Abstract
Food allergy is an increasingly prevalent disease of immune dysregulation directed to a small subset of proteins. Shared structural and functional features of allergens, such as glycosylation, lipid-binding and protease activity may provide insight into the mechanisms involved in the induction of primary Th2 immune responses. Here we review the literature of innate Th2-type immune activation as a context for better understanding the properties of allergens that contribute to the induction of Th2-biased immune responses in at least a subset of individuals. Th2-priming signals have been largely identified in the context of parasite immunity and wound healing. Some of the features of parasite antigens and the innate immune responses to them are now understood to play a role in allergic inflammation as well. These include both exogenous and endogenous activators of innate immunity and subsequent release of key cytokine mediators such as TSLP, IL-25 and IL-33. Moreover, numerous innate immune cells including epithelium, dendritic cells, basophils, innate lymphoid cells and others all interact to shape the adaptive Th2 immune response. Progress toward understanding Th2-inducing innate immune signals more completely may lead to novel strategies for primary prevention and therapy of respiratory and food allergies.
Keywords: Food allergy, innate, Th2, food, respiratory, allergen, parasite, helminth
What is food allergy and how do we define a food allergen?
Food allergy is broadly defined as the subset of adverse reactions to foods that are immune-mediated. The most common subset of these immune-mediated reactions, and what is most typically meant by the use of the term, ‘food allergy’, is the IgE-mediated or immediate hypersensitivity subset of reactions. Even within this IgE-mediated classification, however, there is heterogeneity. The most common form of food allergy in adults and older children, for example, is thought to be the result of a primary immune response to inhalant, not ingested, allergens that subsequently provokes a secondary immune response to cross-reactive allergens upon ingestion [1]. Therefore, while the simplest definition of a food allergen is a substance that causes an allergic reaction when ingested, the definition is vague as it includes both primary and secondary ‘causes’.
In practical terms, allergens are usually defined as those that are recognized by IgE in serum from affected patients. Some allergens defined in this way may not be very potent at inducing primary allergic immune responses, but if IgE capable of binding them is present they may trigger cross-linking of IgE receptors on mast cells and basophils with consequent signs and symptoms of an allergic reaction. Furthermore, some molecules, e.g., some glycans, may be inducers of IgE and are recognized by IgE in binding assays, but may be weak triggers of allergic responses. Therefore, a molecule with the capacity to both induce sensitization and elicit reactions has been termed a ‘complete allergen’ [2]. Ara h 2 from peanut appears to meet this definition. Mal d 1 from apple, by contrast, is an incomplete allergen because it can trigger reactions due to its cross-reactivity with the dominant aeroallergen from birch pollen, Bet v 1, but is not an important immunogen. It should be noted that defining allergens purely on the basis of a humoral immune response may overlook the potential importance of molecules that predominantly stimulate T cells or other immune cells and what role that may have in the pathogenesis of both IgE- and non-IgE-mediated food allergy.
What is a food allergen is greatly influenced by culture and circumstance. Proteins that may not be important at all as food allergens in one culture may be significant in another population [3]. Moreover, proteins that are rarely described as food allergens in patients may be capable of being an allergen under unusual conditions or in experimental models - particularly when combined with strong Th2 adjuvants. Even so, recognizing the significant homology across many plant and animal taxa that are sources of food, some protein families across species are significantly overrepresented (e.g., prolamins) as allergens and some species of plant or animal (e.g., peanut, crustacean) are overrepresented as strongly polarizing allergen sources in many cultures.
A subset of protein families are overrepresented among allergens generally and food allergens in particular
Though it seems not to be the case that simple primary or secondary protein structural features are constrained among allergens [2, 4], some protein families do appear to be overrepresented. Among the >400 described food allergens, 71 structural protein families (pfams) are represented from the total known >13,000 protein families (0.6%). The top 20 of those families by abundance of described allergens -- 0.16% of the known pfams -- account for 80% of all described food allergens [5, 6]. Although we must be cautious in the absence of readily accessible systematic information on the diversity and abundance of pfams in the human diet, this suggests that there are structural and/or functional properties of certain proteins that play a significant role in determining their allergenicity. Furthermore, this apparent constraint on protein families is not limited to food allergens: as of September 2011, fewer than 300 pfams have been described as allergenic from any route of exposure [5, 7].
Much of this constraint is likely due to structural features affecting bioavailability, though it is daunting to try to estimate how much. In the case of food allergens, bioavailability is likely influenced by stability during food processing and digestion. The food-pollen syndrome caused by sensitization to Bet v 1 from birch supports this concept, as it has been shown that the structural instability of the PR-10 family (to which Bet v 1 and homologous proteins in plant foods belong) correlates with the observations that cooking readily destroys their allergenicity and that ingestion of even undenatured PR-10 allergens in raw food is rarely associated with systemic reactions, presumably because of rapid degradation. However, digestibility in general seems not to be a consistent predictor of food allergenicity [8–11]. This poor correlation may be due to the limitations of in vitro systems used to mimic digestion, the effects of the food matrix – the macromolecular complexity of a food that includes an allergen and may affect its digestion, absorption and immune handling – which are lost when assessing purified proteins, the alteration of protein structure during protein preparation, the relative abundance of proteins in whole food, or other reasons. However, regardless of the explanation, we know that IgE-mediated activation of effector cells requires interaction of IgE with multi-valent ligands to induce cross-linking of the high-affinity IgE receptor (FcεRI) and that can only be provided by a relatively complex structure – not highly digested peptides, for example. Therefore, in order for food allergens to provoke immune responses they must either survive or bypass digestion in sufficient amounts. Complete allergens must do this during primary sensitization (i.e. in the absence of specific IgE) as well as during the IgE-dependent secondary immune responses that cause the signs/symptoms of clinical allergic disease.
Many food allergens are glycoproteins and protein glycosylation may contribute to protein stability [12], as well as enhance immunogenicity in other ways. The finding that glycan-specific antibodies are common from human clinical contexts as well as in murine models suggests that glycans enhance immunogenicity [13, 14]. Bencurova et al. directly demonstrated this by creating neo-conjugates of carrier proteins with specific glycans, which increased the allergic immunogenicity of these proteins [15]. Non-mammalian glycans are antigenic structures that are distinct from endogenous glycans, and much like the glycans that underly ABO blood group incompatibility, responses to them are not edited out during immune development. Recognition of some glycans by C-type lectin receptors appears to enhance or modulate their immunogenicity (see below).
A very common feature of both food and respiratory allergens, including many prolamins (non-specific lipid transfer proteins (LTPs), 2S albumins, prolamin storage proteins and α-amylase/trypsin inhibitors) as well as lipocalins and some cupins, is lipid binding [16, 17]. This interaction with lipids is thought to potentially protect them from degradation and enhance their absorption from the GI tract [18]. The lipocalin milk allergen, β-lactoglobulin, has been shown to be more stable when lipid-bound [19]. Other macromolecular aspects of protein structure are also known to be relevant in the context of mucosal immunity. For example, mammalian milk is a complex colloidal fluid in which caseins (α, β, κ) are largely contained in large micelles that are in suspension and are predominantly presented to the immune system via Peyer’s patches, while whey proteins (e.g. β-lactoglobulin and α-lactalbumin) are highly soluble and rapidly transported across the intestinal epithelium [20].
The hypothesis that follows from these observations is that complete food allergens are the subset of ingested proteins that are intrinsically, or by intimate association with other molecules, immunogenic in a manner that favors IgE production and allergic inflammation – in other words, stimulating an allergen-specific Th2-skewed immune response. While it is clear that food allergens induce Th2 immunity in affected patients, whether allergens are generally potent immunogens that are tolerated in healthy individuals through regulatory mechanisms, versus weakly immunogenic molecules that are essentially ignored in the absence of allergic immune dysregulation is still being defined; however, evidence of the former view comes from the prevalence of humoral (IgG) and cell-mediated responses to several food allergens among healthy individuals [21].
The role of Th2 responses in immunity
Differentiation of naive T cells is induced by antigen-presenting cells (APCs), most importantly dendritic cells (DCs). Upon activation, naive CD4+ T cells differentiate into various subsets of T helper cells that can be divided into categories based on their cytokine secretion and effector function. Th1 cells secrete interferon (IFN)-γ, and promote clearance of intracellular pathogens. Th2 cells produce interleukin (IL)-4, IL-5, and IL-13, and are important in the immune response against extracellular, multicellular pathogens. Th17 cells secrete IL-17, enhance neutrophil responses and promote clearance of extracellular bacteria. Regulatory T (Treg) cells produce IL-10, transforming growth factor (TGF)-β, or both, and suppress the other T cell subsets to prevent excess or damaging immune responses. In addition to their involvement in immunity against extracellular parasites, Th2 cells play a key role in sensitization and allergy. IL-4 is important in driving Th2 cell differentiation and induces production of allergen-specific IgE by B cells, which binds to FcεRI on mast cells and basophils [22]. These effector cells are crucial in the immediate clinical symptoms upon re-exposure to allergen. IL-13 functions as an effector molecule that mediates eosinophilic inflammation, airway hyperresponsiveness, and mucus hypersecretion [22]. IL-5 is the major eosinophil-active cytokine, inducing eosinophil proliferation and differentiation and acting as a costimulator for eosinophil activation [23].
Th2 responses are strongly induced by extracellular, multicellular parasites, such as helminths, and likely evolved in response to them. Unlike bacteria, protozoa, fungi and viruses, most helminths do not replicate in the mammalian host. The infective stages must find an opportunity to establish infection and then grow to sexual maturity, producing eggs or live offspring for transmission to the next host. The adult stages of these parasites can live for decades and withstand immune-mediated attack. These distinct features, as well as the multicellular nature of these pathogens, may explain why helminths induce an immune response that is entirely different from the Th1- or Th17-dominated response to unicellular pathogens. Key factors in the human immune response against helminths are the Th2 cytokines IL-4, IL-5, and IL-13, production of IgE, and expansion of eosinophils, basophils, mast cells and alternatively activated macrophages (AAMs) [24].
Th2 cells are central players in immune responses to helminths, as has been shown in murine models, in which immunity to the helminths S. mansoni and N. brasiliensis was found to be dependent on IL-4-expressing CD4+ T cells [25–27]. In addition, innate immune cells such as basophils and the recently identified innate lymphoid cells (ILCs) can produce Th2-type cytokines and thereby contribute to the anti-parasite response. Basophils armed with helminth-specific IgE produce high levels of IL-4 during helminthiasis, and ILCs, which provide a first line of defense against helminths, produce IL-5 and IL-13 [28, 29]. These and other data indicate that multiple cell types contribute crucial cytokines to enhance Th2-type immunity [30]. The involvement of various cell types is likely to be driven by the large variety of parasites and their finely evolved immune evasion strategies. Whereas IL-4 can be produced by innate immune cells such as basophils, these cells cannot substitute for IL-4-producing T cells in providing CD40-mediated co-stimulation of B cells, and induction of IgE production. In humans, there is a strong positive association between levels of helminth-specific IgE and acquired protective immunity to helminth infections, suggesting the biological importance of IgE [31–35]. In mice however, contradictory data have been reported regarding the protective effect of IgE. For example, IgE appears to play an important role in immunity to the helminth T. spiralis [36], but not in immunity to N. brasiliensis and S. mansoni [37, 38]. Other factors may contribute more to anti-helminth immunity in mice, such as helminth-specific IgG and IgA [39], and IgE-independent mast cell responses [40]. Nevertheless, acquired immunity to ticks appears to be dependent on IgE in mice, and FcεRI-expressing basophils are critically involved in the protective immune response to these ectoparasites [41]. Tick bites also induce IgE responses in humans, and an interesting study showed that this IgE is specific to tick-derived proteins, as well as to the oligosaccharide galactose-α-1,3-galactose (alpha-gal). Alpha-gal-specific IgE is related to delayed anaphylaxis to red meat, which has a known distribution similar to that of important tick populations, implicating that tick bites may be relevant triggers of this type of food allergy [42].
