Abstract
Determining how a biomaterial interacts with cells (“structure-function relationship”) reflects its eventual clinical applicability. Therefore, a fundamental understanding of how individual material properties modulate cell-biomaterial interactions is pivotal to improving the efficacy and safety of clinically translatable biomaterial systems. However, due to the coupled nature of material properties, their individual effects on cellular responses are difficult to understand. Structure-function relationships can be more clearly understood by the effective decoupling of each individual parameter. In this article, we discuss three basic decoupling strategies: (1) surface modification, (2) cross-linking, and (3) combinatorial approaches (i.e., copolymerization and polymer blending). Relevant examples of coupled material properties are briefly reviewed in each section to highlight the need for improved decoupling methods. This follows with examples of more effective decoupling techniques, mainly from the perspective of three primary classes of synthetic materials: polyesters, polyethylene glycol, and polyacrylamide. Recent strides in decoupling methodologies, especially surface-patterning and combinatorial techniques, offer much promise in further understanding the structure-function relationships that largely govern the success of future advancements in biomaterials, tissue engineering, and drug delivery.
Introduction
The tunability of chemical synthesis and material fabrication enables polymeric biomaterials to display diverse chemical and mechanical properties. This characteristic is crucial in developing materials for advanced biomedical applications, including tissue engineering, drug delivery, and medical device development. However, significant challenges remain in producing polymeric materials that can provide tissue-specific therapeutic effects for repair and regeneration by intricately controlling cell behavior in an effective manner, while minimizing adverse inflammatory and immunogenic effects. Determining how a polymer interacts with biological systems in vitro and in vivo reflects its clinical applicability, and a fundamental understanding of how individual material properties modulate cell-biomaterial interactions is essential for improving the efficacy and safety of clinically translatable biomaterial systems.1,2
Control over cell behavior through the regulation of cell-material interactions is a powerful approach for understanding how extracellular cues modulate the cellular response, at both the single-cell and tissue levels. When a biomaterial is in contact with a biological environment, water molecules surround the material within nanoseconds; and proteins are adsorbed to the surface in a surface property-dependent manner.3 Cells then sense and attach onto the adsorbed proteins4 by forming focal adhesion (FA) through specific interactions between cell-surface receptors (e.g., integrin) and extracellular ligands (e.g., arginine-glycine-aspartic acid: RGD). The cell-adhesive motifs are contained within extracellular adhesive proteins, such as fibronectin, vitronectin, collagen, and laminin.3,5–7 Receptor-ligand interactions direct cell motility, morphology, and function8,9 by mediating complex bi-directional signaling between cells and extracellular matrix (ECM)10 through various chemo- and mechano-transduction pathways.11–13 It is well established that biomaterial properties, such as chemical,14 mechanical,15,16 and topographical properties,17,18 modulate cell and tissue responses to the biomaterials (e.g., structure-function relationships).19,20 For example, hydrophobic materials tend to bind more proteins in a thermodynamically favorable manner, resulting in improved cell attachment and spreading, compared with hydrophilic materials.21 In addition, the intrinsic mechanical properties of biomaterials have significant effects on cell attachment,22,23 spreading, migration, proliferation,24,25 and differentiation.15,25–30 Cells respond to changes in the mechanical microenvironment in a cell type-specific manner, as the elastic modulus of tissues in the body ranges over several orders of magnitude from ∼100 Pa in the soft tissue of the brain to ∼100 MPa in bone.31,32 Intracellular mechanical forces are generated as cells adhere to and migrate across a surface, resulting in alternation of the structure and composition of FAs.3,30,33,34 A polymeric substrate should be stiff enough to resist these cell traction forces and maintain sufficient cell-material interactions; on the other hand, substrates that are too stiff hinder cell viability, presumably due to a lack of cell-cell communication.3,30,35
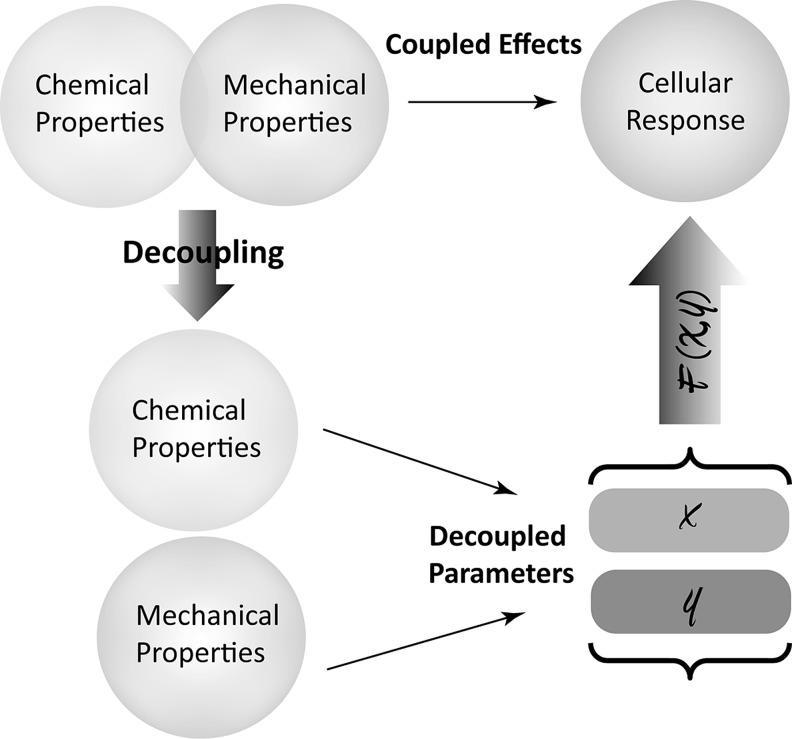
It has become increasingly clear that material properties influence one another and are, therefore, intrinsically coupled. For example, the chemical composition of a polymer largely determines the other resulting material characteristics, including crystallinity, hydrophilicity/hydrophobicity, and degradation. Improved crystallinity inherently correlates with enhanced polymer chain packing, thereby resisting water penetration and hydrolytic degradation. In addition, tightly packed polymer chains usually result in increased material stiffness. These facts indicate that the resulting cellular behavior is actually in response to the coupled material properties rather than individual ones. Therefore, there is an unmet need to decouple the material properties so that the effect of individual material properties on cellular functions can be clearly understood and exploited for an enhanced, precise regulation of cell behavior (Fig. 1).
FIG. 1.
Coupling and decoupling of polymer chemical and mechanical properties determine cellular response.
This article aims at providing a comprehensive review of current methods for altering chemical and mechanical properties of biomaterials, especially in a decoupled manner, to design the most suitable micro-environment for control over cell fate. Three promising decoupling strategies are discussed: (1) surface modification, (2) cross-linking, and (3) combinatorial approaches (i.e., copolymerization and polymer blending). In each section, we first briefly review the relevant literature regarding the cellular response to coupled material properties to highlight the need for decoupling strategies, and then discuss the current strategies for decoupling chemical and mechanical parameters in similar systems. Although other types of material properties such as topography play a role in cell-material interactions17,18,36–43 and some of the decoupling strategies can be extended to handle such delineations, we focus primarily on two dominant properties, chemical and mechanical. The purpose of this article is to reveal the limitations and issues associated with coupled polymer parameters, as they pertain to the control of cell behavior and establish a benchmark for decoupling strategies to foster advancements in biomaterials, tissue engineering, and drug delivery.
