Abstract
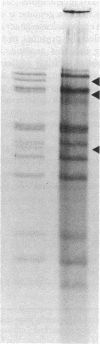
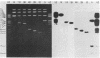
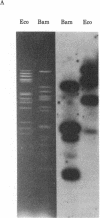
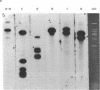
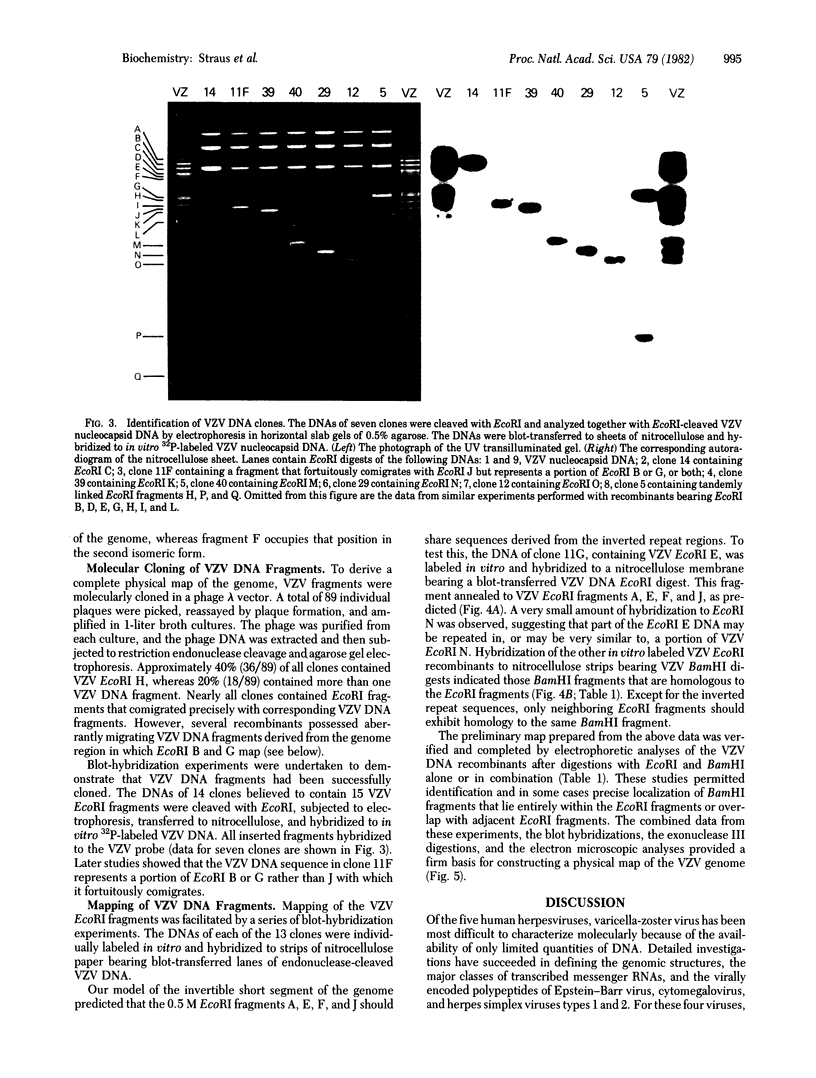
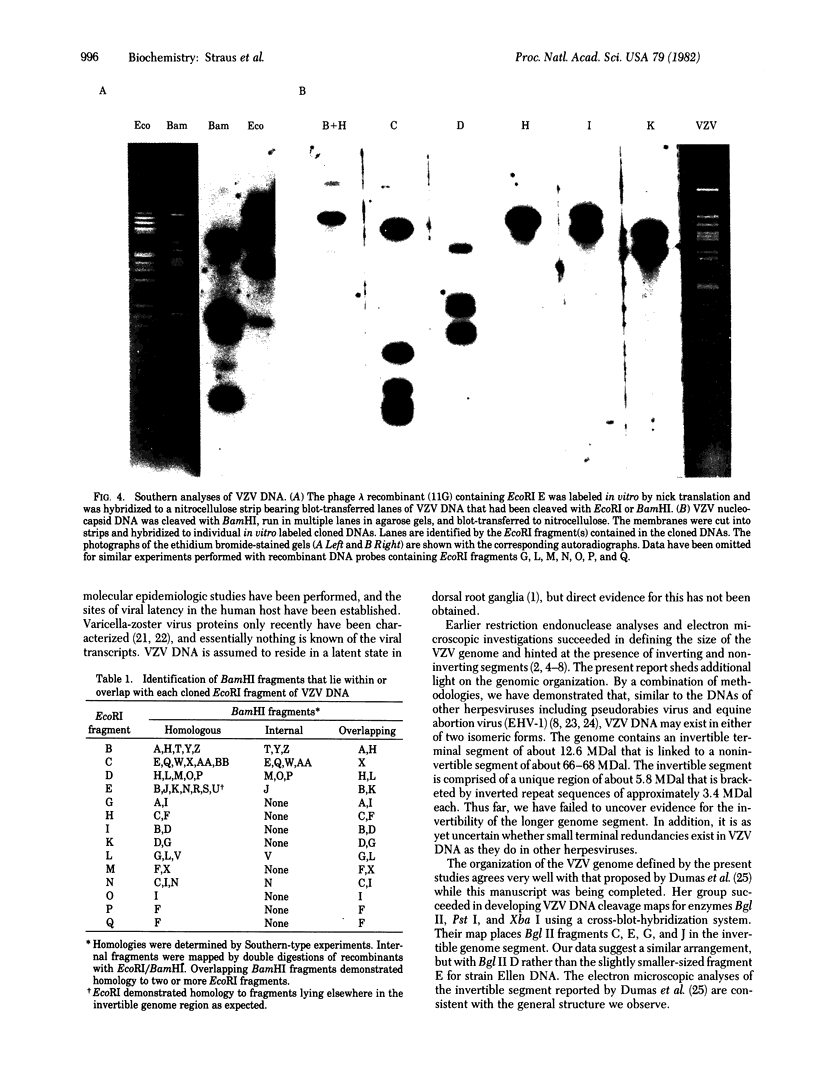
Varicella-zoster virus (VZV) DNA was cleaved with restriction endonuclease EcoRI, and most of the resulting fragments were successfully cloned in the phage vector lambda gtWES . lambda B. Double digestions of cloned fragments with EcoRI and BamHI and hybridizations to blot-transferred BamHI digests of VZV DNA were used to construct a physical map of the genome. The molecular termini of the DNA were identified by restriction enzyme analysis after exonuclease III digestion. The data indicate that VZV DNA exists in two isomeric forms that differ by inversion of one short terminal genome segment. Electron microscopic studies revealed that the short genome segment consists of a terminal revealed that the short genome segment consists of a terminal sequence of about 3.4 X 10(6) daltons that is separated from an internal inverted repeat of itself by a 5.8 X 10(60)-dalton unique DNA segment.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck E., Sommer R., Auerswald E. A., Kurz C., Zink B., Osterburg G., Schaller H., Sugimoto K., Sugisaki H., Okamoto T. Nucleotide sequence of bacteriophage fd DNA. Nucleic Acids Res. 1978 Dec;5(12):4495–4503. doi: 10.1093/nar/5.12.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas A. M., Geelen J. L., Weststrate M. W., Wertheim P., van der Noordaa J. XbaI, PstI, and BglII restriction enzyme maps of the two orientations of the varicella-zoster virus genome. J Virol. 1981 Aug;39(2):390–400. doi: 10.1128/jvi.39.2.390-400.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enquist L. W., Madden M. J., Schiop-Stanley P., Vande Woude G. F. Cloning of herpes simplex type 1 DNA fragments in a bacteriophage lambda vector. Science. 1979 Feb 9;203(4380):541–544. doi: 10.1126/science.216076. [DOI] [PubMed] [Google Scholar]