Recent insights have yielded a better understanding of how Th2-type immunity, including both innate and adaptive components, has evolved to protect the host from fatal parasite infection and the associated tissue damage. A consistent feature of mammalian infection with macropathogens is that complete expulsion or killing of all parasites is rarely achieved, presumably because the costs of achieving sterilizing immunity exceed the benefits [43]. After all, as most helminths do not replicate in the mammalian host, complete eradication is in most cases not necessary for host survival. The costs of sterilizing immunity include not only the energy resources of the immune response itself but also the damage associated with attempting to contain large parasites, which themselves cause extensive tissue disruption while migrating through the host. Thus, Th2-type immunity may have arisen from our innate response to tissue injury, with repair responses isolating and encapsulating macroparasites through the deposition of extracellular matrix proteins while simultaneously resolving localized damage [24]. In regard to energy management during helminth infection, recent studies have reported an unexpected role for IL-4 in regulation of peripheral nutrient metabolism and insulin sensitivity. Activation of the STAT6 signaling pathway by IL-4 improves insulin action, resulting in a decrease in blood glucose concentration [44]. Glucose homeostasis is maintained by AAMs in adipose tissues, and these macrophages are activated by IL-4, which is mainly produced by eosinophils in white adipose tissue. Infection with the helminth N. brasiliensis increases the number of eosinophils in adipose tissue and decreases blood glucose levels [45]. In mice and humans, bacterial infections trigger Toll-like receptor signaling, which interferes with insulin action in liver and adipose tissue, leading to the release of glucose and fatty acids to fuel the activated immune system. However, infection with helminths poses distinct metabolic challenges because these pathogens chronically parasitize host nutrients for their own growth. The IL-4-induced decrease in blood glucose levels during helminth infection may be directed toward inhibiting the growth of helminths and the loss of nutrients to these pathogens during chronic infection. Hence, Th2-type immunity appears to have four major components: multicellular parasite resistance, wound repair, inflammatory control, and metabolic regulation [24].
An interesting recent paper provided a slightly different perspective on the origin of Th2-type immunity, implicating an important role for Th2-type responses in host defense against noxious environmental substances such as venoms and irritants, in addition to the functions described above. The authors suggested that allergic hypersensitivity evolved to elicit anticipatory responses and to promote avoidance of suboptimal environments [46]. Whereas these arguments are thought-provoking, protection against toxins and other environmental stressors does not appear to be dependent on IgE, and nonatopic subjects are not less protected than atopic individuals. Therefore, allergy and its detrimental aspects seem to be the price we pay for the evolution of protection against multicellular parasites [47].
Sensing of foreign and self antigens by the innate immune system
The human immune system recognizes all foreign substances, both pathogenic and benign. The nature of these substances dictates whether an immune response should be aimed at eradication, or rather at maintaining tolerance. Our immune system decides which response is appropriate based on the presence of pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs). PAMPs are conserved microbial ligands, whereas DAMPs are endogenous ligands derived from cells that are stressed or damaged by pathogens or other harmful substances. These patterns are recognized by the innate immune system, which has evolved to sense conserved molecular features and take appropriate action, by activation of innate immune cells such as phagocytes and by inducing adaptive immune responses. Innate imune cells include DCs, monocytes, macrophages, neutrophils, eosinophils, basophils, mast cells, and ILCs, as well as nonprofessional immune cells such as epithelial cells, endothelial cells and fibroblasts. PAMPs and DAMPs are recognized by germline-encoded pattern recognition receptors (PRRs), which are expressed by these cell types, although innate immune cells show a wide variety in expression of the various types of PRRs.
PRRs are expressed as surface receptors in the cell membrane, as well as in various intracellular components, and as secreted proteins. The best-characterized families of cellular PRRs are Toll-like receptors (TLRs), C-type lectin receptors (CLRs), retinoic acid-inducible gene (RIG)-I-like receptors (RLRs), nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), scavenger receptors (SRs), and protease-activated receptors (PARs) [48–50]. Some of these are transmembrane cell surface receptors (TLR1,2,4,5,6, and all CLRs, SRs, and PARs), whereas other PRRs are localized in the endolysosome (TLR3,7,8,9,10) or in the cytoplasm (all RLRs and NLRs). Secreted PRRs include the complement proteins, collectins, mucins, pentraxins, ficolins, and resolvins [51].
Foreign proteins vary widely in their capacity to induce an immune response, and in the type of response that is initiated. An important factor determining the immunogenicity of a protein is the presence of an adjuvant. Immune adjuvants are substances and formulations that have the capacity to increase the immune response to an antigen. Common adjuvants include microbial products or particulate compounds such as mineral salts, oil-in-water emulsions and microparticles. The best defined adjuvants interact with the innate immune system through PRRs, resulting in activation of innate immune cells and initiation of adaptive immune responses [52, 53]. PRRs recognize a wide range of products derived from bacteria, viruses, fungi, protozoa, and parasites. Well-studied examples of these products include lipopolysaccharide (LPS) from Gram-negative bacteria, which binds to TLR4, bacterial and viral unmethylated cytosine-guanine rich nucleotide sequences (CpG DNA), which activates TLR9, and β-glucan from fungi, which binds to the CLRs Dectin-1 and Dectin-2. In addition, particulate adjuvants such as alum target the NLRP3 inflammasome, which is formed by the PRR NLRP3 along with ASC and Caspase-1, and is involved in sensing of crystallized endogenous molecules [54]. The activity of adjuvants, which are predominantly non-antigenic molecules, can thus enhance the immune response to proteins that are associated with them.
A large body of research has shown that activation of PRRs generally results in the production of proinflammatory cytokines such as tumor necrosis factor (TNF), IL-1, IL-6, IL-12, and type I IFNs, giving rise to the induction of Th1, Th17, and cytotoxic T cell differentiation and activation [53, 55]. In contrast, relatively little is known of the mechanisms governing Th2 responses. Recently, this area has received considerable attention, and accumulating evidence indicates that PRRs are also involved in orchestrating Th2-type immunity and allergic inflammation. More knowledge of the innate immune cells and receptors involved in sensing Th2-skewing pathogens and associated adjuvants may provide us with valuable clues as to how Th2 responses to allergens are initiated.
Parasites and adjuvants inducing Th2-type responses through innate immune activation
Differentiation of naïve T cells into the various types of regulatory and effector T cells is induced by professional APCs, which include DCs, macrophages, and B cells. APCs present antigen-derived peptides to T cells via major histocompatibility complex (MHC) I and MHC II molecules, which are bound by the T cell receptor. A defining feature of professional APCs is the expression of costimulatory molecules, which are essential for activation of naïve T cells. DCs express the highest levels of MHC and costimulatory molecules, and have a very large contact surface to their surroundings compared with overall cell volume. These features facilitate their function as the most important APCs [56, 57]. Consistent with these observations, the adaptive Th2 response to helminths is dependent on and driven by DCs [58, 59]. Soluble egg antigens (SEA) from S. mansoni have long been known to induce strong Th2 responses, and have therefore been studied to unravel mechanisms of Th2 immunity. Glycans in SEA were observed to bind to DCs via the CLRs dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN), macrophage galactose-type lectin (MGL), mannose receptor (MR), and Dectin-2, resulting in internalization of the antigens and DC-induced Th2 differentiation [14, 60, 61]. In addition, the SEA-derived glycoprotein and ribonuclease omega-1 was shown to activate DCs to induce Th2-skewing, which was dependent on its enzymatic activity [62, 63] (Fig. 1A).
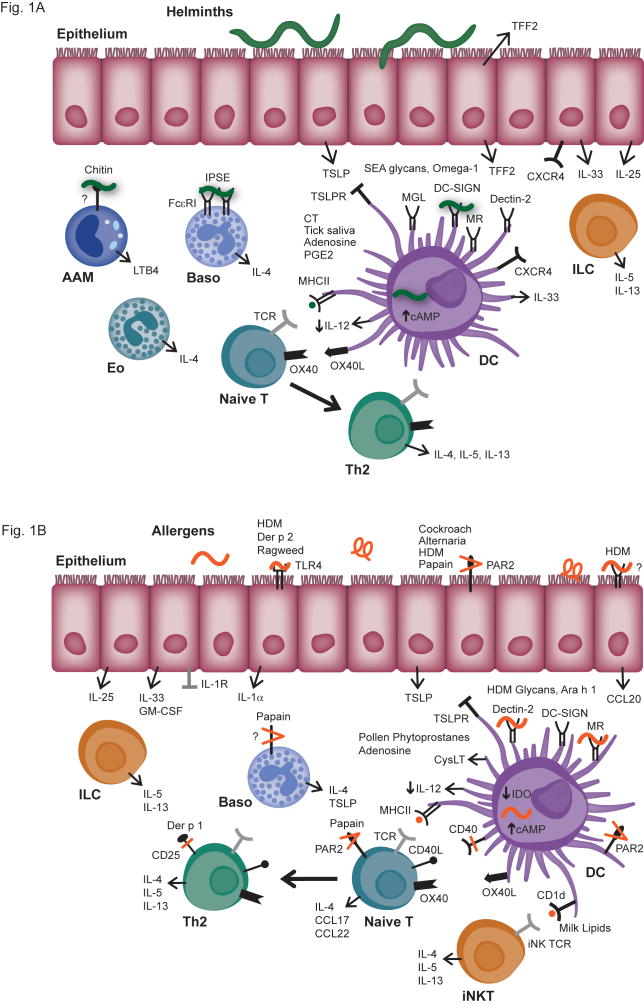
Fig. 1.
Fig. 1A. Pathways of innate immune activation associated with helminth infection.
Epithelial cells of the respiratory and gastrointestinal tract have been shown to produce several factors in response to helminth infection, including trefoil factor 2 (TFF2), TSLP, IL-25, and IL-33, all of which have been implicated in Th2 inflammation. In the airways, TFF2 signals via the putative receptor CXCR4 on respiratory epithelium, alveolar macrophages, and DC subsets. TSLP affects multiple cells, including basophils, DCs and lymphocytes to promote Th2 responses; on DCs, TSLP induces the expression of OX40L which can subsequently lead to Th2 differentiation of naïve T cells. IL-25 and IL-33 have important effects as well on a number of cells, but have particularly been shown to be involved in the activation of innate lymphoid cells (ILCs) to produce IL-5 and IL-13. A number of molecules derived from eggs of the parasite S. mansoni have been shown to directly stimulate innate immune cells, resulting in Th2-type responses. This includes the glycoprotein IPSE/alpha-1, which activates basophils via a non-polymorphic domain of IgE, other glycans that contribute to alternative activation of DCs via C-type lectin receptors (CLRs), and the ribonuclease omega-1 which activates DCs through an undefined receptor, resulting in Th2-skewing. A key event in promoting Th2 differentiation appears to be inhibition of IL-12 production by DCs, which is mediated by TSLP, certain helminth-derived products, as well as by CT and components in tick saliva, which act through induction of cAMP.
Fig. 1B. Pathways of innate immune activation associated with allergen exposure. Several of the same signaling pathways observed to promote Th2 immunity in the context of helminth infection are implicated in allergy. TSLP, IL-25 and IL-33 are induced by exposure to a variety of aeorallergens including those of house dust mite (HDM) and cockroach. Glycoallergens from HDM and peanut are recognized by CLRs expressed on DCs to induce alternative activation and promote Th2-skewing of CD4+ T cells. The proteolytic activity of Der p 1, papain and other allergens appears to be an important Th2-inducing signal, as Der p 1 cleaves CD40 and CD25, and protease allergens signal through protease-activated receptor 2 (PAR2) and other as yet unknown targets on epithelium and other innate immune cells. Der p 2 and ragweed allergen signal through TLR4 on epithelium and DCs, and several allergens inhibit production of IL-12 via induction of TSLP, cAMP, or other pathways, resulting in enhanced Th2 differentiation.
