Abstract
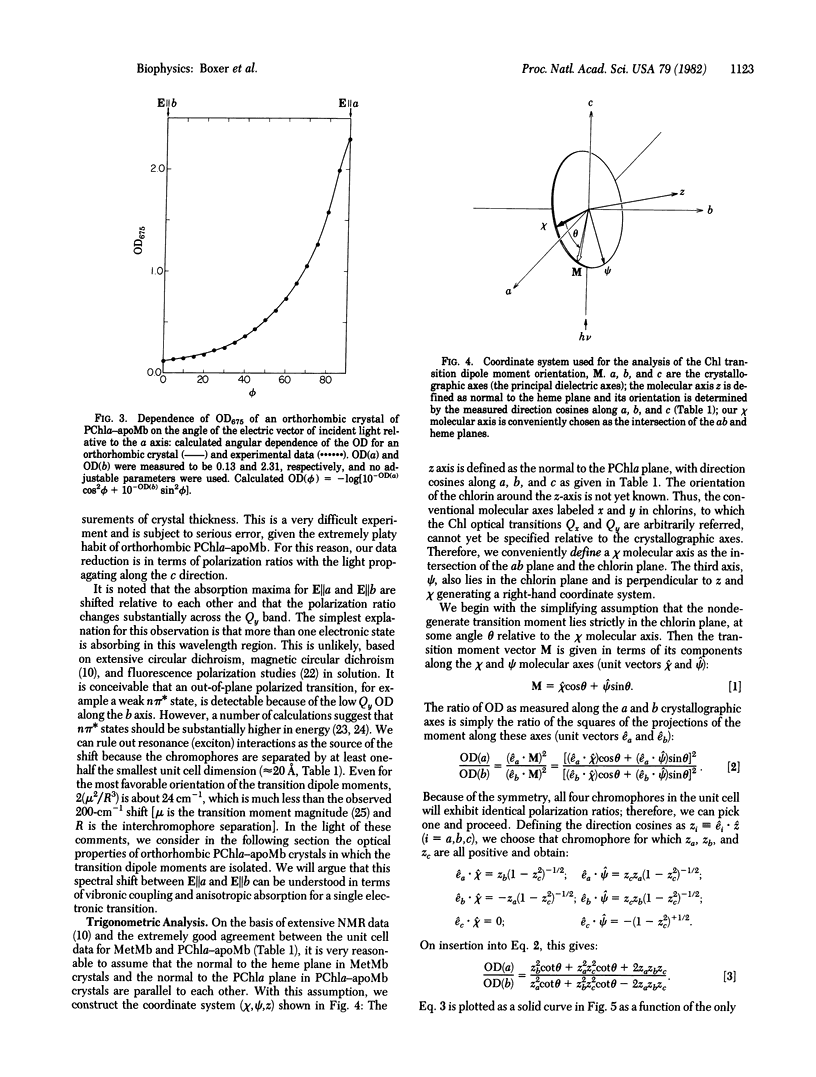
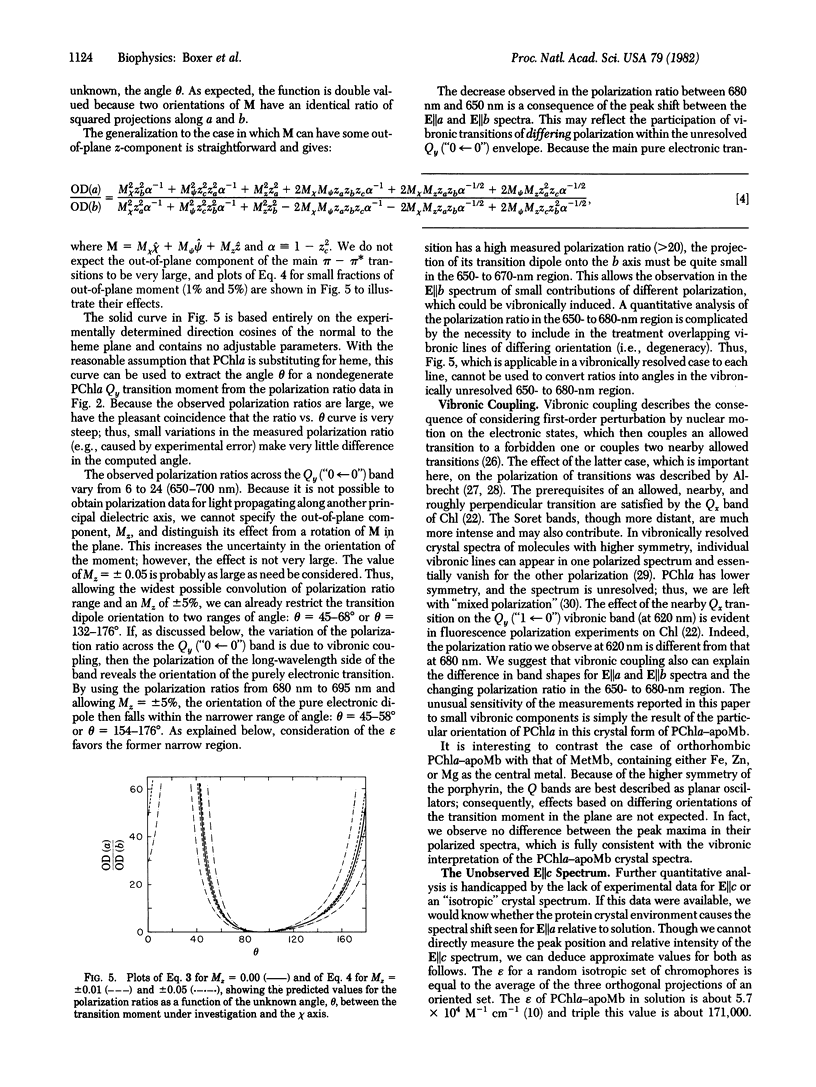
The orientations of the transition dipole moments in chlorophyll (Chl) are among the most useful spectroscopic properties for determining macromolecular architecture in photosynthetic complexes; however, the relationships between these orientations and the Chl molecular geometry are unknown. In order to solve this problem, we have prepared single crystals of the synthetic 1:1 complex between pyrochlorophyllide a and apomyoglobin. The protein crystallizes readily in the orthorhombic (B) form, space group P212121, and the unit cell dimensions are determined to be within 0.5% of those for native MetMb crystals of the same type. These green crystals are highly dichroic, and the strong absorption along the crystallographic a axis in the Qy band is red-shifted by about 9 nm, relative to the corresponding feature in a solution of the protein. Although the crystal structure for native Mb in this space group has not been determined, the direction cosines of the heme normal relative to the crystal axes have been measured. By using these values, an appropriate trigonometric analysis, and the measured polarized single-crystal spectra, the orientation of the Chl transition dipole moment for the Qy transition can be specified relative to the crystal axes. With the completion of the protein crystal structure, this result will lead directly to the orientations of the optical transition dipole moments relative to the molecular geometry. The effects of vibronic coupling and the protein environment on the absorption properties of Chl are discussed in detail.
Keywords: photosynthesis, myoglobin, protein crystal
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boxer S. G., Roelofs M. G. Chromophore organization in photosynthetic reaction centers: High-resolution magnetophotoselection. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5636–5640. doi: 10.1073/pnas.76.11.5636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton W. A., Hochstrasser R. M. Electronic spectrum of single crystals of ferricytochrome-c. J Chem Phys. 1967 Apr 1;46(7):2533–2539. doi: 10.1063/1.1841081. [DOI] [PubMed] [Google Scholar]
- Eaton W. A., Hochstrasser R. M. Single-crystal spectra of ferrimyoglobin complexes in polarized light. J Chem Phys. 1968 Aug 1;49(3):985–995. doi: 10.1063/1.1670263. [DOI] [PubMed] [Google Scholar]
