Abstract
We characterized the novel Schizosaccharomyces pombe genes myo4+ and myo5+, both of which encode myosin-V heavy chains. Disruption of myo4 caused a defect in cell growth and led to an abnormal accumulation of secretory vesicles throughout the cytoplasm. The mutant cells were rounder than normal, although the sites for cell polarization were still established. Elongation of the cell ends and completion of septation required more time than in wild-type cells, indicating that Myo4 functions in polarized growth both at the cell ends and during septation. Consistent with this conclusion, Myo4 was localized around the growing cell ends, the medial F-actin ring, and the septum as a cluster of dot structures. In living cells, the dots of green fluorescent protein-tagged Myo4 moved rapidly around these regions. The localization and movement of Myo4 were dependent on both F-actin cables and its motor activity but seemed to be independent of microtubules. Moreover, the motor activity of Myo4 was essential for its function. These results suggest that Myo4 is involved in polarized cell growth by moving with a secretory vesicle along the F-actin cables around the sites for polarization. In contrast, the phenotype of myo5 null cells was indistinguishable from that of wild-type cells. This and other data suggest that Myo5 has a role distinct from that of Myo4.
INTRODUCTION
Polarized cell growth and cell division are important activities in both unicellular and multicellular organisms (reviewed by Drubin and Nelson, 1996; Keller and Simons, 1997). Establishment of cell polarity consists of at least two steps. First, the cell chooses a specific site at which it will polarize. Second, this site is recognized by a series of proteins, which then form the machinery required for surface extension and/or secretion of substances. These processes require a directed movement of organelles to the site. Microtubules and microfilaments are thought to be involved in this organelle transport, and the roles of motor proteins, including kinesin, dynein, and myosin, have been investigated (reviewed by Schliwa, 1999).
Class V unconventional myosin (myosin-V) is an actin-based motor protein that may be involved in several types of organelle transport (reviewed by Titus, 1997). It is thought to contain two heavy chains, each of which has an N-terminal motor (head) domain. The motor domain is linked to a C-terminal tail domain by a neck domain that contains potent light chain-binding sites (Cheney et al., 1993). The tail domain contains a coiled-coil region, which is thought to mediate dimerization of the heavy chains, and a globular domain, which has been postulated to mediate an interaction between myosin and a secretory cargo (reviewed by Mooseker and Cheney, 1995; Catlett and Weisman, 1998; Schott et al., 1999).
The in vivo function of myosin-V has been studied both in mammalian cells and in budding yeast. dilute mice, which lack myosin-Va, fail to properly localize both pigment granules in melanocytes and smooth endoplasmic reticulum in neurons (Mercer et al., 1991; Provance et al., 1996; Takagishi et al., 1996). In humans, the myosin-5a gene has been identified as the mutant gene in Griscelli syndrome, which is characterized by severe immunodeficiency and partial albinism (Griscelli et al., 1978; Pastural et al., 1997), the latter probably because of an improper transport of pigment-containing organelles. Saccharomyces cerevisiae has two myosin-V heavy chains, Myo2p and Myo4p, which appear to have distinct intracellular roles (reviewed by Titus, 1997). Myo2p appears to be involved in polarized secretion through a role in transport of post-Golgi vesicles (Johnston et al., 1991; Govindan et al., 1995; Karpova et al., 2000). It is localized both to the tip of the developing bud and to the bud neck, the sites at which polarized secretion occurs (Brockerhoff et al., 1994; Lillie and Brown, 1994). In contrast, Myo4p is required for the asymmetric localization of some mRNAs (Bobola et al., 1996; Jansen et al., 1996).
How does myosin-V transport a secretory cargo to the growth sites? The simple “transport” model (Mooseker and Cheney, 1995) proposes that myosin-V travels by its motor activity along an actin filament to the growth sites, carrying the secretory cargo. In the case of S. cerevisiae Myo2p, this model has been supported by localization studies: a mutation in the actin-binding site of Myo2p causes its delocalization (Lillie and Brown, 1994; Schott et al., 1999), and loss of F-actin cables in a tropomyosin mutant leads to a rapid delocalization of Myo2p (Pruyne et al., 1998). However, other studies have indicated that the globular tail domain of Myo2p may play a role in its localization (Catlett and Weisman, 1998; Reck-Peterson et al., 1999). In addition, a fraction of Myo2p localizes to the incipient bud site (Ayscough et al., 1997) and to the bud neck (Karpova et al., 2000) independently of F-actin. It is not understood how the F-actin–dependent localization and tail domain-dependent localization are related and involved in transporting organelles. Furthermore, actual movement of Myo2p to the growth site and a requirement for its motor activity have not been demonstrated.
In vertebrate cells, the actin cytoskeleton and microtubules seem to cooperate with each other in the transport of intracellular organelles. In mouse melanophores (Wu et al., 1998) and neurons (Bridgman, 1999), myosin-Va is thought to interact with actin filaments in the periphery of the cell to generate local movements of melanosomes and synaptic vesicles, respectively. However, these organelles also undergo rapid long-range movements, dependent on microtubules, in the dendritic extension of the melanocyte or in the axon of the neuron. It is not clear how the switch of organelle movement from the microtubule-based system to the actin filament-based one is regulated.
Fission yeast is an attractive organism for studying polarized cell growth because cells are cylindrical and growth occurs exclusively by elongation at the cell ends. This polarized growth begins immediately after cell division and continues until initiation of the next mitosis. F-actin patches and secretory vesicles are concentrated at the growing cell ends during interphase and at the septation site during cell division, and secretion of new cell wall materials occurs at these sites (Kanbe et al., 1989). F-actin cables and microtubules are oriented along the long axis of the cell independently from each other (Kanbe et al., 1989; Arai et al., 1998). F-actin is essential for polarized growth and cytokinesis and may serve as tracks for the polarized transport of secretory vesicles to the growth site (Kanbe et al., 1989, 1993). Unlike the situation in budding yeast (Huffaker et al., 1988; Jacobs et al., 1998), microtubules are involved in maintaining the direction of polarized cell growth, although they are not involved in the growth itself (Toda et al., 1983; Umesono et al., 1983; Hiraoka et al., 1984; Radcliffe et al., 1998; Sawin and Nurse, 1998).
In this report, we characterize two fission yeast myosin-V heavy chains, Myo4 and Myo5. We conclude that Myo4 is involved in polarized cell growth through movements around the growth sites that are dependent on F-actin cables and its motor activity but not on microtubules. Myo5 appears to have a function distinct from that of Myo4.
MATERIALS AND METHODS
Strains, Genetic Techniques, and Chemicals
The Schizosaccharomyces pombe strains used in this study are listed in Table 1. Media used were those described previously (Moreno et al., 1991). Standard procedures for S. pombe genetics were used (Moreno et al., 1991; Alfa et al., 1993). Latrunculin-A (Lat-A) and methyl benzimidazol-2-yl-carbamate (MBC) were purchased from Wako Pure Chemicals (Osaka, Japan). Thiabendazole (TBZ) was purchased from Sigma Chemical (St. Louis, MO). Thiamine was used at 5 μM to repress expression from the nmt1 promoter (Maundrell, 1989, 1993).
Table 1.
Strains used in this study
| Strain | Genotype | Reference/Source |
|---|---|---|
| JY1 | h− wild type | Lab stock |
| JY741 | h− ade6-M216 leu1-32 ura4-D18 | Lab stock |
| JY746 | h+ ade6-M210 leu1-32 ura4-D18 | Lab stock |
| JY747 | h+/h− ade6-M216ura4-D18/ade6-M210 leu1-32/leu1-32 ura4-D18/ura4-D18 | JY741 × JY746 |
| JY333 | h− ade6-M216 leu1-32 | Lab stock |
| PN1419 | h− cdc25-22 leu1-32 | Russell and Nurse (1986) |
| MBcdc4 | h− leu1-32 ura4-D18 cdc4-8 | Nurse et al. (1976) |
| MBcdc8 | h− ade6-M210 leu1-32 ura4-D18 cdc8-110 | Nurse et al. (1976) |
| VScdc7 | h− ura4-D18 cdc7-24 | Nurse et al. (1976) |
| VScdc11 | h− ura4-D18 cdc11-136 | Nurse et al. (1976) |
| VScdc12 | h− ura4-D18 cdc12-112 | Nurse et al. (1976) |
| VScdc14 | h− cdc14-118 | Nurse et al. (1976) |
| VScdc15 | h− ura4-D18 cdc15-140 | Nurse et al. (1976) |
| VScdc16 | h− cdc16-116 | Minet et al. (1979) |
| KM311-110 | h− leu1-32 nda3-KM311 | Umesono et al. (1983) |
| JIcps8 | h− leu1-32 cp18-8 | Ishiguro and Kobayashi (1996) |
| MFP2-4A | h− ade6-M216 leu1-32 ura4-D18 cam1-E14 | Moser et al. (1997) |
| MBY151 | h− ade6-M216 leu1-32 ura4-D18 myo2-E1 | Balasubramanian et al. (1998) |
| FM202* | h− ade6-M216 leu1-32 ura4-D18 myo2∷ura4+ | This study |
| FM301 | h− ade6-M216 leu1-32 ura4-D18 myo3∷ura4+ | Motegi et al. (1997) |
| FM400 | h+ ade6-M216 leu1-32 ura4-D18 myo4∷ura4+ | This study |
| FM401 | h− ade6-M216 leu1-32 ura4-D18 myo4∷ura4+ | This study |
| FM402 | h− leu1-32 ura4-D18 myo4∷ura4+ cdc25-22 | This study |
| FM241* | h− ade6-M216 leu1-32 ura4-D18 myo2∷ura4+ myo4∷ura4+ | This study |
| FM501 | h− ade6-M216 leu1-32 ura4-D18 myo5∷ura4+ | This study |
| FM451 | h− ade6-M216 leu1-32 ura4-D18 myo4∷ura4+ myo5∷ade2+ | This study |
Strains FM202 and FM241 were kept as transformants. Other strains such as various double and triple mutants were constructed by crosses among the strains listed here.