Tick bites induce IgE responses in mice and humans, and components in tick saliva have been observed to possess Th2-skewing properties. Exposure of DCs to tick saliva results in impaired expression of MHC I, MHC II, and costimulatory molecules, as well as enhanced capacity to promote Th2 differentiation [64]. Tick saliva was shown by a different group to inhibit production of TNF and the pivotal Th1-differentiating cytokine IL-12 by DCs, enhance secretion of IL-10, and decrease expression of CD40 and CD86. Two components in tick saliva, adenosine and prostaglandin E2 (PGE2), were found to induce these effects in DCs by enhancing cyclic adenosine monophosphate (cAMP) and activation of the enzyme protein kinase A [65]. Whereas these responses were suggested by the authors to be immunomodulatory, other studies have shown that similar effects can contribute to enhanced Th2 differentiation as well (see below).
In addition to DCs, other innate immune cells have been shown to be activated by helminth-derived products and contribute to Th2-type immune responses. An interesting recent study reported that infection with the hookworm N. brasiliensis induces production of trefoil factor 2 (TFF2) in the lung and intestine. TFF2 is a secreted protein that is mainly produced by epithelial cells and released within the mucus gel layer upon infection-induced injury. It controls the extent of lung injury caused by N. brasiliensis infection, and induces production of the Th2-promoting cytokine IL-33 via the putative TFF2 receptor CXCR4 in lung epithelial cells, alveolar macrophages, and inflammatory DCs. Moreover, TFF2-treated macrophages induced Th2 differentiation in an IL-33-dependent manner. Production of IL-33 was found to be necessary for host immunity against N. brasiliensis. Also in this study, increased expression of TFF2 was observed in the nasal mucosa of children with acute asthma, suggesting that TFF2 and IL-33 play an important role in immunity to helminths, as well as in asthma [66].
Thymic stromal lymphopoietin (TSLP) and IL-25 are two other cytokines that are produced by innate immune cells and have been shown to promote Th2 responses [67]. It was observed that infection with N. brasiliensis upregulates expression of IL-25 mainly in intestinal epithelial cells, and that immunity against N. brasiliensis is dependent on IL-25 [68]. TSLP is also predominantly produced by epithelial cells, and the expression of TSLP as well as IL-33 is induced by the murine helminth T. muris. Exogenous IL-33 also induces TSLP expression [69]. Moreover, TSLP signaling is critical for Th2 responses and immunity to T. muris, and TSLP inhibits production of IL-12 by DCs, which elucidates a mechanism behind the Th2-promoting effect of TSLP [70]. Another effect of TSLP that contributes to induction of Th2 differentiation, as well as maintenance of Th2 memory cells, is upregulation of the costimulatory molecule OX40 ligand (OX40L) on DCs, which binds to OX40 on naïve T cells [71, 72]. Products from various helminths have been shown to induce expression of OX40L on DCs, and OX40L is necessary for induction of Th2-type immunity and worm expulsion [73–75]. Whereas TSLP is an important player in the development of immune responses to some helminths, it has been observed to be redundant for immunity to the helminths N. brasiliensis and H. polygyrus [76]. In this study, it was shown that excretory/secretory products derived from these helminths were capable of directly suppressing DC production of IL-12, thus bypassing the need for TSLP. This effect was independent of signaling via TLR4, IL-10, or MyD88, which is a universal adapter protein and a component of the signaling pathways of all TLRs except TLR3. Together, these data indicate that the innate Th2-promoting cytokines IL-25, IL-33, and TSLP are involved in induction of Th2-type immunity against helminths (Fig. 1A). Nevertheless, the receptors on epithelial cells involved in sensing the helminth-derived products that trigger production of these cytokines are not yet known.
Basophils have been implicated as important inducers of Th2 responses to helminths and occupational allergens by serving as an early source of IL-4 and, at the same time, acting as APCs [77, 78]. More recent studies using superior technical approaches have modified these views and have demonstrated that DCs are the dominant APCs in induction of Th2 immunity to helminths and allergens [58, 79, 80]. However, basophils are the major producers of IL-4 during primary infection with N. brasiliensis, and are important effector cells in the protective response upon secondary infection with this helminth [81, 82]. In addition, the SEA-derived glycoprotein IPSE/alpha-1 induces production of IL-4 by basophils in an IgE-dependent manner, although the IgE does not have to be specific for IPSE/alpha-1 or cross-reactive with this protein. The IgE-dependent, antigen-nonspecific activation of basophils constitutes an innate mechanism for S. mansoni eggs to induce IL-4 production even before the generation of S. mansoni-specific IgE, and is likely to amplify Th2 priming by DCs [83]. Chitin, a biopolymer present in helminths as well as in fungi, crustaceans and insects, induces the accumulation in tissue of IL-4-expressing innate immune cells, including eosinophils and basophils, in a MyD88-independent manner. Recruitment of eosinophils and basophils was dependent on leukotriene B4, which was produced by chitin-induced AAMs, indicating that macrophages are involved in sensing of chitin [84]. In addition, AAMs induced by Th2-cell-derived IL-4 were shown in a different study to function as important effector cells in protective Th2-type immunity and parasite elimination [85]. During helminth infection, basophils may be activated by IL-3, IL-18, IL-33, or TSLP, as well as directly by helminth-derived products such as proteases and glycoproteins, although receptors involved in sensing these products have not yet been found [86, 87]. Recently, multiple types of ILCs have been identified, which are activated by IL-25 and IL-33, and produce IL-5 and IL-13. ILCs are important in the early response to helminth infection and the induction of Th2-type immunity [29] (Fig. 1A). It is currently unknown whether these cells are also directly activated by helminths.
Th2 responses can be induced by parasites, as well as by certain commonly used adjuvants. Aluminum-containing adjuvants, hereafter referred to as alum, have been used in vaccines for over 80 years, and continue to be the most widely used clinical adjuvants. Alum boosts humoral immunity by enhancing APC activity, resulting in increased T cell help to follicular B cells. Injection with alum promotes the local release of uric acid, which activates recruited monocytes via the NLRP3 inflammasome. These monocytes mature into inflammatory DCs, which induce a Th2-biased response [88–90]. Moreover, the particulate nature of alum causes it to stimulate production of the Th2-promoting PGE2 in macrophages [91]. A third mechanism by which alum induces Th2 responses is by causing cell death and the subsequent release of host cell DNA, which acts as a potent endogenous immunostimulatory signal [92]. Damage to host cells and tissues appears to be a potent inducer of Th2 responses, as another study has shown that mechanical injury upregulates TSLP levels in the skin, which polarizes skin DCs to elicit Th2-skewing [93]. The mucosal adjuvant cholera toxin (CT) is widely used in murine models for food allergy. Feeding antigens to mice without adjuvant normally gives rise to oral tolerance, but a combination of antigens and CT administered via the oral route results in antigen-specific Th2 responses and allergic sensitization [94]. CT is composed of an A subunit with ADP-ribosyltransferase activity and a pentameric B subunit that mediates toxin binding to the cell membrane through a high-affinity receptor, the ganglioside GM-1. Once CT is internalized by the cells, the A subunit ADP-ribosylates the α subunit of Gs protein, a GTP-binding protein which regulates the activity of adenylate cyclase, and causes an increase in intracellular cAMP [95]. Cholera toxin activates monocytes and DCs, upregulates expression of MHC and costimulatory molecules such as OX40L, and induces differentiation of monocytes into non-classical DCs which express high CD14 and low CD1a. Moreover, CT enhances production of IL-1β, IL-6, and IL-10, but inhibits release of TNF and IL-12 [73, 96–98].
Allergens inducing Th2-type responses through innate immune activation
Allergens derive from a variety of environmental sources, such as plants, fungi, arthropods, and mammals. Although allergens represent a minute fraction of the proteins that humans are routinely exposed to, allergenicity is a very public phenomenon, with the identical proteins functioning as allergens in a wide variety of allergic patients. These observations suggest that allergens share uncommon structural or functional characteristics that play a significant role in determining their allergenicity. As allergens are derived from complex living organisms, they serve a broad range of functions in their respective hosts, from structural to enzymatic. For instance, the common house dust mite allergens include several enzymes such as cysteine proteases (Der p 1, Der p 3), serine proteases (Der p 3, Der p 6, Der p 9), and chitinases (Der p 15, Der p 18), as well as lipid-binding molecules (Der p 2), and structural molecules such as tropomyosin (Der p 10). It should be kept in mind that natural exposure is not to single, purified proteins, but to complex mixtures of molecules, in which a given component may act as a Th2-skewing adjuvant and induce an IgE response to itself, or rather facilitate sensitization to a protein present in the same mixture. All these factors appear to be important in sensing of allergens by the innate immune system, and the resulting Th2-type response [99, 100]. We will first discuss the innate immune response to respiratory and occupational allergens, and follow with food allergens.
Respiratory and occupational allergens
Clinically important respiratory allergens include those derived from house dust mite (HDM), cockroach, grass and tree pollen, and fungi such as Alternaria and Aspergillus. Of these allergens, HDM extract and purified HDM allergens have been studied most extensively. Glycans in HDM extract were shown to induce Th2 differentiation in mice via binding to the CLR Dectin-2 on DCs and subsequent induction of cysteinyl leukotriene production [101]. The mannose receptor, another CLR, binds to and mediates internalization of a variety of allergens, such as Der p 1 and Der p 2, as well as the cockroach allergen Bla g 2 and the peanut allergen Ara h 1. Moreover, mannose receptor mediated Der p 1-induced Th2-skewing by DCs derived from HDM-sensitized donors through downregulation of indoleamine 2,3-dioxygenase (IDO) activity [102]. Interestingly, a different group reported that Der p 1 suppressed functional IDO in DCs from HDM-sensitized patients with asthma but enhanced IDO activity in DCs from nonatopic patients with asthma. In this study, modulation of IDO activity was dependent on the protease activity of Der p 1. Suppression of IDO in Der p 1-pulsed DCs from HDM-sensitized patients was maintained by the reciprocally induced IL-4 from cocultured autologous Der p 1-specific T cells. Conversely, the upregulation of IDO activity in DCs from nonatopic subjects was maintained by IFN-γ released from autologous Der p 1-specific T cells [103]. HDM extract, Der p 1 and Der p 2 have also been shown to bind to the CLR DC-SIGN, which facilitates their uptake by DCs [104–106]. HDM extract downregulates cell surface DC-SIGN due to endocytosis of the HDM extract-DC-SIGN complex, and inhibits differentiation of monocytes into CD14−CD1a+ DCs [105]. Lastly, airway epithelial cells stimulated with HDM extract showed enhanced secretion of CCL20, a chemokine attractant for immature DCs. This effect was not dependent on protease activity or TLR2/TLR4 signaling, but relied on β-glucan moieties within the HDM extract. Upregulation of CCL20 was not mediated by the CLR Dectin-1, a known receptor for β-glucans, but was dependent on activation of spleen tyrosine kinase (Syk), indicating that β-glucans in HDM are recognized by and signal through an immunoreceptor tyrosine-based activation motif (ITAM) pathway [107]. In sum, these data indicate that HDM glycoproteins and polysaccharides are detected by multiple CLRs, resulting in enhanced allergen uptake by DCs, downregulation of IDO activity in DCs from HDM-sensitized subjects, inhibition of DC maturation, recruitment of immature DCs, and Th2-skewing (Fig. 1B).
