Gold nanoparticles have demonstrated tremendous utility and multifunctionality for the diagnosis and treatment of cancer.[1] Not only can these structures serve as targeted drug delivery vehicles,[2] they can also act as contrast agents for near-infrared (NIR) laser photothermal tumor ablation[3] and as platforms in a range of other biomedical diagnostic[1a,4] and therapeutic[5] applications. The uptake and removal of circulating nanoparticles by the mononuclear phagocyte system (MPS),[6] represents one of the most significant impediments to the efficient delivery of nanoscale structures to solid tumors and to-date, the majority of tumor-targeting strategies for nanoparticles attempt to evade the MPS and increase circulation time. Here, we show that colloidal gold nanorods (AuNRs) can be actively-targeted towards phagocytic macrophages that exhibit high intrinsic accumulation and infiltration into solid tumors. Macrolide-functionalized gold nanorods were preferentially delivered to tumor-associated macrophage (TAM) cells and selectively induced TAM-dependent cytotoxicity towards breast cancer cells in co-culture. Because TAMs migrate freely in circulation,[7] bypass the blood-brain-barrier,[8] and extensively accumulate/infiltrate into breast tumors,[9] these data show that macrophage-targeting gold nanoparticles can serve as promising candidates for targeted cancer therapy.
Although the MPS plays an important physiological function in removing foreign material, cellular debris, and pathogens from circulation, its cells also play a principal role in anti-tumor immunity[10] and as such, TAMs readily accumulate and infiltrate into solid tumors, comprising up to 50% of tumor mass in breast carcinomas.[9] A limited number of studies have investigated the ability of macrophages to deliver nanoscale drugs and imaging agents to solid tumors. Badie and coworkers have shown that TAMs can serve as efficient carriers of cyclodextrin-based nanoparticles (CDPs; fluorescent analogues of CRLX101) into glioma tumors.[7] CDPs were found to preferentially accumulate in TAMs that subsequently migrated into circulation and localized at distant tumor sites. Because TAMs are able to bypass the blood-brain-barrier during pathogenesis,[11] increasingly-specific delivery of camptothecin (CRLX101) to brain tumors is expected from CDPs. Jackson et al. found that circulating, PEG-labeled quantum dots are similarly uptaken by TAMs that readily infiltrate glial tumors.[8] TAMs have also been actively targeted by nanoparticle ligands to facilitate increasingly-specific delivery. Mannan-conjugated lipid nanoparticles have achieved selective gene delivery to alveolar macrophages;[12] folate-targeted iron oxide nanoparticles have exhibited TAM-exclusive accumulation in breast tumors[13] and glutamine-functionalized liposomes have demonstrated TAM-dependent translocation into neuroblastoma tumors.