Abstract
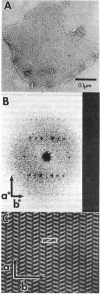
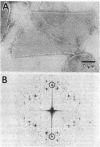
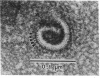
Two-dimensional crystals of rhodopsin have been prepared from purified frog disk membranes by using the detergent Tween 80. The space group of the orthorhombic crystals is p22121; the unit cell dimensions are 47 X 151 A. Projection maps of negatively stained preparations have been calculated to a resolution of approximately 22 A. The rhodopsin molecules are associated as dimers that appear to be slightly sigmoidal and are 20-25 A in width and 70-80 A in length.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert A. D., Litman B. J. Independent structural domains in the membrane protein bovine rhodopsin. Biochemistry. 1978 Sep 19;17(19):3893–3900. doi: 10.1021/bi00612a001. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Chabre M. Diamagnetic anisotropy and orientation of alpha helix in frog rhodopsin and meta II intermediate. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5471–5474. doi: 10.1073/pnas.75.11.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corless J. M., Cobbs W. H., 3rd, Costello M. J., Robertson J. D. On the asymmetry of frog retinal rod outer segment disk membranes. Exp Eye Res. 1976 Sep;23(3):295–324. doi: 10.1016/0014-4835(76)90130-5. [DOI] [PubMed] [Google Scholar]
- Corless J. M., Costello M. J. Isolation, rapid freezing, and freeze-fracture methods for frog retinal photoreceptors. Methods Enzymol. 1982;81:585–593. doi: 10.1016/s0076-6879(82)81082-3. [DOI] [PubMed] [Google Scholar]
- Corless J. M., Costello M. J. Paracrystalline inclusions associated with the disk membranes of frog retinal rod outer segments. Exp Eye Res. 1981 Feb;32(2):217–228. doi: 10.1016/0014-4835(81)90010-5. [DOI] [PubMed] [Google Scholar]
- Dratz E. A., Miljanich G. P., Nemes P. P., Gaw J. E., Schwartz S. The structure of rhodopsin and its disposition in the rod outer segment disk membrane. Photochem Photobiol. 1979 Apr;29(4):661–670. doi: 10.1111/j.1751-1097.1979.tb07746.x. [DOI] [PubMed] [Google Scholar]
- Erickson H. P., Voter W. A., Leonard K. Image reconstruction in electron microscopy: enhancement of periodic structure by optical filtering. Methods Enzymol. 1978;49:39–63. doi: 10.1016/s0076-6879(78)49006-8. [DOI] [PubMed] [Google Scholar]
- Fung B. K., Hubbell W. L. Organization of rhodopsin in photoreceptor membranes. 1. Proteolysis of bovine rhodopsin in native membranes and the distribution of sulfhydryl groups in the fragments. Biochemistry. 1978 Oct 17;17(21):4396–4402. doi: 10.1021/bi00614a007. [DOI] [PubMed] [Google Scholar]
- Fung B. K., Hubbell W. L. Organization of rhodopsin in photoreceptor membranes. 2. Transmembrane organization of bovine rhodopsin: evidence from proteolysis and lactoperoxidase-catalyzed iodination of native and reconstituted membranes. Biochemistry. 1978 Oct 17;17(21):4403–4410. doi: 10.1021/bi00614a008. [DOI] [PubMed] [Google Scholar]
- Henderson R., Unwin P. N. Three-dimensional model of purple membrane obtained by electron microscopy. Nature. 1975 Sep 4;257(5521):28–32. doi: 10.1038/257028a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liebman P. A., Entine G. Lateral diffusion of visual pigment in photorecptor disk membranes. Science. 1974 Aug 2;185(4149):457–459. doi: 10.1126/science.185.4149.457. [DOI] [PubMed] [Google Scholar]