In addition to CLRs, TLRs have been shown to be involved in sensing respiratory allergens and induction of Th2-type responses. Intriguingly, Der p 2 was observed to have structural and functional homology with MD-2, the LPS-binding component of the TLR4 signaling complex. Because of this homology, Der p 2 in the presence of a very low concentration of LPS signals directly through TLR4, which plays an essential role in the induction of sensitization to Der p 2 and experimental allergic asthma [17]. HDM extract, which is naturally contaminated with LPS, was shown in another important study to activate lung epithelial cells through TLR4 and induce these cells to produce the innate proallergic cytokines TSLP, granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-25, and IL-33. In contrast, activation with LPS alone induced only GM-CSF and IL-33. Stimulation of lung epithelial cells with HDM extract resulted in the recruitment and activation of DCs, which initiated the HDM-specific Th2 response. Interestingly, TLR4 expression on lung epithelial cells, but not on DCs, was shown to be necessary and sufficient for DC activation and Th2 priming [108]. Recently, it was reported that bronchial epithelial cells produce IL-1α, but not IL-1β, upon stimulation with HDM extract. This effect is dependent on TLR4 signaling. IL-1α acts in an autocrine manner to trigger the release of GM-CSF and IL-33 by epithelial cells, and all three cytokines are crucial for the development of allergic sensitization to HDM. These findings put IL-1α upstream in the cytokine cascade leading to epithelial and DC activation in response to inhaled HDM allergen [109]. A different allergen, short ragweed pollen, has also been reported to stimulate epithelial cells through TLR4. Activation of epithelium by short ragweed pollen extract induced production of TSLP, which was dependent on TLR4 and resulted in OX40L/OX40 signaling and Th2 responses in the ocular mucosa and draining cervical lymph nodes [110]. Another study indicated that the structure of the HDM allergen Der p 7 has distant homology to a family of proteins involved in recognition of bacterial lipid products by the human innate immune system. These structural features may also facilitate TLR signaling [111].
Several respiratory allergens have cysteine or serine protease activity, and this enzymatic activity has been shown in numerous studies to influence innate immune responses and enhance allergenicity of these proteins [99]. For example, the HDM allergen Der p 1 cleaves the co-stimulatory molecule CD40 on human monocyte-derived DCs (MoDCs), rendering the DCs less responsive to stimulation by CD40 ligand (CD40L)-expressing T cells. This leads to downregulated production of IL-12 by DCs, and an increase in Th2 differentiation of cocultured naïve T cells [112]. Moreover, Der p 1 has been reported to act directly on T cells, by cleaving the α-chain of the IL-2 receptor (CD25). As a result, T cells exposed to Der p 1 show markedly reduced Th1 cytokine production and enhanced Th2 cytokine release [113]. Cleavage of CD25 might also alter regulatory function, as IL-2 stimulation is required for the maintenance of regulatory T cells in the periphery. The overall effect may be to shift the balance of immune responses from a tolerogenic to a Th2-dominated response.
Some allergenic proteases appear to exert Th2-skewing effects through stimulation of the protease-activated receptor 2 (PAR2). This receptor is expressed by numerous cell types in the lung, including airway epithelial cells, fibroblasts, macrophages, and mast cells. Intranasal administration of cockroach extract has been observed to activate PAR2 in the airways, which is necessary for the induction of allergic airway inflammation. PAR2 activation by cockroach extract is dependent on protease activity and acts as an adjuvant for allergic sensitization even in the absence of functional TLR4 [114]. A different study showed that adoptive transfer of cockroach allergen-stimulated DCs from wild-type but not from PAR2-deficient mice could induce Th2-type responses and allergic airway inflammation in wild-type recipients [115]. Protease allergens from the fungi Alternaria and Aspergillus also directly stimulate DCs to induce Th2-skewing, which may be due to relatively low IL-12 production and increased OX40L expression by these DCs [116, 117]. Moreover, airway epithelial cells were reported to increase the expression of TSLP through the activation of PAR2 when stimulated with Alternaria or the occupational allergen papain [118]. As TSLP activates DCs to polarize naïve T cells to a Th2 phenotype, these results suggest that PAR2 activation serves as a link between innate and adaptive immune responses. Interestingly, a recent study showed that both murine and human naïve CD4+ T cells also express PAR2 on the cell surface. Direct activation of T cells through PAR2 by papain induced production of IL-4 and the chemokines CCL17 and CCL22, which are chemoattractants for basophils and Th2 cells [119] (Fig. 1B). The role of PAR2 in sensing allergens appears to be clinically relevant, as expression of PAR2 is increased in bronchial epithelium of patients with asthma [120]. A single polymorphism of PAR2 is positively associated with PAR2 expression, as well as with atopy, serum IgE levels, and total eosinophil counts [121]. Furthermore, expression of PAR2 is increased in submucosal glands of patients with allergic rhinitis or chronic rhinosinusitis, and activation of PAR2 by HDM extract induces glandular secretion of airway surface fluid in these patients, but not in healthy controls [122].
The enzyme papain, also known as papaya proteinase 1, is used in the food industry to break down tough meat fibres, and is an important occupational allergen. Papain has recently been extensively studied in murine models, and was observed to trigger production of TSLP and IL-33 by lung stromal cells, which activated ILCs in the lung to produce IL-5 and IL-13. Wild-type mice and Rag1 knockout mice, which produce no mature T cells or B cells, showed similar levels of IL-5 and IL-13 after papain treatment. Thus, ILCs appear to be an important T cell-independent early source of IL-5 and IL-13 in protease allergen-induced lung inflammation [123]. Moreover, ILCs may be relevant in HDM-induced allergic asthma, because the number of IL-5+ and IL-13+ ILCs in the lung after HDM challenge was shown to be in the same range as found for Th2 cells [124]. In humans, ILCs are present in the lung and gut and are enriched in nasal polyps of patients with chronic rhinosinusitis, suggesting that ILCs also play a role in human allergic inflammation [125]. In addition to stimulating non-hematopoietic cells, papain has been reported to directly activate basophils to produce IL-4, TSLP, and other Th2-associated cytokines and chemokines. Basophils were found to be essential for Th2 responses induced by papain, and appeared to function as APCs in the induction of these responses [78, 126]. However, a more recent study provided a different picture, by showing that immunization with papain results in production of reactive oxygen species in DCs and epithelial cells, which trigger production of TSLP by epithelial cells and suppress release of IL-12 by DCs. Basophils are recruited to the lymph node upon immunization with papain, and cooperate with DCs in induction of Th2 responses, with DCs presenting antigen and basophils providing IL-4 [127] (Fig. 1B). These findings were corroborated by a study showing that induction of Th2 responses to HDM allergen is dependent on inflammatory DCs, although exposure to inhaled HDM extract leads to recruitment of IL-4-competent basophils, and depletion of basophils partially reduces Th2 immunity [80]. Furthermore, recent studies have reported that human basophils do not act as APCs in the induction of T cell responses to the major birch pollen allergen, Bet v 1 [128, 129].
Birch pollen grains contain well-characterized protein allergens, as well as bioactive lipids such as phytoprostanes. It has been observed that pollen-derived E1-phytoprostanes inhibit IL-12 production by DCs, and that these lipids resemble endogenous PGE2 both structurally and functionally, in that they modulate human DC function in a fashion that favors Th2 cell polarization [130]. The inhibitory effect of E1-phytoprostanes on IL-12 production by DCs was shown to be dependent on signaling via peroxisome proliferator-activated receptor-γ (PPAR-γ), leading to inhibition of NF-κB activation [131]. Another pollen-derived component, adenosine, was found to inhibit IL-12 release by DCs through induction of cAMP [132]. Interestingly, this effect mimics the responses to tick saliva and the Th2-skewing adjuvant CT, which also downregulate IL-12 production in DCs by increasing cAMP [65, 133] (Fig. 1B).
NOD-like receptors (NLRs) are present in the cytoplasm and are involved in sensing microbe-derived products as well as endogenous danger signals [134]. Immunization with antigen plus agonists of Nod1 or Nod2 was reported to result in priming of Th2 responses. This effect was dependent on expression of Nod1 and Nod2 within the stromal compartment, TSLP production by stromal cells, and upregulation of the costimulatory molecule OX40L on DCs. Nod activation in DCs did not initiate Th2 immunity but was required for antigen presentation and optimal antigen-specific Th2 responses [135]. So far, direct activation of NLRs by allergens has not yet been reported. Lastly, inhalation of HDM allergen has been shown to trigger the release of uric acid in the airways of asthmatic patients and mice, where it is necessary for mounting Th2 immunity. Uric acid crystals are known to induce acute neutrophilic inflammation through stimulation of the NLRP3 inflammasome and release of IL-1β. Nevertheless, the Th2-skewing effect of uric acid was not dependent on activation of the NLRP3 inflammasome. Rather, uric acid induced Th2 cell immunity by triggering DC activation in a Syk- and PI3-kinase δ-dependent manner [136].
Food allergens
The most allergenic foods are cow’s milk, hen’s egg, peanut, tree nuts, fish, shellfish, wheat, and soy, and these are the cause of the large majority of food allergy cases in the United States [21]. Relative to the wealth of information on innate immunostimulatory properties of respiratory allergens, our understanding of innate sensing of food proteins is still fairly limited, although direct activation of innate immune cells by food allergens has been reported. Our laboratory observed that the glycoprotein Ara h 1, one of the major peanut allergens, stimulates human MoDCs to induce Th2 differentiation in naive T cells. Ara h 1 was further shown to bind to the CLR DC-SIGN, and induce phosphorylation of extracellular signal-regulated kinase (Erk) 1/2 in DCs. Deglycosylated Ara h 1 did not have a Th2-skewing effect, confirming that allergen-bound carbohydrate structures can act as a Th2-promoting adjuvant [137] (Fig. 1B). In agreement with these findings, other studies have reported that glycation of proteins enhances their uptake by DCs and T cell immunogenicity, as well as their ability to induce Th2 differentiation [138–140]. Incubation of DCs with antigen-coupled Lewis-x trisaccharides suppressed production of IL-12, which is probably important in the mechanism of Th2-skewing induced by these glycans [140].
Whereas Ara h 1 has intrinsic adjuvant activity, whole peanut extract possesses much higher immune-stimulating capacity than the purified allergens Ara h 1, Ara h 2, and Ara h 6. Whole peanut extract injected into the footpad of mice without exogenous adjuvant was shown to induce an increase in cell number, cytokine production, and activation of APCs in the popliteal lymph node. Furthermore, the presence of associated components in peanut extract enhanced the immune response to the individual allergens. In contrast, injection with the purified peanut allergens did not have these effects, indicating that other molecules in peanut extract act as adjuvants to promote immune responses to the individual peanut allergens [141]. In this light, we recently observed that a previously undescribed nonglycosylated peanut protein upregulates expression of the rate-limiting retinoic acid-producing enzyme retinaldehyde dehydrogenase 2 (RALDH2) in human myeloid DCs. Retinoic acid produced by these DCs acts on naive T cells to induce expression of the gut-homing α4β7 integrin and production of IL-5 [142]. The RALDH2-inducing peanut protein is not a major allergen, and appears to be a member of the plant glycine-rich protein superfamily [143]. Another as yet unidentified component in peanut extract, probably of low molecular weight (<5 kD), has been shown to activate complement in vitro in mice and humans, and the complement component 3a (C3a) was observed to mediate peanut extract-induced anaphylactic shock in susceptible mice. Platelet-activating factor, as well as macrophages and basophils, play an important role in peanut extract-induced shock. Whereas complement activation by itself is not sufficient to induce shock in normal mice and humans, peanut extract-induced C3a production may act synergistically with IgE/FcεRI-dependent mast cell degranulation to exacerbate anaphylaxis. Extracts from cashew nuts, walnuts, and almonds also activated complement and induced shock, whereas this effect was not observed with cow’s milk and hen’s egg white. In contrast to peanuts and tree nuts, which are associated with severe anaphylaxis, milk and egg white typically cause relatively mild allergic reactions. The association between complement activation and induction of severe allergic symptoms may reflect complement exacerbation of the effector phase of anaphylaxis [144].
Innate Th2-type immune responses can also be induced by components in cow’s milk. Invariant natural killer T (iNKT) cells recognize lipid antigens presented via the nonpolymorphic CD1d molecule on DCs and other APCs, and exhibit characteristics that place them at the border between innate and adaptive immunity. An interesting recent study reported that sphingolipid isolated from cow’s milk induces iNKT cell proliferation and secretion of IL-4, IL-5, and IL-13, whereas stimulation with the well-described iNKT cell agonist α-galactosylceramide results mainly in production of IFN-γ. iNKT cells from children with cow’s milk allergy produced more IL-4 and IL-13 in response to cow’s milk sphingolipid than those from nonallergic children [145]. Thus, cow’s milk sphingolipid-specific iNKT cells could give rise to a proallergic microenvironment which facilitates the induction of cow’s milk protein-specific Th2 cells and sensitization to cow’s milk (Fig. 1B).