[14] Hirschberg and coworkers have further exploited TAMs to preferentially deliver photothermal contrast agents to tumor cells,[15] finding that TAMs efficiently take up PEGylated gold nanoshells and subsequently infiltrate glial tumor spheroids to allow selective NIR laser photothermal ablation therapy (810 nm, ≥ 7 W cm−2, 10 min).
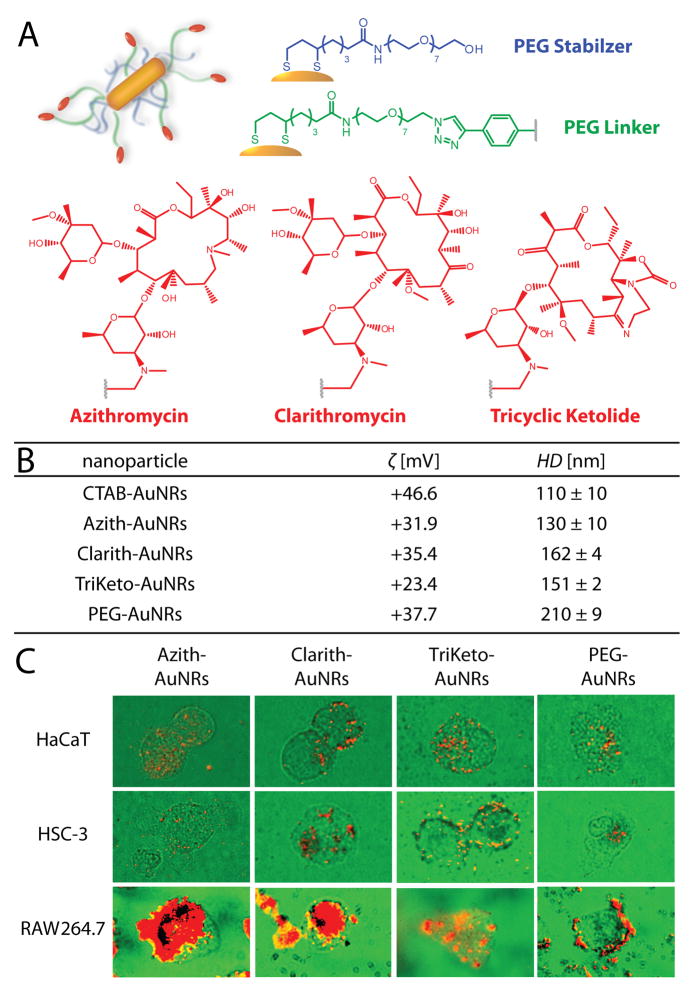
Macrolides are a class of structurally-homologous antibiotics widely administered for more than four decades for the treatment of microbial infections in humans, particularly those of the respiratory tract and soft tissues. In addition to their broad-spectrum antibiotic activity, one notable hallmark of macrolides is their exceptionally high accumulation in phagocytic cells (macrophages) that facilitate increasingly specific delivery of these drugs to sites of inflammation (infection).[16] We hypothesized that macrolide ligands could also facilitate the preferential delivery of gold nanoparticles to inflamed tumor tissues via TAMs, allowing for enhanced TAM anti-tumor potential,[17] increasingly effective laser photothermal therapy,[3] and/or heat shock protein-induced activation of macrophage-mediated anti-tumor immunity.[18] To this end, gold nanorods (AuNRs) were synthesized via seed-mediated growth from chloroauric acid and conjugated with PEG-thiol or mixed self-assembled monolayers of (9:1) PEG-thiol and thiol-PEGylated azithromycin (Zithromax®), clarithromycin (Biaxin®), or tricyclic ketolide (TE-802) (Figure 1A,B; Supporting Information; Figure S1). Each of the nanorods, abbreviated hereafter as PEG-AuNRs, Azith-AuNRs, Clarith-AuNRs, and TriKeto- AuNRs, respectively, were conjugated such that they displayed 1 × 103 macrolide ligands and 9 × 103 PEG-thiol molecules per particle, as well as a NIR absorption maximum ca. 818 nm. PEG-AuNRs displayed 10 × 103 PEG-thiol molecules per particle.
Figure 1.
A) Schematic representation and (B) physiochemical characteristics of the macrolide-gold nanorods used herein. C) Cardioid immersion dark-field scattering microscopy (DFSM) of cell cultures (green) illustrating preferential uptake/accumulation of macrolide-gold nanorods (red) into tumor-associated macrophage cells (RAW 264.7) relative to squamous cell carcinoma (HSC-3) and keratinocyte cells (HaCaT). PEGylated gold nanorods exhibited only nominal cell-surface binding with TAMs. Images in (C) are false color. Gold nanorods used in these studies were ca. 50 ± 8 nm in length and 13 ± 2 nm in width (4.0 ± 0.9 aspect ratio) as measured by TEM.
