Abstract
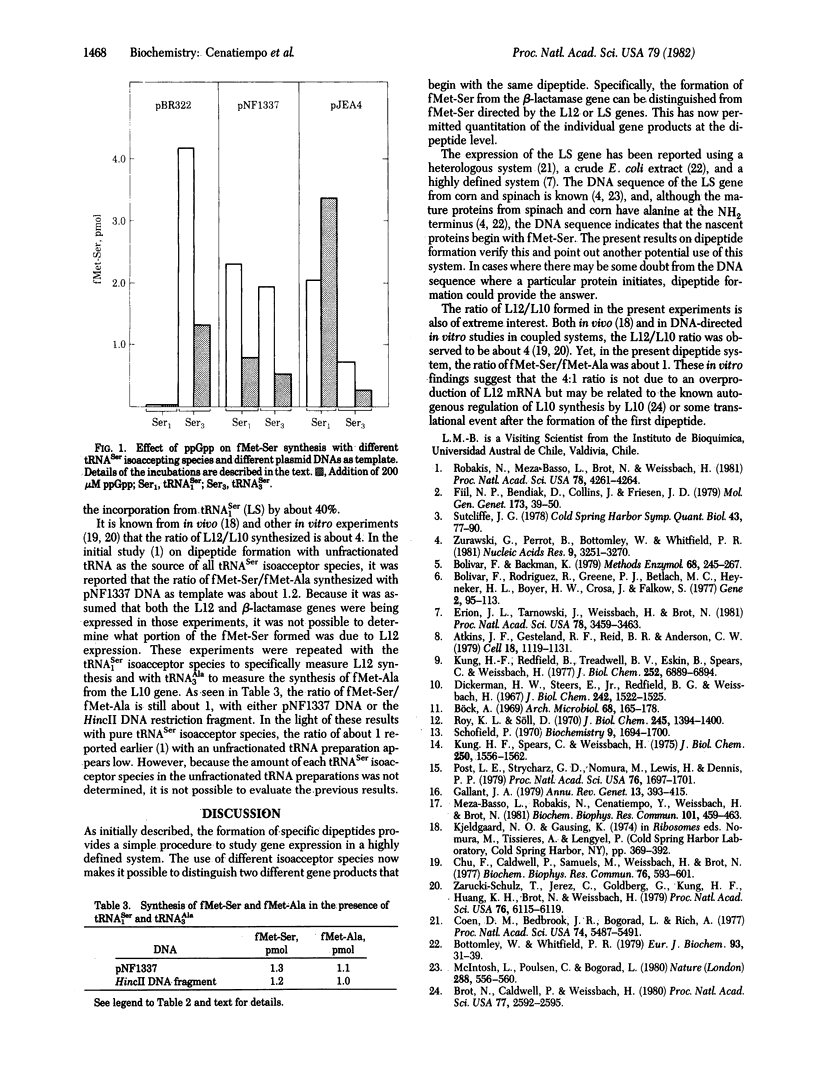
A simplified translation system coupled to DNA transcription that involves assaying the synthesis of the first dipeptide of a gene product has been described recently [Robakis, N., Meza-Basso, L., Brot, N. & Weissbach, H. (1981) Proc. Natl. Acad. Sci. USA 78, 4261--4264]. Using this dipeptide system, we have investigated the expression of genes carried on plasmids coding for beta-lactamase, ribosomal protein L12, and the chloroplast large subunit (LS) of ribulosebisphosphate carboxylase (RbuBPCase). Although all three nascent gene products begin with the sequence fMet-Ser, the formation of fMet-Ser can be used to distinguish between the synthesis of beta-lactamase and either L12 or the LS of RbuBPCase by using different serine isoacceptor tRNA species. In beta-lactamase, the serine codon is AGU, which utilizes the serine isoacceptor species tRNASer3; in L12 and the LS of RbuBPCase, the serine codewords are UCU and UCA, respectively, both of which are recognized by the serine isoacceptor species tRNASer1. By using either pure tRNASer1 or pure tRNASer3, the expression of each gene can be quantitated. In this system, guanosine-5'-diphosphate-3'-diphosphate inhibits the expression of the beta-lactamase and L12 genes but stimulates the synthesis of the LS. In addition, the ratio of fMet-Ser/fMet-Ala (L12/L10) synthesized was about 1 as compared with the ratio of 4 that has been obtained previously in vivo or in vitro protein-synthesizing systems in which the entire gene product was measured.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins J. F., Gesteland R. F., Reid B. R., Anderson C. W. Normal tRNAs promote ribosomal frameshifting. Cell. 1979 Dec;18(4):1119–1131. doi: 10.1016/0092-8674(79)90225-3. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Backman K. Plasmids of Escherichia coli as cloning vectors. Methods Enzymol. 1979;68:245–267. doi: 10.1016/0076-6879(79)68018-7. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Bottomley W., Whitfeld P. R. Cell-free transcription and translation of total spinach chloroplast DNA. Eur J Biochem. 1979 Jan 2;93(1):31–39. doi: 10.1111/j.1432-1033.1979.tb12791.x. [DOI] [PubMed] [Google Scholar]
- Brot N., Caldwell P., Weissbach H. Autogenous control of Escherichia coli ribosomal protein L10 synthesis in vitro. Proc Natl Acad Sci U S A. 1980 May;77(5):2592–2595. doi: 10.1073/pnas.77.5.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böck A. Mutation affecting the charging reaction of alanyl-tRNA synthetase from Escherichia coli K 10. Arch Mikrobiol. 1969 Oct;68(2):165–178. doi: 10.1007/BF00413875. [DOI] [PubMed] [Google Scholar]
- Chu F., Caldwell P., Samuels M., Weissbach H., Brot N. DNA-dependent in vitro synthesis of Escherichia coli ribosomal protein L10 and the formation of an L10L12 complex. Biochem Biophys Res Commun. 1976 May 23;76(2):593–601. doi: 10.1016/0006-291x(77)90765-3. [DOI] [PubMed] [Google Scholar]
- Coen D. M., Bedbrook J. R., Bogorad L., Rich A. Maize chloroplast DNA fragment encoding the large subunit of ribulosebisphosphate carboxylase. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5487–5491. doi: 10.1073/pnas.74.12.5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerman H. W., Steers E., Jr, Redfield B. G., Weissbach H. Methionyl soluble ribonucleic acid transformylase. I. Purification and partial characterization. J Biol Chem. 1967 Apr 10;242(7):1522–1525. [PubMed] [Google Scholar]
