Abstract
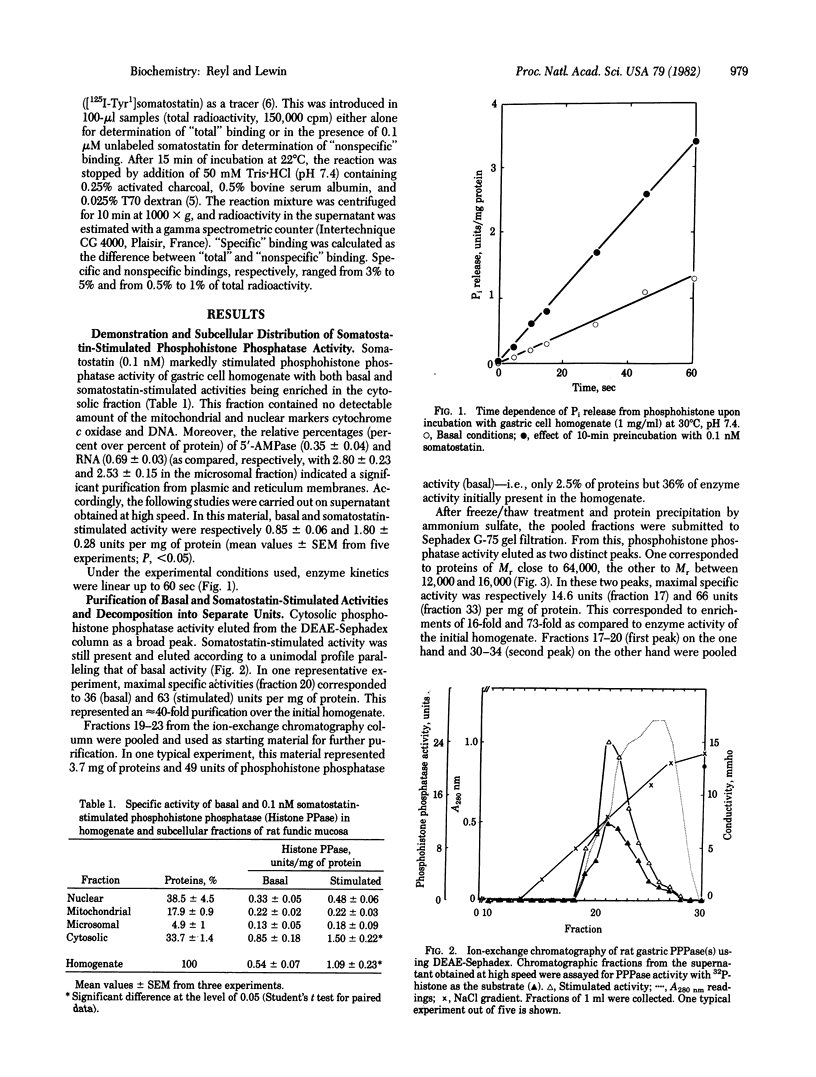
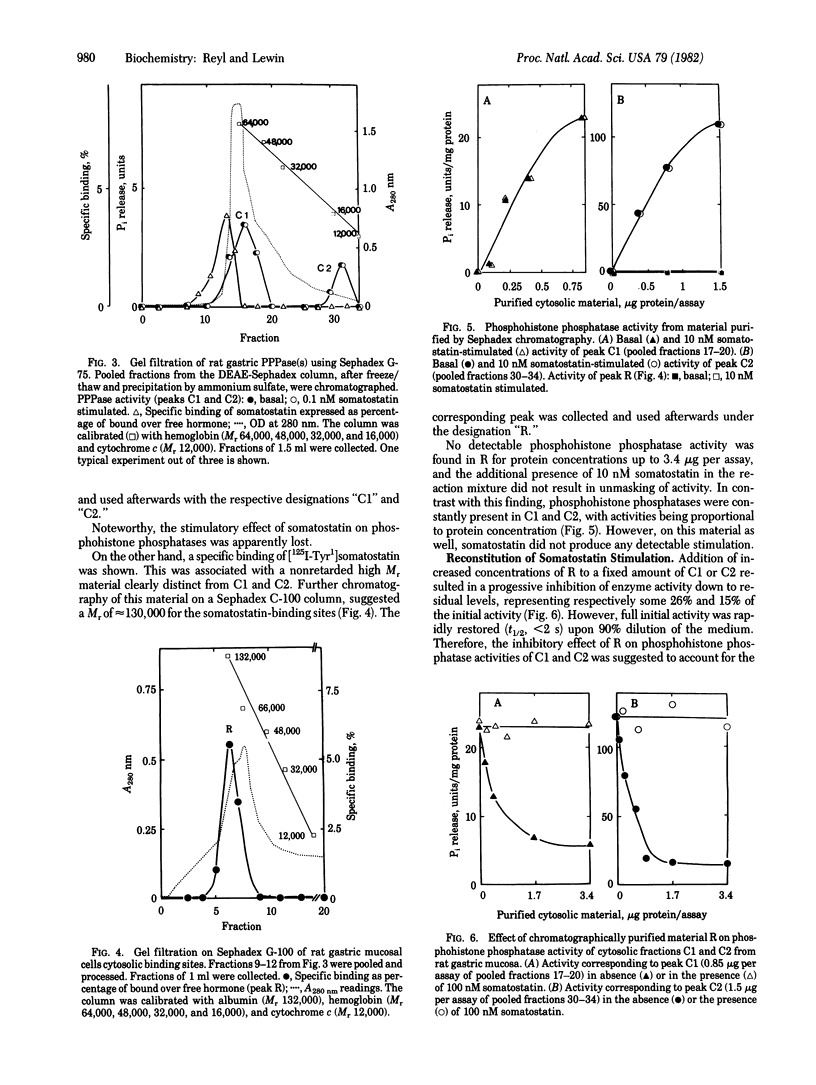
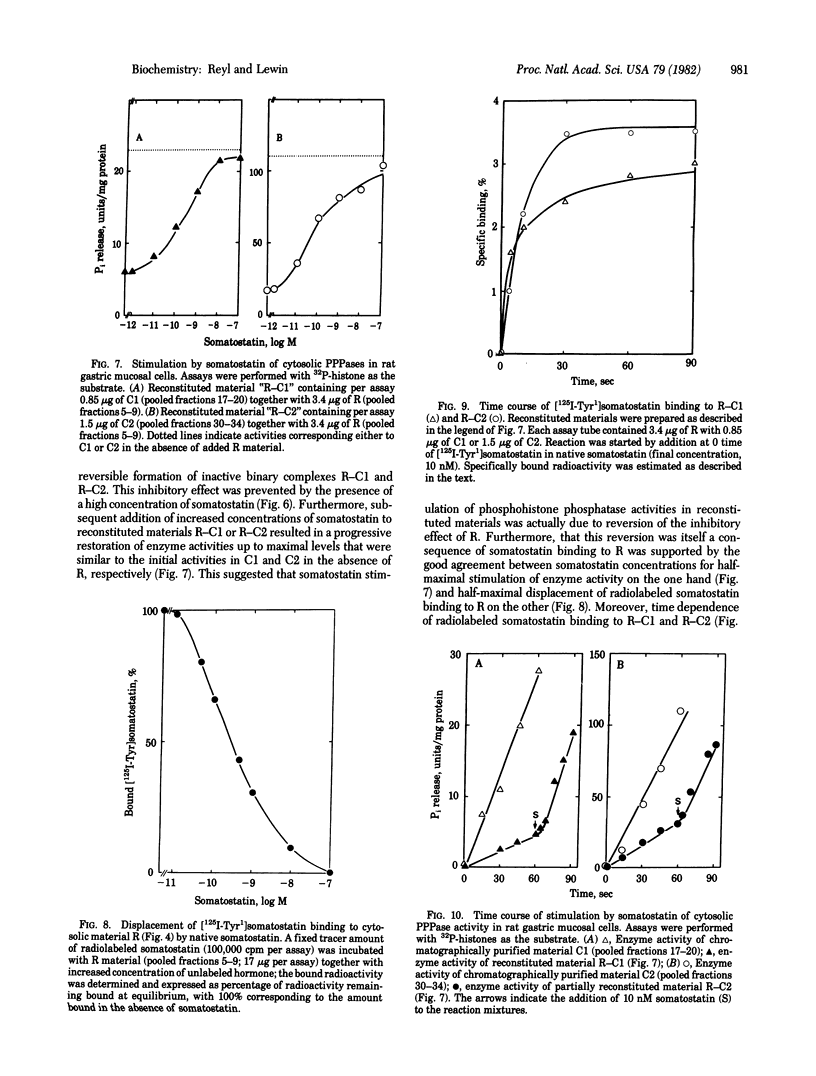
Using 32P-labeled histone as exogenous substrate, we showed a potent stimulatory effect of somatostatin on cytosolic phosphoprotein phosphatases (PPPases; phosphoprotein phosphohydrolase, EC 3.1.3.16) in rat gastric mucosal cells. Partial purification of cytosolic fraction in DEAE-Sephadex ion-exchange chromatography and further gel filtration on Sephadex C-75 and Sephadex G-100 separated somatostatin-dependent PPPases into three distinct molecular species. One corresponding to Mr 130,000 was devoid of any PPPase activity but specifically bound [Tyr1]somatostatin 125I-labeled on the Tyr ([125I-Tyr1]somatostatin) with an apparent equilibrium dissociation constant of 3 x 10(-10) M. The two other molecular species corresponded to Mrs 64,000 and 13,000. They produced catalytic dephosphorylation of 32P-labeled histone, but they were not sensitive to somatostatin and did not show any specific binding to radiolabeled hormone. Mixing of the larger with either of the two smaller molecular species resulted in concentration -dependent inhibition of PPPase activity. However this inhibition was reversed by increased concentrations of somatostatin, with the concentration for half-maximal reactivation on being close to 0.1 nM. Furthermore somatostatin stimulation in reconstituted materials developed