Abstract
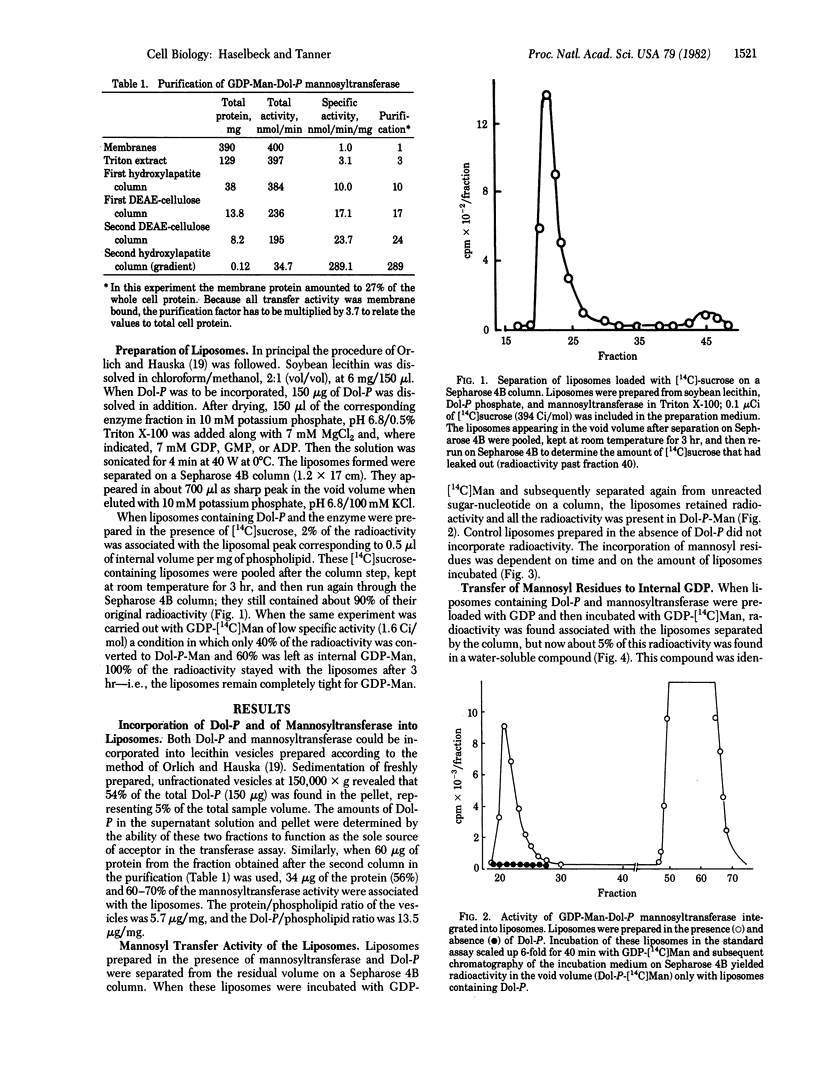
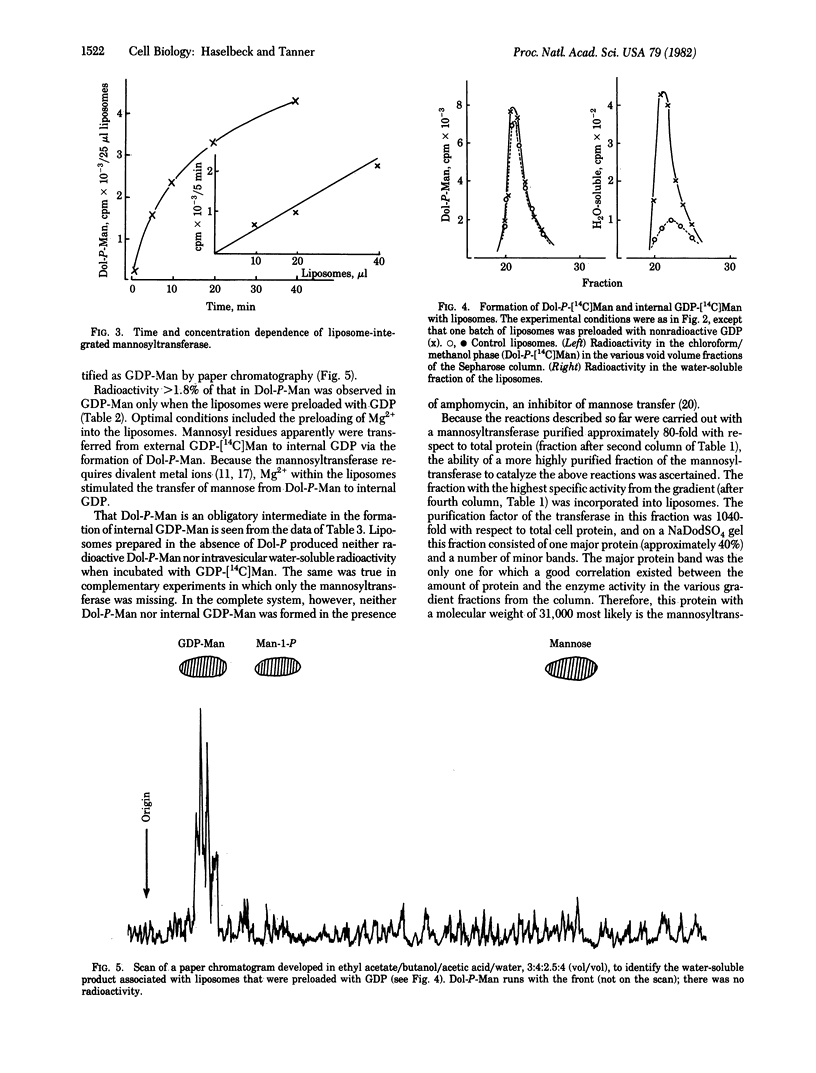
A partially purified (up to 1000-fold) mannosyl transferase that catalyzed the reversible reaction GDP-Man + Dol-P in equilibrium Dol-P-Man + GDP was incorporated into liposomes consisting of soybean lecithin and dolichyl phosphate (Dol-P). The enzyme transferred the mannosyl moiety from external GDP-Man to liposome-associated Dol-P. However, when the liposomes were preloaded with GDP, mannosyl residues were also transferred to the inside, giving rise to internal GDP-Man by the reverse reaction. This transfer of an activated sugar through a membrane required the presence of Dol-P and the enzyme in the liposome. Mannosyl residues were not transferred to the inside when the liposomes were preloaded with ADP or GMP. Amphomycin completely inhibited the formation of Dol-P-Man as well as the transfer of mannose into the liposomes. The results are taken as evidence for the open postulated role of dolichols in sugar translocation through membranes. The data are discussed in relation to glycoprotein synthesis at the endoplasmic reticulum.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babczinski P., Haselbeck A., Tanner W. Yeast mannosyl transferases requiring dolichyl phosphate and dolichyl phosphate mannose as substrate. Partial purification and characterization of the solubilized enzyme. Eur J Biochem. 1980 Apr;105(3):509–515. doi: 10.1111/j.1432-1033.1980.tb04526.x. [DOI] [PubMed] [Google Scholar]
- Babczinski P., Tanner W. Involvement of dolicholmonophosphate in the formation of specific mannosyl-linkages in yeast glycoproteins. Biochem Biophys Res Commun. 1973 Oct 1;54(3):1119–1124. doi: 10.1016/0006-291x(73)90808-5. [DOI] [PubMed] [Google Scholar]
- Ballou C. Structure and biosynthesis of the mannan component of the yeast cell envelope. Adv Microb Physiol. 1976;14(11):93–158. doi: 10.1016/s0065-2911(08)60227-1. [DOI] [PubMed] [Google Scholar]
- Bause E., Lehle L. Enzymatic N-glycosylation and O-glycosylation of synthetic peptide acceptors by dolichol-linked sugar derivatives in yeast. Eur J Biochem. 1979 Nov;101(2):531–540. doi: 10.1111/j.1432-1033.1979.tb19748.x. [DOI] [PubMed] [Google Scholar]
- Bretthauer R. K., Wu S. Synthesis of the mannosyl-O-serine (threonine) linkage of glycoproteins from polyisoprenylphosphate mannose in yeast (Hansenula holstii). Arch Biochem Biophys. 1975 Mar;167(1):151–160. doi: 10.1016/0003-9861(75)90451-8. [DOI] [PubMed] [Google Scholar]