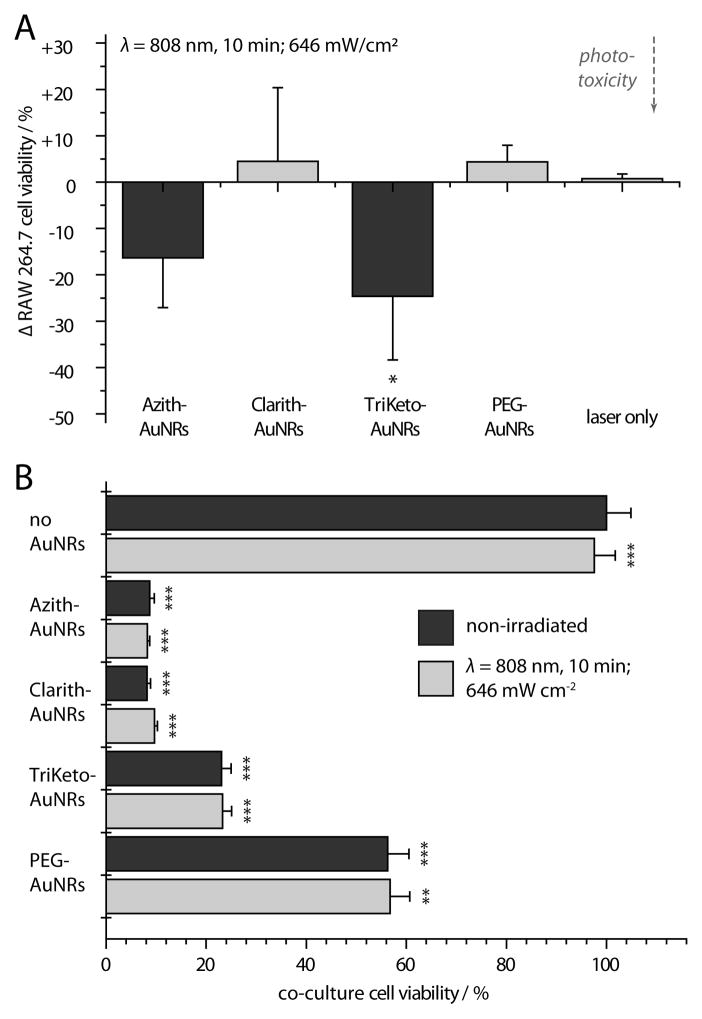
Preferential uptake/accumulation of the macrolide-AuNRs into TAM cells was assessed via cardioid immersion dark-field scattering microscopy (DFSM). TAM cells (RAW 264.7) exhibited substantially higher levels of macrolide-AuNR uptake than either squamous cell carcinoma (HSC-3) or keratinocyte cells (HaCaT) cells (Figure 1C; Supporting Information) and showed only nominal cell-surface binding by PEG-AuNRs.[19] Based on these findings, phototoxicity from NIR laser exposure (λ=808 nm) was assessed using TAMs treated with sub-lethal concentrations of macrolide-AuNRs (10 pm, 24 h; Figure 2A; Supporting Information; Figure S2). NIR laser exposure of TAM cultures washed/immersed in buffer (1.6 mm) showed modest phototoxicity from Azith- and TriKeto-AuNRs, but no significant effects from Clarith-AuNRs, PEG-AuNRs, or laser treatment alone (10 min, 646 mW cm−2). We then evaluated the effects of AuNR-loaded TAMs and NIR laser exposure on cell viability in breast adenocarcinoma co-cultures. TAM cultures were again loaded with sub-lethal concentrations of macrolide-AuNRs (10 pm, 24 h), washed with buffer, and seeded onto MCF7 breast cultures at 50% plating densities to reflect physiological levels of TAM infiltration into breast carcinomas (Supporting Information; Figure S3).[9] After 12 h, co-cultures were washed and immersed in buffer (1.6 mm), NIR laser-exposed (10 min, 646 mW cm−2), and allowed to incubate in complete growth media for an additional 24 h. While no statistically significant cytotoxic effects from NIR laser exposure were observed, we found substantial cytotoxicity in co-cultures containing macrolide-AuNR-treated TAMs and significant, but notably diminished cytotoxicity in those containing PEG-AuNR-treated TAMs (ca. 55%; Figure 2B).
Figure 2.
A) Near-infrared laser photothermal ablation of tumor-associated macrophage (TAM) cells loaded with macrolide-gold nanorods (AuNRs). B) Selective cytotoxicity of AuNR-loaded TAMs co-cultured with MCF7 breast adenocarcinoma cells. Note that (A) is plotted as % change in order to emphasize statistically significant differences in viability observed from monocultures in the presence and absence of laser exposure, effects which were not observed from co-cultures in (B). Error bars represent SD. P-values in (A) reported relative to non-irradiated RAW 264.7 cells; P-values in (B) reported relative to non-irradiated, non-AuNR-treated co-cultures. *P<0.05, **P<0.01, ***P<0.001.