- Eaton W. A., Hofrichter J., Makinen M. W., Andersen R. D., Ludwig M. L. Optical spectra and electronic structure of flavine mononucleotide in flavodoxin crystals. Biochemistry. 1975 May 20;14(10):2146–2151. doi: 10.1021/bi00681a016. [DOI] [PubMed] [Google Scholar]
- Eaton W. A., Lewis T. P. Polarized single-crystal absorption spectrum of 1-methyluracil. J Chem Phys. 1970 Sep 15;53(6):2164–2172. doi: 10.1063/1.1674310. [DOI] [PubMed] [Google Scholar]
- Frank H. A., Bolt J., Friesner R., Sauer K. Magnetophotoselection of the triplet state of reaction centers from Rhodopseudomonas sphaeroides R-26. Biochim Biophys Acta. 1979 Sep 11;547(3):502–511. doi: 10.1016/0005-2728(79)90030-6. [DOI] [PubMed] [Google Scholar]
- HOWLING D. H., FITZGERALD P. J. The nature, significance, and evaluation of the Schwarzschild-Villiger (SV) effect in photometric procedures. J Biophys Biochem Cytol. 1959 Dec;6:313–337. doi: 10.1083/jcb.6.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENDREW J. C. Side-chain interactions in myoglobin. Brookhaven Symp Biol. 1962 Dec;15:216–228. [PubMed] [Google Scholar]
- Rafferty C. N., Clayton R. K. The orientations of reaction center transition moments in the chromatophore membrane of Rhodopseudomonas sphareroides, bases on new linear dichroism and photoselection measurements. Biochim Biophys Acta. 1979 May 9;546(2):189–206. doi: 10.1016/0005-2728(79)90039-2. [DOI] [PubMed] [Google Scholar]
- Shuvalov V. A., Asadov A. A. Arrangement and interaction of pigment molecules in reaction centers of Rhodopseudomonas viridis. Photodichroism and circular dichroism of reaction centers at 100 k. Biochim Biophys Acta. 1979 Feb 8;545(2):296–308. doi: 10.1016/0005-2728(79)90207-x. [DOI] [PubMed] [Google Scholar]
- Vermeglio A., Breton J., Paillotin G., Cogdell R. Orientation of chromophores in reaction centers of Rhodopseudomonas sphaeroides: a photoselection study. Biochim Biophys Acta. 1978 Mar 13;501(3):514–530. doi: 10.1016/0005-2728(78)90118-4. [DOI] [PubMed] [Google Scholar]
- Wright K. A., Boxer S. G. Solution properties of synthetic chlorophyllide--and bacteriochlorophyllide--apomyoglobin complexes. Biochemistry. 1981 Dec 22;20(26):7546–7556. doi: 10.1021/bi00529a033. [DOI] [PubMed] [Google Scholar]