DNA Manipulations and Cloning of myo4+ and myo5+
Standard methods of DNA manipulation were used (Sambrook et al., 1989). A 2.2-kb EcoRV fragment containing a C-terminal region of S. cerevisiae MYO2 (a gift from Dr. Gerald C. Johnston, Dalhousie University) was used as a probe to screen the S. pombe SpeI genomic DNA library (Nakano et al., 1997), in which genomic DNA fragments from strain JY1 were inserted into the SpeI site of the ZAPII vector (Stratagene, La Jolla, CA). Hybridization and washing of filters were performed at 50°C. After plaque purification, the insert from a positive clone was subcloned into pBluescript II SK− by in vivo excision (according to the protocol provided by Stratagene) and sequenced. A 6.0-kb SpeI fragment proved to contain a novel myosin-V heavy chain gene, designated myo4+. The genomic sequence of myo4+ appeared to contain two introns spanning nucleotides 30–90 and 197–272, respectively.
For expression of myo4+, we used vectors carrying the thiamine-repressible nmt1 promoter (Maundrell, 1989, 1993). pREP1 carries the normal nmt1 promoter, whereas pREP81 contains a mutated promoter with 1% of the normal activity. Nucleotides spanning from −3 to 3 of the gene (GTCATG) were replaced by the NdeI target sequence (CATATG) according to a standard protocol for site-directed mutagenesis, and the complete myo4+-coding region downstream of this NdeI site was cloned into the NdeI site of pREP1 and pREP81. To express a Myo4-YFP fusion protein, NdeI and SalI sites were created at the 5′- and 3′-ends of myo4+, respectively, with removal of the stop codon, and the myo4+-coding region was cloned into NdeI- and SalI- digested pREP81. Sequences encoding YFP (Clontech, Palo Alto, CA), at the 5′-end of which a SalI site has been introduced, were cloned into the above plasmid after digestion with SalI and SmaI, yielding plasmid pREP81-myo4YFP. The linkage sequence between the 3′-end of myo4+ and the 5′-end of YFP sequence was GTCGACCAT. To mutate the putative ATP-binding site of Myo4, the nucleotides (GGAGCAGGAAAAACT) encoding amino acids 170–174 (GAGKT) were replaced by two rounds of polymerase chain reaction (PCR) with nucleotides (GCAGCTGCAGCTGCA) encoding amino acids AAAAA. The myo4 sequence between the NdeI and BamHI sites in pREP81-myo4-YFP was then replaced with the mutagenized PCR fragment.
A related gene, designated myo5+, was revealed by the S. pombe genome-sequencing project (http://www.sanger.ac.uk). The genomic sequence of myo5+ appeared to contain two introns spanning nucleotides 30–86 and 4006–4046, respectively. To express Myo5 with the hemagglutinin (HA) epitope fused to its N terminus, an NdeI site was introduced at the initiation codon of a myo5+ cDNA by PCR amplification using an S. pombe cDNA library (Clontech) as a template. The myo5+-coding region was then cloned into the NdeI site of pHA1, an HA-tagging vector that carries the normal nmt1 promoter and one copy of HA, yielding plasmid pHA1-myo5.
Gene Disruption
To disrupt myo4+, a 3.5-kb HincII fragment including the N-terminal 60% of the myo4 open reading frame (ORF; Figure 2A) was replaced by an S. pombe ura4+ cassette. After linearization by digestion with SpeI, this DNA fragment carrying the myo4::ura4+ allele was transformed into diploid strain JY747. Stable Ura+ transformants were selected and analyzed by Southern blotting to verify the proper replacement of one of the chromosomal myo4+ alleles by the myo4::ura4+ allele. A transformed diploid was sporulated and subjected to random spore analysis. To disrupt myo5+, the complete myo5+ ORF was amplified by PCR, using S. pombe genomic DNA as a template, and cloned into pBluescript II SK−. Its 1.5-kb HincII fragment was replaced by an S. pombe ura4+ or ade2+ cassette (Figure 9A). After linearization by digestion with PstI and BgllI, this DNA was transformed into diploid strain JY747, and transformants were analyzed as just described for myo4.
Figure 2.
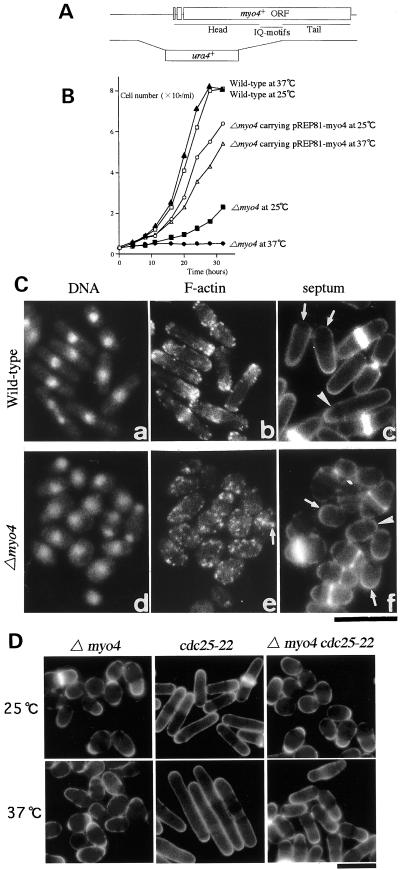
The phenotype of Δmyo4 cells. (A) Map of the genomic myo4 locus. Top open bars indicate the extent of the myo4 ORF; note the two predicted introns. Areas corresponding to the head, neck, and tail regions of Myo4 are indicated. The disruption construct used to generate the chromosomal Δmyo4 allele is shown schematically at the bottom. (B) Growth profiles of wild-type cells (strain JY333) at 25°C (□) and 37°C (▴), of Δmyo4 cells (strain FM401) at 25°C (▪) and 37°C (●), and of Δmyo4 cells (strain FM401) carrying plasmid pREP81-myo4 at 25°C (○) and 37°C (▵). Cultures grown to midlog phase at 25°C were split into two. After reinoculating to 106 cells/ml, one subculture was maintained at 25°C, and the other was shifted to 37°C. At each time point, cell number was counted in a hemacytometer. (C) Morphologies of wild-type (a–c) and Δmyo4 (d–f) cells. Cells of strains JY333 and FM401 were grown to midlog phase at 25°C and then stained with DAPI (a and d) and Bodipy-phallacidin (b and e) or with Calcofluor (c and f). The arrow in e indicates an F-actin ring; the arrows in c and f indicate cells with one growing end; and the arrowheads in c and f indicate cells with two growing ends. Bar, 10 μm. (D) Reduced efficiency of polarized growth at cell ends in the absence of Myo4. Cultures of Δmyo4 cells (strain FM401), cdc25-22 cells (strain PN1419), and Δmyo4 cdc25-22 cells (strain FM402) were grown to midlog phase and split into two. One-half was maintained at 25°C, and the other was shifted to 37°C. After 3 h, the cells were stained with Calcofluor. Bar, 10 μm.
Figure 9.
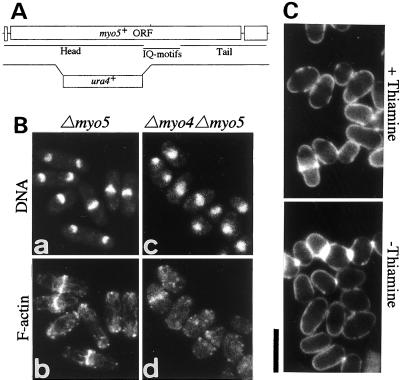
Characterization of Myo5 function. (A) Map of the genomic myo5 locus. Top open bars, the extent of the myo5 ORF; the head, neck, and tail regions of Myo5 are indicated. Bottom line and bar, scheme of the myo5 disruption. (B) The phenotypes of Δmyo5 strain FM501 (a and b) and Δmyo4 Δmyo5 strain FM451 (c and d). Cells were grown at 25°C to midlog phase and then stained simultaneously with DAPI (a and c) and Bodipy-phallacidin (b and d). (C) Effect of overexpression of HA-Myo5 on Δmyo4 cells. Δmyo4 strain FM401 carrying pHA1-myo5 was grown in medium containing thiamine (top) and then transferred to medium without thiamine for 48 h (bottom). Cells were then stained with Calcofluor. Bar, 10 μm.
Preparation of Antibodies and Immunoblotting
A 1.3-kb EcoRI fragment encoding the tail region of Myo4 was expressed as a fusion protein with glutathione S-transferase in Escherichia coli using vector pGEX4T-3 (Amersham Pharmacia Biotech, Uppsala, Sweden). This fusion protein was purified on glutathione-Sepharose 4B (Amersham Pharmacia Biotech) and used as an antigen to raise antibodies in a male rabbit. Antibodies were purified from the antisera using an affinity column in which the antigen was coupled to CNBr-activated Sepharose 4B (Amersham Pharmacia Biotech).
For immunoblotting, total cell extracts of S. pombe cells were subjected to SDS-PAGE and then transferred onto a polyvinylidene difluoride membrane (Millipore, Bedford, MA). This membrane was then incubated with antibodies against Myo4, Myo2 (Motegi et al., 2000), or HA (Boehringer Mannheim, Mannheim, Germany), followed by detection using secondary antibodies coupled to peroxidase.
