Abstract
We conducted a systematic review and meta-analysis of randomized controlled trials to determine whether magnesium sulfate administered to women at risk of preterm delivery before 34 weeks of gestation may reduce the risk of cerebral palsy in their children. Six trials involving 4796 women and 5357 infants were included. Antenatal magnesium sulfate was associated with a significant reduction in the risk of cerebral palsy (relative risk [RR], 0.69; 95% confidence interval [CI], 0.55–0.88]), moderate or severe cerebral palsy (RR, 0.64; 95% CI, 0.44–0.92), and substantial gross motor dysfunction (RR, 0.60; 95% CI, 0.43–0.83). There was no overall difference in the risk of total pediatric mortality (RR, 1.01; 95% CI, 0.89–1.14). Minor side effects were more frequent among women receiving magnesium sulfate. In conclusion, magnesium sulfate administered to women at risk of delivery before 34 weeks of gestation reduces the risk of cerebral palsy.
Keywords: Magnesium sulfate, cerebral palsy, pediatric mortality, meta-analysis, systematic review, prematurity, preterm birth, neurologic handicap, infant development, preterm birth
INTRODUCTION
Cerebral palsy describes a group of disorders affecting the development of movement and posture, causing activity limitation, and which are attributed to non-progressive disturbances. Insults responsible for cerebral palsy are believed to have occurred during fetal development or infancy.1 Cerebral palsy is the most prevalent chronic childhood motor disability with an estimated lifetime cost in 2003 of nearly $1 million per person.2 The prevalence of cerebral palsy reported in recent population-based studies ranges between 1.5 and 3.6 cases per 1000 live births.3–5 The United Cerebral Palsy Foundation estimates that nearly 800,000 children and adults of all ages in the United States have cerebral palsy.6 Secular trends in overall prevalence of cerebral palsy for over the last 40 years shows a modest increase in the frequency which has been attributed to a substantial increase in cerebral palsy in very low birth weigh infants, which, in turn, is attributable to their increased survival resulting from improvements in neonatal intensive care.3, 5
Preterm birth is a major risk factor for cerebral palsy, and the risk increases markedly with decreasing gestational age.7 Currently, infants born at less than 34 weeks of gestation constitute about 25% of all new cases of cerebral palsy.5, 8 Multiple pregnancy is associated with an increased risk of cerebral palsy. This has been attributed, at least in part, to preterm birth. However, fetal or neonatal death of a member of a multiple gestation, twin-to-twin transfusion syndrome, or intrapartum problems also contribute.9, 10
Several observational studies have reported an association of antenatal treatment with magnesium sulfate for preterm labor or preeclampsia with a decreased risk of cerebral palsy in low birth weight or preterm infants. In 1995, Nelson and Grether11 reported a case-control study investigating whether in utero exposure to magnesium sulfate were used to prevent convulsions in preeclampsia or if a tocolytic agent was associated with a lower prevalence of cerebral palsy in infants born weighing <1500 g. Children with cerebral palsy were less likely to have been exposed to magnesium sulfate than were control subjects (odds ratio, 0.14 [95% confidence interval, 0.05 to 0.51]) suggesting a protective effect of magnesium sulfate against cerebral palsy in these very low birth weight infants. Although some observational studies have reported similar findings,12–15 others have reported no association between administration of magnesium sulfate and the subsequent risk of cerebral palsy.16–21
In response to the conflicting evidence from observational studies, several randomized controlled trials of magnesium sulfate administered to mothers for fetal neuroprotection have been performed. A recent systematic review that assessed the effects of magnesium sulfate as a fetal neuroprotective agent when given to women considered at risk of preterm birth concluded that this therapy reduced the risk of cerebral palsy and substantial gross motor dysfunction in early childhood.22 This review, however, based its main conclusions on meta-analysis that included preterm infants less than 37 weeks of gestational age and did not perform any economic evaluation for estimating the cost-effectiveness of this intervention for the prevention of cerebral palsy.
We carried out a systematic review and meta-analysis of all available randomized controlled trials to determine the efficacy and safety of antenatal administration of magnesium sulfate to women at risk of preterm delivery before 34 weeks of gestational age for the prevention of cerebral palsy in their children.
MATERIALS AND METHODS
The systematic review was conducted following a prospectively prepared protocol and reported using the Quality of Reporting of Meta-analysis (QUOROM) guidelines for meta-analysis of randomized controlled trials.23
Search
We searched PubMed, Embase, Cinahl, and Lilacs (all from inception to March 30, 2009), ISI Web of Science (http://www.isiknowledge.com) (1960 to March 30, 2009), the Cochrane Central Register of Controlled Trials (http://www.mrw.interscience.wiley.com/cochrane/cochrane_clcentral_articles_fs.html) (1960 to March 30, 2009), and Research Registers of ongoing trials (www.clinicaltrials.gov, www.controlled-trials.com, www.centerwatch.com, www.actr.org.au, www.nrr.nhs.uk, and www.umin.ac.jp/ctr) using a combination of keywords and text words related to magnesium, cerebral palsy, and neuroprotection. Proceedings of the Society for Maternal-Fetal Medicine and international meetings on cerebral palsy, reference lists of identified studies, textbooks, previously published systematic reviews, and review articles were also searched. No language restrictions were applied. All searches were carried independently by the two authors and results were merged. For studies that resulted in multiple publications, the data from the publication with the largest sample size were used and supplemented if additional information appeared in the other publications.
Study selection
We included randomized controlled trials comparing magnesium sulfate with placebo or no magnesium sulfate for women at risk of preterm birth before 34 weeks of gestation, whose primary aim was to prevent cerebral palsy and other neurologic abnormalities in the unborn baby or if the primary aim was otherwise but data on cerebral palsy were reported for the infants. Quasi-randomized studies were excluded. We classified trials according to aim of the treatment with magnesium sulfate into two groups: “neuroprotection of the fetus” and “other aims”.
All published studies deemed suitable were retrieved and reviewed independently by the two authors to determine inclusion. Disagreements were resolved through consensus.
Outcome measures
The primary outcomes of interest were cerebral palsy and total pediatric mortality (fetal death + any mortality of live births that occurred during the two years of corrected age following the birth). We included total pediatric mortality because it is a competing outcome that would preclude the assessment of cerebral palsy. Pre-specified secondary outcomes for the neonate and infant included mild cerebral palsy, moderate or severe cerebral palsy, grade III or IV intraventricular hemorrhage, periventricular leukomalacia, Apgar score <7 at 5 minutes, neonatal seizures, respiratory distress syndrome, need for supplemental oxygen at 36 weeks, bronchopulmonary dysplasia, mechanical ventilation, necrotizing enterocolitis, substantial gross motor dysfunction, major neurologic disability, any neurological impairment, Bayley mental development index <70 and <85, Bayley psychomotor development index <70 and <85, blindness, and deafness. For the mother, secondary outcomes were death, cardiac or respiratory arrest, pulmonary edema, respiratory depression, hypotension, tachycardia, severe postpartum hemorrhage, cesarean delivery, and clinical and self-assessed maternal side effects of the infusion such as flushing, nausea or vomiting, sweating, problems at injection site, stopping of infusion because of adverse effects adverse effect, and any side effect.
Study quality assessment
We assessed study methodological quality using a modified scoring system proposed by Jadad et al.24 which is based on three items: randomization, blinding, and follow-up. Points were awarded on the basis of the quality of randomization (2 points: computer-generated random numbers or similar; 1 point: not described; 0 points: quasi-randomized or not randomized [we excluded such studies]); double blinding (2 points: neither the person doing the assessments nor the study participant could identify the intervention being assessed; 1 point: not described; 0 points: no blinding or inadequate method), and follow-up (2 points: number or reasons for dropouts and withdrawals described, and assessment of primary outcomes in ≥95% of randomized fetuses; 1 point: number or reasons for dropouts and withdrawals described but assessment of primary outcomes in <95% of randomized fetuses; 0 points: number or reasons for dropouts and withdrawals not described). In addition, we assessed concealment of allocation as follows: 2 points: adequate method (central randomization; or drug containers or opaque, sealed envelopes that were sequentially numbered and opened sequentially only after they have been irreversibly assigned to the participant); 0 points: no concealment of allocation or inadequate method or not described. Thus, the total score ranged from 0 (lowest quality) to 8 (highest quality). The methodological quality of included trials was assessed individually by the two reviewers who were not associated with any of the trials. When differences in scoring existed, a consensus was reached.