Necessity and Strategies of Decoupling Material Properties
Studying cell behavior in response to coupled material properties hinders the development of materials that can actively control cell function, because individual material properties could be more effectively optimized to modulate cell functions if understood as separate components. Decoupling strategies are, therefore, imperative to achieve a better control over cell functions by incrementally varying the chemical or mechanical properties while minimizing the collateral effects on other properties in order to elucidate the independent effects of each property. The decoupling approaches discussed next provide an insight for further improvement of these strategies and will enable the increased isolation of the effects of individual material properties on cell behavior, thereby providing a rational basis for the design and implementation of polymer scaffolds for specific applications.
Surface modifications
Covalent conjugation or simple blending of bioactive molecules into bulk polymer materials (e.g., scaffold) has been widely used to improve cell functions. For example, RGD can be covalently tethered to polyethylene glycol (PEG) chains to improve cell attachment,44–46 and protease-sensitive peptides originating from collagen or fibrinogen can be conjugated to PEG cross-linkers for hydrogel degradation and remodeling by matrix metalloproteases (MMPs).47,48 It is usually assumed that the impact of such peptide/protein modification on bulk scaffold properties is negligible, and, therefore, the enhanced cellular response is solely the result of chemical changes imparted by the surface modification; however, there are often appreciable changes in bulk scaffold properties.49–52 For example, Zustiak et al. reported that the covalent attachment of ECM peptides (e.g., RGDS, IKVAV, and YIGSR) to PEG hydrogels changed the modulus, mesh size, swelling ratio, and albumin diffusivity of the bulk material.51 Blending poly(lactic-co-glycolic acid) (PLGA) or poly-L-lactide (PLLA) with ECM-derived components (e.g., collagen, gelatin, and elastin) has been shown to improve cell adhesion and proliferation by changing the chemical composition, but resulted in changes to the mechanical properties as well.53–57 In another example, collagen was incorporated into a poly(ɛ-caprolactone) (PCL) scaffold at different ratios to enhance human dermal fibroblast and epidermal keratinocyte proliferation.58 Although similar morphological properties were observed as the fiber diameters remained constant for scaffolds containing 1% and 30% PCL, the stiffness of the nanofibrous scaffolds increased from 8.4×10−3 to 2.3×10−2 N/mm, while both cell proliferation and type IV collagen synthesis decreased significantly, indicating coupled chemical and mechanical effects on cell behavior.58
One way to address this interdependency is to introduce a coating of functional groups on the surface of a scaffold, generating a thin, activated, superficial layer that can minimize the alteration of bulk scaffold properties, as the modification only occurs at the surface. Chemical properties can be further fine tuned on this surface to enable efficient decoupling while maintaining bulk scaffold properties. The immobilization of bioactive molecules (e.g., ECM-derived peptides or growth factors), addition of chemical groups, or introduction of micro-patterns to the scaffold surface can, in many cases, dramatically alter surface properties and cell behavior without significantly changing bulk mechanical properties, indicating the successful decoupling of the surface chemical and bulk mechanical properties, as discussed next (Fig. 2).
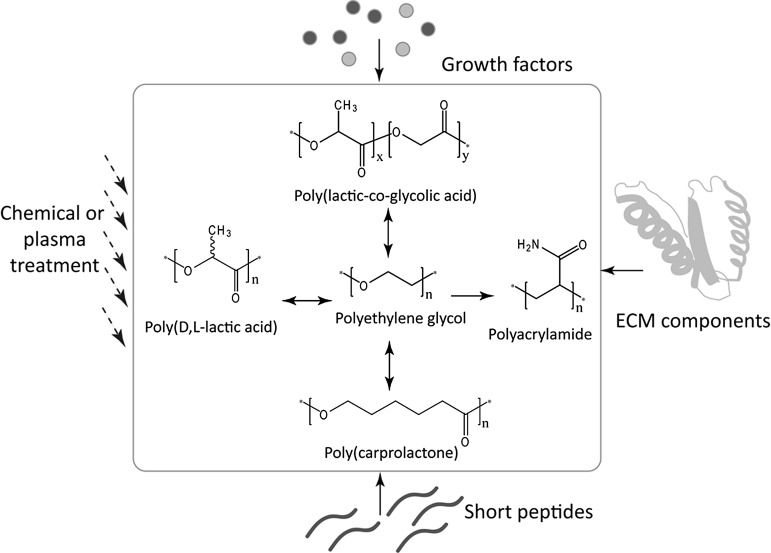
FIG. 2.
Structures of polyethylene glycol (PEG), polyesters (polylactic acid, poly(lactic-co-glycolic acid), poly(ɛ-caprolactone)), and polyacrylamide (PA) as discussed in the article. Among these polymers, PEG plays a central role in copolymerization and also in tuning the properties of polyesters and PA gel. Polymer chemical properties can be further modified either by chemical or plasma treatment to introduce additional functional groups, or by incorporating extracellular matrix components, short peptides, and growth factors onto polymer chains.
Surface biofunctionalization with peptides and proteins
Several methods exist for chemically attaching peptides and proteins to the surface of a biomaterial scaffold. Chitosan and heparin have been covalently immobilized onto the scaffold surface of PLGA films using N-(3-dimethylaminopropyl)-N-ethylcarbodiimide (EDC) and N-hydroxysuccinimide (NHS), which improved hepatocyte adhesion and proliferation and decreased coagulation.59 In another study, chitosan was immobilized to the surface of a PLGA scaffold by a photochemical reaction of azide-chitosan followed by the conjugation of gelatin, which strongly supported 3T3 fibroblast adhesion.60 The same group reported that the surface modification of a PLGA scaffold with collagen could also be achieved by reacting with 1,8-diaminooctane and glutaraldehyde, which subsequently improved porcine smooth muscle cell proliferation.61 Various growth factors, such as basic fibroblast growth factor and vascular endothelial growth factor (VEGF), can be further adsorbed to surface-immobilized heparin and collagen to provide the sustained release of these biomolecules.62–64 The surface of PCL scaffolds has been functionalized with amine groups, which are further used to conjugate RGD motifs for the improved attachment of rat bone marrow stromal cells65 and L929 fibroblasts.66 Similarly, RGD has been covalently tethered to the surface of PEG scaffolds to improve cell attachment.44–46 Polyacrylamide (PA), as an inert and anti-adhesive hydrogel,67,68 can be used to decouple chemical and mechanical effects on cell behavior via the copolymerization of an active ester acrylate followed by the hydrolysis or replacement of the N-succinimidyl group with ECM-derived peptides, such as RGD.69 Alternatively, ECM-derived proteins immobilized to the PA hydrogel surface via sulfo-SANPAH directed the osteogenic and myogenic differentiation of mesenchymal stem cells.25
Scaffold surface functionalization by plasma and chemical treatments
Instead of binding biofunctional molecules, the scaffold surface can be modified by adding chemical groups to improve cellular responses to biomaterial scaffolds. Various plasma treatments (e.g., O2, Ar or NH3) have been applied to change the surface-free energy and introduce hydroxyl, peroxyl, amine, or other functional groups to the surface of PLGA films, each of which subsequently impacted cell adhesion and proliferation.70–72 Similarly, adhesion and proliferation of umbilical cord perivascular cells to a PCL scaffold was enhanced by increasing the density of surface carboxyl groups by grafting with poly(acrylic acid) using low-pressure plasma immobilization.73 Apart from plasma treatment, chemical modification of the PCL scaffold surface using sodium hydroxide was shown to enhance surface hydrophilicity and improved the proliferation of endothelial and smooth muscle cells.74,75 Finally, hydroxyl, carboxyl, or anionic surfaces have been introduced to derivatizable PA hydrogel surfaces to study cellular response to chemical cues.76