according to a rapid time course (t1/2, less than 5 sec), consistent with that observed for binding of [125I-Tyr1]somatostatin. These results strongly argue for the presence of an intracellular somatostatin receptor in gastric mucosal cells and characterize this receptor as a PPPase regulatory subunit. Thus, substrate dephosphorylation could be the primary event triggering physiological effects of somatostatin in stomach and perhaps other organs of the digestive tract [Reyl, F. & Lewin, M. J.l M. (1981) Biochim. Biophys. Acta 675, 297-300].
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brazeau P., Vale W., Burgus R., Ling N., Butcher M., Rivier J., Guillemin R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973 Jan 5;179(4068):77–79. doi: 10.1126/science.179.4068.77. [DOI] [PubMed] [Google Scholar]
- Khandelwal R. L., Vandenheede J. R., Krebs E. G. Purification, properties, and substrate specificities of phosphoprotein phosphatase(s) from rabbit liver. J Biol Chem. 1976 Aug 25;251(16):4850–4858. [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Ogawa N., Thompson T., Friesen H. G. Characteristics of a somatostatin-binding protein. Can J Physiol Pharmacol. 1978 Feb;56(1):48–53. doi: 10.1139/y78-007. [DOI] [PubMed] [Google Scholar]
- Reyl F., Lewin M. J. Somatostatin is a potent activator of phosphoprotein phosphatases in the digestive tract. Biochim Biophys Acta. 1981 Jul;675(2):297–300. doi: 10.1016/0304-4165(81)90241-5. [DOI] [PubMed] [Google Scholar]