Data abstraction
One reviewer (AC-A) scanned abstracts and titles. Potentially relevant articles were acquired and data were extracted in duplicate from all reports and recorded on a piloted form independently by the two reviewers. There was no blinding by authorship. All outcome data were further verified with the original articles. Information was extracted on study characteristics (randomization procedure, blind assessment at baseline and follow up, follow up period, intention to treat analysis, and losses to follow up), participants (inclusion and exclusion criteria, numbers of mothers and infants in randomized groups, baseline characteristics, and country and date of recruitment), details of intervention (aim, loading dose, maintenance dose, median total dose, duration, and retreatment), and outcomes (number of outcome events including mortality and morbidity). The number of infants was used as the denominator for primary (cerebral palsy and pediatric mortality), neonatal, and infant neurodevelopmental outcomes. When maternal outcomes were presented, numerators and denominators were calculated based on the number of mothers. In an attempt to obtain additional data, we contacted three authors by e-mail of whom one responded. Disagreements in extracted data were resolved by discussion among reviewers.
Statistical analysis
Statistical analysis was done according to the guidelines of the Cochrane Collaboration.25 We analyzed outcomes on an intend-to-treat basis. If this was not clear from the original article, then we carried out re-analysis where possible. If data for similar outcomes from two or more separate studies were available, we combined the data in a meta-analysis and calculated a summary relative risk (RR) with associated 95% confidence interval (CI). Heterogeneity of the results among studies was tested with the quantity I2, which describes the percentage of total variation across studies that is due to heterogeneity rather than chance.26 A value of 0% indicates no observed heterogeneity whereas I2 values of 50% or more indicate a substantial level of heterogeneity.26 We planned to pool data across studies using a fixed-effects model if substantial statistical heterogeneity was not present. We used random-effects models to pool data across studies if the I2 values were ≥50%.
We conducted sensitivity analyses to explore the robustness of findings for the primary outcomes according to statistical model (fixed-effects vs. random-effects), modified Jadad quality score (>4 vs ≤4), and completeness of follow-up of randomized fetuses (≥95% vs <95%). Further subgroup analyses were planned to assess the primary outcomes by primary aim of the treatment with magnesium sulfate (neuroprotective vs other aims), median total dose of magnesium sulfate used (≤4 grams vs >4 grams), gestational age at trial entry (<34, <32, and <30 weeks), and plurality (singleton and multiple pregnancy). The meta-analysis by plurality of pregnancy, however, was not possible because there were not sufficient data available from the great majority of studies.
We assessed publication and related biases visually by examining the symmetry of funnel plots and statistically by using the Egger test.27 The larger the deviation of the intercept of the regression line from zero, the greater was the asymmetry and the more likely it was that the meta-analysis would yield biased estimates of effect. As suggested by Egger, we considered P<0.1 to indicate significant asymmetry.
We also calculated the number needed to treat (NNT) for an additional beneficial outcome and the NNT for an additional harmful outcome with their 95% CIs for outcomes in which the treatment effect was significant at the 5% level (the 95% CI for the absolute risk difference did not include zero).28 NNT was computed from the results of meta-analysis of relative risks as follows:
In this review, NNT for an additional beneficial outcome is the number of women at risk of preterm delivery before 34 weeks of gestation who need to be treated with magnesium sulfate rather than with placebo to prevent one case of cerebral palsy. The NNT for an additional harmful outcome is the number of women at risk of preterm delivery before 34 weeks of gestation who need to be treated with magnesium sulfate rather than with placebo for one additional woman to be harmed by an adverse event.
We estimated the hypothetical impact of universal use of magnesium sulfate in American women at high risk of preterm delivery before 34 weeks of gestational age to prevent cerebral palsy in their children. Initially, we calculated the total number of new cases of cerebral palsy diagnosed each year in the US among infants born before 34 weeks of gestational age using published data on the total number of new cases of cerebral palsy recognized each year in the US and the percentage of infants with cerebral palsy born before 34 weeks of gestational age. Then, the total number of new cases of cerebral palsy among infants born before 34 weeks of gestational age was multiplied by the summary RR and the upper and lower bounds of its 95% CI obtained in our meta-analysis to estimate the hypothetical number of new cases of cerebral palsy with corresponding 95% CI that could be prevented annually using this intervention.
Finally, we performed an economic evaluation to calculate the incremental cost of preventing one case of cerebral palsy in the US through use of antenatal magnesium sulfate in women at risk of preterm delivery before 34 weeks of gestational age. First, we searched data published recently on the total cost per patient of administrating magnesium sulfate as a tocolytic agent in the US. Then, the incremental cost of preventing one case of cerebral palsy (with corresponding 95% CI) by using antenatal magnesium sulfate was estimated by multiplying the total cost per patient of administering magnesium sulfate by the NNT for benefit and the upper and lower bounds of its 95% CI obtained in our analysis. All statistical analyses were performed with the StatsDirect version 2.7.2 (StatsDirect Ltd, United Kingdom).
RESULTS
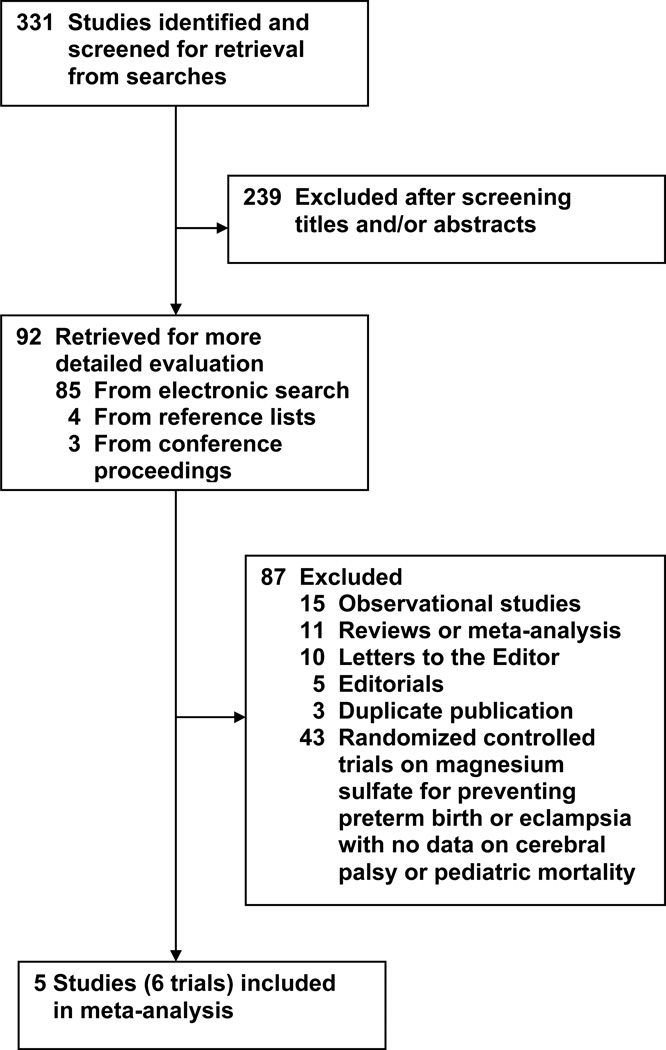
The flow of the search is shown in Figure 1. Of the 331 potentially relevant citations identified, 5 studies (6 trials) published in 7 articles fulfilled the inclusion criteria after a detailed review of 92 studies.29–35 Because the study by Mittendorf et al29, 30 had two arms (tocolytic and neuroprotective), it was considered as two separate trials in this review. Of the other four studies included, three31, 33, 35 evaluated magnesium sulfate as an infant neuroprotective agent, and one32 assessed the efficacy of magnesium sulfate for preventing eclampsia. Eighty-seven studies were excluded, the main reason being the lack of data on cerebral palsy and/or pediatric mortality in randomized controlled trials on magnesium sulfate for preventing preterm birth or eclampsia. Overall agreement on the inclusion of studies was 100% (κ, 1.00). The 6 trials included a total of 4796 women and 5357 infants.
Figure 1.
Study selection process.
The characteristics of included studies are presented in Table 1. Two studies were performed in the United States,29, 35 one each in France,33 Australia and New Zealand,31 and the remaining one was conducted in 19 countries across five continents.32 Trials included women with gestational ages <34 weeks29 (165 infants), <33 weeks33 (688 women), <32 weeks35 (2444 infants), and <30 weeks31 (1255 infants). The Magpie trial32 reported data for 3283 infants whose mothers were randomized before delivery. Of these, 805 infants were randomized before 34 weeks of gestational age and 788 between 34 and 36 weeks. Data for the 805 infants randomized before 34 weeks of gestational age included in our meta-analyses were extracted from the review by Doyle et al.22 Multiple pregnancies were included in all trials. Two trials31, 33 included women at high risk of preterm delivery because of expected or planned birth within 24 hours. The trial by Rouse et al35 included women at high risk for spontaneous delivery because of premature rupture of membranes or advanced preterm labor and women with indicated preterm delivery within 2 to 24 hours. The main reasons for preterm birth were preterm labor and premature rupture of the membranes (63 and 9% in the study by Crowther et al,31 85 and 61% in the study by Marret et al,33 and 10 and 87% in the study by Rouse et al,35 respectively), followed by antepartum hemorrhage and chorioamnionitis (14 and 14% in the study by Crowther et al,31 and 19 and 11% in the study by Marret et al,33 respectively). In the study by Crowther et al,31 15% of women enrolled had preeclampsia. One study only included women in active preterm labor with cervical dilation ≤4 cm (tocolytic arm) or >4 cm (neuroprotective arm).29 The Magpie trial32 included only women with preeclampsia. Three trials excluded women with preeclampsia.29, 33, 35 The definition and diagnostic criteria of cerebral palsy were clearly reported in four trials.31, 32, 34, 35 The study by Mittendorf et al30 did not report a definition or details of the diagnosis. A pediatrician made the diagnosis of cerebral palsy in all trials at a corrected age of at least 18 months30, 32 or 24 months.31, 34, 35
TABLE 1.