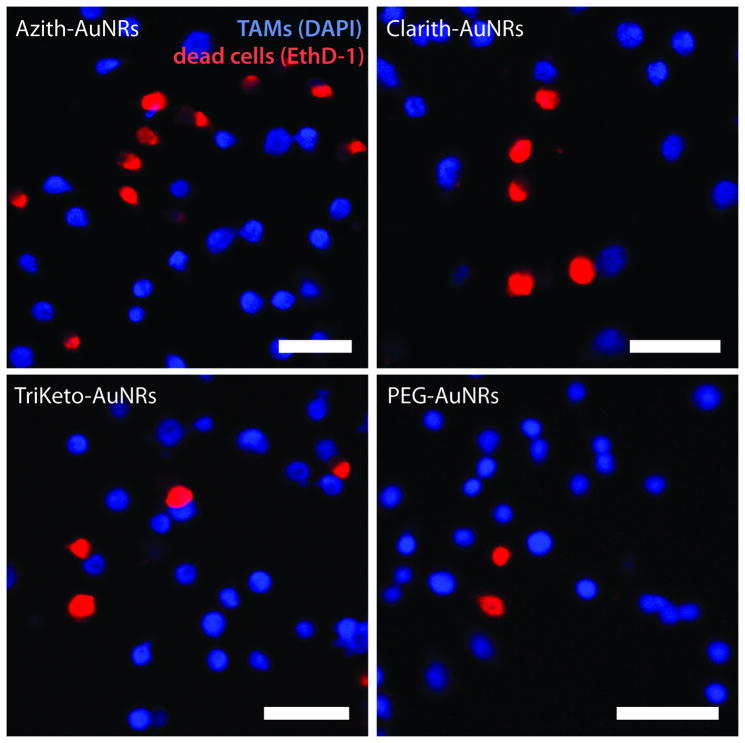
In order to delineate which cells contributed to co-culture cell death, AuNR-treated TAMs were labeled with 4′,6-diamidino-2-phenylindole (DAPI), a membrane-permeable fluorescent nuclear marker, prior to passage into co-culture. DAPI-labeled, AuNR-loaded TAM cells were thoroughly rinsed with buffer and again seeded at 50% plating densities with MCF7 breast adenocarcinoma cells. After 12 h, culture media were removed and replaced with fresh growth media. Following an additional 24 h of incubation, adherent co-culture cells were labeled with ethidium homodimer-1 (EthD-1), a membrane-impermeable fluorescent nuclear marker for apoptotic/necrotic cells. Confocal fluorescence microscopy of the co-cultures revealed that MCF7 breast cancer cells contributed near exclusively to the observed cell death, with no colocalization of DAPI-labeled TAM fluorescence with the EthD-1 apoptotic/necrotic cell marker (Figure 3; Supporting Information).
Figure 3.
Tumor-associated macrophages (TAMs) treated with macrolide-gold nanorods (AuNRs) induce cell death in co-cultured breast adenocarcinoma cells. Macrolide-AuNR-loaded TAM nuclei were labeled with 4′,6-diamidino-2-phenylindole (DAPI, blue) and seeded onto MCF7 breast cell cultures at 50% plating densities. Confocal fluorescence microscopy using the apoptotic/necrotic nuclear marker, ethidium homodimer-1 (EthD-1, red), shows cytotoxicity exclusive to breast adenocarcinoma cells. Scale bar represents 50μm.
Although macrophage cytokine activation by gold nanoparticles (AuNPs) is well-documented,[17] we believe this to be the first report on the subsequent effects of AuNP-activated macrophages on nearby cells. Tsai and coworkers observed upregulation of cytotoxic tumor necrosis factor (TNF-α) and interlukin (IL) 1/6 in response to the administration of untargeted AuNPs.[17a] In an excellent study by Puntes and coworkers, enhanced upregulation of cytotoxic TNF-α and IL-1/6 was also observed in macrophage cells treated with peptide-conjugated gold nanoparticles designed to mimic virus-like particles through epitope repetition.[17b] Groll and coworkers more recently observed that PEGylated AuNRs can likewise increase cytotoxic TNF-α and IL-1/6 protein levels in AuNR-treated macrophages.[17c] Together, these findings suggest that AuNP-activated macrophages may enhance the innate cytotoxic responses of TAMs towards the tumors which they infiltrate. Moreover, macrolide-AuNRs that actively target TAMs may further augment anti-tumor response and achieve increasingly preferential delivery due to the size-dependent enhanced permeability and retention (EPR) effect.[20] Clinical trials involving the systemic administration of therapeutic TNF-α-AuNPs may additionally synergize with tumor-specific cytotoxic effects from AuNP-activated TAMs (CYT-6091, CytImmune Sciences, Inc.).[2]
Unwanted MPS uptake of nanostructures can be mitigated in a variety of ways including saturation by “decoy” nanoparticles (e.g. 3.4–8.5 nmol dosages in rats),[8] transient depletion of circulating macrophages (e.g. by anti-α CSF1[13] and/or liposomal clodronate[10]), and surface-functionalization with protein-repellant polymers (e.g. PEG, POx),[21] complement inhibitors (e.g. heparin),[22] and/or “markers of self” (e.g. CD47[23] and CD200). In spite of these efforts, biodistribution profiles of “stealth” AuNPs typically remain high in MPS organs such as the spleen and liver.[1b,24] Jeong and coworkers observed that intravenously administered PEG-AuNPs sequester in splenic macrophages and neutrophil- infiltrated liver tissues, resulting in tissue-specific inflammation (i.e. upregulation of TNF-α, IL-1/6/10/12).[25] While potentially beneficial in tumor tissues, the deleterious effects of AuNP-activated TAMs described here could result significant impacts to healthy tissues and warrant further investigation.