Surface patterning
Cellular responses can also be controlled by patterning the scaffold surface with cell-adhesive or nonadhesive molecules. The inert nature of PEG makes it an excellent substrate on which cell-adhesive or bioactive molecules can be patterned to confine cell adhesion, migration, and differentiation to patterned areas. For example, RGD-modified islands were created by UV irradiation of PEG diacrylate-casted film surfaces through a photomask. Cells, therefore, only adhered and grew on the RGD-modified islands surrounded by nonadhesive cross-linked PEG.77 The spatial neurite extension of cortical neurons was also controlled by confining the shape of cell-adhesive regions of poly-D-lysine with cell-resistant PEG materials (e.g., PEG-PDLA) lined up as barriers.78 Interestingly, glass surfaces patterned with PEG dimethacrylate (PEGDMA) micro-wells promoted the differentiation of preadipocytes with increasing lipid accumulation, though it significantly increased the contact angle and decreased surface energy, making the surface less cell adhesive than unpatterned glass.79
Cross-linking
The degree of cross-linking can be varied to study the effects of substrate stiffness on cell behavior. However, in many cases, the manipulation of cross-linking often results in the change of chemical properties as well as the physical and topographical makeup of a material. For example, the concentration of EDC cross-linkers was varied to alter the elastic modulus in poly-L-lysine/hyaluronan (PLL/HA) multi-electrolyte films, but unexpected changes to surface roughness and cell adhesion were observed in a coupled fashion.80 Modulating the stiffness of PA gels by changing the bis-acrylamide cross-linker concentration has been used to create substrates with a physiological range of stimuli to mimic the stiffness of native tissue.15 It was demonstrated that soft PA gels produced more dynamic, irregularly shaped FAs, whereas stiff gel stabilized FAs and reduced the migration of rat kidney epithelial and 3T3 fibroblast cells.81 However, varying acrylamide and bis-acrylamide concentrations may expose different amounts of chemical moieties to cells, leading to unexpected cell responses (e.g., the cytotoxicity of acrylamide in vivo82). In addition, as the cross-linking degree increases, the pore size decreases within the gel network,83,84 indicating the coupling of cross-linking and structural parameters.85 In parallel, the intrinsic properties of PEG-containing hydrogels are coupled, as mesh size,85 modulus,86,87 and degradation rates88,89 change with PEG content. A previous study demonstrated that PEGylation of fibrinogen reduced the MMP production and altered the normal spindle shape of neonatal human foreskin fibroblasts, which was likely due to increased hydrophilicty and repellence in coupling with reduced storage modulus.90 In addition, changes in stiffness can be coupled with cell repellence and hydration in PEG-based hydrogels, which can drastically affect cell behavior.86,91 The degree of cross-linking, PEG molecular weight (Mw),92 and the type of cross-linker (i.e., PEG diacrylate [PEGDA])85 can be altered to tune the modulus of PEG hydrogels. However, as stiffness increases, the swelling and diffusivity of PEG hydrogel decreases.93 These coupled effects have been shown to impact ECM production and differentiation of mesenchymal stem cells,94 illustrating another example of the need to decouple these properties for studying cell behavior.
The chemical and mechanical properties of cross-linked hydrogels can be decoupled by altering the types of the cross-linkers or the Mw of the polymers. For example, alginate hydrogels covalently cross-linked with adipic dihydrazide, methyl ester L-lysine, or PEG-diamine (1,000 Da) showed similar shear moduli within 31.1 to 36.9 kPa.95 Alternatively, relatively constant stiffness can be obtained by varying the percentage of PEG monoacrylate (PEGMA) cross-linkers or the Mw of PEGDA in PEG hydrogels.96 The Mw and hydrophilicity of PEGMA cross-linkers further refined this decoupling strategy to provide a tunable template for the independent modulation of stiffness and mesh size. Similarly, the incorporation of 4-arm PEG acrylate cross-linkers at various concentrations with the simultaneous tuning of the concentration and Mw of PEGDA enabled the decoupling of either the gel modulus or mesh size from a chemical composition.97 VEGF expression and viability of fibroblasts was reported to be affected by both elastic modulus and mesh size, and the effect of the individual properties were identified through the aforementioned decoupling methods.97 In another study, PEG methacrylate and poly(N-hydroxymethyl acrylamide) were cross-linked with oxidized methacrylic alginate (OMA) to control modulus and degradation rates.98 Although degradation rate usually decreases with increased modulus, varying the number of oxidized uronic residues on OMA enabled controlled degradation rates with a relatively constant stiffness. Protein-release rates and subsequent angiogenesis in vivo were then regulated as a function of the OMA cross-linking.98
Combinatorial approaches: copolymerization and polymer blending
Altering the polymer composition is considered an important strategy that is employed for modulating matrix stiffness. However, a significant challenge still remains, as changing the polymer composition often results in simultaneous changes to hydrophobicity, crystallinity, and water uptake. For example, varying the blending ratio of PLGA and PLLA created a mechanical gradient to determine a “threshold stiffness” for optimal cell organization, myotubule formation, and myoblast differentiation.30 The two polymers, however, have inherently different chemical properties that change simultaneously with the blending ratio, indicating coupling between chemical and mechanical properties. Similarly, PCL was blended with poly(ether sulfone) (PES) to study the influence of polymer stiffness on the differentiation of embryonic mesenchymal progenitor cells. A stiffer PES-PCL scaffold (modulus=30.6 MPa) promoted osteogenesis, while a softer, pure PCL scaffold (modulus=7.1 MPa) promoted chondrogenesis.99 However, the change in chemical composition via altering the blending ratio was coupled with stiffness and should also be considered.