Characteristics of studies included in the systematic review
| Study, year | Location | Inclusion/exclusion criteria | No of infants |
Magnesium sulfate |
Cerebral palsy |
||||
|---|---|---|---|---|---|---|---|---|---|
| Magnesium group |
No magnesium group |
Loading dose (g) |
Maintenance dose |
Median total dose (g) received by women in magnesium group |
Definition and/or diagnostic criteria |
Health professional who made the diagnosis and age at diagnosis |
|||
| Mittendorf et al,29, 301997 | United States, Single center |
1. Neuroprotective arm Inclusion: women with single or twin pregnancy in preterm labor between 25 and 33 weeks of gestation with or without premature rupture of the membranes, reassuring fetal assessment, and cervical dilatation >4 cm. Exclusion: preeclampsia, infection |
30 | 29 | 4 | None | 4 | Not reported | Developmental pediatrician after 18 months of corrected age |
| 2. Tocolytic arm Inclusion: women with single or twin pregnancy in preterm labor between 25 and 33 weeks of gestation with or without premature rupture of the membranes, reassuring fetal assessment, and cervical dilatation ≤4 cm. Exclusion: preeclampsia, infection |
55 | 51 | 4 | 2–3 g/h; Duration not reported |
49.8 | Not reported | Developmental pediatrician after 18 months of corrected age |
||
| Crowther et al,31 2003 | Australia and New Zealand, 16 centers |
Inclusion: women with single, twin, triplet or quadruplet fetuses at risk of preterm delivery before 30 weeks' gestation because of planned or expected birth within 24 hours. Exclusion: women in second stage of labor, if they had received magnesium sulfate in this pregnancy, and contraindications to magnesium sulfate |
629 | 626 | 4 | 1 g/h until birth (if occurred within 24 hours) or up to 24 hours |
≈10.5 | Abnormalities of muscle tone and loss of motor function. Mild cerebral palsy: disability in ambulant children that interfered only slightly with normal daily activities; moderate cerebral palsy: children attempting to walk at two years with or without appliances; Severe cerebral palsy: children permanently non ambulant |
Developmental pediatrician and psychologist at 24 months of corrected age |
| Magpie32 2007a |
19 countries across five continents, 125 centers |
Inclusion: women with singleton or multiple pregnancy with preeclampsia who had not given birth or were 24 hours or less postpartum and uncertainty about whether to use magnesium sulfate to prevent eclampsia, irrespective of whether they had received previously magnesium sulfate or other anticonvulsant. Exclusion: hypersensitivity to magnesium, hepatic coma with a risk of renal failure, or myasthenia gravis. |
404 | 401 | 4 | Either 1 g/h intravenously for 24 hours or 5 g every four hours intramuscularly for 24 hours |
18.0b | Severe cerebral palsy: not walking or unlikely to walk unaided by 24 months; non severe cerebral palsy: not defined |
Pediatrician at 18 months of corrected age |
| Marret etal, 33, 34 2007 | France, 13 centers |
Inclusion: Inclusion: women with single, twin or triplet fetuses at risk of preterm delivery before 33 weeks' gestation because of planned or expected birth within 24 hours. Exclusion: fetus with severe malformations or chromosomal abnormalities and women with pregnancy-associated vascular disease (preeclampsia, growth restriction, HELLP syndrome, retroplacental hematoma) or with at least one of the following criteria: hypotension, cardiac rhythm abnormalities, hydroelectrolyte abnormalities, renal insufficiency, ingestion during the last 24 hours of calcium channel blockers, digitalins or indomethacin, persistent signs of cardiovascular toxicity or tachycardia >1 hour after cessation of tocolytic intake, myasthenia, or indication for emergency cesarean section. |
352 | 336 | 4 | None | 4 | European Cerebral Palsy Network definition |
Pediatrician by clinical examination (77% of survivors) or parent telephone interview (22% of survivors) at 24 months of age |
| Rouse et al,35 2008 | United States, 20 centers |
Inclusion: women carrying singletons or twins at 24 through 31 weeks of gestation and at high risk for spontaneous delivery because of premature rupture of the membranes, advanced preterm labor with dilatation of 4 to 8 cm and intact membranes, or indicated preterm delivery anticipated within 2 to 24 hours. Exclusion: delivery anticipated within less than 2 hours, cervical dilatation >8 cm, rupture of the membranes before 22 weeks, unwillingness of the obstetrician to intervene for the benefit of the fetus, major fetal anomalies or death, maternal hypertension or preeclampsia, maternal contraindications to magnesium sulfate, and receipt of intravenous magnesium sulfate within the previous 12 hours. |
1188 | 1256 | 6 | 2 g/hc | 31.5 | Presence of two or more of the following three features: (1) a delay of 30% or more in gross motor developmental milestones; (2) abnormality in muscle tone, 4+ or absent deep tendon reflexes, or movement abnormality; (3) persistence of primitive reflexes or absence of protective reflexes |
Pediatrician or pediatric neurologist at or beyond 24 months of corrected age |
Data for children whose mothers were less than 34 weeks of gestation and undelivered at randomization extracted from Doyle et al.’s review22
For women of all gestational ages and undelivered at randomization
If delivery had not occurred after 12 hours and was no longer considered imminent, the infusion was discontinued and resumed when delivery was deemed imminent again. If at least 6 hours had passed since the discontinuation of the study medication, another loading dose was given
The studies included a range of dosing schedules. In five trials,29, 31–33 women received a loading dose of 4 g of intravenous magnesium sulfate. In the trial by Rouse et al5 women received a loading infusion of 6 g. There was no maintenance infusion of magnesium sulfate in two trials (neuroprotective arm of the study by Mittendorf et al29 and the study by Marret el al33). The maintenance doses of magnesium sulfate used in four trials were 1 g/h,31 2 g/h,35 2–3 g/h (tocolytic arm of the study by Mittendorf et al. study29), and either 1 g/h intravenously or 5 g every four hours intramuscularly.32 The maintenance infusion was continued until birth (if it occurred within 24 hours) or up to 24 hours in the study by Crowther et al,31 or until 24 hours in the Magpie trial.32
In the study by Rouse et al,35 if delivery had not occurred after 12 hours and was no longer considered imminent, the infusion was discontinued and resumed when delivery was deemed imminent again. If at least 6 hours had passed since the discontinuation of the study medication, another loading dose was given. Retreatment with magnesium sulfate was not allowed in three trials31–33 and in the neuroprotective arm of the study by Mittendorf et al.30 There was no information on duration of maintenance infusion and whether retreatment was permitted in the tocolytic arm of the study by Mittendorf et al.30 The median total dose of magnesium sulfate received by women in the magnesium group ranged between 4 and 49.8 g.
The quality assessment of trials is shown in Table 2. The overall quality of the trials was relatively good. All studies were randomized with the appropriate method to generate the sequence of randomization (computer-generated). Five trials, including the neuroprotective arm of the study by Mittendorf et al,30 were double-blinded in which a placebo was used, and the outcomes were evaluated by individuals who were blinded to treatment group allocation. The tocolytic arm of the study by Mittendorf et al,30 was not double-blinded because the women were randomized to receive magnesium sulfate or other tocolytic therapy such as ritodrine, terbutaline, indomethacin, or nifedipine, although diagnosis of cerebral palsy was made by a pediatrician who was masked to the antenatal treatment. Four trials had adequate methods of allocation sequence concealment.31, 32, 34, 35 The tocolytic arm of the study by Mittendorf et al30 had no concealment of allocation whereas the neuroprotective arm did not report on this item. Information on withdrawals and dropouts was available for four trials.31, 32, 34, 35 In the two trials performed by Mittendorf et al30 there was no statement on numbers and reasons for withdrawals in each group. Three trials reported assessment of primary outcomes in ≥95% of randomized fetuses.31, 34, 35 The Magpie trial32 evaluated the primary outcomes in only 47.4% of fetuses of all gestational ages randomized before delivery. Four trials had a modified Jadad score of 7 or more.31, 32, 34, 35 The two trials performed by Mittendorf et al30 had scores of 2 (tocolytic arm) and 4 (neuroprotective arm).
TABLE 2.