In conclusion, we have synthesized a novel gold nanoparticle conjugate which targets and activates anti-tumor potential in macrophage cells that exhibit high accumulation[7] and infiltration[9] into solid tumors. Macrolide-gold nanorods (AuNRs) preferentially accumulated in tumor-associated macrophage cells (TAMs) that exhibited selectively-enhanced cytotoxicity towards breast adenocarcinoma cells in co-culture. Although modest near-infrared photothermal ablation response was observed in monocultures of AuNR-activated TAMs, we observed no additive cytotoxic effects from photothermally-treated TAMs in co-culture over the time course of these experiments – as would be expected from heat-shock protein-induced activation or photothermal ablation. Taken together, the ability of TAMs to migrate freely in circulation,[7] bypass the blood-brain-barrier,[8] and preferentially accumulate and infiltrate into solid tumors[9] make macrolide-functionalized gold nanoparticles promising candidates for targeted cancer drug delivery to breast and brain tumors. Enhanced anti-tumor potential by tumor-localized, AuNR-activated TAMs may further synergize with chemotherapeutic treatment regimens and warrant further investigation.
Acknowledgments
This work was supported by the U.S. National Institutes of Health (1U01CA151802–01, R01CA131217) and the Korean Ministry of Education, Science, and Technology (Grant No. 08K1501–01910). The authors also thank S.C. Hayden and H. Murakami for editing assistance.
Footnotes
Supporting Information
Supporting Information is available on the WWW under http://www.small-journal.com or from the author.
Contributor Information
Erik C. Dreaden, Laser Dynamics Laboratory Department of Chemistry and Biochemistry Georgia Institute of Technology 901 Atlantic Drive NW, Atlanta, GA 30332–0400, USA
Dr. Sandra C. Mwakwari, Petit Institute for Bioengineering and Biosciences Department of Chemistry and Biochemistry Georgia Institute of Technology 315 Ferst Drive NW, Atlanta, GA 30332–0230, USA
Lauren A. Austin, Laser Dynamics Laboratory Department of Chemistry and Biochemistry Georgia Institute of Technology 901 Atlantic Drive NW, Atlanta, GA 30332–0400, USA
Matthew J. Kieffer, Laser Dynamics Laboratory Department of Chemistry and Biochemistry Georgia Institute of Technology 901 Atlantic Drive NW, Atlanta, GA 30332–0400, USA
Prof. Adegboyega K. Oyelere, Email: aoyelere@gatech.edu, Petit Institute for Bioengineering and Biosciences Department of Chemistry and Biochemistry Georgia Institute of Technology 315 Ferst Drive NW, Atlanta, GA 30332–0230, USA
Prof. Mostafa A. El-Sayed, Email: melsayed@gatech.edu, Laser Dynamics Laboratory Department of Chemistry and Biochemistry Georgia Institute of Technology 901 Atlantic Drive NW, Atlanta, GA 30332–0400, USA
References