Although copolymerizing and blending different monomers or polymers often leads to simultaneous changes in other properties, these techniques can be used to decouple the chemical and mechanical properties by creating a combinatorial library of polymers and selecting compositions that display similar mechanical properties. Our recent study demonstrated that the wet elastic moduli of polymeric nanofibrous scaffolds were maintained within a tight range while altering the chemical compositions of co- or ter-polymers through combinatorial synthesis and electrospinning techniques.100 This unique approach enabled the synthesis of six polymer formulations with unique chemical characteristics but similar wet moduli. When cultured with murine embryonic stem cells, they induced different levels of expression of intracellular hydrogen peroxide and cardiomyocyte markers, suggesting the effect of polymer composition on cell behavior, as the mechanical strength was maintained constant and decoupled. In addition, the copolymerization of PCL with other polyesters, such as polyglycolic acid and polylactic acid, have been shown to alter degradation, hydrophobicity, and crystallinity, while maintaining the elasticity exhibited by the pure PCL scaffold.101–104 These examples demonstrate that the combinatorial synthesis of polymers is a promising approach for decoupling scaffold properties. The scale of the combinatorial library can be expanded to further tune a particular type of material property while minimizing the changes in other properties. A library of combinatorial polymers with 112 different compositions was produced from copolymerizing 14 different tyrosine-derived diphenols and eight aliphatic diacid monomers.105 Changes in their chemical compositions were found to affect modulus, glass transition temperature (Tg), and contact angle. Polymers with a similar property (e.g., stiffness or Tg) could be grouped to study the cell response affected by chemical composition, demonstrating the effective decoupling of these properties.105 Similarly, a library of poly(β-amino esters) (PBAEs) provided tunable mechanical, degradation, and adhesion properties. Degradation and mechanical properties were decoupled by varying Mw and branching structure.106,107 Selected PBAE macromers were also cross-linked during electrospinning to improve the tunability of mechanical and degradation properties.108 Such an approach was used to elucidate polymer structure-function relationships, thereby improving the safety and efficacy of their applications for gene delivery.109,110 Similar approaches have been adapted to fine tune cellular responses to PCL acrylates111 and methacrylate terpolymers.112 An excellent review of combinatorial polymer synthesis techniques, high-throughput assessment of material properties, and material-cell interactions is thoroughly covered elsewhere.113
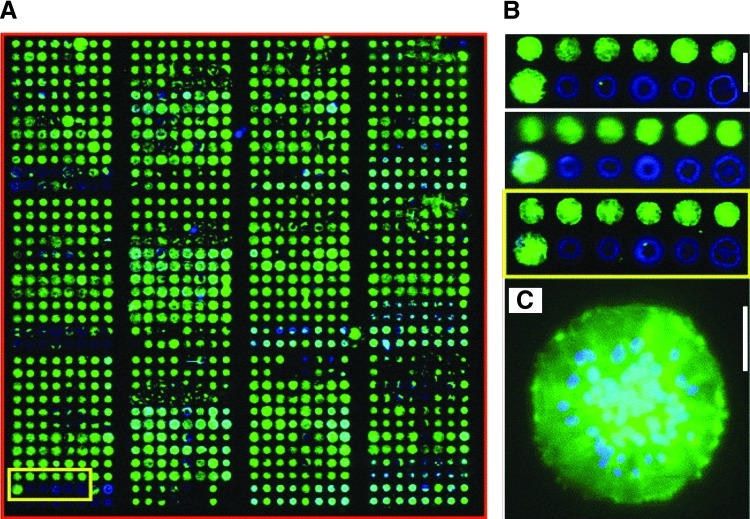
These combinatorial synthesis techniques offer distinct advantages over the traditional synthesis used for “one-measurement for one-sample” in terms of time, resources, and information to be obtained. However, there are still drawbacks and challenges associated with these methodologies, including the preservation of sample purity, data handling, and availability of such resources.114 It is more challenging to use high-throughput schemes for sophisticated syntheses that require purification, and, in many cases, it is more worthwhile to test discrete polymer ratios to obtain data by a potentially more reliable method. Polymer blends can be used to circumvent such issues with synthesis, for instance, by observing cellular responses to gradient changes of polymer properties in a two-dimensional combinatorial library (e.g., temperature and polymer composition). For example, Sung et al. reported that a large library of PCL/PLGA blends with different stiffness, surface roughness, and/or crystallinity has been generated by varying the PCL-to-PLGA ratio and the annealing temperature, which provides an applicable approach for decoupling by selecting a group of blends similar in one property (e.g., stiffness) while differing in another (e.g., chemical composition). This study further demonstrated that a mid-to-high relative composition of PCL in a PCL/PLGA blend was ideal for smooth muscle cell adhesion, proliferation, and protein production.115 Similarly, biomaterial microarrays can be used to decouple stiffness, cross-linking density, and crystallinity (Fig. 3)116 and examine other chemical and topographical influences on various cell functions.117–120 These combinatorial approaches offer much promise in elucidating the distinct mechanisms that govern cell-material interaction to further advance biomaterials and tissue-engineering technologies.
FIG. 3.
Human mesenchymal stem cells grown on a microarray of combinatorial polymer blendings with various ratios of different polymers (adapted from reference 116). (A–C) cells on polymer microarray were fixed, and immunohistochemistry staining was performed for actin (green). Nuclei were stained with Hoechst (blue). (B) Close view of triplicates of polymer blendings highlighted in yellow squares in (A), scale bar=500 μm (C) Close view of polymer spots highlighted in yellow squares in (B), scale bar=100 μm. Color images available online at www.liebertpub.com/teb
Conclusion and Future Direction
The field of biomaterials has witnessed a drastic expansion over the last few decades, but significant hurdles remain in clearly understanding structure-function relationships. A major reason is that coupled material properties limit our understanding of how individual properties influence cellular response. For example, when the chemical composition of a polymer scaffold is changed by grafting biomolecules or altering the cross-linking density, other material properties such as stiffness, hydrophobicity, crystallinity, mesh size, and topography change simultaneously. Cellular responses to a material are regulated by the coupled effects of these interrelated material properties, making it difficult to interpret structure-function relationships. The decoupling of material properties is critical in determining how a change of a material property influences cell behavior. Although we primarily focus on decoupling chemical and mechanical properties, other influences such as physical and topographical properties are also vital to our understanding of cell-biomaterial interactions.
Promising approaches that are involved in decoupling chemical and mechanical properties are discussed and evaluated, which include (1) surface modification to create a superficial layer of functional groups, bioactive molecules, or micro-patterns; (2) cross-linking to specifically control chemical, mechanical, or physical properties independently; and (3) copolymerization to produce a library of combinatorial polymers with various chemical compositions, or blending of multiple polymers to create an array. Surface modification through chemical alternation (e.g., plasma or sodium hydroxide treatment) or the conjugation of bioactive molecules (e.g., ECM-derived ligands or growth factors) to create a superficial layer with distinct chemical properties compared with the bulk material often allows for the conservation of stiffness to isolate specific chemical influences on cell behavior.59–61,70–75 Surface patterning, in addition to the decoupling of chemical and mechanical properties, provides a promising strategy to further alter the topography of the interface in cell-biomaterial interactions.77,78 By incorporating additional cross-linkers, relatively constant stiffness can be obtained with PEG hydrogels, demonstrating the decoupling of chemical and mechanical properties.98,121 Copolymerizing multiple polymer units in varying ratios provides another way for decoupling by producing more than one polymer type that exhibits the same mechanical properties but different chemical compositions.100,105–108,111,112 The copolymerization and polymer blending combinatorial library techniques enable a high-throughput approach that is utilized in elucidating cell-material relationships.115–120 Other strategies such as using nanomaterials of different chemical compositions to achieve similar mechanical properties100 could be considered as further alternatives to the decoupling approaches discussed here.
Although significant progress has been made, it should be understood that elucidating structure-function relationships is not easy, because decoupling two properties often results in an unexpected change of another property due to the highly interdependent nature of material properties. It should also be noted that the cellular response can also alter the material properties, effectively creating a complex feedback mechanism (“function-structure relationship”) that changes in real time as the biomaterial interacts with the surrounding cells and tissues in a highly dynamic fashion.19,122 It is, therefore, of great importance to keep improving the methods to more precisely and accurately decouple material parameters and to thoroughly verify the improvement by investigating how the strategies affect scaffold properties and cellular responses before and after decoupling. This effort will provide a broader insight into structure-function and function-structure relationships, as well as property-property relationships, thereby establishing a whole-picture view of cell-biomaterial interactions. This is an urgent task for modern biomaterial scientists and tissue engineers in order to advance the development of instructive scaffolds for tissue engineering, regenerative medicine, and drug delivery.
Acknowledgments
The authors acknowledge grant supports from the National Science Foundation (NSF CAREER CBET 1056046, NSF DMR 1006558) and the National Institutes of Health (NIH HL091465).
Disclosure Statement
No competing financial interests exist.