Modified Jadad score for assessment of methodological quality of included studies
| Item | Mittendorf, 199729, 30 | Crowther, 200331 | Magpie, 200732 | Marret, 200733, 34 | Rouse, 200835 | |
|---|---|---|---|---|---|---|
| Tocolytic arm |
Neuroprotective arm |
|||||
| Randomization | Yes | Yes | Yes | Yes | Yes | Yes |
| Method to generate randomization clear and appropriate |
Yes | Yes | Yes | Yes | Yes | Yes |
| Double blind | No | Yes | Yes | Yes | Yes | Yes |
| Methods for blinding appropriate | No | Yes | Yes | Yes | Yes | Yes |
| Method of allocation concealmenta | No | Unreported | Adequate | Adequate | Adequate | Adequate |
| Description of withdrawal or dropout |
No | No | Yes | Yes | Yes | Yes |
| Completeness of follow-up of randomized fetuses (%)b |
Unreported | Unreported | 98.9 | 47.4c | 98.5 | 95.6 |
| Total score | 2 | 4 | 8 | 7 | 8 | 8 |
Yes=1 point, No=zero points; Scores: 0=lowest quality, 8=highest quality
Adequate=2 points, no concealment of allocation or inadequate method or unreported=zero points
Follow-up ≥95%=1 point, follow-up <95% or unreported=zero points
For fetuses of all gestational ages and undelivered at randomization
Primary outcomes
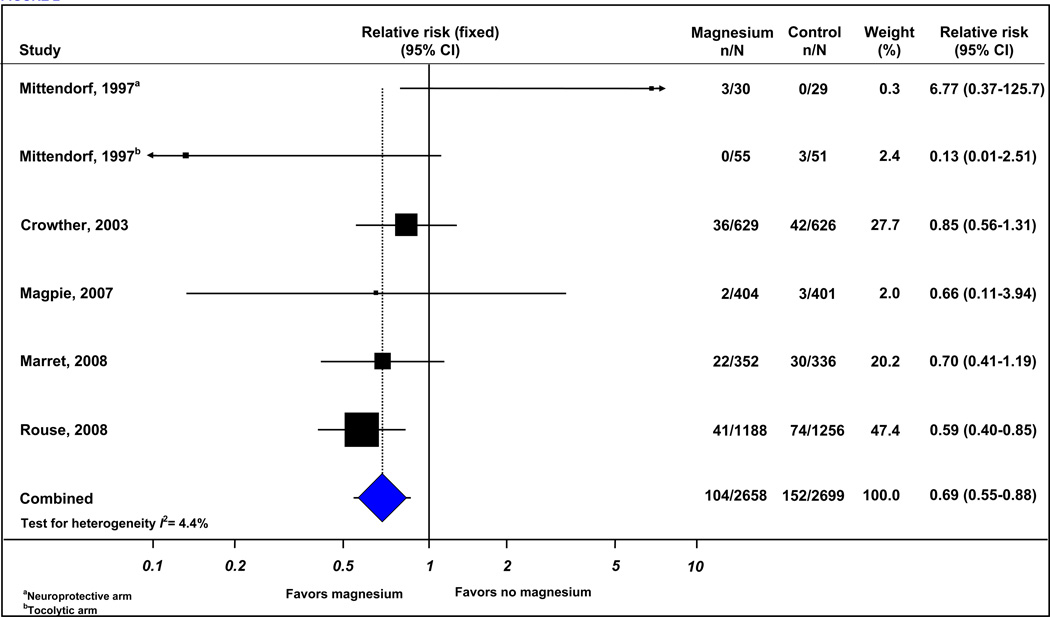
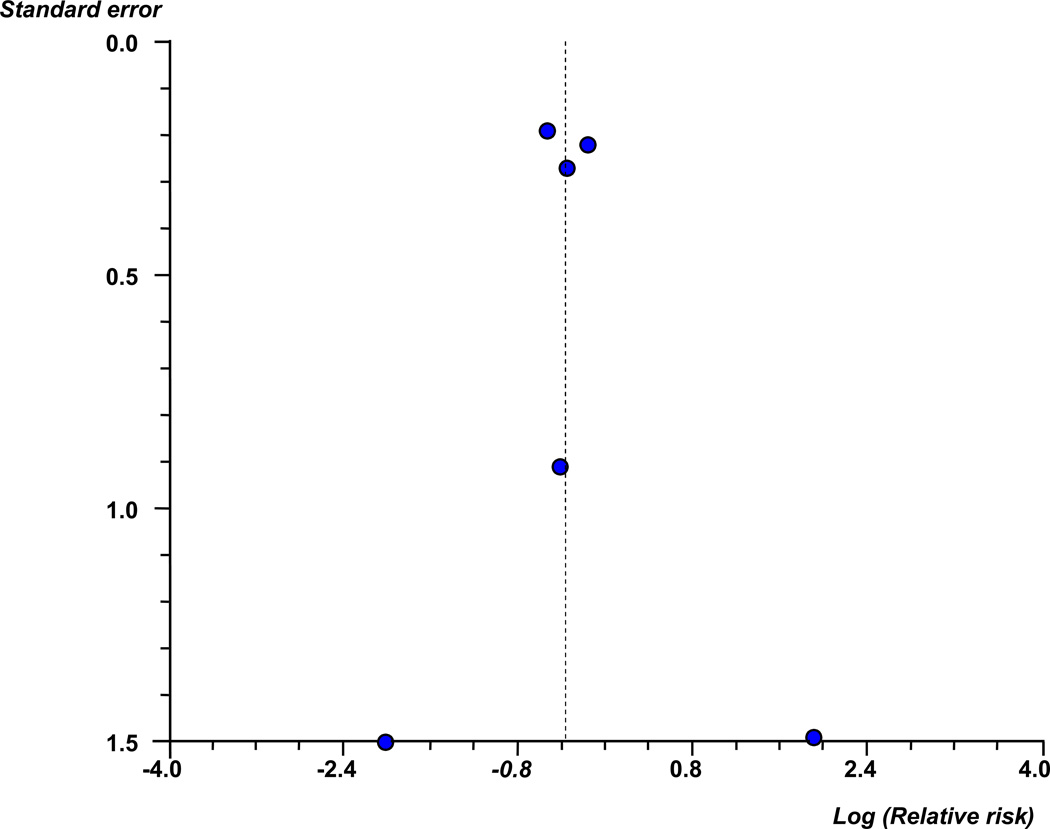
Six trials (5357 infants) reported cerebral palsy, total pediatric mortality, and the combined outcome of death or cerebral palsy. The risk of giving birth to an infant subsequently receiving a diagnosis of cerebral palsy was significantly lower in the group of women who received magnesium sulfate than among women who did not receive magnesium sulfate (3.9% vs 5.6%; RR, 0.69 [95% CI, 0.55–0.88]) (Figure 2) (Table 3). There was evidence of low statistical heterogeneity among the trials included in this analysis (I2=4.4%). The funnel plot of trials of antenatal magnesium sulfate in the prevention of cerebral palsy appeared symmetrical either visually or in terms of statistical significance (intercept, 0.23 [95% CI, −1.97 to 2.43]; P = 0.79) (Figure 3) suggesting that there was no evidence of either publication or related biases.
Figure 2.
Effect of magnesium sulfate on cerebral palsy.
TABLE 3.
Effect of magnesium sulfate on cerebral palsy and pediatric mortality
| Outcome | No of trials |
Number of events/total number | Relative risk (95% Cl) |
I2 (%) |
|
|---|---|---|---|---|---|
| Magnesium | No magnesium | ||||
| Cerebral palsy | 630–32, 34, 35 | 104/2658 | 152/2699 | 0.69 (0.55–0.88) | 4.4 |
| Moderate/severe cerebral palsy | 331,34,35 | 45/2169 | 72/2218 | 0.64 (0.44–0.92) | 0.0 |
| Mild cerebral palsy | 331,34,35 | 54/2169 | 74/2218 | 0.74(0.52–1.04) | 0.0 |
| Total pediatric mortality | 629,31,32,34,35 | 401/2658 | 400/2699 | 1.01 (0.89–1.14) | 38.9 |
| Fetal mortality | 529,31,34,35 | 17/2254 | 22/2298 | 0.78(0.42–1.46) | 0.0 |
| Under two years of corrected age mortality |
529,31,34,35 | 217/2254 | 220/2298 | 1.00(0.84–1.19) | 47.3 |
| Death or cerebral palsy | 630–32,34,35 | 505/2658 | 551/2699 | 0.92(0.83–1.02) | 43.3 |
Cl, confidence interval
Figure 3.
Funnel plot of trials of antenatal magnesium sulfate in the prevention of cerebral palsy. Circles indicate log (relative risks) from trials included in meta-analysis.
The number of women at risk for preterm delivery less than 34 weeks of gestation who needed to be treated with magnesium sulfate rather than with placebo to prevent one case of cerebral palsy in their children was 52 (95% CI, 31–154). The random-effects analysis of the primary outcome of cerebral palsy yielded effect sizes similar in magnitude and direction to those obtained from the fixed-effects analysis (Table 4).
TABLE 4.