- 1.a) Jokerst JV, Miao Z, Zavaleta C, Cheng Z, Gambhir SS. Small. 2011;7:625. doi: 10.1002/smll.201002291. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Zavaleta CL, Hartman KB, Miao Z, James ML, Kempen P, Thakor AS, Nielsen CH, Sinclair R, Cheng Z, Gambhir SS. Small. 2011;7:2232. doi: 10.1002/smll.201002317. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Cheng Y, Samia AC, Meyers JD, Panagopoulos I, Fei B, Burda C. J Am Chem Soc. 2008;130:10643. doi: 10.1021/ja801631c. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Cheng Y, Meyers JD, Broome AM, Kenney ME, Basilion JP, Burda C. J Am Chem Soc. 2011;133:2583. doi: 10.1021/ja108846h. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Perrault SD, Walkey C, Jennings T, Fischer HC, Chan WCW. Nano Lett. 2009;9:1909. doi: 10.1021/nl900031y. [DOI] [PubMed] [Google Scholar]; f) Kim BYS, Rutka JT, Chan WCW. New Engl J Med. 2010;363:2434. doi: 10.1056/NEJMra0912273. [DOI] [PubMed] [Google Scholar]
- 2.Libutti SK, Paciotti GF, Byrnes AA, Alexander HR, Gannon WE, Walker M, Seidel GD, Yuldasheva N, Tamarkin L. Clin Cancer Res. 2010;16:6139. doi: 10.1158/1078-0432.CCR-10-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.a) Hirsch LR, Stafford RJ, Bankson JA, Sershen SR, Rivera B, Price RE, Hazle JD, Halas NJ, West JL. Proc Natl Acad Sci USA. 2003;100:13549. doi: 10.1073/pnas.2232479100. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Dickerson EB, Dreaden EC, Huang X, El-Sayed IH, Chu H, Pushpanketh S, McDonald JF, El-Sayed MA. Cancer Lett. 2008;269:57. doi: 10.1016/j.canlet.2008.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Lu W, Xiong C, Zhang G, Huang Q, Zhang R, Zhang JZ, Li C. Clin Cancer Res. 2009;15:876. doi: 10.1158/1078-0432.CCR-08-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) von Maltzahn G, Park JH, Agrawal A, Bandaru NK, Das SK, Sailor MJ, Bhatia SN. Cancer Res. 2009;69:3892. doi: 10.1158/0008-5472.CAN-08-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Chen J, Glaus C, Laforest R, Zhang Q, Yang M, Gidding M, Welch MJ, Xia Y. Small. 2010;6:811. doi: 10.1002/smll.200902216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.a) Bajaj A, Miranda OR, Kim IB, Phillips RL, Jerry DJ, Bunz UHF, Rotello VM. Proc Natl Acad Sci USA. 2009;106:10912. doi: 10.1073/pnas.0900975106. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Millstone JE, Park S, Shuford KL, Qin L, Schatz GC, Mirkin CA. J Am Chem Soc. 2005;127:5312. doi: 10.1021/ja043245a. [DOI] [PubMed] [Google Scholar]
- 5.a) Giljohann DA, Seferos DS, Prigodich AE, Patel PC, Mirkin CA. J Am Chem Soc. 2009;131:2072. doi: 10.1021/ja808719p. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Arvizo RR, Miranda OR, Moyano DF, Walden CA, Giri K, Bhattacharya R, Robertson JD, Rotello VM, Reid JM, Mukherjee P. PLoS ONE. 2011;6:e24374. doi: 10.1371/journal.pone.0024374. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Hao L, Patel PC, Alhasan AH, Giljohann DA, Mirkin CA. Small. 2011;7:3158. doi: 10.1002/smll.201101018. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Hainfeld JF, Dilmanian FA, Zhong Z, Slatkin DN, Kalef-Ezra JA, Smilowitz HM. Phys Med Biol. 2010;55:3045. doi: 10.1088/0031-9155/55/11/004. [DOI] [PubMed] [Google Scholar]
- 6.Formerly referred to as the reticuloendothelial system (RES).