- Sachs G., Chang H. H., Rabon E., Schackman R., Lewin M., Saccomani G. A nonelectrogenic H+ pump in plasma membranes of hog stomach. J Biol Chem. 1976 Dec 10;251(23):7690–7698. [PubMed] [Google Scholar]
- Schlessinger J., Shechter Y., Willingham M. C., Pastan I. Direct visualization of binding, aggregation, and internalization of insulin and epidermal growth factor on living fibroblastic cells. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2659–2663. doi: 10.1073/pnas.75.6.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soumarmon A., Abastado M., Bonfils S., Lewin M. J. C1-transport in gastric micorsomes. An ATP-dependent influx sensitive to membrane potential and to protein kinase inhibitor. J Biol Chem. 1980 Dec 25;255(24):11682–11687. [PubMed] [Google Scholar]
- Soumarmon A., Lewin M., Cheret A. M., Bonfils S. Gastric HCO3-stimulated ATPase: evidence against its microsomal localization in rat fundus mucosa. Biochim Biophys Acta. 1974 Mar 29;339(3):403–414. doi: 10.1016/0005-2736(74)90167-9. [DOI] [PubMed] [Google Scholar]
- Vale W., Brazeau P., Grant G., Nussey A., Burgus R., Rivier J., Ling N., Guillemin R. Premières observations sur le mode d'action de la somatostatine, un facteur hypothalamique qui inhibe la sécrétion de l'hormone de croissance. C R Acad Sci Hebd Seances Acad Sci D. 1972 Dec 18;275(25):2913–2916. [PubMed] [Google Scholar]