References
- 1.Byers B.A. Baksh D. Translation of new tissue engineering materials to clinical application. In: Burdick J.A., editor; Mauck R.L., editor. Biomaterials for Tissue Engineering Applications: A Review of the Past and Future Trends. Germany: SpringerWienNewYork; 2011. pp. 541–562. [Google Scholar]
- 2.Lendlein A. Neffe A.T. Pierce B.F. Vienken J. Why are so few degradable polymeric biomaterials currently established in clinical applications? Int J Artif Organs. 2011;34:71. doi: 10.5301/ijao.2011.6422. [DOI] [PubMed] [Google Scholar]
- 3.Roach P. Eglin D. Rohde K. Perry C.C. Modern biomaterials: a review - bulk properties and implications of surface modifications. J Mater Sci Mater Med. 2007;18:1263. doi: 10.1007/s10856-006-0064-3. [DOI] [PubMed] [Google Scholar]
- 4.Mogilner A. Oster G. Cell motility driven by actin polymerization. Biophys J. 1996;71:3030. doi: 10.1016/S0006-3495(96)79496-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siebers M.C. ter Brugge P.J. Walboomers X.F. Jansen J.A. Integrins as linker proteins between osteoblasts and bone replacing materials. A critical review. Biomaterials. 2005;26:137. doi: 10.1016/j.biomaterials.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 6.Bokel C. Brown N.H. Integrins in development: moving on, responding to, and sticking to the extracellular matrix. Dev Cell. 2002;3:311. doi: 10.1016/s1534-5807(02)00265-4. [DOI] [PubMed] [Google Scholar]
- 7.Humphries J.D. Byron A. Humphries M.J. Integrin ligands at a glance. J Cell Sci. 2006;119:3901. doi: 10.1242/jcs.03098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geiger B. Spatz J.P. Bershadsky A.D. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol. 2009;10:21. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- 9.Dey T. Mann M.C. Goldmann W.H. Comparing mechano-transduction in fibroblasts deficient of focal adhesion proteins. Biochem Biophys Res Commun. 2011;413:541. doi: 10.1016/j.bbrc.2011.08.133. [DOI] [PubMed] [Google Scholar]
- 10.Brunton V.G. MacPherson I.R. Frame M.C. Cell adhesion receptors, tyrosine kinases and actin modulators: a complex three-way circuitry. Biochim Biophys Acta. 2004;1692:121. doi: 10.1016/j.bbamcr.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 11.Holle A.W. Engler A.J. More than a feeling: discovering, understanding, and influencing mechanosensing pathways. Curr Opin Biotechnol. 2011;22:648. doi: 10.1016/j.copbio.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takada Y. Ye X. Simon S. The integrins. Genome Biol. 2007;8:215. doi: 10.1186/gb-2007-8-5-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aplin A.E. Howe A. Alahari S.K. Juliano R.L. Signal transduction and signal modulation by cell adhesion receptors: the role of integrins, cadherins, immunoglobulin-cell adhesion molecules, and selectins. Pharmacol Rev. 1998;50:197. [PubMed] [Google Scholar]
- 14.Castner D.G. Ratner B.D. Biomedical surface science: foundations to frontiers. Surface Science. 2002;500:28. [Google Scholar]
- 15.Engler A.J. Sen S. Sweeney H.L. Discher D.E. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 16.Discher D.E. Janmey P. Wang Y.L. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 17.Curtis A. Wilkinson C. Topographical control of cells. Biomaterials. 1997;18:1573. doi: 10.1016/s0142-9612(97)00144-0. [DOI] [PubMed] [Google Scholar]
- 18.Curtis A.S.G. Wilkinson C.D.W. Reactions of cells to topography. J Biomater Sci Polym Ed. 1998;9:1313. doi: 10.1163/156856298x00415. [DOI] [PubMed] [Google Scholar]
- 19.Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]
- 20.Wong J.Y. Leach J.B. Brown X.Q. Balance of chemistry, topography, and mechanics at the cell-biomaterial interface: issues and challenges for assessing the role of substrate mechanics on cell response. Surface Sci. 2004;570:119. [Google Scholar]
- 21.Temenoff J.S. Mikos A.G. Biomaterials The Intersection of Biology and Materials Science. New Jersey: Pearson Education, Inc.; 2008. [Google Scholar]
- 22.Yeung T., et al. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton. 2005;60:24. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
- 23.Irwin E.F., et al. Modulus-dependent macrophage adhesion and behavior. J Biomater Sci Polym Ed. 2008;19:1363. doi: 10.1163/156856208786052407. [DOI] [PubMed] [Google Scholar]
- 24.Hsiong S.X. Carampin P. Kong H.J. Lee K.Y. Mooney D.J. Differentiation stage alters matrix control of stem cells. J Biomed Mater Res A. 2008;85:145. doi: 10.1002/jbm.a.31521. [DOI] [PubMed] [Google Scholar]
- 25.Rowlands A.S. George P.A. Cooper-White J.J. Directing osteogenic and myogenic differentiation of MSCs: interplay of stiffness and adhesive ligand presentation. Am J Physiol Cell Physiol. 2008;295:C1037. doi: 10.1152/ajpcell.67.2008. [DOI] [PubMed] [Google Scholar]
- 26.Zajac A.L. Discher D.E. Cell differentiation through tissue elasticity-coupled, myosin-driven remodeling. Curr Opin Cell Biol. 2008;20:609. doi: 10.1016/j.ceb.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guilak F., et al. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell. 2009;5:17. doi: 10.1016/j.stem.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Discher D.E. Mooney D.J. Zandstra P.W. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324:1673. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winer J.P. Janmey P.A. McCormick M.E. Funaki M. Bone marrow-derived human mesenchymal stem cells become quiescent on soft substrates but remain responsive to chemical or mechanical stimuli. Tissue Eng Part A. 2009;15:147. doi: 10.1089/ten.tea.2007.0388. [DOI] [PubMed] [Google Scholar]
- 30.Levy-Mishali M. Zoldan J. Levenberg S. Effect of scaffold stiffness on myoblast differentiation. Tissue Eng Part A. 2009;15:935. doi: 10.1089/ten.tea.2008.0111. [DOI] [PubMed] [Google Scholar]
- 31.Park J.S., et al. The effect of matrix stiffness on the differentiation of mesenchymal stem cells in response to TGF-beta. Biomaterials. 2011;32:3921. doi: 10.1016/j.biomaterials.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gribova V. Crouzier T. Picart C. A material's point of view on recent developments of polymeric biomaterials: control of mechanical and biochemical properties. J Mater Chem. 2011;21:14354. doi: 10.1039/C1JM11372K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zimerman B., et al. Formation of focal adhesion-stress fibre complexes coordinated by adhesive and non-adhesive surface domains. IEE Proc Nanobiotechnol. 2004;151:62. doi: 10.1049/ip-nbt:20040474. [DOI] [PubMed] [Google Scholar]
- 34.Beningo K.A. Dembo M. Kaverina I. Small J.V. Wang Y.L. Nascent focal adhesions are responsible for the generation of strong propulsive forces in migrating fibroblasts. J Cell Biol. 2001;153:881. doi: 10.1083/jcb.153.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sazonova O.V., et al. Cell-cell interactions mediate the response of vascular smooth muscle cells to substrate stiffness. Biophys J. 2011;101:622. doi: 10.1016/j.bpj.2011.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lim J.Y. Donahue H.J. Cell sensing and response to micro- and nanostructured surfaces produced by chemical and topographic patterning. Tissue Eng. 2007;13:1879. doi: 10.1089/ten.2006.0154. [DOI] [PubMed] [Google Scholar]
