genet. 1972 Nov;36(2):141–152. doi: 10.1111/j.1469-1809.1972.tb00765.x. [DOI] [PubMed] [Google Scholar]
- Voss R., Lerer I., Povey S., Solomon E., Bobrow M. Confirmation and further regional assignment of aminoacylase 1 (acy-1) on human chromosome 3 using a simplified detection method. Ann Hum Genet. 1980 Jul;44(Pt 1):1–9. doi: 10.1111/j.1469-1809.1980.tb00940.x. [DOI] [PubMed] [Google Scholar]
- Weiss M. C., Chaplain M. Expression of differentiated functions in hepatoma cell hybrids: reappearance of tyrosine aminotransferase inducibility after the loss of chromosomes. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3026–3030. doi: 10.1073/pnas.68.12.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A., Lieberman J., Gaidulis L., Ewing C. Molecular abnormality of human alpha1-antitrypsin variant (Pi-ZZ) associated with plasma activity deficiency. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1324–1328. doi: 10.1073/pnas.73.4.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Someren H., Beijersbergen van Henegouwen H., Los W., Wurzer-Figurelli E., Doppert B., Vervloet M., Meera Khan P. Enzyme electrophoresis on cellulose acetate gel. II. Zymogram patterns in man-Chinese hamster somatic cell hybrids. Humangenetik. 1974;25(3):189–201. doi: 10.1007/BF00281426. [DOI] [PubMed] [Google Scholar]