Microscopy
Electron microscopy and immunofluorescence staining of S. pombe cells were carried out as described by Arai et al. (1998). Affinity-purified antibodies against Myo4 (see above) or green fluorescence protein (GFP; Molecular Probes, Eugene, OR) or monoclonal antibodies against α-tubulin (Sigma) were used as primary antibodies. Secondary antibodies were rhodamine- or Bodipy-conjugated goat immunoglobulin (IgG) against rabbit IgG or Bodipy-conjugated goat IgG against mouse IgG (Molecular Probes). The cells were finally stained with 4′,6-diamidino-2-phenylindole (DAPI) and Bodipy-phallacidin or rhodamine-phalloidin (Molecular Probes).
For time-lapse microscopy of living cells, cells that had been growing on plates were suspended in liquid medium. The cell suspension (1 μl) was then placed on a coverslip and sealed with Valap (vaseline/lanolin/paraffin, 1:1:1). Observations were performed at 23–24°C.
Fluorescence images were recorded using two approaches. Conventional two-dimensional images were obtained using T-Max ASA 400 film (Kodak, Rochester, NY) and a Zeiss Axioskop fluorescence microscope with a Plan Apochromat 63× lens (Carl Zeiss, Oberkochen, Jena, Germany). Three-dimensional images were obtained using a Delta Vision system (Applied Precision, Issaquah, WA) attached to an Olympus IX-70-SIF microscope with a UPlanApo100× oil lens (Olympus, Tokyo, Japan). Images were captured with a cooled charge-coupled device camera (Photometrics, Munich, Germany). Thirty 0.2-μm-thick optical sections from top to bottom of a single S. pombe cell were recorded, and out-of-focus light was removed by iterative deconvolution using a Silicon Graphics (Mountain View, CA) IRIX work station. Three-dimensional images were constructed from the deconvoluted two-dimensional images.
RESULTS
Identification of S. pombe Myosin-V Heavy Chain Genes
Screening of an S. pombe genomic library using the S. cerevisiae MYO2 gene as a probe (see MATERIALS AND METHODS) yielded 11 positive clones. All clones contained a 6-kb insert whose nucleotide sequence was similar to those of genes encoding myosin heavy chains but distinct from those of the known S. pombe myosin-II heavy chain genes myo2+ (Kitayama et al., 1997; May et al., 1997) and myp2+/myo3+ (Bezanilla et al., 1997; Motegi et al., 1997). We designated this gene myo4+. myo4+ encodes a protein of 1516 amino acids and a calculated molecular mass of 175 kDa. The N-terminal 820-amino acid region of Myo4 showed high similarity to the head domains of other myosins, including a putative ATP-binding motif (Figure 1). Over its full sequence, Myo4 is most similar to S. cerevisiae Myo2p (38% identity), S. cerevisiae Myo4p (37%), and human Myo5A (37%), all of which belong to the myosin-V heavy chain family. Furthermore, Myo4 shows several structural motifs that are characteristic of myosin-V heavy chains. First, the neck domain of Myo4 contains six 23- to 25-amino acid repeats referred to as IQ motifs (Figure 1), which are potent binding sites for calmodulin and other light chains (Mooseker and Cheney, 1995). Second, the N-terminal half of the tail domain contains several segments predicted to form α-helical coiled-coil structures.
Figure 1.
The deduced amino acid sequence of Myo4. Numbering of the amino acid residues is given on the right. The putative ATP-binding site is shown in italics, the putative IQ motifs are boxed, and the putative coiled-coil regions are underlined. The nucleotide sequence data are in the DDBJ, EMBL, and GenBank nucleotide sequence database under accession number SPCC1919.10c.
In addition, a search of the S. pombe Genome Database for sequences similar to that of S. cerevisiae Myo2p revealed a second ORF encoding a myosin-V heavy chain-like protein. We designated this gene myo5+. It encodes a protein of 1471 amino acids and a predicted molecular mass of 168 kDa. Myo5 also possessed several domains characteristic of myosin-V heavy chains, namely, an N-terminal motor domain, six IQ repeats, and predicted coiled-coil regions in the C-terminal tail domain. Over its full sequence, Myo5 is most similar to S. pombe Myo4 (35% identity), S. cerevisiae Myo4p (34%), and S. cerevisiae Myo2p (33%). Its head and tail domains are 47 and 20% identical, respectively, to those of S. pombe Myo4.
myo4 Null Cells Have a Defect in Polarized Growth
myo4+ was disrupted as described in MATERIALS AND METHODS (Figure 2A). This revealed that myo4+ is not essential for viability. However, the Δmyo4 cells grew slowly at 25°C in liquid medium and their growth was severely inhibited at 37°C (Figure 2B). The Δmyo4 cells even lost viability at 37°C: about one-half of the cells incubated at 37°C for 6 h could not form colonies when shifted down to 25°C (Motegi and Mabuchi, unpublished result). The growth and morphology (see below) defects of the Δmyo4 cells could be rescued by expression of full-length myo4+ from pREP81-myo4 (Figure 2B), whereas truncated versions of myo4+ that lacked the head or tail domain sequences could not rescue these defects (Motegi and Mabuchi, unpublished result). Thus, the defects are due to the loss of Myo4 function.
At 25°C, the Δmyo4 cells were nearly round (Figure 2C, e and f); they averaged 5.1 μm in length and 3.5 μm in diameter during mitosis, whereas wild-type cells averaged 10.1 μm in length and 3.1 μm in diameter (Figure 2C, b and c). This suggests that elongation of the cells at their ends during interphase is inefficient in Δmyo4 cells. Consistent with this conclusion, the F-actin patches were dispersed throughout the cell (Figure 2Ce). F-actin rings were seen between segregating nuclei during mitosis (Figure 2Ce); as expected (Kitayama et al., 1997), these rings were shown to contain Myo2 by immunofluorescence microscopy (Motegi and Mabuchi, unpublished result).
In S. pombe, primary septa and growing cell ends where new cell wall deposition is occurring stain brightly with Calcofluor, whereas nongrowing areas are not stained. In the Δmyo4 cells, primary septa were seen at the cell middles, and cells with one or two Calcofluor-stained ends were both seen (Figure 2Cf). Thus, despite the inefficient growth of the cell ends and the lack of F-actin patch polarity, the growth zones appear to be polarized in the Δmyo4 cells as they are in wild-type cells.
To explore this issue further, we examined the effect of myo4 deletion on cell growth in a cdc25-22 strain, in which the cell cycle is arrested in G2 with continuing bipolar growth (Russell and Nurse, 1986). Cells of the two single mutants and the double mutant were cultured at 25°C and then transferred to 37°C. For 3 h after the shift, the growth rate of the Δmyo4 cells was similar to that at 25°C, and they showed no significant change in cell shape (Figure 2D). At 25°C, both the growth rate and morphology of the Δmyo4 cdc25-22 cells were similar to those of the Δmyo4 cells (Figure 2D). Within 3 h after the shift, the cdc25-22 cells elongated from an average of 10.9 μm to an average of 24.2 μm (Figure 2D). The Δmyo4 cdc25-22 cells also became cylindrical after 3 h at 37°C but were shorter than the cdc25-22 cells; the double-mutant cells elongated from an average of 6.2 μm to an average of 8.6 μm (Figure 2D). A similar result was obtained with a Δmyo4 cdc11-119 double mutant (Motegi and Mabuchi, unpublished result). These results support the conclusion that Myo4 is not involved in establishment of the polarized sites but is required for efficient growth at the cell ends.
Apparent Accumulation of Secretory Vesicles in the myo4 Mutant
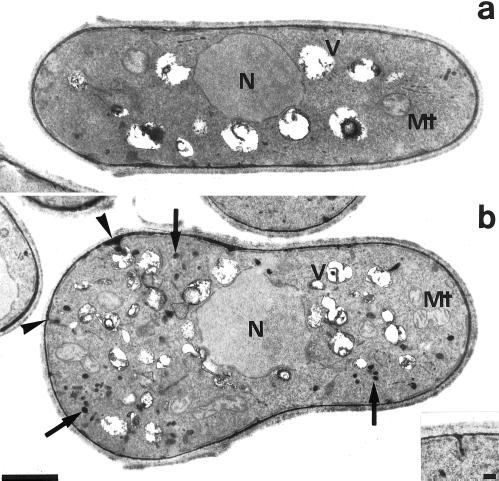
Because the sites of polarization were established in the Δmyo4 cells, their defect in polarized growth might be due to an impairment in transport or polarized secretion of materials necessary for cell elongation. Thus, the Δmyo4 cells were examined by thin-section electron microscopy. Many electron-dense vesicles of ∼0.1 μm diameter were seen throughout the cytoplasm (Figure 3b), whereas wild-type cells contained few such vesicles (Figure 3a). These vesicles were occasionally continuous with the extracellular electron-dense layer, suggesting that they contain extracellular materials. Thus, the delivery of secretory vesicles to the sites of polarized growth may be impaired in the Δmyo4 cells. Another feature of the Δmyo4 cells is that vacuoles were smaller and more numerous than in wild-type cells (Figure 3, a and b).
Figure 3.
Electron microscopic images of a wild-type cell (a; strain JY333) and a Δmyo4 cell (b; strain FM401). Both strains were grown at 25°C in YEPD liquid medium. Arrows, electron-dense granules; arrowheads, granules continuous with the extracellular electron-dense layer. N, nucleus. Mt, mitochondrion. V, vacuole. Bar, 1 μm. Inset, an enlarged view of a part of a Δmyo4 cell showing continuity of a granule with the extracellular layer. Bar, 0.1 μm.