- 37.Chen C.S. Geometric Control of Cell Life and Death. Science. 1997;276:1425. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 38.Gonzalez-Garcia C. Sousa S.R. Moratal D. Rico P. Salmeron-Sanchez M. Effect of nanoscale topography on fibronectin adsorption, focal adhesion size and matrix organisation. Colloids Surf B Biointerfaces. 2010;77:181. doi: 10.1016/j.colsurfb.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 39.Lim J.Y., et al. The regulation of integrin-mediated osteoblast focal adhesion and focal adhesion kinase expression by nanoscale topography. Biomaterials. 2007;28:1787. doi: 10.1016/j.biomaterials.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 40.McNamara L.E., et al. Nanotopographical control of stem cell differentiation. J Tissue Eng. 2010;2010:120623. doi: 10.4061/2010/120623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yim E.K. Darling E.M. Kulangara K. Guilak F. Leong K.W. Nanotopography-induced changes in focal adhesions, cytoskeletal organization, and mechanical properties of human mesenchymal stem cells. Biomaterials. 2010;31:1299. doi: 10.1016/j.biomaterials.2009.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dunn G.A. Brown A.F. Alignment of fibroblasts on grooved surfaces described by a simple geometric transformation. J Cell Sci. 1986;83:313. doi: 10.1242/jcs.83.1.313. [DOI] [PubMed] [Google Scholar]
- 43.Frisman I. Seliktar D. Bianco-Peled H. Nanostructuring PEG-fibrinogen hydrogels to control cellular morphogenesis. Biomaterials. 2011;32:7839. doi: 10.1016/j.biomaterials.2011.06.078. [DOI] [PubMed] [Google Scholar]
- 44.DeLong S.A. Gobin A.S. West J.L. Covalent immobilization of RGDS on hydrogel surfaces to direct cell alignment and migration. J Control Release. 2005;109:139. doi: 10.1016/j.jconrel.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 45.Hern D.L. Hubbell J.A. Incorporation of adhesion peptides into nonadhesive hydrogels useful for tissue resurfacing. J Biomed Mater Res. 1998;39:266. doi: 10.1002/(sici)1097-4636(199802)39:2<266::aid-jbm14>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 46.Schmidt D.R. Kao W.J. Monocyte activation in response to polyethylene glycol hydrogels grafted with RGD and PHSRN separated by interpositional spacers of various lengths. J Biomed Mater Res A. 2007;83A:617. doi: 10.1002/jbm.a.31270. [DOI] [PubMed] [Google Scholar]
- 47.Lee S.H. Miller J.S. Moon J.J. West J.L. Proteolytically degradable hydrogels with a fluorogenic substrate for studies of cellular proteolytic activity and migration. Biotechnol Prog. 2005;21:1736. doi: 10.1021/bp0502429. [DOI] [PubMed] [Google Scholar]
- 48.West J.L. Hubbell J.A. Polymeric biomaterials with degradation sites for proteases involved in cell migration. Macromolecules. 1999;32:241. [Google Scholar]
- 49.Gunn J.W. Turner S.D. Mann B.K. Adhesive and mechanical properties of hydrogels influence neurite extension. J Biomed Mater Res A. 2005;72A:91. doi: 10.1002/jbm.a.30203. [DOI] [PubMed] [Google Scholar]
- 50.Skornia S.L. Bledsoe J.G. Kelso B. Kuntz Willitz R. Mechanical properties of layered poly (ethylene glycol) gels. J Appl Biomater Biomech. 2007;5:176. [PubMed] [Google Scholar]
- 51.Zustiak S.P. Durbal R. Leach J.B. Influence of cell-adhesive peptide ligands on poly(ethylene glycol) hydrogel physical, mechanical and transport properties. Acta Biomater. 2010;6:3404. doi: 10.1016/j.actbio.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marquardt L. Willits R.K. Student award winner in the undergraduate's degree category for the society for biomaterials 35th annual meeting, Orlando, Florida, April 13–16, 2011. J Biomed Mater Res A. 2011;98A:1. doi: 10.1002/jbm.a.33044. [DOI] [PubMed] [Google Scholar]
- 53.Chen G., et al. Culturing of skin fibroblasts in a thin PLGA-collagen hybrid mesh. Biomaterials. 2005;26:2559. doi: 10.1016/j.biomaterials.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 54.Chen G. Ushida T. Tateishi T. A biodegradable hybrid sponge nested with collagen microsponges. J Biomed Mater Res. 2000;51:273. doi: 10.1002/(sici)1097-4636(200008)51:2<273::aid-jbm16>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 55.Han J., et al. Co-electrospun blends of PLGA, gelatin, and elastin as potential nonthrombogenic scaffolds for vascular tissue engineering. Biomacromolecules. 2011;12:399. doi: 10.1021/bm101149r. [DOI] [PubMed] [Google Scholar]
- 56.Jose M.V. Thomas V. Dean D.R. Nyairo E. Fabrication and characterization of aligned nanofibrous PLGA/Collagen blends as bone tissue scaffolds. Polymer. 2009;50:3778. [Google Scholar]
- 57.Li M., et al. Co-electrospun poly(lactide-co-glycolide), gelatin, and elastin blends for tissue engineering scaffolds. J Biomed Mater Res A. 2006;79:963. doi: 10.1002/jbm.a.30833. [DOI] [PubMed] [Google Scholar]
- 58.Powell H.M. Boyce S.T. Engineered human skin fabricated using electrospun collagen-pcl blends: morphogenesis and mechanical properties. Tissue Eng A. 2009;15:2177. doi: 10.1089/ten.tea.2008.0473. [DOI] [PubMed] [Google Scholar]
- 59.Wang X.H., et al. Covalent immobilization of chitosan and heparin on PLGA surface. Int J Biol Macromol. 2003;33:95. doi: 10.1016/s0141-8130(03)00072-2. [DOI] [PubMed] [Google Scholar]
- 60.Zhu A.P. Fang N. Chan-Park M.B. Chan V. Adhesion contact dynamics of 3T3 fibroblasts on poly (lactide-co-glycolide acid) surface modified by photochemical immobilization of biomacromolecules. Biomaterials. 2006;27:2566. doi: 10.1016/j.biomaterials.2005.11.039. [DOI] [PubMed] [Google Scholar]
- 61.Zhu Y. Chan-Park M.B. Sin Chian K. The growth improvement of porcine esophageal smooth muscle cells on collagen-grafted poly(DL-lactide-co-glycolide) membrane. J Biomed Mater Res B Appl Biomater. 2005;75:193. doi: 10.1002/jbm.b.30305. [DOI] [PubMed] [Google Scholar]
- 62.Cai S. Liu Y. Zheng Shu X. Prestwich G.D. Injectable glycosaminoglycan hydrogels for controlled release of human basic fibroblast growth factor. Biomaterials. 2005;26:6054. doi: 10.1016/j.biomaterials.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 63.Tae G. Scatena M. Stayton P.S. Hoffman A.S. PEG-cross-linked heparin is an affinity hydrogel for sustained release of vascular endothelial growth factor. J Biomater Sci-Polym Ed. 2006;17:187. doi: 10.1163/156856206774879090. [DOI] [PubMed] [Google Scholar]