- McCaslin D. R., Tanford C. Different states of aggregation for unbleached and bleached rhodopsin after isolation in two different detergents. Biochemistry. 1981 Sep 1;20(18):5212–5221. doi: 10.1021/bi00521a018. [DOI] [PubMed] [Google Scholar]
- McCaslin D. R., Tanford C. Effects of detergent micelles on the recombination reaction of opsin and 11-cis-retinal. Biochemistry. 1981 Sep 1;20(18):5207–5212. doi: 10.1021/bi00521a017. [DOI] [PubMed] [Google Scholar]
- Michel-Villaz M., Saibil H. R., Chabre M. Orientation of rhodopsin alpha-helices in in retinal rod outer segment membranes studied by infrared linear dichroism. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4405–4408. doi: 10.1073/pnas.76.9.4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel H., Oesterhelt D., Henderson R. Orthorhombic two-dimensional crystal form of purple membrane. Proc Natl Acad Sci U S A. 1980 Jan;77(1):338–342. doi: 10.1073/pnas.77.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne H. B., Sardet C., Michel-Villaz M., Chabre M. Structural study of rhodopsin in detergent micelles by small-angle neutron scattering. J Mol Biol. 1978 Aug 5;123(2):177–206. doi: 10.1016/0022-2836(78)90320-0. [DOI] [PubMed] [Google Scholar]
- Papermaster D. S., Schneider B. G., Zorn M. A., Kraehenbuhl J. P. Immunocytochemical localization of a large intrinsic membrane protein to the incisures and margins of frog rod outer segment disks. J Cell Biol. 1978 Aug;78(2):415–425. doi: 10.1083/jcb.78.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo M., Cone R. A. Lateral diffusion of rhodopsin in the photoreceptor membrane. Nature. 1974 Feb 15;247(5441):438–441. doi: 10.1038/247438a0. [DOI] [PubMed] [Google Scholar]
- Rothschild K. J., Sanches R., Hsiao T. L., Clark N. A. A spectroscopic study of rhodopsin alpha-helix orientation. Biophys J. 1980 Jul;31(1):53–64. doi: 10.1016/S0006-3495(80)85040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardet C., Tardieu A., Luzzati V. Shape and size of bovine rhodopsin: a small-angle x-ray scattering study of a rhodopsin-detergent complex. J Mol Biol. 1976 Aug 15;105(3):383–407. doi: 10.1016/0022-2836(76)90100-5. [DOI] [PubMed] [Google Scholar]
- Smith H. G., Jr, Stubbs G. W., Litman B. J. The isolation and purification of osmotically intact discs from retinal rod outer segments. Exp Eye Res. 1975 Mar;20(3):211–217. doi: 10.1016/0014-4835(75)90134-7. [DOI] [PubMed] [Google Scholar]
- Unwin P. N., Henderson R. Molecular structure determination by electron microscopy of unstained crystalline specimens. J Mol Biol. 1975 May 25;94(3):425–440. doi: 10.1016/0022-2836(75)90212-0. [DOI] [PubMed] [Google Scholar]
- Wrigley N. G. The lattice spacing of crystalline catalase as an internal standard of length in electron microscopy. J Ultrastruct Res. 1968 Sep;24(5):454–464. doi: 10.1016/s0022-5320(68)80048-6. [DOI] [PubMed] [Google Scholar]
- Wu C. W., Stryer L. Proximity relationships in rhodopsin. Proc Natl Acad Sci U S A. 1972 May;69(5):1104–1108. doi: 10.1073/pnas.69.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeager M., Schoenborn B., Engelman D., Moore P., Stryer L. Neutron diffraction analysis of the structure of rod photoreceptor membranes in intact retinas. J Mol Biol. 1980 Mar 5;137(3):315–348. doi: 10.1016/0022-2836(80)90319-8. [DOI] [PubMed] [Google Scholar]
- Zorn M., Futterman S. Extraction, regeneration after bleaching, and ion-exchange chromatography of rhodopsin in Tween 80. Arch Biochem Biophys. 1973 Jul;157(1):91–99. doi: 10.1016/0003-9861(73)90393-7. [DOI] [PubMed] [Google Scholar]