Delayed Septation in Δmyo4 Cells
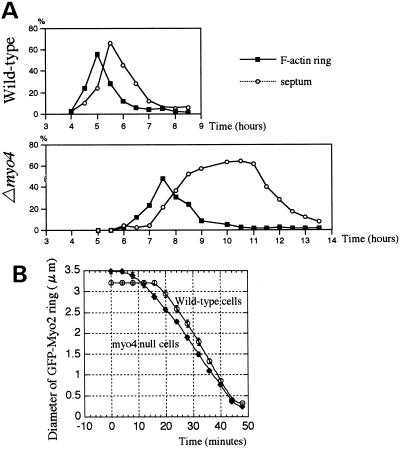
We also investigated the possible role of Myo4 in cell division, when polarized cell-surface growth occurs at the middle of the cell. Cells that had been synchronized in G1 by nitrogen starvation were examined at intervals after refeeding to determine the proportions of cells having an F-actin ring or forming a septum. The formation of the F-actin ring was delayed in the Δmyo4 cells relative to wild-type cells, although the eventual kinetics of ring formation and contraction were similar (Figure 4A). The proportion of septating cells increased after F-actin ring formation in a similar manner in the wild-type and Δmyo4 cells. However, the proportion of septating Δmyo4 cells remained high for several hours, in contrast to its rapid decrease in wild-type cells (Figure 4A). These results suggest that the completion of septation or of cell separation is inefficient in the absence of Myo4.
Figure 4.
Cell division in Δmyo4 mutant. (A) Kinetics of F-actin ring formation and septation in Δmyo4 (strain FM401) and wild-type (strain JY333) cells that had been grown in medium without nitrogen for 24 h and then transferred to medium containing nitrogen. The cells were then grown at 25°C and stained at 0.5-h intervals with Bodipy-phallacidin or Calcofluor to determine the percentages of cells having an F-actin ring or a septum. (B) Kinetics of contraction of GFP-Myo2 rings in Δmyo2 (strain FM202) or Δmyo2 Δmyo4 (strain FM241) cells expressing GFP-Myo2. Ring diameters were measured at 4-min intervals during cytokinesis in 23 wild-type cells and 21 Δmyo4 cells. The averaged values are plotted.
To investigate further whether Myo4 functions in the contraction of the F-actin ring, the speed of contraction of GFP-Myo2 rings in living Δmyo4 cells was analyzed by time-lapse microscopy. The reduction in the diameter versus time was linear and had an average speed of 0.29 μm/min, compared with an average of 0.33 μm/min in wild-type cells (Figure 4B). Thus, Myo4 does not appear to be significantly involved in the contraction of the F-actin ring.
Localization of Myo4 to the Growth Sites and the F-Actin Ring
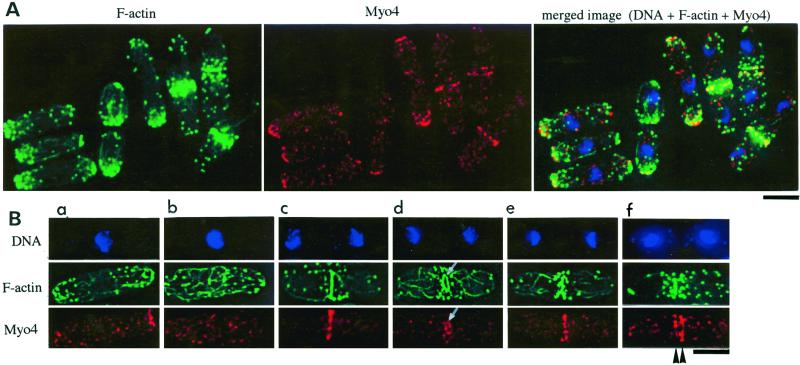
The intracellular localization of Myo4 was investigated by immunofluorescence microscopy. The antibodies against Myo4 did not show specific staining in the Δmyo4 cells, and preimmune serum showed no specific staining in wild-type cells (Motegi and Mabuchi, unpublished result). In interphase wild-type cells, Myo4 was detected as dot structures located mainly at the growing cell ends, where F-actin patches were also concentrated (Figure 5, A and Ba). However, the Myo4 dots and the F-actin patches did not in general coincide. In early mitotic cells, the F-actin cables accumulated around the middle of the cell, and the Myo4 dots were scattered throughout the cell and not concentrated at the cell ends (Figure 5Bb). In anaphase cells, the Myo4 dots formed a discontinuous ring that colocalized approximately with the F-actin ring (Figure 5, A and Bc), and this relationship seemed to be maintained during cytokinetic contraction of the F-actin ring (Figure 5Bd). As septation proceeded, the Myo4 dots seemed to be aligned again at the division plane (Figure 5Be), and, at a still later stage, the dots at the division plane split into two groups (Figure 5Bf). Throughout the cell cycle, Myo4 was also detected as small dots sparsely distributed throughout the cell (Figure 5, A and B); these dots did not colocalize with F-actin patches.
Figure 5.
Localization of Myo4 and F-actin in wild-type cells. Cells of strain JY1 were stained simultaneously with DAPI (blue), Bodipy-phallacidin (green), and antibodies against Myo4 (red). (A) Three-dimensional images reconstructed from deconvoluted sectional images. (B) Deconvoluted sectional images focused near the surface of cells at various cell-cycle stages, as judged from the distribution of F-actin (R. Arai and I. Mabuchi, unpublished data). a, interphase; b, prophase; c, anaphase; d, cytokinesis; e, after cytokinesis; f, late septation. Arrows, a contracting F-actin ring and the corresponding localization of Myo4. This F-actin ring is contained in the single section because of compression of the cell by a coverslip. Arrowheads, Myo4 accumulation at new cell ends. Bar, 3.3 μm.
Maintenance and Relocalization of Myo4 at the Growth Sites Require F-Actin Cables but Not Microtubules
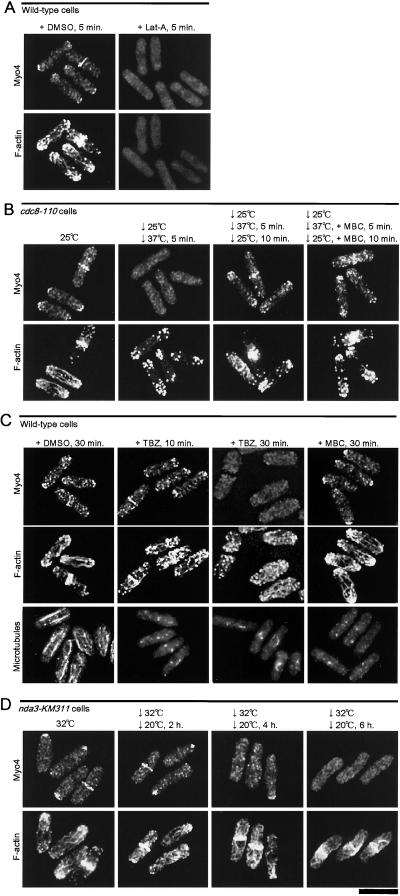
We investigated whether F-actin is involved in the localization of Myo4 using Lat-A, which quickly depolymerizes F-actin. When wild-type cells were treated for 5 min with Lat-A in DMSO, no visible F-actin staining was seen. In these cells, localization of Myo4 both to the growing cell ends during interphase and to the medial regions during mitosis was abolished (Figure 6A). In contrast, cells treated with DMSO alone showed normal F-actin and Myo4 localization (Figure 6A). Lat-A did not affect the expression level of Myo4 (Motegi and Mabuchi, unpublished result).
Figure 6.
Localization of Myo4 during loss of actin cytoskeleton or of microtubules. (A) Effect of Lat-A on the localization of Myo4. A culture of wild-type cells (strain JY1) was grown at 30°C and split into two. DMSO (final concentration, 0.1%) was added to one-half, and Lat-A in DMSO (final concentrations, 10 μM Lat-A and 0.1% DMSO) was added to the other. After 5 min, the cells were fixed and stained simultaneously for Myo4 (top) and F-actin (bottom). (B) Kinetics of delocalization and relocalization of Myo4 in cdc8-110 cells. A culture of strain MBcdc8 was grown at 25°C and split into two. One-half was transferred to 37°C for 5 min and then returned to 25°C for 10 min. MBC (final concentration, 30 μM) was added to the other half, which was then transferred to 37°C for 5 min and then returned to 25°C for 10 min. The cells were fixed and stained simultaneously for Myo4 (top) and F-actin (bottom). (C) Effect of TBZ or MBC on the localization of Myo4. Strain JY1 was grown at 30°C and incubated with DMSO (1%) for 30 min, 0.1 mM TBZ (and 1% DMSO) for 10 or 30 min, or 30 μM MBC (and 1% DMSO) for 30 min. The cells were then fixed and stained simultaneously for Myo4 (top) and F-actin (middle) or stained for microtubules (bottom). (D) Localization of Myo4 in nda3-KM311 cells. A culture of strain KM311-110 was grown at 30°C, transferred to 20°C, and sampled 0, 2, 4, and 6 h after the shift. The cells were fixed and stained simultaneously for Myo4 (top) and F-actin (bottom). Bar, 10 μm.