- 64.Zieris A., et al. FGF-2 and VEGF functionalization of starPEG-heparin hydrogels to modulate biomolecular and physical cues of angiogenesis. Biomaterials. 2010;31:7985. doi: 10.1016/j.biomaterials.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 65.Zhang H. Lin C.Y. Hollister S.J. The interaction between bone marrow stromal cells and RGD-modified three-dimensional porous polycaprolactone scaffolds. Biomaterials. 2009;30:4063. doi: 10.1016/j.biomaterials.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Karakecili A. Satriano C. Gumusderelioglu M. Marletta G. Relationship between the fibroblastic behaviour and surface properties of RGD-immobilized PCL membranes. J Mater Sci Mater Med. 2007;18:317. doi: 10.1007/s10856-006-0695-4. [DOI] [PubMed] [Google Scholar]
- 67.Georges P.C. Janmey P.A. Cell type-specific response to growth on soft materials. J Appl Physiol. 2005;98:1547. doi: 10.1152/japplphysiol.01121.2004. [DOI] [PubMed] [Google Scholar]
- 68.Moshayedi P., et al. Mechanosensitivity of astrocytes on optimized polyacrylamide gels analyzed by quantitative morphometry. J Phys Condens Matter. 2010;22:194114. doi: 10.1088/0953-8984/22/19/194114. [DOI] [PubMed] [Google Scholar]
- 69.Reinhart-King C.A. Dembo M. Hammer D.A. Endothelial cell traction forces on RGD-derivatized polyacrylamide substrata. Langmuir. 2003;19:1573. [Google Scholar]
- 70.Wan Y. Yang J. Bei J. Wang S. Cell adhesion on gaseous plasma modified poly-(L-lactide) surface under shear stress field. Biomaterials. 2003;24:3757. doi: 10.1016/s0142-9612(03)00251-5. [DOI] [PubMed] [Google Scholar]
- 71.Wan Y., et al. Characterization of surface property of poly(lactide-co-glycolide) after oxygen plasma treatment. Biomaterials. 2004;25:4777. doi: 10.1016/j.biomaterials.2003.11.051. [DOI] [PubMed] [Google Scholar]
- 72.Hasirci N. Endogan T. Vardar E. Kiziltay A. Hasirci V. Effect of oxygen plasma on surface properties and biocompatibility of PLGA films. Surface and Interface Analysis. 2010;42:486. [Google Scholar]
- 73.Chong M.S. Chan J. Choolani M. Lee C.N. Teoh S.H. Development of cell-selective films for layered co-culturing of vascular progenitor cells. Biomaterials. 2009;30:2241. doi: 10.1016/j.biomaterials.2008.12.056. [DOI] [PubMed] [Google Scholar]
- 74.Serrano M.C., et al. Vascular endothelial and smooth muscle cell culture on NaOH-treated poly(epsilon-caprolactone) films: a preliminary study for vascular graft development. Macromol Biosci. 2005;5:415. doi: 10.1002/mabi.200400214. [DOI] [PubMed] [Google Scholar]
- 75.Serrano M.C., et al. Nitric oxide production by endothelial cells derived from blood progenitors cultured on NaOH-treated polycaprolactone films: a biofunctionality study. Acta Biomater. 2009;5:2045. doi: 10.1016/j.actbio.2009.02.034. [DOI] [PubMed] [Google Scholar]
- 76.Brandley B.K. Weisz O.A. Schnaar R.L. Cell attachment and long-term growth on derivatizable polyacrylamide surfaces. J Biol Chem. 1987;262:6431. [PubMed] [Google Scholar]
- 77.Lee H.J. Kim D.N. Park S. Lee Y. Koh W.G. Micropatterning of a nanoporous alumina membrane with poly(ethylene glycol) hydrogel to create cellular micropatterns on nanotopographic substrates. Acta Biomater. 2011;7:1281. doi: 10.1016/j.actbio.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 78.Yang I.H. Co C.C. Ho C.C. Controlling neurite outgrowth with patterned substrates. J Biomed Mater Res A. 2011;97:451. doi: 10.1002/jbm.a.33082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fozdar D.Y. Wu X. Patrick C.W., Jr. Chen S. Micro-well texture printed into PEG hydrogels using the FILM nanomanufacturing process affects the behavior of preadipocytes. Biomed Microdevices. 2008;10:839. doi: 10.1007/s10544-008-9198-z. [DOI] [PubMed] [Google Scholar]
- 80.Schneider A., et al. Polyelectrolyte multilayers with a tunable young's modulus: influence of film stiffness on cell adhesion. Langmuir. 2005;22:1193. doi: 10.1021/la0521802. [DOI] [PubMed] [Google Scholar]
- 81.Pelham R.J., Jr. Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci U S A. 1997;94:13661. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Matsuoka M. Igisu H. Lin J. Inoue N. Effects of acrylamide and N,N′-methylene-bis-acrylamide on creatine kinase activity. Brain Res. 1990;507:351. doi: 10.1016/0006-8993(90)90297-o. [DOI] [PubMed] [Google Scholar]
- 83.Ueda M. Oana H. Baba Y. Doi M. Yoshikawa K. Electrophoresis of long DNA molecules in linear polyacrylamide solutions. Biophys Chem. 1998;71:113. doi: 10.1016/s0301-4622(98)00093-3. [DOI] [PubMed] [Google Scholar]
- 84.Russell S.M. Carta G. Mesh size of charged polyacrylamide hydrogels from partitioning measurements. Ind Eng Chem Res. 2005;44:8213. [Google Scholar]
- 85.Bryant S.J. Chowdhury T.T. Lee D.A. Bader D.L. Anseth K.S. Crosslinking density influences chondrocyte metabolism in dynamically loaded photocrosslinked poly(ethylene glycol) hydrogels. Ann Biomed Eng. 2004;32:407. doi: 10.1023/b:abme.0000017535.00602.ca. [DOI] [PubMed] [Google Scholar]
- 86.Peyton S.R. Raub C.B. Keschrumrus V.P. Putnam A.J. The use of poly(ethylene glycol) hydrogels to investigate the impact of ECM chemistry and mechanics on smooth muscle cells. Biomaterials. 2006;27:4881. doi: 10.1016/j.biomaterials.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 87.Stegemann J.P. Hong H. Nerem R.M. Mechanical, biochemical, and extracellular matrix effects on vascular smooth muscle cell phenotype. J Appl Physiol. 2005;98:2321. doi: 10.1152/japplphysiol.01114.2004. [DOI] [PubMed] [Google Scholar]
- 88.Bryant S.J. Anseth K.S. Controlling the spatial distribution of ECM components in degradable PEG hydrogels for tissue engineering cartilage. J Biomed Mater Res A. 2003;64A:70. doi: 10.1002/jbm.a.10319. [DOI] [PubMed] [Google Scholar]
- 89.Munoz-Pinto D.J. Bulick A.S. Hahn M.S. Uncoupled investigation of scaffold modulus and mesh size on smooth muscle cell behavior. J Biomed Mater Res A. 2009;90:303. doi: 10.1002/jbm.a.32492. [DOI] [PubMed] [Google Scholar]
- 90.Gonen-Wadmany M. Goldshmid R. Seliktar D. Biological and mechanical implications of PEGylating proteins into hydrogel biomaterials. Biomaterials. 2011;32:6025. doi: 10.1016/j.biomaterials.2011.04.055. [DOI] [PubMed] [Google Scholar]
- 91.Shapira-Schweitzer K. Seliktar D. Matrix stiffness affects spontaneous contraction of cardiomyocytes cultured within a PEGylated fibrinogen biomaterial. Acta Biomater. 2007;3:33. doi: 10.1016/j.actbio.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 92.Temenoff J.S. Athanasiou K.A. Lebaron R.G. Mikos A.G. Effect of poly(ethylene glycol) molecular weight on tensile and swelling properties of oligo(poly(ethylene glycol) fumarate) hydrogels for cartilage tissue engineering. J Biomed Mater Res. 2002;59:429. doi: 10.1002/jbm.1259. [DOI] [PubMed] [Google Scholar]
- 93.Kloxin A.M. Kloxin C.J. Bowman C.N. Anseth K.S. Mechanical Properties of Cellularly Responsive Hydrogels and Their Experimental Determination. Adv Mater. 2010;22:3484. doi: 10.1002/adma.200904179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Buxton A.N., et al. Design and characterization of poly(ethylene glycol) photopolymerizable semi-interpenetrating networks for chondrogenesis of human mesenchymal stem cells. Tissue Eng. 2007;13:2549. doi: 10.1089/ten.2007.0075. [DOI] [PubMed] [Google Scholar]