Sensitivity and subgroup palsy and pediatric mortality analyses of metaanalysis on effect of magnesium sulfate on cerebral
| Subgroup | No of trials | Relative risk (95% Cl) |
I2 (%) |
|
|---|---|---|---|---|
| CEREBRAL PALSY | ||||
| Statistical model | ||||
| Fixed effects | 630–33,35 | 0.69 (0.55–0.88) | 4.4 | |
| Random effects | 630–33, 35 | 0.70 (0.54–0.90) | NA | |
| Modified Jadad quality score | ||||
| >4 | 4 31–33,35 | 0.69 (0.54–0.88) | 0.0 | |
| ≤4 | 230a,b | 0.95(0.02–44.91) | 71.1 | |
| Completeness of follow-up of randomized fetuses | ||||
| ≥95% | 331,34,35 | 0.69 (0.54–088) | 0.0 | |
| <95% or unreported | 330,32 | 0.83 (0.28–2.43) | 43.5 | |
| Aim of the treatment | ||||
| Neuroprotective | 430a,31,33,35 | 0.71 (0.55–0.91) | 25.2 | |
| Other aims | 230b, 32 | 0.37(0.09–1.58) | 0.0 | |
| Median total dose of magnesium sulfate | ||||
| ≤4 grams | 230a, 33 | 1.37(0.18–10.70) | 56.5 | |
| >4 grams | 430b,31,32,35 | 0.67(0.51–0.88) | 0.0 | |
| Gestational age at trial entry | ||||
| <34 weeks | 630–33,35 | 0.69 (0.55–0.88) | 4.4 | |
| <32 weeks | 331,32,35 | 0.69(0.52–0.91) | 0.0 | |
| <30 weeks | 231;32 | 0.86(0.56–1.31) | 0.0 | |
| PEDIATRIC MORTALITY | ||||
| Statistical model | ||||
| Fixed effects | 629,31–33,35 | 1.01 (0.89–1.14) | 38.9 | |
| Random effects | 629,31–33,35 | 0.99(0.82–1.19) | NA | |
| Modified Jadad quality score | ||||
| >4 | 431–33,35 | 0.99(0.87–1.11) | 27.9 | |
| ≤<4 | 229a,b | 6.61 (1.21–36.11) | 31.0 | |
| Completeness of follow-up of randomized fetuses | ||||
| ≥95% | 331,34,35 | 0.94(0.79–1.12) | 40.7 | |
| <95% | 329,32 | 1.10(0.93–1.30) | 49.5 | |
| Aim of the treatment | ||||
| Neuroprotective | 429a,31,33,35 | 0.95(0.80–1.12) | 19.6 | |
| Other aims | 229b,32 | 2.83(0.21–38.80) | 72.9 | |
| Median total dose of magnesium sulfate | ||||
| ≤4 grams | 229a,33 | 0.88(0.57–1.35) | 0.0 | |
| >4 grams | 429b,31,32,35 | 1.01 (0.80–1.28) | 59.3 | |
| Gestational age at trial entry | ||||
| <34 weeks | 629,31–33,35 | 1.01 (0.89–1.14) | 38.9 | |
| <32 weeks | 331;32;35 | 1.03(0.83–1.28) | 65.5 | |
| <30 weeks | 231,32 | 0.97(0.67–1.41) | 83.0 | |
Antenatal magnesium sulfate significantly decreased the risk of cerebral palsy in sensitivity analyses limited to the 4 trials with modified Jadad quality score >4 (RR, 0.69 [95% CI, 0.54–0.88]; I2=0.0%) and to the 3 trials with completeness of follow-up of randomized fetuses ≥95% (RR, 0.69 [95% CI, 0.54–0.88]; I2=0.0%). The RR for the subgroup of 4 trials (4,446 infants) whose primary aim was neuroprotection was 0.71 (95% CI, [0.55–0.91]; I2=25.2%) and for the subgroup of 2 trials (911 infants) whose primary aim was tocolysis or to prevent eclampsia was 0.37 (95% CI, [0.09–1.58]; I2=0.0%). The lowered risk of cerebral palsy was demonstrated even in the subgroup of 3 trials (3981 infants) that included women with gestational age <32 weeks at trial entry and in the subgroup of 4 trials (4610 infants) that used a median total dose of magnesium sulfate greater than 4 grams. No statistically significant differences between groups treated with antenatal magnesium sulfate and controls in cerebral palsy were seen in the subgroups of 2 trials that used a median total dose of magnesium sulfate ≤4 grams and that included women with gestational age <30 weeks at trial entry.
Three trials (4,387 infants) reported cerebral palsy according to severity.31, 34, 35 Moderate or severe cerebral palsy was significantly reduced in the group who received magnesium sulfate (2.1% vs 3.2%; RR, 0.64 [95% CI, 0.44–0.92]; I2=0.0%; NNT for benefit, 74 [95% CI, 41–373]). The risk of mild cerebral palsy was reduced by 26% in the group allocated magnesium sulfate rather than placebo, although this did not achieve statistical significance (RR, 0.74 [95% CI, 0.52–1.04]).
There was no overall difference in the risk of total pediatric mortality, including fetal mortality and under two years of corrected age mortality, between infants exposed to magnesium sulfate and those non exposed (15.1% versus 14.8%; RR, 1.01 [95% CI, 0.89–1.14; I2 = 38.9%) (Table 3). The lack of evidence for any overall effect of magnesium sulfate on total pediatric mortality was found in all sensitivity and subgroup analyses performed except one (subgroup of two trials by Mittendorf et al30 with modified Jadad quality score ≤4 (RR, 6.61; 95% CI, 1.12–36.11; I2=31.0%) (Table 4). The combined outcome of death or cerebral palsy was slightly lower for children in the magnesium sulfate group (19.0% versus 20.4%), although this was not a statistically significant difference.
Secondary outcomes
There were no significant differences between the groups in the risk of adverse neonatal outcomes (Table 5), although a non-significant increase was seen in the risk of necrotizing enterocolitis in the magnesium group compared with the control group (7.1% vs 5.9%; RR, 1.23 [95% CI, 0.98–1.54]; I2=0.0%)
TABLE 5.
Effect of magnesium sulfate on neonatal outcomes
| Outcome | No of trials |
Number of events/total number | Relative risk (95% Cl) |
I2 (%) |
|
|---|---|---|---|---|---|
| Magnesium | No magnesium | ||||
| Intraventricular hemorrhage (all grades) |
530,31,33,35 | 467/2254 | 493/2298 | 0.96(0.86–1.08) | 20.1 |
| Grade III/IV intraventricular hemorrhage |
430,31,35 | 74/1902 | 91/1962 | 0.83(0.61 1.11) | 0.0 |
| Periventricular leukomalacia | 530,31,33,35 | 71/2254 | 76/2298 | 0.93(0.68–1.28) | 0.0 |
| Apgar score <7 at 5 minutes | 331,33,35 | 351/2169 | 351/2218 | 1.03(0.90– 1.18) | 7.3 |
| Neonatal seizures | 331,33,35 | 55/2169 | 70/2218 | 0.80(0.56–1.13) | 0 |
| Respiratory distress syndrome | 233,35 | 730/1540 | 779/1592 | 1.01 (0.85–1.19) | 65.8 |
| Need for supplemental oxygen at 36 weeks |
231,33 | 220/981 | 195/962 | 1.12(0.95–1.32) | 23.1 |
| Bronchopulmonary dysplasia | 135 | 213/1188 | 218/1256 | 1.03(0.87–1.23) | NA |
| Mechanical ventilation | 331,33,35 | 1381/2169 | 1446/2218 | 0.99(0.89–1.09) | 82.1 |
| Necrotizing enterocolitis | 331,33,35 | 155/2169 | 131/2218 | 1.23(0.98–1.54) | 0.0 |
Cl, confidence interval; NA, not applicable
With regard to infant neurodevelopmental outcomes, the risk of substantial gross motor dysfunction at the corrected age of 2 years was significantly lower among children whose mothers received magnesium sulfate than among children whose mothers did not receive magnesium sulfate (3 trials; 4387 infants; 2.6% vs 4.2%; RR, 0.60 [95% CI, 0.43–0.83]; I2=0.0%; NNT for benefit, 53 [95% CI, 32–146]). There were no significant effects of antenatal magnesium sulfate treatment on other infant neurodevelopmental outcomes such as major neurologic disability, any neurological impairment, Bayley mental and psychomotor development indexes, blindness, and deafness (Table 6).
TABLE 6.