The localization of Myo4 was also investigated in two temperature-sensitive mutants that have defects in F-actin organization. In a cdc8-110 tropomyosin mutant, cells grown at 25°C showed localized F-actin patches at their ends, longitudinal F-actin cables, and mitotic F-actin rings, as in wild-type cells (Figure 6B). However, F-actin cables were not detectable in these cells by 5 min after a shift to 36°C; at this time, the distribution of the F-actin patches appeared unchanged, whereas Myo4 was found to be delocalized in interphase (Figure 6B) and mitotic (Motegi, Arai, and Mabuchi, unpublished result) cells. The F-actin patches gradually also became delocalized during the next 10 min (Motegi and Mabuchi, unpublished result). When the cells were returned to 25°C, the F-actin cables were reformed and Myo4 also became relocalized within 10 min (Figure 6B). In a cdc4-8 myosin-light-chain mutant, F-actin organization is normal during interphase but the F-actin ring cannot form during mitosis (McCollum et al., 1995). Correspondingly, Myo4 localization to the growing cell ends during interphase was not affected by this mutation, but its localization to the medial region of mitotic cells was abolished (Motegi and Mabuchi, unpublished result).
We next investigated whether microtubules are necessary for the polarized localization of Myo4. During a 10-min treatment of wild-type cells with the tubulin-binding drug TBZ, cytoplasmic microtubules became very short, but the localizations of Myo4, F-actin cables, and F-actin patches remained almost normal (Figure 6C). In contrast, after 30 min of TBZ treatment, the F-actin patches were scattered in the cell and the cables seemed to lose their directionality, and Myo4 was delocalized (Figure 6C). To test whether this delocalization of Myo4 was due to the alteration of the actin cytoskeleton, we also examined the effects of MBC, another anti-tubulin drug. During a 30-min treatment with MBC, the cytoplasmic microtubules disappeared, but Myo4, F-actin cables, and F-actin patches all remained localized (Figure 6C). Neither TBZ nor MBC affected the expression level of Myo4 (Motegi and Mabuchi, unpublished result).
We also investigated whether microtubules are involved in the relocalization of Myo4 in cdc8-110 cells after the temperature shifts. After 5 min at 36°C in the presence of MBC, both F-actin cables and cytoplasmic microtubules had disappeared, and Myo4 had become delocalized (Motegi and Mabuchi, unpublished result). When the cells were returned to 25°C and incubated for an additional 10 min in the presence of MBC, the F-actin cables reappeared and Myo4 was relocalized (Figure 6B), despite the continued absence of microtubules.
The localization of Myo4 was also examined in the cold-sensitive β-tubulin mutant nda3-KM311 (Hiraoka et al., 1984). After 2 h at 20°C, the restrictive temperature, cytoplasmic microtubules had almost disappeared (Motegi and Mabuchi, unpublished result), but the actin cytoskeleton and Myo4 localized normally (Figure 6D). After 4–6 h, most cells were arrested in mitosis and showed the F-actin ring at the medial cortex. However, Myo4 was delocalized in these cells (Figure 6D). When the arrested cells were returned to 32°C, the cells underwent mitosis and cytokinesis. Myo4 was delocalized during these processes but then relocalized to the septation site as septation proceeded (Motegi and Mabuchi, unpublished result).
Dynamic Properties of Myo4-YFP around the Growth Sites
To investigate the dynamics of Myo4 during the cell cycle, we expressed a YFP-tagged Myo4 in living cells. The tagged protein appeared to be functional: its expression level in Δmyo4 cells was similar to that of endogenous Myo4 in wild-type cells (Figure 7A), and its expression rescued both the rate of growth (Figure 8B) and the morphology of the Δmyo4 cells (Figure 7). Moreover, the localization of Myo4-YFP in Δmyo4 cells was similar to that of Myo4 in wild-type cells as seen by immunofluorescence microscopy (Figures 5B and 7). Thus, the localization of Myo4-YFP should represent the normal localization of Myo4.
Figure 7.
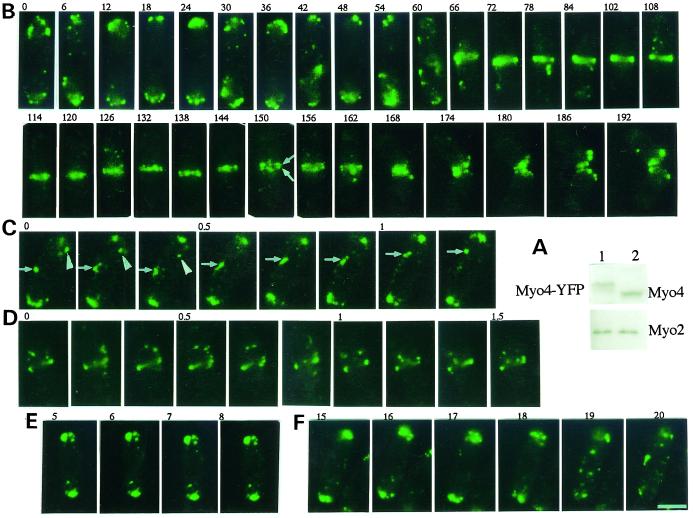
In vivo dynamics of Myo4-YFP. (A) Immunoblot comparing the expression levels of Myo4-YFP in Δmyo4 strain FM401 carrying pREP81-myo4YFP (lane 1) and of Myo4 in wild-type strain JY333 (lane 2). Both strains were grown in a medium without thiamine, and extracts were analyzed by SDS-PAGE followed by immunoblotting using antibodies against Myo4 (top) and Myo2 (bottom). (B) Localization of Myo4-YFP in strain FM401 carrying pREP81-myo4YFP from G2 to cell division at 23–24°C. Time-lapse images were obtained in a fixed focal plane close to the center of the cell at 6-min intervals. Arrows indicate the splitting of the cluster of Myo4-YFP dots into two groups on opposite sides of the septum. (C and D) Rapid movements of Myo4-YFP dots in interphase (C) and mitotic (D) cells. Images were obtained at 10-s intervals with focus at the cell cortex. Arrowheads, a short-range movement; arrows, a directed movement along the cell's long axis. (E and F) Movements of Myo4-YFP dots in cells treated with Lat-A (E) or TBZ (F). Images were obtained at 1-min intervals beginning 5 min after the Lat-A treatment (E) or 15 min after the TBZ treatment (F). In all panels, the numbers indicate the elapsed time in minutes. Bar, 3.3 μm.
Figure 8.
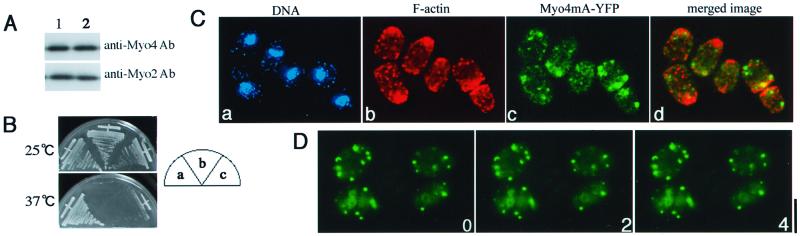
Effects of a mutation in the ATP-binding site of Myo4 on its function and localization. (A) Immunoblot comparing the expression levels of Myo4-YFP and Myo4mA-YFP in Δmyo4 strain FM401 carrying pREP81-myo4YFP (lane 1) or pREP81-myo4mAYFP (lane 2). Cells were grown and proteins were analyzed as described for Figure 7A. (B) Growth of Δmyo4 strain FM401 carrying plasmid pREP81-myo4YFP (a), pREP81 (b), or pREP81-myo4mAYFP (c). The cells were grown without thiamine at 25 or 37°C. (C) Localization of F-actin and Myo4mA-YFP in strain FM401 carrying plasmid pREP81-myo4mAYFP. Cells cultured in the absence of thiamine were stained simultaneously with DAPI (a), rhodamine-phalloidin (b), and antibodies against GFP (c). Three-dimensional reconstructed images are shown; d shows a merger of the F-actin and GFP images. (D) In vivo dynamics of Myo4mA-YFP. Strain FM401 expressing Myo4mA-YFP was cultured in the absence of thiamine and images were captured at 2-min intervals. Bar, 3.3 μm.
In one set of experiments, we collected images at 6-min intervals in a fixed focal plane. In G2 cells, most Myo4-YFP clustered as dots at the two cell ends (Figure 7B, 0–54 min). The distributions of the dots differed in every image, indicating that they were moving rapidly. Therefore, we monitored the movements by collecting images every 10 s. At least two discrete patterns of motility were observed in G2 cells: short-range movements within limited areas near the cell ends and seemingly directed long-range movements along the long axis of the cell (Figure 7C). The speed of the long-range movements was 0.5–0.7 μm/s (n = 15). During early mitosis, all dots at the cell ends began to translocate to the medial region and eventually formed a ring structure in the medial cortex (Figure 7B, 60–72 min). Some Myo4-YFP dots were seen to move rapidly around the ring (Figure 7D). The diameter of the Myo4-YFP ring then decreased as cytokinesis proceeded (Figure 7B, 108–120 min), and the Myo4-YFP dots concentrated at the septation site during septum formation (Figure 7B, 132 min). As septation proceeded, the cluster of Myo4-YFP dots split into two groups on opposite sides of the septum (Figure 7B, 150–162 min). During septation, the Myo4-YFP dots continued to move in the area near the septation site (Figure 7B, 132–168 min). During cell separation, the Myo4-YFP dots were localized to the new ends of the cells (Figure 7B, 156–192 min).
Disruption of F-actin by Lat-A appeared to immobilize the Myo4-YFP dots (Figure 7E). In contrast, although disruption of microtubules by TBZ gradually delocalized the dots, it did not seem to affect their rapid movement during the first 20 min after its application (Figure 7F).