Whereas a limited number of food allergens have been shown to induce innate immune responses that may add to their allergenicity, other food components rather have an innate immunosuppressive effect. Isoflavones are anti-inflammatory molecules that occur naturally in soybean. In a murine model for peanut allergy, oral exposure to these isoflavones reduced allergic symptoms upon challenge with peanut. Moreover, isoflavones inhibited CT-induced DC maturation in the mesenteric lymph nodes and in human MoDCs, and decreased production of Th2 cytokines in cocultures of CT-primed DCs and CD4+ naive T cells. The immunosuppressive capacity of isoflavones might account for the fact that soy is less allergenic than peanut, whereas proteins from both species share extensive amino acid sequence homology [146].
In murine models for food allergy, a combination of antigen with a Th2-skewing adjuvant, such as CT or staphylococcal enterotoxin B (SEB), is generally used to establish food allergy through the oral route of exposure. These adjuvants have profound effects on innate immune cells in the gastrointestinal tract, most importantly on DCs. DCs play a crucial role in the development of food protein-specific Th2 responses and allergy to food [147–149]. Oral administration of CT induces maturation and expression of OX40L in mesenteric lymph node DCs. Binding of OX40L to OX40 on naïve T cells is necessary for CT-induced Th2 differentiation [98]. In addition, both CT and SEB have been shown to increase expression of T cell immunoglobulin and mucin domain-containing protein (TIM)4 on DCs, and induction of Th2 responses to coadministered antigens is dependent on ligation of TIM4 with TIM1 on naïve T cells [150, 151]. Although it is currently unknown whether certain food allergens can independently induce expression of OX40L and/or TIM4, these observations indicate that induction of these surface molecules on DCs may be important in the development of food allergy.
Conclusion
Th2 immunity is thought to have evolved primarily in response to chronic parasitism by multicellular organisms and perhaps because of this it is both complexly regulated and integrated with other homeostatic processes such as wound repair and metabolism. Understanding the multifarious pathways that innate immune cells use to integrate external and intrinsic signals to shape adaptive immunity, as well as the intrinsically allergenic features of allergens or intimately associated molecules that are recognized by those cells, will likely provide new insights into primary prevention and treatment of food allergy.
Footnotes
This article is published as part of the Special Issue on Food Allergy [34:6]
References
- 1.Skypala IJ, Calderon MA, Leeds AR, Emery P, Till SJ, Durham SR. Development and validation of a structured questionnaire for the diagnosis of oral allergy syndrome in subjects with seasonal allergic rhinitis during the UK birch pollen season. Clin Exp Allergy. 2011;41:1001–11. doi: 10.1111/j.1365-2222.2011.03759.x. [DOI] [PubMed] [Google Scholar]
- 2.Aalberse RC. Structural biology of allergens. J Allergy Clin Immunol. 2000;106:228–38. doi: 10.1067/mai.2000.108434. [DOI] [PubMed] [Google Scholar]
- 3.Sicherer SH. Epidemiology of food allergy. J Allergy Clin Immunol. 2011;127:594–602. doi: 10.1016/j.jaci.2010.11.044. [DOI] [PubMed] [Google Scholar]
- 4.Aalberse RC, Stadler BM. In silico predictability of allergenicity: from amino acid sequence via 3-D structure to allergenicity. Mol Nutr Food Res. 2006;50:625–7. doi: 10.1002/mnfr.200500270. [DOI] [PubMed] [Google Scholar]
- 5.Radauer C, Bublin M, Wagner S, Mari A, Breiteneder H. Allergens are distributed into few protein families and possess a restricted number of biochemical functions. J Allergy Clin Immunol. 2008;121:847–52. e7. doi: 10.1016/j.jaci.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 6.Ivanciuc O, Garcia T, Torres M, Schein CH, Braun W. Characteristic motifs for families of allergenic proteins. Mol Immunol. 2009;46:559–68. doi: 10.1016/j.molimm.2008.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.http://www.meduniwien.ac.at/allergens/allfam/
- 8.Astwood JD, Leach JN, Fuchs RL. Stability of food allergens to digestion in vitro. Nat Biotech. 1996;14:1269–73. doi: 10.1038/nbt1096-1269. [DOI] [PubMed] [Google Scholar]
- 9.Bannon GA. What makes a food protein an allergen? Curr Allergy Asthma Rep. 2004;4:43–6. doi: 10.1007/s11882-004-0042-0. [DOI] [PubMed] [Google Scholar]
- 10.Fu TJ, Abbott UR, Hatzos C. Digestibility of food allergens and nonallergenic proteins in simulated gastric fluid and simulated intestinal fluid-a comparative study. J Agric Food Chem. 2002;50:7154–60. doi: 10.1021/jf020599h. [DOI] [PubMed] [Google Scholar]
- 11.Herman RA, Woolhiser MM, Ladics GS, Korjagin VA, Schafer BW, Storer NP, Green SB, Kan L. Stability of a set of allergens and non-allergens in simulated gastric fluid. Int J Food Sci Nutr. 2007;58:125–41. doi: 10.1080/09637480601149640. [DOI] [PubMed] [Google Scholar]
- 12.Pedrosa C, De Felice FG, Trisciuzzi C, Ferreira ST. Selective neoglycosylation increases the structural stability of vicilin, the 7S storage globulin from pea seeds. Arch Biochem Biophys. 2000;382:203–10. doi: 10.1006/abbi.2000.2024. [DOI] [PubMed] [Google Scholar]
- 13.Altmann F. The role of protein glycosylation in allergy. Int Arch Allergy Immunol. 2007;142:99–115. doi: 10.1159/000096114. [DOI] [PubMed] [Google Scholar]
- 14.Faveeuw C, Mallevaey T, Paschinger K, Wilson IB, Fontaine J, Mollicone R, Oriol R, Altmann F, Lerouge P, Capron M, Trottein F. Schistosome N-glycans containing core alpha 3-fucose and core beta 2-xylose epitopes are strong inducers of Th2 responses in mice. Eur J Immunol. 2003;33:1271–81. doi: 10.1002/eji.200323717. [DOI] [PubMed] [Google Scholar]
- 15.Bencurova M, Hemmer W, Focke-Tejkl M, Wilson IB, Altmann F. Specificity of IgG and IgE antibodies against plant and insect glycoprotein glycans determined with artificial glycoforms of human transferrin. Glycobiology. 2004;14:457–66. doi: 10.1093/glycob/cwh058. [DOI] [PubMed] [Google Scholar]
- 16.Breiteneder H, Mills EN. Molecular properties of food allergens. J Allergy Clin Immunol. 2005;115:14–23. doi: 10.1016/j.jaci.2004.10.022. quiz 4. [DOI] [PubMed] [Google Scholar]
- 17.Trompette A, Divanovic S, Visintin A, Blanchard C, Hegde RS, Madan R, Thorne PS, Wills-Karp M, Gioannini TL, Weiss JP, Karp CL. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature. 2009;457:585–8. doi: 10.1038/nature07548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mills EN, Jenkins JA, Alcocer MJ, Shewry PR. Structural, biological, and evolutionary relationships of plant food allergens sensitizing via the gastrointestinal tract. Crit Rev Food Sci Nutr. 2004;44:379–407. doi: 10.1080/10408690490489224. [DOI] [PubMed] [Google Scholar]
- 19.Considine T, Patel HA, Singh H, Creamer LK. Influence of binding of sodium dodecyl sulfate, all-trans-retinol, palmitate, and 8-anilino-1-naphthalenesulfonate on the heat-induced unfolding and aggregation of beta-lactoglobulin B. J Agric Food Chem. 2005;53:3197–205. doi: 10.1021/jf0481756. [DOI] [PubMed] [Google Scholar]
- 20.Roth-Walter F, Berin MC, Arnaboldi P, Escalante CR, Dahan S, Rauch J, Jensen-Jarolim E, Mayer L. Pasteurization of milk proteins promotes allergic sensitization by enhancing uptake through Peyer’s patches. Allergy. 2008;63:882–90. doi: 10.1111/j.1398-9995.2008.01673.x. [DOI] [PubMed] [Google Scholar]
- 21.Boyce JA, Assa’ad A, Burks AW, Jones SM, Sampson HA, Wood RA, Plaut M, Cooper SF, Fenton MJ, Arshad SH, Bahna SL, Beck LA, Byrd-Bredbenner C, Camargo CA, Jr, Eichenfield L, Furuta GT, Hanifin JM, Jones C, Kraft M, Levy BD, Lieberman P, Luccioli S, McCall KM, Schneider LC, Simon RA, Simons FE, Teach SJ, Yawn BP, Schwaninger JM. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126:S1–58. doi: 10.1016/j.jaci.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wills-Karp M, Finkelman FD. Untangling the complex web of IL-4- and IL-13-mediated signaling pathways. Sci Signal. 2008;1:pe55. doi: 10.1126/scisignal.1.51.pe55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takatsu K, Kouro T, Nagai Y. Interleukin 5 in the link between the innate and acquired immune response. Adv Immunol. 2009;101:191–236. doi: 10.1016/S0065-2776(08)01006-7. [DOI] [PubMed] [Google Scholar]
- 24.Allen JE, Maizels RM. Diversity and dialogue in immunity to helminths. Nat Rev Immunol. 2011;11:375–88. doi: 10.1038/nri2992. [DOI] [PubMed] [Google Scholar]
- 25.Vignali DA, Crocker P, Bickle QD, Cobbold S, Waldmann H, Taylor MG. A role for CD4+ but not CD8+ T cells in immunity to Schistosoma mansoni induced by 20 krad-irradiated and Ro 11–3128-terminated infections. Immunology. 1989;67:466–72. [PMC free article] [PubMed] [Google Scholar]
- 26.Katona IM, Urban JF, Jr, Finkelman FD. The role of L3T4+ and Lyt-2+ T cells in the IgE response and immunity to Nippostrongylus brasiliensis. J Immunol. 1988;140:3206–11. [PubMed] [Google Scholar]
- 27.Mohrs M, Shinkai K, Mohrs K, Locksley RM. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 2001;15:303–11. doi: 10.1016/s1074-7613(01)00186-8. [DOI] [PubMed] [Google Scholar]
- 28.Mitre E, Taylor RT, Kubofcik J, Nutman TB. Parasite antigen-driven basophils are a major source of IL-4 in human filarial infections. J Immunol. 2004;172:2439–45. doi: 10.4049/jimmunol.172.4.2439. [DOI] [PubMed] [Google Scholar]
- 29.Spits H, Cupedo T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Ann Rev Immunol. 2012;30:647–75. doi: 10.1146/annurev-immunol-020711-075053. [DOI] [PubMed] [Google Scholar]
- 30.Paul WE, Zhu J. How are T(H)2-type immune responses initiated and amplified? Nat Rev Immunol. 2010;10:225–35. doi: 10.1038/nri2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dunne DW, Butterworth AE, Fulford AJ, Kariuki HC, Langley JG, Ouma JH, Capron A, Pierce RJ, Sturrock RF. Immunity after treatment of human schistosomiasis: association between IgE antibodies to adult worm antigens and resistance to reinfection. Eur J Immunol. 1992;22:1483–94. doi: 10.1002/eji.1830220622. [DOI] [PubMed] [Google Scholar]
- 32.Hagan P, Blumenthal UJ, Dunn D, Simpson AJ, Wilkins HA. Human IgE, IgG4 and resistance to reinfection with Schistosoma haematobium. Nature. 1991;349:243–5. doi: 10.1038/349243a0. [DOI] [PubMed] [Google Scholar]
- 33.Jiz M, Friedman JF, Leenstra T, Jarilla B, Pablo A, Langdon G, Pond-Tor S, Wu HW, Manalo D, Olveda R, Acosta L, Kurtis JD. Immunoglobulin E (IgE) responses to paramyosin predict resistance to reinfection with Schistosoma japonicum and are attenuated by IgG4. Infect Immun. 2009;77:2051–8. doi: 10.1128/IAI.00012-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Black CL, Muok EM, Mwinzi PN, Carter JM, Karanja DM, Secor WE, Colley DG. Increases in levels of schistosome-specific immunoglobulin E and CD23(+) B cells in a cohort of Kenyan children undergoing repeated treatment and reinfection with Schistosoma mansoni. J Infect Dis. 2010;202:399–405. doi: 10.1086/653828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Capron M, Capron A. Immunoglobulin E and effector cells in schistosomiasis. Science. 1994;264:1876–7. doi: 10.1126/science.8009216. [DOI] [PubMed] [Google Scholar]