- Erion J. L., Tarnowski J., Weissbach H., Brot N. Cloning, mapping, and in vitro transcription-translation of the gene for the large subunit of ribulose-1,5-bisphosphate carboxylase from spinach chloroplasts. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3459–3463. doi: 10.1073/pnas.78.6.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiil N. P., Bendiak D., Collins J., Friesen J. D. Expression of Escherichia coli ribosomal protein and RNA polymerase genes cloned on plasmids. Mol Gen Genet. 1979 May 23;173(1):39–50. doi: 10.1007/BF00267689. [DOI] [PubMed] [Google Scholar]
- Gallant J. A. Stringent control in E. coli. Annu Rev Genet. 1979;13:393–415. doi: 10.1146/annurev.ge.13.120179.002141. [DOI] [PubMed] [Google Scholar]
- Kung H. F., Redfield B., Treadwell B. V., Eskin B., Spears C., Weissbach H. DNA-directed in vitro synthesis of beta-galactosidase. Studies with purified factors. J Biol Chem. 1977 Oct 10;252(19):6889–6894. [PubMed] [Google Scholar]
- Kung H., Spears C., Weissbach H. Purification and properties of a soluble factor required for the deoxyribonucleic acid-directed in vitro synthesis of beta-galactosidase. J Biol Chem. 1975 Feb 25;250(4):1556–1562. [PubMed] [Google Scholar]
- Meza-Basso L., Robakis N., Cenatiempo Y., Weissbach H., Brot N. Guanosine-5'-diphosphate-3'-diphosphate inhibits the in vitro synthesis of beta-lactamase from pBR322 DNA. Biochem Biophys Res Commun. 1981 Jul 30;101(2):459–463. doi: 10.1016/0006-291x(81)91282-1. [DOI] [PubMed] [Google Scholar]
- Post L. E., Strycharz G. D., Nomura M., Lewis H., Dennis P. P. Nucleotide sequence of the ribosomal protein gene cluster adjacent to the gene for RNA polymerase subunit beta in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1697–1701. doi: 10.1073/pnas.76.4.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robakis N., Meza-Basso L., Brot N., Weissbach H. Translational control of ribosomal protein L10 synthesis occurs prior to formation of first peptide bond. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4261–4264. doi: 10.1073/pnas.78.7.4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy K. L., Söll D. Purification of five serine transfer ribonucleic acid species from Escherichia coli and their acylation by homologous and heterologous seryl transfer ribonucleic acid synthetases. J Biol Chem. 1970 Mar 25;245(6):1394–1400. [PubMed] [Google Scholar]
- Schofield P. Isolation and some properties of methionine transfer ribonucleic acid from Escherichia coli. Biochemistry. 1970 Apr 14;9(8):1694–1700. doi: 10.1021/bi00810a007. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- Zarucki-Schulz T., Jerez C., Goldberg G., Kung H. F., Huang K. H., Brot N., Weissbach H. DNA-directed in vitro synthesis of proteins involved in bacterial transcription and translation. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6115–6119. doi: 10.1073/pnas.76.12.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Perrot B., Bottomley W., Whitfeld P. R. The structure of the gene for the large subunit of ribulose 1,5-bisphosphate carboxylase from spinach chloroplast DNA. Nucleic Acids Res. 1981 Jul 24;9(14):3251–3270. doi: 10.1093/nar/9.14.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]