A Myo4 Mutant in the ATP-binding Site Cannot Localize to the Growth Sites
To investigate whether Myo4 functions as an actin-based motor protein, we analyzed the function and localization of a mutant Myo4 (Myo4mA) in which the domain presumed to be responsible for ATP binding (amino acids 170-GAGKT-174) was replaced with alanines. Immunoblotting indicated that the expression level of Myo4mA-YFP in Δmyo4 cells transformed with pREP81-myo4mA-YFP was similar to that of Myo4-YFP in the Δmyo4 cells expressing Myo4-YFP (Figure 8A). However, the cells expressing Myo4mA-YFP showed temperature-sensitive growth (Figure 8B) and a round morphology during growth at 25°C (Figure 8C) like the Δmyo4 cells, whereas the Myo4-YFP–expressing cells could grow at 37°C (Figure 8B) and showed a normal morphology at 25°C (Figure 7). Myo4mA-YFP was not localized specifically to the ends of interphase cells (Figure 8C) and the medial region of mitotic cells (Motegi and Mabuchi, unpublished result); instead, it was dispersed randomly in the cytoplasm as dot structures of various size. In living cells, these dots did not move detectably (Figure 8D). These results suggest that both the function and the proper localization and dynamics of Myo4 depend on its motor activity.
Genetic Interactions of myo4+
To gain additional insights into the function of Myo4, we investigated possible genetic interactions between myo4+ and other genes that are involved in cytoskeletal function and/or septation. A Δmyo4 cdc8-110 double mutant was synthetically lethal even at 25°C. Double mutants containing Δmyo4 and cps8, cdc4-8, myo2-E1, cdc7-24, or cdc14-118 also grew very poorly at 25°C. The cells were aberrantly large and swollen, and a high percentage were dead. Double mutants containing Δmyo4 and cdc12-112, cdc15-140, nda3-KM311, cam1-E14, or Δmyo3 also grew slowly and showed a swollen morphology at 25°C. No particular phenotype was observed in double mutants containing Δmyo4 and either cdc11-136 or cdc16-116.
Myo5 Plays a Role Distinct from That of Myo4
myo5+ was disrupted as described in MATERIALS AND METHODS (Figure 9A). The Δmyo5 cells were viable, and their cell morphology, growth rate, and distribution of F-actin were indistinguishable from those of wild-type cells at all temperatures tested (Figure 9B, a and b). In addition, the phenotype of a Δmyo4 Δmyo5 double mutant was similar to that of the Δmyo4 single mutant (Figure 9B, c and d), suggesting that Myo4 and Myo5 do not overlap in function.
We also examined whether the phenotype of the Δmyo4 mutant could be suppressed by the overexpression of Myo5. Δmyo4 cells carrying pHA1-myo5 were grown in medium containing thiamine and transferred to medium without thiamine. The cell morphology 48 h after the transfer was similar to that before the transfer (Figure 10C). An increased expression of HA-Myo5 after the transfer was confirmed by immunoblotting using antibodies against HA (Motegi and Mabuchi, unpublished result). Thus, the function of Myo4 can apparently not be replaced by overexpression of Myo5. Moreover, when a Δmyo4 Δmyo5 cdc25-22 triple mutant was arrested in the bipolar growth phase by the cdc25-22 mutation, the cells became cylindrical as did the arrested Δmyo4 cdc25-22 double mutant (Motegi and Mabuchi, unpublished result), suggesting that Myo5 does not contribute to the polarized growth.
DISCUSSION
Involvement of Myo4 in Polarized Cell Growth and Septation
During interphase, Myo4 was localized as a cluster of dots near the growing cell ends, suggesting a role in polarized cell growth. Consistent with this hypothesis, Δmyo4 cells grew slowly and were rounder than normal, although they were not perfectly round like the cdc42 mutant that completely loses cell polarity (Miller and Johnson, 1994). In addition, the growth areas that are marked by Calcofluor staining seemed to be established in a polarized manner in Δmyo4 cells during interphase, and Δmyo4 cdc25-22 cells arrested in the bipolar growth phase could grow slowly in a polarized manner, so that the cell shape changed from round to cylindrical. However, elongation of the cell ends was less efficient in the absence of Myo4 function. Electron-dense vesicles that appear to be secretory vesicles increased abnormally in number throughout the cytoplasm of the Δmyo4 cells, suggesting that transport of vesicles to the growth sites is impaired. Taken together, the results suggest that Myo4 contributes to the growth at the cell ends by delivering secretory vesicles to those sites but does not play a role in determining the sites for cell growth.
Myo4 was also localized around the septation site during septum formation. Although the Δmyo4 cells formed septa at the middle of the cell and with the proper orientation, they took longer to complete septation than did wild-type cells. Moreover, myo4+ showed strong genetic interactions with cdc7+ and cdc14+, genes that are involved in septation (Nurse et al., 1976; Fankhauser and Simanis, 1993, 1994), and overexpression of Myo4 resulted in aberrant accumulation of septum materials in the cell and the appearance of cells with multiple septa (Motegi and Mabuchi, unpublished result). These results suggest that Myo4 is involved in septation and/or cell separation.
The Calcofluor stainability at the middle of Δmyo4 cells increased after F-actin ring formation in a manner similar to that seen in wild-type cells, but the increased stainability was maintained for a longer period in the mutant. This suggests that the primary septum is formed properly but that formation of the secondary septum and/or disintegration of the primary septum after secondary septum formation occurs less effectively in the mutant cells than in wild type. These defects likely reflect a role of Myo4 in transportation of secretory vesicles to the septation site.
It is not clear at present why Myo4 colocalizes with the F-actin ring, because it does not seem to be involved in either formation or contraction of the ring, which appears and contracts with similar kinetics in Δmyo4 cells and in wild-type cells. However, the fact that myo4+ showed strong genetic interactions with both myo2+ and cdc4+, which encode subunits of a type II myosin essential for the function of the F-actin ring, suggests that Myo4 may play some role in the function of this ring.
F-Actin Dependence of Myo4 Localization and Movement
During both interphase and mitosis, the Myo4 dots were located in the areas where F-actin patches were also concentrated. However, the Myo4 dots and F-actin patches did not coincide, indicating that Myo4 is not a component of the F-actin patch. Nonetheless, the localization of Myo4 was dependent on F-actin, because Myo4 was delocalized within 5 min by treatment with Lat-A. Moreover, when cdc8-110 tropomyosin mutant cells were shifted to the restrictive temperature, F-actin cables disappeared and Myo4 became delocalized within 5 min, whereas the F-actin patches remained polarized. Upon return to permissive temperature, both the F-actin cables and Myo4 localization were restored. Because tropomyosin is required to stabilize the F-actin cables in fission yeast (Arai et al., 1998), these data suggest that the F-actin cables play an important role in targeting Myo4 to the growth sites, as has been found also for Myo2p in S. cerevisiae (Pruyne et al., 1998).
The localization of Myo4 to the medial ring during mitosis was specifically abolished in the cdc4-8 mutant cells, which cannot form the F-actin ring. Thus, the localization of Myo4 to this region may be dependent on the F-actin ring.
Myo4-YFP dots were capable of moving rapidly around the growth sites. We could not estimate the speed of these movements, because we could only find short-range movements in the fixed focal plane. However, the speed of the directed movements of Myo4-YFP dots along the long axis of the cell could be estimated (0.5–0.7 μm/s) and was found to be somewhat faster than that of actin filament movement on purified chick brain myosin-V (0.33–0.38 μm/s, Cheney et al., 1993) or on vesicle-associated chick brain myosin-V (0.34 μm/s, Evans et al., 1998). The movement of Myo4-YFP dots was completely inhibited by treatment with Lat-A, and the motor activity of Myo4 seemed to be required for its proper localization and rapid movement. These results suggest that Myo4 uses its motor activity to walk along the F-actin cables and carry secretory vesicles to the growth sites. This is consistent with the “transport” model of myosin-V function (see INTRODUCTION). However, we cannot rule out the possibility that Myo4 uses its motor activity to target to an unknown structure, which then moves in the cell carrying Myo4 and Myo4-associated secretory vesicles.
It is interesting to speculate as to why the Myo4 dots move around the growth sites. Perhaps a Myo4–secretory vesicle complex builds up as it translocates to these areas and becomes large enough to be detected by fluorescence microscopy. The dynamic movement of a Myo4 dot may thus represent the emergence of an enlarged mass of this complex, its migration to the cell surface, and the subsequent exocytosis causing disappearance of the dot.
Microtubule Independence of Myo4 Localization during Cell Growth
Microtubules are not required to maintain Myo4 at the growth sites, as evidenced by the maintenance of Myo4 localization under several conditions that lead to a loss of cytoplasmic microtubules (see RESULTS). Moreover, both the F-actin cables and Myo4 localization were restored in the absence of intact microtubules when MBC-treated cdc8-110 cells were returned from restrictive to permissive temperature. However, Myo4 did become delocalized after 30 min of treatment with TBZ. Because F-actin organization was considerably altered by this time, the delocalization of Myo4 is probably secondary to the alteration of the F-actin organization.
Myo4 localization was also abolished in the tubulin mutant cells incubated at the restrictive temperature for 6 h. Under these conditions, the cells are arrested in mitosis without a mitotic spindle (Hiraoka et al., 1984). Even after the mitotic arrest was released, Myo4 continued to be delocalized during mitosis and the following cytokinesis. Both F-actin (Hiraoka et al., 1984; Chang et al., 1996) and Myo2 (Motegi et al., 2000) are accumulated at the medial cortex of the arrested cells, suggesting that the delocalization of Myo4 in these cells is not due to an alteration of the actin cytoskeleton. Thus, it is possible that the accumulation of Myo4 at the cell middle during mitosis may require microtubule function. Further work is required to clarify this issue.