- Chapman A., Fujimoto K., Kornfeld S. The primary glycosylation defect in class E Thy-1-negative mutant mouse lymphoma cells is an inability to synthesize dolichol-P-mannose. J Biol Chem. 1980 May 25;255(10):4441–4446. [PubMed] [Google Scholar]
- Hanover J. A., Lennarz W. J. The topological orientation of N,N'-diacetylchitobiosylpyrophosphoryldolichol in artificial and natural membranes. J Biol Chem. 1979 Sep 25;254(18):9237–9246. [PubMed] [Google Scholar]
- Jung P., Tanner W. Identification of the lipid intermediate in yeast mannan biosynthesis. Eur J Biochem. 1973 Aug 1;37(1):1–6. doi: 10.1111/j.1432-1033.1973.tb02949.x. [DOI] [PubMed] [Google Scholar]
- Kang M. S., Spencer J. P., Elbein A. D. Amphomycin inhibition of mannose and GlcNAc incorporation into lipid-linked saccharides. J Biol Chem. 1978 Dec 25;253(24):8860–8866. [PubMed] [Google Scholar]
- Katz F. N., Rothman J. E., Lingappa V. R., Blobel G., Lodish H. F. Membrane assembly in vitro: synthesis, glycosylation, and asymmetric insertion of a transmembrane protein. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3278–3282. doi: 10.1073/pnas.74.8.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehle L., Bauer F., Tanner W. The formation of glycosidic bonds in yeast glycoproteins. Intracellular localisation of the reactions. Arch Microbiol. 1977 Jul 26;114(1):77–81. doi: 10.1007/BF00429634. [DOI] [PubMed] [Google Scholar]
- Lehle L. Biosynthesis of the core region of yeast mannoproteins. Formation of a glucosylated dolichol-bound oligosaccharide precursor, its transfer to protein and subsequent modification. Eur J Biochem. 1980 Aug;109(2):589–601. doi: 10.1111/j.1432-1033.1980.tb04832.x. [DOI] [PubMed] [Google Scholar]
- Li E., Tabas I., Kornfeld S. The synthesis of complex-type oligosaccharides. I. Structure of the lipid-linked oligosaccharide precursor of the complex-type oligosaccharides of the vesicular stomatitis virus G protein. J Biol Chem. 1978 Nov 10;253(21):7762–7770. [PubMed] [Google Scholar]
- Marriott M., Tanner W. Localization of dolichyl phosphate- and pyrophosphate-dependent glycosyl transfer reactions in Saccharomyces cerevisiae. J Bacteriol. 1979 Aug;139(2):566–572. doi: 10.1128/jb.139.2.566-572.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloskey M. A., Troy F. A. Paramagnetic isoprenoid carrier lipids. 2. Dispersion and dynamics in lipid membranes. Biochemistry. 1980 May 13;19(10):2061–2066. doi: 10.1021/bi00551a009. [DOI] [PubMed] [Google Scholar]