- Fiers W., Contreras R., Haegemann G., Rogiers R., Van de Voorde A., Van Heuverswyn H., Van Herreweghe J., Volckaert G., Ysebaert M. Complete nucleotide sequence of SV40 DNA. Nature. 1978 May 11;273(5658):113–120. doi: 10.1038/273113a0. [DOI] [PubMed] [Google Scholar]
- Grose C., Edmond B. J., Friedrichs W. E. Immunogenic glycoproteins of laboratory and vaccine strains of Varicella-Zoster virus. Infect Immun. 1981 Mar;31(3):1044–1053. doi: 10.1128/iai.31.3.1044-1053.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iltis J. P., Oakes J. E., Hyman R. W., Rapp F. Comparison of the DNAs of varicella-zoster viruses isolated from clinical cases of varicella and herpes zoster. Virology. 1977 Oct 15;82(2):345–352. doi: 10.1016/0042-6822(77)90009-5. [DOI] [PubMed] [Google Scholar]
- Kelly R. B., Cozzarelli N. R., Deutscher M. P., Lehman I. R., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. XXXII. Replication of duplex deoxyribonucleic acid by polymerase at a single strand break. J Biol Chem. 1970 Jan 10;245(1):39–45. [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes J. E., Iltis J. P., Hyman R. W., Rapp F. Analysis by restriction enzyme cleavage of human varicella-zoster virus DNAs. Virology. 1977 Oct 15;82(2):353–361. doi: 10.1016/0042-6822(77)90010-1. [DOI] [PubMed] [Google Scholar]
- Rapp F., Iltis J. P., Oakes J. E., Hyman R. W. A novel approach to study the DNA of herpes zoster virus. Intervirology. 1977;8(5):272–280. doi: 10.1159/000148902. [DOI] [PubMed] [Google Scholar]