Effect of magnesium sulfate on infant neurodevelopmental outcomes
| Outcome | No of trials | Number of events/total number | Relative risk (95% Cl) |
I2(%) | |
|---|---|---|---|---|---|
| Magnesium | No magnesium | ||||
| Substantial gross motor dysfunction |
331,34,35 | 56/2169 | 94/2218 | 0.60 (0.43–0.83) | 0.0 |
| Major neurologic disability | 231,32 | 93/1033 | 85/1027 | 1.09(0.83–1.43) | 15.3 |
| Any neurological impairment | 231,32 | 198/1033 | 194/1027 | 1.02(0.86–1.20) | 0.0 |
| Bayley mental development index <85 |
331,34,35 | 639/2169 | 660/2218 | 1.00(0.91–1.09) | 0.0 |
| Bayley mental development index <70 |
135 | 165/1188 | 171/1256 | 1.02(0.84–1.24) | NA |
| Bayley psychomotor development index <85 |
135 | 299/1188 | 315/1256 | 1.00(0.88–1.15) | NA |
| Bayley psychomotor development index <70 |
135 | 134/1188 | 144/1256 | 0.98(0.79–1.23) | NA |
| Blindness | 231,34 | 2/981 | 2/962 | 0.97(0.14–6.90) | 0.0 |
| Deafness | 231,34 | 8/981 | 11/962 | 0.51 (0.05–4.96) | 58.7 |
Cl, confidence interval; NA, not; applicable
There was no evidence of an effect of magnesium sulfate on the risk of maternal death (3 trials; 3,867 women), cardiac or respiratory arrest (3 trials; 3867 women), pulmonary edema (1 trial; 2,241 women), respiratory depression (2 trials; 3,303 women), severe postpartum hemorrhage (2 trials; 1626 women), and cesarean section (3 trials; 3,867 women) (Table 7). Compared with women receiving placebo, those exposed to magnesium sulfate had about a 50% increased risk of both hypotension and tachycardia (RR, 1.51 [95% CI, 1.09–2.09]; NNT for harm, 30 [95% CI, 17–156] and RR, 1.53 [95% CI, 1.03–2.29; NNT for harm, 28 [95% CI, 14–379], respectively). Maternal side effects secondary to study medication were significantly more common among women allocated magnesium sulfate rather than placebo, including flushing (58.4% vs 8.3%; NNT for harm, 2 [95% CI, 2–2]), nausea or vomiting (16.3% vs 3.9%; NNT for harm, 8 [95% CI, 7–10]), sweating (25.2% vs 3.4%; NNT for harm, 5 [95% CI, 4–5]), problems at injection site (37.6% vs 4.1%; NNT for harm, 3 [95% CI, 3–3]), stopping of infusion because of adverse effects (7.5% vs 2.6%; NNT for harm, 20 [95% CI, 16–29]), and any side effect (70.7% vs 17.6%; NNT for harm, 2 [95% CI, 2–2]). All funnel plots showed no asymmetry, either visually or in terms of statistical significance (P>.10 for all, by Egger test).
TABLE 7.
Effect of magnesium sulfate on maternal outcomes
| Outcome | No of trials |
Number of events/total number |
Relative risk (95% Cl) |
I2 (%) |
||
|---|---|---|---|---|---|---|
| Magnesium | No magnesium | |||||
| Death | 331,33,35 | 0/1917 | 1/1950 | 0.32(0.01–7.92) | 0.0 | |
| Cardiac or respiratory arrest | 331,33,35 | 0/1917 | 0/1950 | Not estimable | NA | |
| Pulmonary edema | 135 | 8/1096 | 3/1145 | 2.79(0.74–10.47) | NA | |
| Respiratory depression | 231,35 | 41/1631 | 31/1672 | 1.31 (0.83–2.07) | 0.0 | |
| Hypotension | 231,33 | 80/821 | 52/805 | 1.51 (1.09–2.09) | 3.6 | |
| Tachycardia | 131 | 56/535 | 36/527 | 1.53(1.03–2.29) | NA | |
| Severe postpartum hemorrhage | 231,33 | 28/821 | 26/805 | 1.06(0.63–1.79) | 0.0 | |
| Cesarean section | 331,33,35 | 822/1917 | 834/1950 | 1.00(0.93–1.07) | 21.6 | |
| Clinical and self-assessed maternal side effects of the infusion |
||||||
| Flushing | 331,33,35 | 1119/1917 | 162/1950 | 7.56(3.39–16.88) | 93.8 | |
| Nausea or vomiting |
331,33,35 | 312/1917 | 76/1950 | 4.60(1.54–13.75) | 91.5 | |
| Sweating | 231,35 | 411/1631 | 57/1672 | 6.37(1.96–20.68) | 94.6 | |
| Problems at injection site |
231,35 | 614/1631 | 68/1672 | 9.12(7.19–11.57) | 0.0 | |
| Stopping of infusion because of adverse effects |
231,35 | 123/1631 | 44/1672 | 2.81 (2.01–3.93) | 0.0 | |
| Any side effect | 331,33,35 | 1356/1917 | 343/1950 | 5.05(2.06–12.39) | 98.3 | |
Cl, confidence interval; NA, not applicable
Impact and economic evaluation of the intervention
The United Cerebral Palsy Foundation has estimated that about 8,000 babies and infants are diagnosed with cerebral palsy each year in the US6 of which about 25% (n=2,000) are born before 34 weeks of gestational age.5, 8 Therefore, if all women who deliver before 34 weeks of gestation receive antenatal magnesium sulfate, the estimated number of new cases of cerebral palsy that hypothetically could be prevented annually is 620 (95% CI, 240–900).
The number of women at risk of preterm delivery before 34 weeks of gestation who needed to receive magnesium sulfate to prevent one case of cerebral palsy was 52 (95% CI, 31–154). A recent cost decision analysis from the US revealed that the total cost for administrating magnesium sulfate as a tocolytic agent, including costs attributable to the evaluation and treatment of its adverse events, was $197.90 per patient in 2005.36 Thus, if magnesium sulfate was given to all women at risk of preterm delivery before 34 weeks of gestation, the incremental cost of preventing one case of cerebral palsy would be $10,291 (95% CI, 6,135–30,477).
COMMENT
In this systematic review, we found persuasive evidence that magnesium sulfate administered to women at high risk of delivery before 34 weeks of gestation reduces the risk of cerebral palsy in their children. The evidence was strongest for the subgroup of trials that specifically evaluated the use of antenatal magnesium sulfate in preventing cerebral palsy. In addition, this therapy was also associated with a significantly decreased risk of moderate or severe cerebral palsy and substantial gross motor dysfunction without any statistically significant effect on the risk of total pediatric mortality. This last finding suggests the reduced risk for cerebral palsy does not appear to be due to selective mortality of magnesium sulfate-exposed infants. Overall, magnesium sulfate therapy had no significant effect on the risk of major maternal complications, adverse neonatal outcomes, and other infant neurodevelopmental outcomes, although a trend was found towards increased risk of necrotizing enterocolitis. About 70% of women reported any minor side effect associated with the infusion of magnesium sulfate mainly flushing, problems at injection site, and sweating.
The strength of our findings is based on its compliance with stringent criteria for performing a rigorous systematic review of randomized controlled trials, the inclusion of a relatively large number of women and infants in these meta-analyses, the relatively narrow confidence intervals obtained which made our results more precise, the evidence of clinical and statistical homogeneity in the results of the trials for cerebral palsy, the sensitivity analysis restricted to high quality trials that upheld our main results, and the symmetrical funnel plots that suggested absence of publication and related biases in our meta-analyses.
We were unable to determine whether antenatal magnesium sulfate is more effective to prevent cerebral palsy in infants of singleton pregnancies than in infants of multiple pregnancies. Specific data for the use of magnesium sulfate according to plurality of pregnancy were reported only by two authors.31, 35 Crowther et al31 found that magnesium sulfate had no a significant effect on the risk of cerebral palsy in both infants of singleton pregnancies (RR, 1.01 [95 CI, 0.61–1.68]) and infants of multiple gestations (RR, 0.52 [95 CI, 0.21–1.25]). Rouse et al35 reported that magnesium sulfate reduced the risk of moderate or severe cerebral palsy in infants of singleton pregnancies (RR, 0.52 [95 CI, 0.27–0.98]) but not in infants of twin pregnancy (RR, 0.73; 95% CI, 0.27–1.92]).
A recent meta-analysis22 reported similar findings to those found in our review. In fact, antenatal magnesium sulfate given to women at risk of preterm birth before 37 weeks of gestational age reduced the risk of cerebral palsy (RR, 0.68 [95% CI, 0.54–0.87]) and substantial gross motor dysfunction (RR, 0.61 [95% CI, 0.44–0.85]) in their child without a statistically significant effect on pediatric mortality (RR, 1.04 [95% CI, 0.92–1.17]). Notwithstanding, our findings need to be distinguished from those of this meta-analysis. We specifically evaluated the effect of antenatal magnesium sulfate in preventing cerebral palsy in preterm infants whose mothers were less than 34 weeks of gestational age at randomization. The meta-analyses by Doyle et al22 included 5,357 preterm infants whose mothers were less than 34 weeks of gestation at randomization plus 788 infants from the Magpie trial32 whose mothers were between 34 0/7 and 36 6/7 weeks of gestation when treated with magnesium sulfate or placebo. In this last subgroup of preterm infants, cerebral palsy was diagnosed in 0 of 394 infants in the magnesium sulfate group (0.0%) and in 2 of 394 infants in the placebo group (RR, 0.20 [0.01–4.15]). Thus, there is no evidence that antenatal magnesium sulfate administered to women at risk of preterm delivery between 34 0/7 and 36 6/7 weeks of gestation decreases the risk of cerebral palsy in their infants. In addition, the Magpie trial was not primarily designed to evaluate the neuroprotective role of magnesium sulfate and had a low rate of follow-up. Finally, the systematic review by Doyle et al.22 did not perform an economic evaluation to assess the cost-effectiveness of this intervention for the prevention of cerebral palsy or evaluate some important neonatal and maternal outcomes such as necrotizing enterocolitis, maternal pulmonary edema, and some maternal side effects of the infusion (e.g. flushing, nausea or vomiting, sweating, and problems at injection site).