- 7.Alizadeh D, Zhang L, Hwang J, Schluep T, Badie B. Nanomedicine. 2010;6:382. doi: 10.1016/j.nano.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jackson H, Muhammad O, Daneshvar H, Nelms J, Popescu A, Vogelbaum MA, Bruchez M, Toms SA. Neurosurgery. 2007;60:524. doi: 10.1227/01.NEU.0000255334.95532.DD. [DOI] [PubMed] [Google Scholar]
- 9.Lewis C, Leek R, Harris A, McGee J. J Leuk Bio. 1995;57:747. doi: 10.1002/jlb.57.5.747. [DOI] [PubMed] [Google Scholar]
- 10.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL, Torigian DA, O’Dwyer PJ, Vonderheide RH. Science. 2011;331:1612. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Djukic M, Mildner A, Schmidt H, Czesnik D, Brück W, Priller J, Nau R, Prinz M. Brain. 2006;129:2394. doi: 10.1093/brain/awl206. [DOI] [PubMed] [Google Scholar]
- 12.Yu W, Liu C, Liu Y, Zhang N, Xu W. Pharm Res. 2010;27:1584. doi: 10.1007/s11095-010-0149-z. [DOI] [PubMed] [Google Scholar]
- 13.Daldrup-Link HE, Golovko D, Ruffell B, DeNardo DG, Castaneda R, Ansari C, Rao J, Tikhomirov GA, Wendland MF, Corot C, Coussens LM. Clin Cancer Res. 2011;17:5695. doi: 10.1158/1078-0432.CCR-10-3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castelli DD, Terreno E, Cabella C, Chaabane L, Lanzardo S, Tei L, Visigalli M, Aime S. NMR in Biomedicine. 2009;22:1084. doi: 10.1002/nbm.1416. [DOI] [PubMed] [Google Scholar]
- 15.a) Madsen SJ, Baek SK, Makkouk AR, Krasieva T, Hirschberg H. Ann Biomed Eng. 2011;1 doi: 10.1007/s10439-011-0415-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Baek SK, Makkouk A, Krasieva T, Sun CH, Madsen S, Hirschberg H. J Neurooncol. 2011;104:439. doi: 10.1007/s11060-010-0511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.a) Bryskier AJ. Macrolides: chemistry, pharmacology and clinical uses. Wiley-Blackwell; Paris, France: 1993. [Google Scholar]; b) Schönfeld W, Kirst HA. Macrolide antibiotics. Birkhäuser Verlag; Basel, Switzerland: 2002. [Google Scholar]
- 17.a) Yen H-J, Hsu S-h, Tsai C-L. Small. 2009;5:1553. doi: 10.1002/smll.200900126. [DOI] [PubMed] [Google Scholar]; b) Bastús NG, Sánchez-Tilló E, Pujals S, Farrera C, López C, Giralt E, Celada A, Lloberas J, Puntes V. ACS Nano. 2009;3:1335. doi: 10.1021/nn8008273. [DOI] [PubMed] [Google Scholar]; c) Bartneck M, Keul HA, Singh S, Czaja K, Bornemann Jr, Bockstaller M, Moeller M, Zwadlo-Klarwasser G, Groll Jr. ACS Nano. 2010;4:3073. doi: 10.1021/nn100262h. [DOI] [PubMed] [Google Scholar]
- 18.Nau GJ, Richmond JFL, Schlesinger A, Jennings EG, Lander ES, Young RA. Proc Natl Acad Sci USA. 2002;99:1503. doi: 10.1073/pnas.022649799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dreaden EC, Mwakwari SC, Austin LA, Humphries WH, IV, Oyelere AK, El-Sayed MA. J Controlled Release. 2012 submitted to. [Google Scholar]
- 20.Jain RK, Stylianopoulos T. Nat Rev Clin Oncol. 2010;7:653. doi: 10.1038/nrclinonc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.a) Schipper ML, Iyer G, Koh AL, Cheng Z, Ebenstein Y, Aharoni A, Keren S, Bentolila LA, Li J, Rao J, Chen X, Banin U, Wu AM, Sinclair R, Weiss S, Gambhir SS. Small. 2009;5:126. doi: 10.1002/smll.200800003. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Wang J, Byrne JD, Napier ME, DeSimone JM. Small. 2011;7:1919. doi: 10.1002/smll.201100442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Socha M, Bartecki P, Passirani C, Sapin A, Damgé C, Lecompte T, Barré J, El Ghazouani F, Maincent P. J Drug Targeting. 2009;17:575. doi: 10.1080/10611860903112909. [DOI] [PubMed] [Google Scholar]
- 23.Hsu YC, Acuña M, Tahara SM, Peng CA. Pharm Res. 2003;20:1539. doi: 10.1023/a:1026114713035. [DOI] [PubMed] [Google Scholar]
- 24.Semmler-Behnke M, Kreyling WG, Lipka J, Fertsch S, Wenk A, Takenaka S, Schmid G, Brandau W. Small. 2008;4:2108. doi: 10.1002/smll.200800922. [DOI] [PubMed] [Google Scholar]
- 25.Cho WS, Cho M, Jeong J, Choi M, Cho HY, Han BS, Kim SH, Kim HO, Lim YT, Chung BH, Jeong J. Toxicol Appl Pharmacol. 2009;236:16. doi: 10.1016/j.taap.2008.12.023. [DOI] [PubMed] [Google Scholar]