- 36.Gurish MF, Bryce PJ, Tao H, Kisselgof AB, Thornton EM, Miller HR, Friend DS, Oettgen HC. IgE enhances parasite clearance and regulates mast cell responses in mice infected with Trichinella spiralis. J Immunol. 2004;172:1139–45. doi: 10.4049/jimmunol.172.2.1139. [DOI] [PubMed] [Google Scholar]
- 37.Watanabe N, Katakura K, Kobayashi A, Okumura K, Ovary Z. Protective immunity and eosinophilia in IgE-deficient SJA/9 mice infected with Nippostrongylus brasiliensis and Trichinella spiralis. PNAS. 1988;85:4460–2. doi: 10.1073/pnas.85.12.4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.El Ridi R, Ozaki T, Kamiya H. Schistosoma mansoni infection in IgE-producing and IgE-deficient mice. J Parasitol. 1998;84:171–4. [PubMed] [Google Scholar]
- 39.McCoy KD, Stoel M, Stettler R, Merky P, Fink K, Senn BM, Schaer C, Massacand J, Odermatt B, Oettgen HC, Zinkernagel RM, Bos NA, Hengartner H, Macpherson AJ, Harris NL. Polyclonal and specific antibodies mediate protective immunity against enteric helminth infection. Cell Host Microbe. 2008;4:362–73. doi: 10.1016/j.chom.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 40.Hepworth MR, Danilowicz-Luebert E, Rausch S, Metz M, Klotz C, Maurer M, Hartmann S. Mast cells orchestrate type 2 immunity to helminths through regulation of tissue-derived cytokines. PNAS. 2012;109:6644–9. doi: 10.1073/pnas.1112268109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wada T, Ishiwata K, Koseki H, Ishikura T, Ugajin T, Ohnuma N, Obata K, Ishikawa R, Yoshikawa S, Mukai K, Kawano Y, Minegishi Y, Yokozeki H, Watanabe N, Karasuyama H. Selective ablation of basophils in mice reveals their nonredundant role in acquired immunity against ticks. J Clin Invest. 2010;120:2867–75. doi: 10.1172/JCI42680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Commins SP, James HR, Kelly LA, Pochan SL, Workman LJ, Perzanowski MS, Kocan KM, Fahy JV, Nganga LW, Ronmark E, Cooper PJ, Platts-Mills TA. The relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2011;127:1286–93. e6. doi: 10.1016/j.jaci.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Behnke JM, Barnard CJ, Wakelin D. Understanding chronic nematode infections: evolutionary considerations, current hypotheses and the way forward. Int J Parasitol. 1992;22:861–907. doi: 10.1016/0020-7519(92)90046-n. [DOI] [PubMed] [Google Scholar]
- 44.Ricardo-Gonzalez RR, Red Eagle A, Odegaard JI, Jouihan H, Morel CR, Heredia JE, Mukundan L, Wu D, Locksley RM, Chawla A. IL-4/STAT6 immune axis regulates peripheral nutrient metabolism and insulin sensitivity. PNAS. 2010;107:22617–22. doi: 10.1073/pnas.1009152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–7. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palm NW, Rosenstein RK, Medzhitov R. Allergic host defences. Nature. 2012;484:465–72. doi: 10.1038/nature11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Artis D, Maizels RM, Finkelman FD. Forum: Immunology: Allergy challenged. Nature. 2012;484:458–9. doi: 10.1038/484458a. [DOI] [PubMed] [Google Scholar]
- 48.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–20. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 49.Shpacovitch V, Feld M, Hollenberg MD, Luger TA, Steinhoff M. Role of protease-activated receptors in inflammatory responses, innate and adaptive immunity. J Leukoc Biol. 2008;83:1309–22. doi: 10.1189/jlb.0108001. [DOI] [PubMed] [Google Scholar]
- 50.Areschoug T, Gordon S. Scavenger receptors: role in innate immunity and microbial pathogenesis. Cell Microbiol. 2009;11:1160–9. doi: 10.1111/j.1462-5822.2009.01326.x. [DOI] [PubMed] [Google Scholar]
- 51.Minnicozzi M, Sawyer RT, Fenton MJ. Innate immunity in allergic disease. Immunol Rev. 2011;242:106–27. doi: 10.1111/j.1600-065X.2011.01025.x. [DOI] [PubMed] [Google Scholar]
- 52.Akira S. Innate immunity and adjuvants. Philos Trans R Soc Lond B Biol Sci. 2011;366:2748–55. doi: 10.1098/rstb.2011.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–50. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 54.De Gregorio E, D’Oro U, Wack A. Immunology of TLR-independent vaccine adjuvants. Curr Opin Immunol. 2009;21:339–45. doi: 10.1016/j.coi.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 55.Palm NW, Medzhitov R. Pattern recognition receptors and control of adaptive immunity. Immunol Rev. 2009;227:221–33. doi: 10.1111/j.1600-065X.2008.00731.x. [DOI] [PubMed] [Google Scholar]
- 56.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 57.Itano AA, Jenkins MK. Antigen presentation to naive CD4 T cells in the lymph node. Nat Immunol. 2003;4:733–9. doi: 10.1038/ni957. [DOI] [PubMed] [Google Scholar]
- 58.Phythian-Adams AT, Cook PC, Lundie RJ, Jones LH, Smith KA, Barr TA, Hochweller K, Anderton SM, Hammerling GJ, Maizels RM, MacDonald AS. CD11c depletion severely disrupts Th2 induction and development in vivo. J Exp Med. 2010;207:2089–96. doi: 10.1084/jem.20100734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.MacDonald AS, Pearce EJ. Cutting edge: polarized Th cell response induction by transferred antigen-pulsed dendritic cells is dependent on IL-4 or IL-12 production by recipient cells. J Immunol. 2002;168:3127–30. doi: 10.4049/jimmunol.168.7.3127. [DOI] [PubMed] [Google Scholar]
- 60.van Liempt E, van Vliet SJ, Engering A, Garcia Vallejo JJ, Bank CM, Sanchez-Hernandez M, van Kooyk Y, van Die I. Schistosoma mansoni soluble egg antigens are internalized by human dendritic cells through multiple C-type lectins and suppress TLR-induced dendritic cell activation. Mol Immunol. 2007;44:2605–15. doi: 10.1016/j.molimm.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 61.Ritter M, Gross O, Kays S, Ruland J, Nimmerjahn F, Saijo S, Tschopp J, Layland LE, Prazeres da Costa C. Schistosoma mansoni triggers Dectin-2, which activates the Nlrp3 inflammasome and alters adaptive immune responses. PNAS. 2010;107:20459–64. doi: 10.1073/pnas.1010337107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Steinfelder S, Andersen JF, Cannons JL, Feng CG, Joshi M, Dwyer D, Caspar P, Schwartzberg PL, Sher A, Jankovic D. The major component in schistosome eggs responsible for conditioning dendritic cells for Th2 polarization is a T2 ribonuclease (omega-1) J Exp Med. 2009;206:1681–90. doi: 10.1084/jem.20082462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Everts B, Perona-Wright G, Smits HH, Hokke CH, van der Ham AJ, Fitzsimmons CM, Doenhoff MJ, van der Bosch J, Mohrs K, Haas H, Mohrs M, Yazdanbakhsh M, Schramm G. Omega-1, a glycoprotein secreted by Schistosoma mansoni eggs, drives Th2 responses. J Exp Med. 2009;206:1673–80. doi: 10.1084/jem.20082460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Skallova A, Iezzi G, Ampenberger F, Kopf M, Kopecky J. Tick saliva inhibits dendritic cell migration, maturation, and function while promoting development of Th2 responses. J Immunol. 2008;180:6186–92. doi: 10.4049/jimmunol.180.9.6186. [DOI] [PubMed] [Google Scholar]
- 65.Oliveira CJ, Sa-Nunes A, Francischetti IM, Carregaro V, Anatriello E, Silva JS, Santos IK, Ribeiro JM, Ferreira BR. Deconstructing tick saliva: non-protein molecules with potent immunomodulatory properties. J Biol Chem. 2011;286:10960–9. doi: 10.1074/jbc.M110.205047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wills-Karp M, Rani R, Dienger K, Lewkowich I, Fox JG, Perkins C, Lewis L, Finkelman FD, Smith DE, Bryce PJ, Kurt-Jones EA, Wang TC, Sivaprasad U, Hershey GK, Herbert DR. Trefoil factor 2 rapidly induces interleukin 33 to promote type 2 immunity during allergic asthma and hookworm infection. J Exp Med. 2012;209:607–22. doi: 10.1084/jem.20110079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oliphant CJ, Barlow JL, McKenzie AN. Insights into the initiation of type 2 immune responses. Immunology. 2011;134:378–85. doi: 10.1111/j.1365-2567.2011.03499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao A, Urban JF, Jr, Sun R, Stiltz J, Morimoto M, Notari L, Madden KB, Yang Z, Grinchuk V, Ramalingam TR, Wynn TA, Shea-Donohue T. Critical role of IL-25 in nematode infection-induced alterations in intestinal function. J Immunol. 2010;185:6921–9. doi: 10.4049/jimmunol.1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Humphreys NE, Xu D, Hepworth MR, Liew FY, Grencis RK. IL-33, a potent inducer of adaptive immunity to intestinal nematodes. J Immunol. 2008;180:2443–9. doi: 10.4049/jimmunol.180.4.2443. [DOI] [PubMed] [Google Scholar]
- 70.Taylor BC, Zaph C, Troy AE, Du Y, Guild KJ, Comeau MR, Artis D. TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J Exp Med. 2009;206:655–67. doi: 10.1084/jem.20081499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, Qin FX, Yao Z, Cao W, Liu YJ. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202:1213–23. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang YH, Ito T, Homey B, Watanabe N, Martin R, Barnes CJ, McIntyre BW, Gilliet M, Kumar R, Yao Z, Liu YJ. Maintenance and polarization of human TH2 central memory T cells by thymic stromal lymphopoietin-activated dendritic cells. Immunity. 2006;24:827–38. doi: 10.1016/j.immuni.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 73.de Jong EC, Vieira PL, Kalinski P, Schuitemaker JH, Tanaka Y, Wierenga EA, Yazdanbakhsh M, Kapsenberg ML. Microbial compounds selectively induce Th1 cell-promoting or Th2 cell-promoting dendritic cells in vitro with diverse th cell-polarizing signals. J Immunol. 2002;168:1704–9. doi: 10.4049/jimmunol.168.4.1704. [DOI] [PubMed] [Google Scholar]
- 74.Ekkens MJ, Liu Z, Liu Q, Whitmire J, Xiao S, Foster A, Pesce J, VanNoy J, Sharpe AH, Urban JF, Gause WC. The role of OX40 ligand interactions in the development of the Th2 response to the gastrointestinal nematode parasite Heligmosomoides polygyrus. J Immunol. 2003;170:384–93. doi: 10.4049/jimmunol.170.1.384. [DOI] [PubMed] [Google Scholar]
- 75.Balic A, Harcus Y, Holland MJ, Maizels RM. Selective maturation of dendritic cells by Nippostrongylus brasiliensis-secreted proteins drives Th2 immune responses. Eur J Immunol. 2004;34:3047–59. doi: 10.1002/eji.200425167. [DOI] [PubMed] [Google Scholar]