Myo5 Has a Function Distinct from that of Myo4
Myo5 is a second myosin-V heavy chain that is similar in sequence to Myo4 in the motor domain and less similar in the tail. The Δmyo5 cells showed no defects in cell morphology, growth rate, or distribution of F-actin at any temperature tested, and several other observations also suggest that Myo5 has a function distinct from that of Myo4. First, a Δmyo4 Δmyo5 double mutant had no obvious synthetic defect, and a Δmyo4 Δmyo5 cdc25-22 mutant arrested in the bipolar growth phase showed a polarized cylindrical shape, suggesting that Myo5 does not contribute to the polarized cell growth in the absence of Myo4. Moreover, overexpression of Myo5 did not rescue the Δmyo4 mutant defects. However, the intracellular role of Myo5 is not known at present.
An interesting question is how polarized secretion occurs in the absence of both Myo4 and Myo5. Perhaps there is some other system, not involving myosin-V, or a third myosin-V heavy chain that can function in polarized secretion in S. pombe.
ACKNOWLEDGMENTS
We are grateful to Dr. Gerald C. Johnston (Dalhousie University) for the S. cerevisiae MYO2 gene and strains; to Dr. Susan S. Brown (University of Michigan Medical School, Ann Arbor, MI) for the S. cerevisiae MYO4 gene; to Drs. Trisha N. Davis and Mark R. Flory (University of Washington) for an S. pombe MFP2-4A strain; to Dr. D.-Qiao Ding (Kansai Advanced Research Center) for the TC58 clone of S. pombe cDNA; to an anonymous reviewer for several very helpful suggestions. We thank Drs. Jeremy S. Hyams, and Thein Z. Win (University College, London, UK) for discussion. We also thank Mrs. Michiko Nakajima for technical assistance and members of our laboratory for encouragement and many stimulating discussions. This work was supported by a Research Fellowship of the Japan Society for the Promotion of Science for Young Scientists to F.M. and a research grant from the Ministry of Education, Science and Culture of Japan (10213202) to I.M.
Note added in proof:
Recently a paper describing myosin-V heavy chains in S. pombe has appeared independently (Win, T.Z., Gachet, Y., Mulvihill, D.P., May, K.M., and Hyams, J.S. (2001). J. Cell Sci. 114, 69–70). Myo52 and Myo51 in their paper correspond to Myo4 and Myo5 in the present paper, respectively.
REFERENCES
- Adams AEM, Pringle JR. Relationship of actin and tubulin distribution to bud growth in wild-type and morphogenetic-mutant Saccharomyces cerevisiae. J Cell Biol. 1984;98:934–945. doi: 10.1083/jcb.98.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfa C, Fantes P, Hyams J, McLeod M, Warbrick E. Experiments with Fission Yeast: A Laboratory Course Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1993. pp. 28–34. [Google Scholar]
- Arai R, Nakano K, Mabuchi I. Subcellular localization and possible function of actin, tropomyosin and actin-related protein 3 (Arp3) in the fission yeast Schizosaccharomyces pombe. Eur J Cell Biol. 1998;76:288–295. doi: 10.1016/S0171-9335(98)80007-1. [DOI] [PubMed] [Google Scholar]
- Ayscough KR, Stryker J, Pokala N, Sanders M, Crews P, Drubin DG. High rates of actin filament turnover in budding yeast and roles for actin in establishment and maintenance of cell polarity revealed using the actin inhibitor Latrunculin-A. J Cell Biol. 1997;137:399–416. doi: 10.1083/jcb.137.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian MK, Helfman DM, Hemmingsen SM. A new tropomyosin essential for cytokinesis in the fission yeast S. pombe. Nature. 1992;360:84–87. doi: 10.1038/360084a0. [DOI] [PubMed] [Google Scholar]
- Balasubramanian MK, McCollum D, Chang L, Wong KC, Naqvi NI, He X, Sazer S, Gould KL. Isolation and characterization of new fission yeast cytokinesis mutants. Genetics. 1998;149:1265–1275. doi: 10.1093/genetics/149.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla M, Forsburg SL, Pollard TD. Identification of a second myosin-II in S. pombe: Myp2p is conditionally required for cytokinesis. Mol Biol Cell. 1997;8:2693–2705. doi: 10.1091/mbc.8.12.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobola N, Jansen RP, Shin TH, Nasmyth K. Asymmetric accumulation of Ash1p in postanaphase nuclei depends on a myosin and restricts yeast mating-type switching to mother cells. Cell. 1996;84:699–709. doi: 10.1016/s0092-8674(00)81048-x. [DOI] [PubMed] [Google Scholar]
- Bridgman PC. Myosin Va movements in normal and dilute-lethal axons provide support for a dual filament motor complex. J Cell Biol. 1999;146:1045–1060. doi: 10.1083/jcb.146.5.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockerhoff SE, Stevens RC, Davis TN. The unconventional myosin, Myo2p, is a calmodulin target at sites of cell growth in Saccharomyces cerevisiae. J Cell Biol. 1994;124:315–323. doi: 10.1083/jcb.124.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlett NL, Weisman LS. The terminal tail region of a yeast myosin-V mediates its attachment to vacuole membranes and sites of polarized growth. Proc Natl Acad Sci USA. 1998;95:14799–14804. doi: 10.1073/pnas.95.25.14799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang F, Woollard A, Nurse P. Isolation and characterization of fission yeast mutants defective in the assembly and placement of the contractile actin ring. J Cell Sci. 1996;109:131–142. doi: 10.1242/jcs.109.1.131. [DOI] [PubMed] [Google Scholar]
- Cheney RE, O'Shea MK, Heuser JE, Coelho MV, Wolenski JS, Espreafico EM, Forscher P, Larson RE, Mooseker MS. Brain myosin-V is a two-headed unconventional myosin with motor activity. Cell. 1993;75:13–23. doi: 10.1016/S0092-8674(05)80080-7. [DOI] [PubMed] [Google Scholar]
- Drubin DG, Nelson WJ. Origins of cell polarity. Cell. 1996;84:335–344. doi: 10.1016/s0092-8674(00)81278-7. [DOI] [PubMed] [Google Scholar]
- Evans LL, Lee AJ, Bridgman PC, Mooseker MS. Vesicle-associated brain myosin-V can be activated to catalyze actin-based transport. J Cell Sci. 1998;111:2055–2066. doi: 10.1242/jcs.111.14.2055. [DOI] [PubMed] [Google Scholar]
- Fankhauser C, Simanis V. The Schizosaccharomyces pombe cdc14 gene is required for septum formation and can also inhibit nuclear division. Mol Biol Cell. 1993;4:531–539. doi: 10.1091/mbc.4.5.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fankhauser C, Simanis V. The cdc7 protein kinase is a dosage-dependent regulator of septum formation in fission yeast. EMBO J. 1994;13:3011–3019. doi: 10.1002/j.1460-2075.1994.tb06600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindan B, Bowser R, Novick P. The role of Myo2, a yeast class V myosin, in vesicular transport. J Cell Biol. 1995;128:1055–1068. doi: 10.1083/jcb.128.6.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griscelli C, Durandy A, Guy-Grand D, Daguillard F, Herzog C, Prunieras M. A syndrome associating partial albinism and immunodeficiency. Am J Med. 1978;65:691–702. doi: 10.1016/0002-9343(78)90858-6. [DOI] [PubMed] [Google Scholar]
- Hiraoka Y, Toda T, Yanagida M. The NDA3 gene of fission yeast encodes β-tubulin: a cold sensitive nda3 mutation reversibly blocks spindle formation and chromosome movement in mitosis. Cell. 1984;39:349–358. doi: 10.1016/0092-8674(84)90013-8. [DOI] [PubMed] [Google Scholar]
- Huang JD, Brady ST, Richards BW, Stenoien D, Resau JH, Copeland NG, Jenkins NA. Direct interaction of microtubule- and actin-based transport motors. Nature. 1999;397:267–270. doi: 10.1038/16722. [DOI] [PubMed] [Google Scholar]
- Huffaker TC, Thomas JH, Botstein D. Diverse effects of beta-tubulin mutations on microtubule formation and function. J Cell Biol. 1988;106:1997–2010. doi: 10.1083/jcb.106.6.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro J, Kobayashi W. An actin point-mutation neighboring the 'hydrophobic plug' causes defects in the maintenance of cell polarity and septum organization in the fission yeast Schizosaccharomyces pombe. FEBS Lett. 1996;392:237–241. doi: 10.1016/0014-5793(96)00819-8. [DOI] [PubMed] [Google Scholar]
- Jacobs CW, Adams AEM, Szaniszlo PJ, Pringle JR. Functions of microtubules in the Saccharomyces cerevisiae cell cycle. J Cell Biol. 1998;107:1409–1426. doi: 10.1083/jcb.107.4.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen RP, Dowzer C, Michaelis C, Galova M, Nasmyth K. Mother cell-specific HO expression in budding yeast depends on the unconventional myosin Myo4p and other cytoplasmic proteins. Cell. 1996;84:687–697. doi: 10.1016/s0092-8674(00)81047-8. [DOI] [PubMed] [Google Scholar]