- Richards J. C., Hyman R. W., Rapp F. Analysis of the DNAs from seven varicella-zoster virus isolates. J Virol. 1979 Dec;32(3):812–821. doi: 10.1128/jvi.32.3.812-821.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robberson D., Aloni Y., Attardi G., Davidson N. Expression of the mitochondrial genome in HeLa cells. VI. Size determination of mitochondrial ribosomal RNA by electron microscopy. J Mol Biol. 1971 Sep 28;60(3):473–484. doi: 10.1016/0022-2836(71)90182-3. [DOI] [PubMed] [Google Scholar]
- Roizman B. The structure and isomerization of herpes simplex virus genomes. Cell. 1979 Mar;16(3):481–494. doi: 10.1016/0092-8674(79)90023-0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Friedmann T., Air G. M., Barrell B. G., Brown N. L., Fiddes J. C., Hutchison C. A., 3rd, Slocombe P. M., Smith M. The nucleotide sequence of bacteriophage phiX174. J Mol Biol. 1978 Oct 25;125(2):225–246. doi: 10.1016/0022-2836(78)90346-7. [DOI] [PubMed] [Google Scholar]
- Shemer Y., Leventon-Kriss S., Sarov I. Isolation and polypeptide characterization of varicella-zoster virus. Virology. 1980 Oct 15;106(1):133–140. doi: 10.1016/0042-6822(80)90228-7. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sternberg N., Tiemeier D., Enquist L. In vitro packaging of a lambda Dam vector containing EcoRI DNA fragments of Escherichia coli and phage P1. Gene. 1977 May;1(3-4):255–280. doi: 10.1016/0378-1119(77)90049-x. [DOI] [PubMed] [Google Scholar]
- Stevely W. S. Inverted repetition in the chromosome of pseudorabies virus. J Virol. 1977 Apr;22(1):232–234. doi: 10.1128/jvi.22.1.232-234.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vande Woude G. F., Oskarsson M., Enquist L. W., Nomura S., Sullivan M., Fischinger P. J. Cloning of integrated Moloney sarcoma proviral DNA sequences in bacteriophage lambda. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4464–4468. doi: 10.1073/pnas.76.9.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]