- Nilsson O. S., DePierre J. W., Dallner G. Investigation of the transverse topology of the microsomal membrane using combinations of proteases and the non-penetrating reagent diazobenzene sulfonate. Biochim Biophys Acta. 1978 Jul 20;511(1):93–104. doi: 10.1016/0005-2736(78)90067-6. [DOI] [PubMed] [Google Scholar]
- Orlich G., Hauska G. Reconstitution of photosynthetic energy conservation. I. Proton movements in liposomes containing reaction center of photosystem I from spinach chloroplasts. Eur J Biochem. 1980 Oct;111(2):525–533. doi: 10.1111/j.1432-1033.1980.tb04968.x. [DOI] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Parodi A. J., Leloir L. F. The role of lipid intermediates in the glycosylation of proteins in the eucaryotic cell. Biochim Biophys Acta. 1979 Apr 23;559(1):1–37. doi: 10.1016/0304-4157(79)90006-6. [DOI] [PubMed] [Google Scholar]
- Robbins P. W., Hubbard S. C., Turco S. J., Wirth D. F. Proposal for a common oligosaccharide intermediate in the synthesis of membrane glycoproteins. Cell. 1977 Dec;12(4):893–900. doi: 10.1016/0092-8674(77)90153-2. [DOI] [PubMed] [Google Scholar]
- Sharma C. B., Babczinski P., Lehle L., Tanner W. The role of dolicholmonophosphate in glycoprotein biosynthesis in Saccharomyces cerevisiae. Eur J Biochem. 1974 Jul 1;46(1):35–41. doi: 10.1111/j.1432-1033.1974.tb03594.x. [DOI] [PubMed] [Google Scholar]
- Sharma C. B., Lehle L., Tanner W. N-Glycosylation of yeast proteins. Characterization of the solubilized oligosaccharyl transferase. Eur J Biochem. 1981 May;116(1):101–108. doi: 10.1111/j.1432-1033.1981.tb05306.x. [DOI] [PubMed] [Google Scholar]
- Snider M. D., Sultzman L. A., Robbins P. W. Transmembrane location of oligosaccharide-lipid synthesis in microsomal vesicles. Cell. 1980 Sep;21(2):385–392. doi: 10.1016/0092-8674(80)90475-4. [DOI] [PubMed] [Google Scholar]
- Staneloni R. J., Tolmasky M. E., Petriella C., Ugalde R. A., Leloir L. F. Presence in a plant of a compound similar to the dolichyl diphosphate oligosaccharide of animal tissue. Biochem J. 1980 Oct 1;191(1):257–260. doi: 10.1042/bj1910257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble R. B., Maley F., Tarentino A. L. Characterization of large oligosaccharide-lipids synthesized in vitro by microsomes from Saccharomyces cerevisiae. J Biol Chem. 1980 Nov 10;255(21):10232–10238. [PubMed] [Google Scholar]