- 76.Massacand JC, Stettler RC, Meier R, Humphreys NE, Grencis RK, Marsland BJ, Harris NL. Helminth products bypass the need for TSLP in Th2 immune responses by directly modulating dendritic cell function. PNAS. 2009;106:13968–73. doi: 10.1073/pnas.0906367106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Perrigoue JG, Saenz SA, Siracusa MC, Allenspach EJ, Taylor BC, Giacomin PR, Nair MG, Du Y, Zaph C, van Rooijen N, Comeau MR, Pearce EJ, Laufer TM, Artis D. MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nat Immunol. 2009;10:697–705. doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sokol CL, Chu NQ, Yu S, Nish SA, Laufer TM, Medzhitov R. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat Immunol. 2009;10:713–20. doi: 10.1038/ni.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kool M, Hammad H, Lambrecht BN. Cellular networks controlling Th2 polarization in allergy and immunity F1000. Biol Rep. 2012;4:6. doi: 10.3410/B4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hammad H, Plantinga M, Deswarte K, Pouliot P, Willart MA, Kool M, Muskens F, Lambrecht BN. Inflammatory dendritic cells--not basophils--are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J Exp Med. 2010;207:2097–111. doi: 10.1084/jem.20101563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ohnmacht C, Schwartz C, Panzer M, Schiedewitz I, Naumann R, Voehringer D. Basophils orchestrate chronic allergic dermatitis and protective immunity against helminths. Immunity. 2010;33:364–74. doi: 10.1016/j.immuni.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 82.van Panhuys N, Prout M, Forbes E, Min B, Paul WE, Le Gros G. Basophils are the major producers of IL-4 during primary helminth infection. J Immunol. 2011;186:2719–28. doi: 10.4049/jimmunol.1000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schramm G, Mohrs K, Wodrich M, Doenhoff MJ, Pearce EJ, Haas H, Mohrs M. Cutting edge: IPSE/alpha-1, a glycoprotein from Schistosoma mansoni eggs, induces IgE-dependent, antigen-independent IL-4 production by murine basophils in vivo. J Immunol. 2007;178:6023–7. doi: 10.4049/jimmunol.178.10.6023. [DOI] [PubMed] [Google Scholar]
- 84.Reese TA, Liang HE, Tager AM, Luster AD, Van Rooijen N, Voehringer D, Locksley RM. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature. 2007;447:92–6. doi: 10.1038/nature05746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Anthony RM, Urban JF, Jr, Alem F, Hamed HA, Rozo CT, Boucher JL, Van Rooijen N, Gause WC. Memory T(H)2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat Med. 2006;12:955–60. doi: 10.1038/nm1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Siracusa MC, Comeau MR, Artis D. New insights into basophil biology: initiators, regulators, and effectors of type 2 inflammation. Ann N Y Acad Sci. 2011;1217:166–77. doi: 10.1111/j.1749-6632.2010.05918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Siracusa MC, Saenz SA, Hill DA, Kim BS, Headley MB, Doering TA, Wherry EJ, Jessup HK, Siegel LA, Kambayashi T, Dudek EC, Kubo M, Cianferoni A, Spergel JM, Ziegler SF, Comeau MR, Artis D. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature. 2011;477:229–33. doi: 10.1038/nature10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kool M, Soullie T, van Nimwegen M, Willart MA, Muskens F, Jung S, Hoogsteden HC, Hammad H, Lambrecht BN. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med. 2008;205:869–82. doi: 10.1084/jem.20071087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kool M, Petrilli V, De Smedt T, Rolaz A, Hammad H, van Nimwegen M, Bergen IM, Castillo R, Lambrecht BN, Tschopp J. Cutting edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J Immunol. 2008;181:3755–9. doi: 10.4049/jimmunol.181.6.3755. [DOI] [PubMed] [Google Scholar]
- 90.Lambrecht BN, Kool M, Willart MA, Hammad H. Mechanism of action of clinically approved adjuvants. Curr Opin Immunol. 2009;21:23–9. doi: 10.1016/j.coi.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 91.Kuroda E, Ishii KJ, Uematsu S, Ohata K, Coban C, Akira S, Aritake K, Urade Y, Morimoto Y. Silica crystals and aluminum salts regulate the production of prostaglandin in macrophages via NALP3 inflammasome-independent mechanisms. Immunity. 2011;34:514–26. doi: 10.1016/j.immuni.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 92.Marichal T, Ohata K, Bedoret D, Mesnil C, Sabatel C, Kobiyama K, Lekeux P, Coban C, Akira S, Ishii KJ, Bureau F, Desmet CJ. DNA released from dying host cells mediates aluminum adjuvant activity. Nat Med. 2011;17:996–1002. doi: 10.1038/nm.2403. [DOI] [PubMed] [Google Scholar]
- 93.Oyoshi MK, Larson RP, Ziegler SF, Geha RS. Mechanical injury polarizes skin dendritic cells to elicit a T(H)2 response by inducing cutaneous thymic stromal lymphopoietin expression. J Allergy Clin Immunol. 2010;126:976–84. 84, e1–5. doi: 10.1016/j.jaci.2010.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Berin MC. Mechanisms of allergic sensitization to foods: bypassing immune tolerance pathways. Immunol Allergy Clin North Am. 2012;32:1–10. doi: 10.1016/j.iac.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 95.Spangler BD. Structure and function of cholera toxin and the related Escherichia coli heat-labile enterotoxin. Microbiol Rev. 1992;56:622–47. doi: 10.1128/mr.56.4.622-647.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gagliardi MC, Sallusto F, Marinaro M, Langenkamp A, Lanzavecchia A, De Magistris MT. Cholera toxin induces maturation of human dendritic cells and licences them for Th2 priming. Eur J Immunol. 2000;30:2394–403. doi: 10.1002/1521-4141(2000)30:8<2394::AID-IMMU2394>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 97.Veglia F, Sciaraffia E, Riccomi A, Pinto D, Negri DR, De Magistris MT, Vendetti S. Cholera toxin impairs the differentiation of monocytes into dendritic cells, inducing professional antigen-presenting myeloid cells. Infect Immun. 2011;79:1300–10. doi: 10.1128/IAI.01181-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Blazquez AB, Berin MC. Gastrointestinal dendritic cells promote Th2 skewing via OX40L. J Immunol. 2008;180:4441–50. doi: 10.4049/jimmunol.180.7.4441. [DOI] [PubMed] [Google Scholar]
- 99.Wills-Karp M, Nathan A, Page K, Karp CL. New insights into innate immune mechanisms underlying allergenicity. Mucosal Immunol. 2010;3:104–10. doi: 10.1038/mi.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Berin MC, Shreffler WG. T(H)2 adjuvants: implications for food allergy. J Allergy Clin Immunol. 2008;121:1311–20. doi: 10.1016/j.jaci.2008.04.023. quiz 21–2. [DOI] [PubMed] [Google Scholar]
- 101.Barrett NA, Rahman OM, Fernandez JM, Parsons MW, Xing W, Austen KF, Kanaoka Y. Dectin-2 mediates Th2 immunity through the generation of cysteinyl leukotrienes. J Exp Med. 2011;208:593–604. doi: 10.1084/jem.20100793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Royer PJ, Emara M, Yang C, Al-Ghouleh A, Tighe P, Jones N, Sewell HF, Shakib F, Martinez-Pomares L, Ghaemmaghami AM. The mannose receptor mediates the uptake of diverse native allergens by dendritic cells and determines allergen-induced T cell polarization through modulation of IDO activity. J Immunol. 2010;185:1522–31. doi: 10.4049/jimmunol.1000774. [DOI] [PubMed] [Google Scholar]
- 103.Maneechotesuwan K, Wamanuttajinda V, Kasetsinsombat K, Huabprasert S, Yaikwawong M, Barnes PJ, Wongkajornsilp A. Der p 1 suppresses indoleamine 2, 3-dioxygenase in dendritic cells from house dust mite-sensitive patients with asthma. J Allergy Clin Immunol. 2009;123:239–48. doi: 10.1016/j.jaci.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 104.Emara M, Royer PJ, Mahdavi J, Shakib F, Ghaemmaghami AM. Retagging identifies dendritic cell-specific intercellular adhesion molecule-3 (ICAM3)-grabbing non-integrin (DC-SIGN) protein as a novel receptor for a major allergen from house dust mite. J Biol Chem. 2012;287:5756–63. doi: 10.1074/jbc.M111.312520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Huang HJ, Lin YL, Liu CF, Kao HF, Wang JY. Mite allergen decreases DC-SIGN expression and modulates human dendritic cell differentiation and function in allergic asthma. Mucosal Immunol. 2011;4:519–27. doi: 10.1038/mi.2011.17. [DOI] [PubMed] [Google Scholar]
- 106.Hsu SC, Chen CH, Tsai SH, Kawasaki H, Hung CH, Chu YT, Chang HW, Zhou Y, Fu J, Plunkett B, Su SN, Vieths S, Lee RT, Lee YC, Huang SK. Functional interaction of common allergens and a C-type lectin receptor, dendritic cell-specific ICAM3-grabbing non-integrin (DC-SIGN), on human dendritic cells. J Biol Chem. 2010;285:7903–10. doi: 10.1074/jbc.M109.058370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nathan AT, Peterson EA, Chakir J, Wills-Karp M. Innate immune responses of airway epithelium to house dust mite are mediated through beta-glucan-dependent pathways. J Allergy Clin Immunol. 2009;123:612–8. doi: 10.1016/j.jaci.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med. 2009;15:410–6. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Willart MA, Deswarte K, Pouliot P, Braun H, Beyaert R, Lambrecht BN, Hammad H. Interleukin-1α controls allergic sensitization to inhaled house dust mite via the epithelial release of GM-CSF and IL-33. J Exp Med. 2012;209(8):1505–17. doi: 10.1084/jem.20112691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li DQ, Zhang L, Pflugfelder SC, De Paiva CS, Zhang X, Zhao G, Zheng X, Su Z, Qu Y. Short ragweed pollen triggers allergic inflammation through Toll-like receptor 4-dependent thymic stromal lymphopoietin/OX40 ligand/OX40 signaling pathways. J Allergy Clin Immunol. 2011;128:1318–25. e2. doi: 10.1016/j.jaci.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mueller GA, Edwards LL, Aloor JJ, Fessler MB, Glesner J, Pomes A, Chapman MD, London RE, Pedersen LC. The structure of the dust mite allergen Der p 7 reveals similarities to innate immune proteins. J Allergy Clin Immunol. 2010;125:909–17. e4. doi: 10.1016/j.jaci.2009.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ghaemmaghami AM, Gough L, Sewell HF, Shakib F. The proteolytic activity of the major dust mite allergen Der p 1 conditions dendritic cells to produce less interleukin-12: allergen-induced Th2 bias determined at the dendritic cell level. Clin Exp Allergy. 2002;32:1468–75. doi: 10.1046/j.1365-2745.2002.01504.x. [DOI] [PubMed] [Google Scholar]
- 113.Ghaemmaghami AM, Robins A, Gough L, Sewell HF, Shakib F. Human T cell subset commitment determined by the intrinsic property of antigen: the proteolytic activity of the major mite allergen Der p 1 conditions T cells to produce more IL-4 and less IFN-gamma. Eur J Immunol. 2001;31:1211–6. doi: 10.1002/1521-4141(200104)31:4<1211::aid-immu1211>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 114.Arizmendi NG, Abel M, Mihara K, Davidson C, Polley D, Nadeem A, El Mays T, Gilmore BF, Walker B, Gordon JR, Hollenberg MD, Vliagoftis H. Mucosal allergic sensitization to cockroach allergens is dependent on proteinase activity and proteinase-activated receptor-2 activation. J Immunol. 2011;186:3164–72. doi: 10.4049/jimmunol.0903812. [DOI] [PubMed] [Google Scholar]