- 95.Lee K.Y., et al. Controlling mechanical and swelling properties of alginate hydrogels independently by cross-linker type and cross-linking density. Macromolecules. 2000;33:4291. [Google Scholar]
- 96.Cha C. Jeong J.H. Shim J. Kong H. Tuning the dependency between stiffness and permeability of a cell encapsulating hydrogel with hydrophilic pendant chains. Acta Biomater. 2011;7:3719. doi: 10.1016/j.actbio.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 97.Browning M.B. Wilems T. Hahn M. Cosgriff-Hernandez E. Compositional control of poly(ethylene glycol) hydrogel modulus independent of mesh size. J Biomed Mater Res A. 2011;98:268. doi: 10.1002/jbm.a.33109. [DOI] [PubMed] [Google Scholar]
- 98.Cha C. Kohman R.H. Kong H. Biodegradable polymer crosslinker: independent control of stiffness, toughness, and hydrogel degradation rate. Adv Funct Mater. 2009;19:3056. [Google Scholar]
- 99.Nam J. Johnson J. Lannutti J.J. Agarwal S. Modulation of embryonic mesenchymal progenitor cell differentiation via control over pure mechanical modulus in electrospun nanofibers. Acta Biomater. 2011;7:1516. doi: 10.1016/j.actbio.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gupta M.K., et al. Combinatorial polymer electrospun matrices promote physiologically-relevant cardiomyogenic stem cell differentiation. PloS one. 2011;6:e28935. doi: 10.1371/journal.pone.0028935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.He W., et al. Tubular nanofiber scaffolds for tissue engineered small-diameter vascular grafts. J Biomed Mater Res A. 2009;90:205. doi: 10.1002/jbm.a.32081. [DOI] [PubMed] [Google Scholar]
- 102.Zhang K., et al. Fabrication of silk fibroin blended P(LLA-CL) nanofibrous scaffolds for tissue engineering. J Biomed Mater Res A. 2010;93:984. doi: 10.1002/jbm.a.32504. [DOI] [PubMed] [Google Scholar]
- 103.Engelhardt E.M., et al. A collagen-poly(lactic acid-co-varepsilon-caprolactone) hybrid scaffold for bladder tissue regeneration. Biomaterials. 2011;32:3969. doi: 10.1016/j.biomaterials.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 104.Liu J.J. Wang C.Y. Wang J.G. Ruan H.J. Fan C.Y. Peripheral nerve regeneration using composite poly(lactic acid-caprolactone)/nerve growth factor conduits prepared by coaxial electrospinning. J Biomed Mater Res A. 2011;96:13. doi: 10.1002/jbm.a.32946. [DOI] [PubMed] [Google Scholar]
- 105.Brocchini S. James K. Tangpasuthadol V. Kohn J. Structure-property correlations in a combinatorial library of degradable biomaterials. J Biomed Mater Res. 1998;42:66. doi: 10.1002/(sici)1097-4636(199810)42:1<66::aid-jbm9>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 106.Brey D.M. Erickson I. Burdick J.A. Influence of macromer molecular weight and chemistry on poly(β-amino ester) network properties and initial cell interactions. JBiomed Mater Res A. 2008;85A:731. doi: 10.1002/jbm.a.31494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Brey D.M. Ifkovits J.L. Mozia R.I. Katz J.S. Burdick J.A. Controlling poly(β-amino ester) network properties through macromer branching. Acta Biomater. 2008;4:207. doi: 10.1016/j.actbio.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Metter R.B., et al. Biodegradable fibrous scaffolds with diverse properties by electrospinning candidates from a combinatorial macromer library. Acta Biomater. 2010;6:1219. doi: 10.1016/j.actbio.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Green J.J. Langer R. Anderson D.G. A combinatorial polymer library approach yields insight into nonviral gene delivery. Acc Chem Res. 2008;41:749. doi: 10.1021/ar7002336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Eltoukhy A.A., et al. Effect of molecular weight of amine end-modified poly(β-amino ester)s on gene delivery efficiency and toxicity. Biomaterials. 2012;33:3594. doi: 10.1016/j.biomaterials.2012.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cai L. Wang S. Poly(ɛ-caprolactone) acrylates synthesized using a facile method for fabricating networks to achieve controllable physicochemical properties and tunable cell responses. Polymer. 2010;51:164. [Google Scholar]
- 112.Joy A., et al. Control of Surface Chemistry, Substrate Stiffness, and Cell Function in a Novel Terpolymer Methacrylate Library. Langmuir. 2011;27:1891. doi: 10.1021/la103722m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Peters A. Brey D.M. Burdick J.A. High-throughput and combinatorial technologies for tissue engineering applications. Tissue Eng Part B Rev. 2009;15:225. doi: 10.1089/ten.TEB.2009.0049. [DOI] [PubMed] [Google Scholar]
- 114.Webster D.C. Meier M.A.R. Polymer libraries: preparation and applications. Adv Polym Sci. 2010;225:1. [Google Scholar]
- 115.Sung H.J., et al. The use of temperature-composition combinatorial libraries to study the effects of biodegradable polymer blend surfaces on vascular cells. Biomaterials. 2005;26:4557. doi: 10.1016/j.biomaterials.2004.11.034. [DOI] [PubMed] [Google Scholar]
- 116.Anderson D.G. Putnam D. Lavik E.B. Mahmood T.A. Langer R. Biomaterial microarrays: rapid, microscale screening of polymer–cell interaction. Biomaterials. 2005;26:4892. doi: 10.1016/j.biomaterials.2004.11.052. [DOI] [PubMed] [Google Scholar]
- 117.Dolatshahi-Pirouz A. Nikkhah M. Kolind K. Dokmeci M.R. Khademhosseini A. Micro- and nanoengineering approaches to control stem cell-biomaterial interactions. J Funct Biomater. 2011;2:88. doi: 10.3390/jfb2030088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Spokoyny A.M. Kim D. Sumrein A. Mirkin C.A. Infinite coordination polymer nano- and microparticle structures. Chem Soc Rev. 2009;38:1218. doi: 10.1039/b807085g. [DOI] [PubMed] [Google Scholar]
- 119.Simon C.G., Jr., et al. Combinatorial screening of cell proliferation on poly(l-lactic acid)/poly(d,l-lactic acid) blends. Biomaterials. 2005;26:6906. doi: 10.1016/j.biomaterials.2005.04.050. [DOI] [PubMed] [Google Scholar]
- 120.Chatterjee K. Sun L. Chow L.C. Young M.F. Simon C.G., Jr Combinatorial screening of osteoblast response to 3D calcium phosphate/poly(ɛ-caprolactone) scaffolds using gradients and arrays. Biomaterials. 2011;32:1361. doi: 10.1016/j.biomaterials.2010.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Scott R.A. Elbert D.L. Willits R.K. Modular poly(ethylene glycol) scaffolds provide the ability to decouple the effects of stiffness and protein concentration on PC12 cells. Acta Biomater. 2011;7:3841. doi: 10.1016/j.actbio.2011.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Le D.M. Kulangara K. Adler A.F. Leong K.W. Ashby V.S. Dynamic topographical control of mesenchymal stem cells by culture on responsive poly(epsilon-caprolactone) surfaces. Adv Mater. 2011;23:3278. doi: 10.1002/adma.201100821. [DOI] [PMC free article] [PubMed] [Google Scholar]