The molecular mechanisms by which magnesium sulfate can prevent cerebral palsy in preterm infants are not fully understood. The most prevalent pathological lesion seen in cerebral palsy is periventricular white matter injury resulting from vulnerability of the immature preoligodendrocytes before 32 weeks of gestation.37 Preoligodendrocytes are the precursors of myelinating oligodendrocytes, which constitute a major glial population in the white matter. Oxidative stress and excitotoxicity resulting from excessive stimulation of ionotropic glutamate receptors on preoligodendrocytes are the most prominent molecular mechanisms for periventricular white matter injury.38, 39 In addition, recent studies have identified functional N-methyl-D-aspartate (NMDA) glutamatergic receptors in oligodendroglial injury processes.40, 41 Antagonists of the NMDA receptors for glutamate are potent neuroprotective agents in several animal models of perinatal brain lesions.42 Growing evidence suggests that magnesium sulfate may reverse the harmful effects of hypoxic/ischemic brain damage by blocking the NMDA receptors and, as a calcium antagonist, hindering calcium influx into the cells.43, 44 In addition, magnesium sulfate could also reverse the destructive action of oxygen radicals and excitatory amino acids.45, 46
The finding that antenatal magnesium sulfate significantly reduces cerebral palsy compared with placebo is a strong argument for its use in women at risk of preterm delivery before 34 weeks of gestation. Some limitations must be noted, however, when interpreting the results of this review. First, there were variations in the inclusion and exclusion criteria, gestational age at which magnesium sulfate was administered, loading and maintenance doses, duration of the intervention, and use of retreatment. In addition, the optimal time to administer magnesium sulfate was unclear. Second, two of the studies included in our review30, 32 made the assessment of cerebral palsy at 18 months of corrected age, and other34 was made by parent telephone interview in 22% of surviving infants. For reasons yet poorly understood, many children, especially those born very preterm, exhibit a variety of neurologic abnormalities in the first 18 months of life that suggest the diagnosis of cerebral palsy which then can resolve later.47 Therefore, it has been suggested that the diagnosis of cerebral palsy should be assigned cautiously before the age of 24 months (or 24 months after the expected date of delivery in the case of preterm infants), unless the disorder is exceptionally severe.48 Third, not all of the trials reported included important secondary outcomes such as adverse neonatal and maternal outcomes, as well as infant neurodevelopmental outcomes. Finally, the results of the studies we summarized are directly applicable only to the women and infants included in those studies. Whether the results are applicable to women and infants with other clinical conditions is unknown.
The antenatal administration of magnesium sulfate to women at high risk of delivery before 34 weeks of gestation could be a cost-effective intervention. In 2003, Honeycutt et al2 estimated the average lifetime costs (direct and indirect) per person were $921,000 for persons with cerebral palsy. We have estimated that if magnesium sulfate is administered to all women at high risk of preterm delivery before 34 weeks of gestation, the incremental cost of preventing one case of cerebral palsy would be $10,291 (95% CI, $ 6,135 to $ 29,685). It should be noted that we did not estimate the cost-effectiveness of this intervention in terms of the marginal cost per quality-adjusted life year (QALY) gained.
Antenatal magnesium sulfate should be considered for use in women at high risk of delivery before 34 weeks of gestation, mainly in those with premature rupture of membranes, labor in active phase, and planned delivery within 24 hours. Loading and maintenance doses, and the duration of the treatment should not normally exceed 6 g, 1–2 g/h, and 24 hours, respectively. Further studies and/or meta-analysis of individual patient data from the available trials are required to assess the minimum effective dose of magnesium sulfate, the optimal time to administer it, the need for retreatment, the efficacy of intervention in singleton and multiple pregnancies, the cost-effectiveness of intervention, the effect on the risk of necrotizing enterocolitis, and the long term consequences of exposure for the women and their children.
In summary, our results suggest that antenatal magnesium sulfate could be used for the primary prevention of cerebral palsy in preterm infants less than 34 weeks of gestational age. However, since cerebral palsy is a result of multiple interacting risk factors rather than of a single cause, it is unlikely that antenatal magnesium sulfate administration alone can prevent all cases of this illness in preterm infants.
Acknowledgments
This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Rosenbaum P, Paneth N, Leviton A, Goldstein M, Bax M, Damiano D, et al. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol. 2007;109(Suppl):8–14. [PubMed] [Google Scholar]
- 2.Honeycutt A, Dunlap L, Chen H, al Homsi G, Grosse S, Schendel D. Economic costs associated with mental retardation, cerebral palsy, hearing loss, and vision impairment: United States, 2003. MMWR. 2004;53:57–59. [PubMed] [Google Scholar]
- 3.Paneth N, Hong T, Korzeniewski S. The descriptive epidemiology of cerebral palsy. Clin Perinatol. 2006;33:251–267. doi: 10.1016/j.clp.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Yeargin-Allsopp M, Van Naarden Braun K, Doernberg NS, Benedict RE, Kirby RS, Durkin MS. Prevalence of cerebral palsy in 8-year-old children in three areas of the United States in 2002: a multisite collaboration. Pediatrics. 2008;121:547–554. doi: 10.1542/peds.2007-1270. [DOI] [PubMed] [Google Scholar]
- 5.Moster D, Lie RT, Markestad T. Long-term medical and social consequences of preterm birth. N Engl J Med. 2008;359:262–273. doi: 10.1056/NEJMoa0706475. [DOI] [PubMed] [Google Scholar]
- 6.United Cerebral Palsy. Press room. Vocabulary tips. Cerebral Palsy - Facts & Figures. [Accessed January 20, 2009]; http://www.ucp.org/ucp_generaldoc.cfm/1/9/37/37/447.