- 115.Lewkowich IP, Day SB, Ledford JR, Zhou P, Dienger K, Wills-Karp M, Page K. Protease-activated receptor 2 activation of myeloid dendritic cells regulates allergic airway inflammation. Respir Res. 2011;12:122. doi: 10.1186/1465-9921-12-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kobayashi T, Iijima K, Radhakrishnan S, Mehta V, Vassallo R, Lawrence CB, Cyong JC, Pease LR, Oguchi K, Kita H. Asthma-related environmental fungus, Alternaria, activates dendritic cells and produces potent Th2 adjuvant activity. J Immunol. 2009;182:2502–10. doi: 10.4049/jimmunol.0802773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lamhamedi-Cherradi SE, Martin RE, Ito T, Kheradmand F, Corry DB, Liu YJ, Moyle M. Fungal proteases induce Th2 polarization through limited dendritic cell maturation and reduced production of IL-12. J Immunol. 2008;180:6000–9. doi: 10.4049/jimmunol.180.9.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kouzaki H, O’Grady SM, Lawrence CB, Kita H. Proteases induce production of thymic stromal lymphopoietin by airway epithelial cells through protease-activated receptor-2. J Immunol. 2009;183:1427–34. doi: 10.4049/jimmunol.0900904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liang G, Barker T, Xie Z, Charles N, Rivera J, Druey KM. Naive T cells sense the cysteine protease allergen papain through protease-activated receptor 2 and propel T(H)2 immunity. J Allergy Clin Immunol. 2012;129:1377–86. doi: 10.1016/j.jaci.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Knight DA, Lim S, Scaffidi AK, Roche N, Chung KF, Stewart GA, Thompson PJ. Protease-activated receptors in human airways: upregulation of PAR-2 in respiratory epithelium from patients with asthma. J Allergy Clin Immunol. 2001;108:797–803. doi: 10.1067/mai.2001.119025. [DOI] [PubMed] [Google Scholar]
- 121.Lee JH, Kim KW, Gee HY, Lee J, Lee KH, Park HS, Kim SH, Kim SW, Kim MN, Kim KE, Kim KH, Lee MG, Sohn MH. A synonymous variation in protease-activated receptor-2 is associated with atopy in Korean children. J Allergy Clin Immunol. 2011;128:1326–34. e3. doi: 10.1016/j.jaci.2011.06.036. [DOI] [PubMed] [Google Scholar]
- 122.Cho HJ, Lee HJ, Kim SC, Kim K, Kim YS, Kim CH, Lee JG, Yoon JH, Choi JY. Protease-activated receptor 2-dependent fluid secretion from airway submucosal glands by house dust mite extract. J Allergy Clin Immunol. 2012;129:529–35. 35, e1–5. doi: 10.1016/j.jaci.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 123.Halim TY, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012;36:451–63. doi: 10.1016/j.immuni.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 124.Wolterink RG, Kleinjan A, van Nimwegen M, Bergen I, de Bruijn M, Levani Y, Hendriks RW. Pulmonary innate lymphoid cells are major producers of IL-5 and IL-13 in murine models of allergic asthma. Eur J Immunol. 2012;42:1106–16. doi: 10.1002/eji.201142018. [DOI] [PubMed] [Google Scholar]
- 125.Mjosberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, Fokkens WJ, Cupedo T, Spits H. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12:1055–62. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 126.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9:310–8. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tang H, Cao W, Kasturi SP, Ravindran R, Nakaya HI, Kundu K, Murthy N, Kepler TB, Malissen B, Pulendran B. The T helper type 2 response to cysteine proteases requires dendritic cell-basophil cooperation via ROS-mediated signaling. Nat Immunol. 2010;11:608–17. doi: 10.1038/ni.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kitzmuller C, Nagl B, Deifl S, Walterskirchen C, Jahn-Schmid B, Zlabinger GJ, Bohle B. Human blood basophils do not act as antigen-presenting cells for the major birch pollen allergen Bet v 1. Allergy. 2011;67:593–600. doi: 10.1111/j.1398-9995.2011.02764.x. [DOI] [PubMed] [Google Scholar]
- 129.Eckl-Dorna J, Ellinger A, Blatt K, Ghanim V, Steiner I, Pavelka M, Valent P, Valenta R, Niederberger V. Basophils are not the key antigen-presenting cells in allergic patients. Allergy. 2012;67:601–8. doi: 10.1111/j.1398-9995.2012.02792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Traidl-Hoffmann C, Mariani V, Hochrein H, Karg K, Wagner H, Ring J, Mueller MJ, Jakob T, Behrendt H. Pollen-associated phytoprostanes inhibit dendritic cell interleukin-12 production and augment T helper type 2 cell polarization. J Exp Med. 2005;201:627–36. doi: 10.1084/jem.20041065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gilles S, Mariani V, Bryce M, Mueller MJ, Ring J, Jakob T, Pastore S, Behrendt H, Traidl-Hoffmann C. Pollen-derived E1-phytoprostanes signal via PPAR-gamma and NF-kappaB-dependent mechanisms. J Immunol. 2009;182:6653–8. doi: 10.4049/jimmunol.0802613. [DOI] [PubMed] [Google Scholar]
- 132.Gilles S, Fekete A, Zhang X, Beck I, Blume C, Ring J, Schmidt-Weber C, Behrendt H, Schmitt-Kopplin P, Traidl-Hoffmann C. Pollen metabolome analysis reveals adenosine as a major regulator of dendritic cell-primed T(H) cell responses. J Allergy Clin Immunol. 2011;127:454–61. e1–9. doi: 10.1016/j.jaci.2010.12.1082. [DOI] [PubMed] [Google Scholar]
- 133.la Sala A, He J, Laricchia-Robbio L, Gorini S, Iwasaki A, Braun M, Yap GS, Sher A, Ozato K, Kelsall B. Cholera toxin inhibits IL-12 production and CD8alpha+ dendritic cell differentiation by cAMP-mediated inhibition of IRF8 function. J Exp Med. 2009;206:1227–35. doi: 10.1084/jem.20080912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Robertson SJ, Rubino SJ, Geddes K, Philpott DJ. Examining host-microbial interactions through the lens of NOD: From plants to mammals. Semin Immunol. 2012;24:9–16. doi: 10.1016/j.smim.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 135.Magalhaes JG, Rubino SJ, Travassos LH, Le Bourhis L, Duan W, Sellge G, Geddes K, Reardon C, Lechmann M, Carneiro LA, Selvanantham T, Fritz JH, Taylor BC, Artis D, Mak TW, Comeau MR, Croft M, Girardin SE, Philpott DJ. Nucleotide oligomerization domain-containing proteins instruct T cell helper type 2 immunity through stromal activation. PNAS. 2011;108:14896–901. doi: 10.1073/pnas.1015063108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kool M, Willart MA, van Nimwegen M, Bergen I, Pouliot P, Virchow JC, Rogers N, Osorio F, Reis e Sousa C, Hammad H, Lambrecht BN. An unexpected role for uric acid as an inducer of T helper 2 cell immunity to inhaled antigens and inflammatory mediator of allergic asthma. Immunity. 2011;34:527–40. doi: 10.1016/j.immuni.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 137.Shreffler WG, Castro RR, Kucuk ZY, Charlop-Powers Z, Grishina G, Yoo S, Burks AW, Sampson HA. The major glycoprotein allergen from Arachis hypogaea, Ara h 1, is a ligand of dendritic cell-specific ICAM-grabbing nonintegrin and acts as a Th2 adjuvant in vitro. J Immunol. 2006;177:3677–85. doi: 10.4049/jimmunol.177.6.3677. [DOI] [PubMed] [Google Scholar]
- 138.Ilchmann A, Burgdorf S, Scheurer S, Waibler Z, Nagai R, Wellner A, Yamamoto Y, Yamamoto H, Henle T, Kurts C, Kalinke U, Vieths S, Toda M. Glycation of a food allergen by the Maillard reaction enhances its T-cell immunogenicity: role of macrophage scavenger receptor class A type I and II. J Allergy Clin Immunol. 2010;125:175–83. e1–11. doi: 10.1016/j.jaci.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 139.Hilmenyuk T, Bellinghausen I, Heydenreich B, Ilchmann A, Toda M, Grabbe S, Saloga J. Effects of glycation of the model food allergen ovalbumin on antigen uptake and presentation by human dendritic cells. Immunology. 2010;129:437–45. doi: 10.1111/j.1365-2567.2009.03199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hsu SC, Tsai TH, Kawasaki H, Chen CH, Plunkett B, Lee RT, Lee YC, Huang SK. Antigen coupled with Lewis-x trisaccharides elicits potent immune responses in mice. J Allergy Clin Immunol. 2007;119:1522–8. doi: 10.1016/j.jaci.2007.01.034. [DOI] [PubMed] [Google Scholar]
- 141.van Wijk F, Nierkens S, Hassing I, Feijen M, Koppelman SJ, de Jong GA, Pieters R, Knippels LM. The effect of the food matrix on in vivo immune responses to purified peanut allergens. Toxicol Sci. 2005;86:333–41. doi: 10.1093/toxsci/kfi187. [DOI] [PubMed] [Google Scholar]
- 142.Ruiter B, Grishina G, den Hartog Jager CF, Knol EF, Ozias-Akins P, Sampson HA, Shreffler WG. Human dendritic cells stimulated with a novel peanut protein express high levels of RALDH2 and induce RA-sensitive genes in naive T cells. J Allergy Clin Immunol. 2012;129:AB243. [Google Scholar]
- 143.Keller B, Sauer N, Lamb CJ. Glycine-rich cell wall proteins in bean: gene structure and association of the protein with the vascular system. EMBO J. 1988;7:3625–33. doi: 10.1002/j.1460-2075.1988.tb03243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Khodoun M, Strait R, Orekov T, Hogan S, Karasuyama H, Herbert DR, Kohl J, Finkelman FD. Peanuts can contribute to anaphylactic shock by activating complement. J Allergy Clin Immunol. 2009;123:342–51. doi: 10.1016/j.jaci.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jyonouchi S, Abraham V, Orange JS, Spergel JM, Gober L, Dudek E, Saltzman R, Nichols KE, Cianferoni A. Invariant natural killer T cells from children with versus without food allergy exhibit differential responsiveness to milk-derived sphingomyelin. J Allergy Clin Immunol. 2011;128:102–9. e13. doi: 10.1016/j.jaci.2011.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Masilamani M, Wei J, Bhatt S, Paul M, Yakir S, Sampson HA. Soybean isoflavones regulate dendritic cell function and suppress allergic sensitization to peanut. J Allergy Clin Immunol. 2011;128:1242–50. doi: 10.1016/j.jaci.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 147.Chambers SJ, Bertelli E, Winterbone MS, Regoli M, Man AL, Nicoletti C. Adoptive transfer of dendritic cells from allergic mice induces specific immunoglobulin E antibody in naive recipients in absence of antigen challenge without altering the T helper 1/T helper 2 balance. Immunology. 2004;112:72–9. doi: 10.1111/j.1365-2567.2004.01846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.van Wijk F, Nierkens S, de Jong W, Wehrens EJ, Boon L, van Kooten P, Knippels LM, Pieters R. The CD28/CTLA-4-B7 signaling pathway is involved in both allergic sensitization and tolerance induction to orally administered peanut proteins. J Immunol. 2007;178:6894–900. doi: 10.4049/jimmunol.178.11.6894. [DOI] [PubMed] [Google Scholar]
- 149.Ruiter B, Shreffler WG. The role of dendritic cells in food allergy. J Allergy Clin Immunol. 2012;129:921–8. doi: 10.1016/j.jaci.2012.01.080. [DOI] [PubMed] [Google Scholar]
- 150.Yang PC, Xing Z, Berin CM, Soderholm JD, Feng BS, Wu L, Yeh C. TIM-4 expressed by mucosal dendritic cells plays a critical role in food antigen-specific Th2 differentiation and intestinal allergy. Gastroenterology. 2007;133:1522–33. doi: 10.1053/j.gastro.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 151.Feng BS, Chen X, He SH, Zheng PY, Foster J, Xing Z, Bienenstock J, Yang PC. Disruption of T-cell immunoglobulin and mucin domain molecule (TIM)-1/TIM4 interaction as a therapeutic strategy in a dendritic cell-induced peanut allergy model. J Allergy Clin Immunol. 2008;122:55–61. e1–7. doi: 10.1016/j.jaci.2008.04.036. [DOI] [PubMed] [Google Scholar]