- Johnston GC, Prendergast JA, Singer RA. The Saccharomyces cerevisiae MYO2 gene encodes an essential myosin for vectorial transport of vesicles. J Cell Biol. 1991;113:539–551. doi: 10.1083/jcb.113.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanbe T, Akashi T, Tanaka K. Effect of cytochalasin A on actin distribution in the fission yeast Schizosaccharomyces pombe studied by fluorescent and electron microscopy. Protoplasma. 1993;176:24–32. [Google Scholar]
- Kanbe T, Kobayashi I, Tanaka K. Dynamics of cytoplasmic organelles in the cell cycle of the fission yeast S. pombe: three-dimensional reconstruction from serial sections. J Cell Sci. 1989;94:647–656. doi: 10.1242/jcs.94.4.647. [DOI] [PubMed] [Google Scholar]
- Karpova TS, Reck-Peterson SL, Elkind NB, Mooseker MS, Novick PJ, Cooper JA. Role of actin and Myo2p in polarized secretion and growth of Saccharomyces cerevisiae. Mol Biol Cell. 2000;11:1727–1737. doi: 10.1091/mbc.11.5.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller P, Simons K. Post-Golgi biosynthetic traffickers. J Cell Sci. 1997;110:3001–3009. doi: 10.1242/jcs.110.24.3001. [DOI] [PubMed] [Google Scholar]
- Kilmartin JV, Adams AEM. Structural rearrangements of tubulin and actin during the cell cycle of the yeast Saccharomyces. J Cell Biol. 1984;98:922–933. doi: 10.1083/jcb.98.3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama C, Sugimoto A, Yamamoto M. Type II myosin heavy chain encoded by the myo2 gene composes the contractile ring during cytokinesis in Schizosaccharomyces pombe. J Cell Biol. 1997;137:1309–1319. doi: 10.1083/jcb.137.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillie SH, Brown SS. Immunofluorescence localization of the unconventional myosin, Myo2p, and the putative kinesin-related protein, Smy1p, to the same regions of polarized growth in Saccharomyces cerevisiae. J Cell Biol. 1994;125:825–842. doi: 10.1083/jcb.125.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maundrell K. nmt1 of fission yeast: a highly transcribed gene completely repressed by thiamine. J Biol Chem. 1989;265:10857–10864. [PubMed] [Google Scholar]
- Maundrell K. Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene. 1993;123:127–130. doi: 10.1016/0378-1119(93)90551-d. [DOI] [PubMed] [Google Scholar]
- May KM, Watts FZ, Jones N, Hyams JS. Type II myosin involved in cytokinesis in the fission yeast Schizosaccharomyces pombe. Cell Motil Cytoskeleton. 1997;38:385–396. doi: 10.1002/(SICI)1097-0169(1997)38:4<385::AID-CM8>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- McCollum D, Balasubramanian MK, Pelcher LE, Hemmingsen SM, Gould KL. Schizosaccharomyces pombe cdc4+ gene encodes a novel EF-hand protein essential for cytokinesis. J Cell Biol. 1995;130:651–660. doi: 10.1083/jcb.130.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer JA, Seperake PK, Strobel MC, Copeland NG, Jenkins NA. Novel myosin heavy chain encoded by murine dilute coat color locus. Nature. 1991;349:709–713. doi: 10.1038/349709a0. [DOI] [PubMed] [Google Scholar]
- Miller PJ, Johnson DI. Cdc42p GTPase is involved in controlling polarized cell growth in Schizosaccharomyces pombe. Mol Cell Biol. 1994;14:1075–1083. doi: 10.1128/mcb.14.2.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minet M, Nurse P, Thuriaux P, Mitchison JM. Uncontrolled septation in a cell division cycle mutant of the fission yeast Schizosaccharomyces pombe. J Bacteriol. 1979;137:440–446. doi: 10.1128/jb.137.1.440-446.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooseker MS, Cheney RE. Unconventional myosins. Annu Rev Cell Dev Biol. 1995;11:633–675. doi: 10.1146/annurev.cb.11.110195.003221. [DOI] [PubMed] [Google Scholar]
- Moreno S, Klar A, Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Moser MJ, Flory MR, Davis TN. Calmodulin localizes to the spindle pole body of Schizosaccharomyces pombe and performs an essential function in chromosome segregation. J Cell Sci. 1997;110:1805–1812. doi: 10.1242/jcs.110.15.1805. [DOI] [PubMed] [Google Scholar]
- Motegi F, Nakano K, Kitayama C, Yamamoto M, Mabuchi I. Identification of Myo3, a second type-II myosin heavy chain in the fission yeast Schizosaccharomyces pombe. FEBS Lett. 1997;420:161–166. doi: 10.1016/s0014-5793(97)01510-x. [DOI] [PubMed] [Google Scholar]
- Motegi F, Nakano K, Mabuchi I. Molecular mechanism of myosin-II assembly at the division site in Schizosaccharomyces pombe. J Cell Sci. 2000;113:1813–1825. doi: 10.1242/jcs.113.10.1813. [DOI] [PubMed] [Google Scholar]
- Nakano K, Arai R, Mabuchi I. The small GTP-binding protein Rho1 is a multifunctional protein that regulates actin localization, cell polarity, and septum formation in the fission yeast Schizosaccharomyces pombe. Genes Cells. 1997;2:679–694. doi: 10.1046/j.1365-2443.1997.1540352.x. [DOI] [PubMed] [Google Scholar]
- Novick P, Botstein D. Phenotypic analysis of temperature-sensitive yeast actin mutants. Cell. 1985;40:405–416. doi: 10.1016/0092-8674(85)90154-0. [DOI] [PubMed] [Google Scholar]
- Nurse P, Thuriaux P, Nasmyth K. Genetic control of the cell division cycle in the fission yeast Schizosaccharomyces pombe. Mol Gen Genet. 1976;146:167–178. doi: 10.1007/BF00268085. [DOI] [PubMed] [Google Scholar]
- Pastural E, Barrat FJ, Dufourcq-Lagelouse R, Certain S, Sanal O, Jabado N, Seger R, Griscelli C, Fischer A, de Saint Basile G. Griscelli disease maps to chromosome 15q21 and is associated with mutations in the myosin-Va gene. Nat Genetics. 1997;16:289–292. doi: 10.1038/ng0797-289. [DOI] [PubMed] [Google Scholar]
- Provance DW, Jr, Wei M, Ipe V, Mercer JA. Cultured melanocytes from dilute mutant mice exhibit dendritic morphology and altered melanosome distribution. Proc Natl Acad Sci USA. 1996;93:14554–14558. doi: 10.1073/pnas.93.25.14554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruyne DW, Schott DH, Bretscher A. Tropomyosin-containing actin cables direct the Myo2p-dependent polarized delivery of secretory vesicles in budding yeast. J Cell Biol. 1998;143:1931–1945. doi: 10.1083/jcb.143.7.1931. [DOI] [PubMed] [Google Scholar]
- Radcliffe P, Hirata D, Childs D, Vardy L, Toda T. Identification of novel temperature-sensitive lethal alleles in essential beta-tubulin and nonessential alpha 2-tubulin genes as fission yeast polarity mutants. Mol Biol Cell. 1998;9:1757–1771. doi: 10.1091/mbc.9.7.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reck-Peterson SL, Novick PJ, Mooseker MS. The tail of yeast class V myosin, Myo2p, functions as a localization domain. Mol Biol Cell. 1999;10:1001–1017. doi: 10.1091/mbc.10.4.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell P, Nurse P. cdc25+ functions as an inducer in the mitotic control of fission yeast. Cell. 1986;45:145–153. doi: 10.1016/0092-8674(86)90546-5. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sawin KE, Nurse P. Regulation of cell polarity by microtubules in fission yeast. J Cell Biol. 1998;142:457–471. doi: 10.1083/jcb.142.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliwa M. Molecular motors join forces. Nature. 1999;397:204–205. doi: 10.1038/16577. [DOI] [PubMed] [Google Scholar]
- Schott DS, Ho J, Pruyne D, Bretscher A. The COOH-terminal domain of Myo2p, a yeast myosin V, has a direct role in secretory vesicle targeting. J Cell Biol. 1999;147:791–807. doi: 10.1083/jcb.147.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagishi Y, Oda S-I, Hayasaka S, Dekker-Ohno K, Shikata T, Inouye M, Yamamura H. The dilute-lethal (dl) gene attacks a Ca2+ store in the dendritic spine of Purkinje cells in mice. Neurosci Lett. 1996;215:169–172. doi: 10.1016/0304-3940(96)12967-0. [DOI] [PubMed] [Google Scholar]
- Titus MA. Myosin V: the multi-purpose transport motor. Curr Biol. 1997;7:301–304. doi: 10.1016/s0960-9822(06)00143-6. [DOI] [PubMed] [Google Scholar]
- Toda T, Umesono K, Hirata A, Yanagida M. Cold-sensitive nuclear division arrest mutants of the fission yeast Schizosaccharomyces pombe. J Mol Biol. 1983;168:251–270. doi: 10.1016/s0022-2836(83)80017-5. [DOI] [PubMed] [Google Scholar]
- Umesono K, Toda T, Hayashi S, Yanagida M. Two cell division cycle genes nda2 and nda3 of the fission yeast Schizosaccharomyces pombe control microtubular organization and sensitivity to anti-mitotic benzimidazole compounds. J Mol Biol. 1983;168:271–284. doi: 10.1016/s0022-2836(83)80018-7. [DOI] [PubMed] [Google Scholar]
- Wu X, Bowers B, Rao K, Qin W, Hammer JA., III Visualization of melanosome dynamics within wild-type and dilute melanocytes suggests a paradigm for myosin V function in vivo. J Cell Biol. 1998;143:1899–1918. doi: 10.1083/jcb.143.7.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]