- 7.Himpens E, Van den Broeck C, Oostra A, Calders P, Vanhaesebrouck P. Prevalence, type, distribution, and severity of cerebral palsy in relation to gestational age: a meta-analytic review. Dev Med Child Neurol. 2008;50:334–340. doi: 10.1111/j.1469-8749.2008.02047.x. [DOI] [PubMed] [Google Scholar]
- 8.Himmelmann K, Hagberg G, Beckung E, Hagberg B, Uvebrant P. The changing panorama of cerebral palsy in Sweden. IX. Prevalence and origin in the birth-year period 1995–1998. Acta Paediatr. 2005;94:287–294. doi: 10.1111/j.1651-2227.2005.tb03071.x. [DOI] [PubMed] [Google Scholar]
- 9.Petterson B, Blair E, Watson L, Stanley F. Adverse outcome after multiple pregnancy. Baillieres Clin Obstet Gynaecol. 1998;12:1–17. doi: 10.1016/s0950-3552(98)80036-9. [DOI] [PubMed] [Google Scholar]
- 10.Scher AI, Petterson B, Blair E, Ellenberg JH, Grether JK, Haan E, et al. The risk of mortality or cerebral palsy in twins: a collaborative population-based study. Pediatr Res. 2002;52:671–681. doi: 10.1203/00006450-200211000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Nelson KB, Grether JK. Can magnesium sulfate reduce the risk of cerebral palsy in very low birthweight infants? Pediatrics. 1995;95:263–269. [PubMed] [Google Scholar]
- 12.Hauth JC, Goldenberg RL, Nelson KG, DuBard MB, Peralta MA, Gaudier FL. Reduction of cerebral palsy with maternal MgSO4 treatment in newborns weighing 500–1000 g [abstract] Am J Obstet Gynecol. 1995;172:419. [Google Scholar]
- 13.Schendel DE, Berg CJ, Yeargin-Allsopp M, Boyle CA, Decoufle P. Prenatal magnesium sulfate exposure and the risk for cerebral palsy or mental retardation among very low-birth-weight children aged 3 to 5 years. JAMA. 1996;276:1805–1810. [PubMed] [Google Scholar]
- 14.Wiswell TE, Graziani LJ, Caddell JL, Vecchione N, Stanley C, Spitzer AR. Maternally administered megnesium sulphate protects against early brain injury and long-term adverse neurodevelopmental outcomes in preterm infants: a prospective study [abstract] Pediatr Res. 1996;39:253. [Google Scholar]
- 15.Matsuda Y, Kouno S, Hiroyama Y, Kuraya K, Kamitomo M, Ibara S, et al. Intrauterine infection, magnesium sulfate exposure and cerebral palsy in infants born between 26 and 30 weeks of gestation. Eur J Obstet Gynecol Reprod Biol. 2000;91:159–164. doi: 10.1016/s0301-2115(99)00256-0. [DOI] [PubMed] [Google Scholar]
- 16.Paneth N, Jetton J, Pinto-Martin J, Susser M. Magnesium sulfate in labor and risk of neonatal brain lesions and cerebral palsy in low birth weight infants. The Neonatal Brain Hemorrhage Study Analysis Group. Pediatrics. 1997;99:E1. doi: 10.1542/peds.99.5.e1. [DOI] [PubMed] [Google Scholar]
- 17.O'Shea TM, Klinepeter KL, Dillard RG. Prenatal events and the risk of cerebral palsy in very low birth weight infants. Am J Epidemiol. 1998;147:362–369. doi: 10.1093/oxfordjournals.aje.a009458. [DOI] [PubMed] [Google Scholar]
- 18.Wilson-Costello D, Borawski E, Friedman H, Redline R, Fanaroff AA, Hack M. Perinatal correlates of cerebral palsy and other neurologic impairment among very low birth weight children. Pediatrics. 1998;102(2 Pt 1):315–322. doi: 10.1542/peds.102.2.315. [DOI] [PubMed] [Google Scholar]
- 19.Boyle CA, Yeargin-Allsopp M, Schendel DE, Holmgreen P, Oakley GP. Tocolytic magnesium sulfate exposure and risk of cerebral palsy among children with birth weights less than 1,750 grams. Am J Epidemiol. 2000;152:120–124. doi: 10.1093/aje/152.2.120. [DOI] [PubMed] [Google Scholar]
- 20.Grether JK, Hoogstrate J, Walsh-Greene E, Nelson KB. Magnesium sulfate for tocolysis and risk of spastic cerebral palsy in premature children born to women without preeclampsia. Am J Obstet Gynecol. 2000;183:717–725. doi: 10.1067/mob.2000.106581. [DOI] [PubMed] [Google Scholar]
- 21.Costantine MM, How HY, Coppage K, Maxwell RA, Sibai BM. Does peripartum infection increase the incidence of cerebral palsy in extremely low birthweight infants? Am J Obstet Gynecol. 2007;196:e6–e8. doi: 10.1016/j.ajog.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 22.Doyle LW, Crowther CA, Middleton P, Marret S, Rouse D. Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus. Cochrane Database Syst Rev. 2009;(1):CD004661. doi: 10.1002/14651858.CD004661.pub3. [DOI] [PubMed] [Google Scholar]
- 23.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet. 1999;354:1896–1900. doi: 10.1016/s0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- 24.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 25.Chapter 9: Analysing data and undertaking meta-analyses. In: Deeks JJ, Higgins JPT, Altman DG, editors; Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.1 [updated September 2008] The Cochrane Collaboration; 2008. [Google Scholar]
- 26.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analyses detected by a simple graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Altman DG. Confidence intervals for the number needed to treat. BMJ. 1998;317:1309–1312. doi: 10.1136/bmj.317.7168.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mittendorf R, Covert R, Boman J, Khoshnood B, Lee KS, Siegler M. Is tocolytic magnesium sulphate associated with increased total paediatric mortality? Lancet. 1997;350:1517–1518. doi: 10.1016/s0140-6736(97)24047-x. [DOI] [PubMed] [Google Scholar]
- 30.Mittendorf R, Dambrosia J, Pryde PG, Lee KS, Gianopoulos JG, Besinger RE, Tomich PG. Association between the use of antenatal magnesium sulfate in preterm labor and adverse health outcomes in infants. Am J Obstet Gynecol. 2002;186:1111–1118. doi: 10.1067/mob.2002.123544. [DOI] [PubMed] [Google Scholar]
- 31.Crowther CA, Hiller JE, Doyle LW, Haslam RR. Australasian Collaborative Trial of Magnesium Sulphate (ACTOMg SO4) Collaborative Group. Effect of magnesium sulfate given for neuroprotection before preterm birth: a randomized controlled trial. JAMA. 2003;290:2669–2676. doi: 10.1001/jama.290.20.2669. [DOI] [PubMed] [Google Scholar]
- 32.Magpie Trial Follow-Up Study Collaborative Group. The Magpie Trial: a randomised trial comparing magnesium sulphate with placebo for pre-eclampsia. Outcome for children at 18 months. BJOG. 2007;114:289–299. doi: 10.1111/j.1471-0528.2006.01165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marret S, Marpeau L, Zupan-Simunek V, Eurin D, Lévêque C, Hellot MF, et al. PREMAG trial group. Magnesium sulphate given before very-preterm birth to protect infant brain: the randomised controlled PREMAG trial*. BJOG. 2007;114:310–318. doi: 10.1111/j.1471-0528.2006.01162.x. [DOI] [PubMed] [Google Scholar]
- 34.Marret S, Marpeau L, Bénichou J. Benefit of magnesium sulfate given before very preterm birth to protect infant brain. Pediatrics. 2008;121:225–226. doi: 10.1542/peds.2007-2971. [DOI] [PubMed] [Google Scholar]
- 35.Rouse DJ, Hirtz DG, Thom E, Varner MW, Spong CY, Mercer BM, et al. A randomized, controlled trial of magnesium sulfate for the prevention of cerebral palsy. N Engl J Med. 2008;359:895–905. doi: 10.1056/NEJMoa0801187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayes E, Moroz L, Pizzi L, Baxter J. A cost decision analysis of 4 tocolytic drugs. Am J Obstet Gynecol. 2007;197:383.e1–383.e6. doi: 10.1016/j.ajog.2007.06.052. [DOI] [PubMed] [Google Scholar]
- 37.Back SA. Perinatal white matter injury: the changing spectrum of pathology and emerging insights into pathogenetic mechanisms. Ment Retard Dev Disabil Res Rev. 2006;12:129–140. doi: 10.1002/mrdd.20107. [DOI] [PubMed] [Google Scholar]
- 38.Folkerth RD. Neuropathologic substrate of cerebral palsy. J Child Neurol. 2005;20:940–949. doi: 10.1177/08830738050200120301. [DOI] [PubMed] [Google Scholar]
- 39.Jensen FE. Role of glutamate receptors in periventricular leukomalacia. J Child Neurol. 2005;20:950–959. doi: 10.1177/08830738050200120401. [DOI] [PubMed] [Google Scholar]
- 40.Salter MG, Fern R. NMDA receptors are expressed in developing oligodendrocyte processes and mediate injury. Nature. 2005;438:1167–1171. doi: 10.1038/nature04301. [DOI] [PubMed] [Google Scholar]
- 41.Micu I, Jiang Q, Coderre E, Ridsdale A, Zhang L, Woulfe J, et al. NMDA receptors mediate calcium accumulation in myelin during chemical ischaemia. Nature. 2006;439:988–992. doi: 10.1038/nature04474. [DOI] [PubMed] [Google Scholar]
- 42.Johnston MV, Nakajima W, Hagberg H. Mechanisms of hypoxic neurodegeneration in the developing brain. Neuroscientist. 2002;8:212–220. doi: 10.1177/1073858402008003007. [DOI] [PubMed] [Google Scholar]
- 43.Antonov SM, Johnson JW. Permeant ion regulation of N-methyl-D-aspartate receptor channel block by Mg(2+) Proc Natl Acad Sci U S A. 1999;96:14571–14576. doi: 10.1073/pnas.96.25.14571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gathwala G. Neuronal protection with magnesium. Indian J Pediatr. 2001;68:417–419. doi: 10.1007/BF02723017. [DOI] [PubMed] [Google Scholar]
- 45.Golan H, Kashtutsky I, Hallak M, Sorokin Y, Huleihel M. Maternal hypoxia during pregnancy delays the development of motor reflexes in newborn mice. Dev Neurosci. 2004;26:24–29. doi: 10.1159/000080708. [DOI] [PubMed] [Google Scholar]
- 46.Zylinska L, Gulczynska E, Kozaczuk A. Changes in erythrocyte glutathione and plasma membrane calcium pump in preterm newborns treated antenatally with MgSO4. Neonatology. 2008;94:272–278. doi: 10.1159/000151646. [DOI] [PubMed] [Google Scholar]
- 47.Bracewell M, Marlow N. Patterns of motor disability in very preterm children. Ment Retard Dev Disabil Res Rev. 2002;8:241–248. doi: 10.1002/mrdd.10049. [DOI] [PubMed] [Google Scholar]
- 48.Paneth N. Establishing the diagnosis of cerebral palsy. Clin Obstet Gynecol. 2008;51:742–748. doi: 10.1097/GRF.0b013e318187081a. [DOI] [PubMed] [Google Scholar]