Abstract
Normal epithelial cells undergo apoptosis when they are denied contact with the extracellular matrix, in a process termed “anoikis.” Conversely, malignant epithelial cells typically acquire anchorage independence, i.e., the capacity to survive and grow in the absence of matrix interaction. Here we asked the question whether anoikis is affected by signaling through the EGF receptor (EGFR). We focused on the EGFR because EGFR signaling is frequently deregulated in malignant epithelial cells. We demonstrate that EGFR activation markedly alleviated the requirement of matrix engagement for survival of primary and immortalized human keratinocytes in suspension culture. Protection of epithelial cells through EGFR activation against anoikis was associated with and required sustained MAPK phosphorylation during the early phase of suspension culture. Interestingly, high levels of MAPK phosphorylation were not only required for EGFR-mediated protection against anoikis but also occurred as a consequence of caspase activation at later stages of suspension culture. These results demonstrate that EGFR activation contributes to anchorage-independent epithelial cell survival and identify MAPK activation as an important mechanism in this process.
INTRODUCTION
Normal epithelial cells require contact with extracellular matrix components to survive. In the absence of matrix attachment, these cells die exhibiting molecular characteristics of programmed cell death or apoptosis (Meredith et al., 1993; Frisch and Francis, 1994). This form of cell death has been termed anoikis (Frisch and Francis, 1994) and is likely to preclude dissemination of normal epithelial cells to inappropriate sites. Malignant transformation by oncogenic forms of Ras and Src confers resistance of cultured epithelial cells to anoikis (Frisch and Francis, 1994; Rosen et al., 2000). Activation of proto-oncogenic forms of Ras and Src in epithelial cells occurs not only during integrin engagement but also in response to soluble growth factors, including ligands for the EGF receptor (EGFR) (Luttrell et al., 1994; Walker et al., 1998). Our previous work has implicated the EGFR in protecting normal human keratinocytes against apoptosis of adherent cells in vitro (Rodeck et al., 1997a,b; Jost et al., 1999). Together, these findings led us to investigate whether signaling from the EGFR may also affect survival of human keratinocytes in the absence of matrix engagement. This is an important question because effective growth factor receptor signaling has been shown in fibroblasts to be contingent on adhesion receptor signaling (Lin et al., 1997; Renshaw et al., 1997; Bottazzi et al., 1999; Roovers et al., 1999). Thus, it may be argued that EGFR activation is not likely to affect survival of cells in forced suspension culture.
By contrast, we demonstrate here that activation of the EGFR by endogenous and exogenous ligands substantially delayed death of normal human keratinocytes held in forced suspension. We describe that EGFR-dependent protection of keratinocytes against anoikis required sustained MAP kinase/ERK kinase (MEK) activity during the early stages of suspension culture. Remarkably, the apoptotic process itself was accompanied by high levels of MAPK phosphorylation that required caspase activity.
MATERIALS AND METHODS
Chemicals and Reagents
MAb 425 is an EGFR antagonistic mAb that binds exclusively to a protein epitope on the extracellular domain of the EGFR (Murthy et al., 1987). It has no known agonist activity on the EGFR but inhibits EGFR autophosphorylation (Murthy et al., 1987), Ca2+ mobilization (Murthy et al., 1990), and EGFR-dependent generation of inositol-(1,4,5)-triphosphate (Murthy et al., 1990). PD98059, LY294002, tyrphostins AG1295 and AG1478, and Ac-DEVD-CHO were purchased from Calbiochem (San Diego, CA). Antibodies to MAPK, phosphoMAPK, cleaved PARP (p85), and Elk-1 and Gsk-β phosphorylation kits were obtained from New England Biolabs (Beverly, MA). Antibodies to α-tubulin and hemagglutinin were from Oncogene (Boston, MA) and Covance (Richmond, CA), respectively. Purified mouse EGF was from Collaborative Research (Bedford, MA).
Cells
Human neonatal foreskin keratinocyte cultures were initiated and propagated in MCDB153 complete medium as described (McNeill and Jensen, 1990). The culture medium designated in the following as MCDB base medium consisted of MCDB153 (Sigma, St. Louis, MO) containing 30 μM Ca2+ and supplemented with amino acids, ethanolamine, phosphorylethanolamine, and hydrocortisone (all from Sigma). HaCaT cells are immortalized but nontumorigenic human keratinocytes (Boukamp et al., 1988) and were kindly provided by Dr. N. Fusenig (German Cancer Research Center, Heidelberg, Germany). These cells were maintained in W489 medium (Rodeck et al., 1987) consisting of four parts MCDB153 and one part L15 media supplemented with 2% fetal calf serum. Using a tetracycline regulatable episomally maintained expression system (Jost et al., 1997), we generated HaCaT cells that conditionally overexpressed Bcl-xL (Jost et al., 1999) or the dominant negative MEK construct MKK1–8E (Holmstrom et al., 1999; Jost et al., 2001) or a dominant negative Akt construct (Dudek et al., 1997). Mock-transfected HaCaT keratinocytes expressed an empty pCEPTetP vector (Jost et al., 1997).
Suspension Culture
Suspension cultures were initiated by seeding keratinocytes onto 0.9% agarose gels prepared using MCDB base medium supplemented with 0.2% (vol/vol) BSA–FAF (fatty-acid-free) and free of protein growth factors unless stated otherwise. After various time periods, cell aliquots were removed and processed for viability/apoptosis assays or protein extraction for Western blot analyses. In these assays, EGF was used at 2 nM (10 ng/ml), EGFR antagonistic mAb 425 was used at 66 nM (10 μg/ml), and AG1478 was used at 10 μM unless stated otherwise.
Viability and Apoptosis Assays
To determine cell death in suspension culture, aliquots of cells retrieved from suspension cultures were subjected to flow cytometric analysis of terminal deoxynucleotidyl transferase-mediated biotinylated dUTP nick end labeling (TUNEL)-positive cells. Briefly, cells were fixed in 70% ethanol at 4°C. Before staining with either biotin-16-dUTP or FITC-12-dUTP (both from Roche Molecular Biochemicals, Indianapolis, IN), cells were washed in PBS containing 1% BSA. Staining was performed using TdT enzyme and buffers from Roche Molecular Biochemicals for 1 h at 37°C. When biotin-16-dUTP was used, cells were incubated with FITC-avidin (2.5 μg/ml; Vector Laboratories, Burlingame, CA) in 4× SCC and washed once with PBS containing 1% BSA and 0.1% Triton X-100. Cells were analyzed immediately by flow cytometry using a FACScan cytometer (Becton Dickinson, Fullerton, CA).
To assess loss of cell viability and metabolic competence, the proportion of nonviable cells was determined by trypan blue exclusion assay. To assess the proportion of cells that not only survived but regained proliferative competence, aliquots of cells retrieved from suspension cultures after various time periods were reseeded at low cell densities on tissue culture-treated plastic in either fully supplemented MCDB153 medium (primary keratinocytes) or W489 medium containing 2% FCS (HaCaT keratinocytes). The clonogenic and proliferative potential of cells that reattached after forced suspension culture was determined 3–5 d after reseeding by staining with crystal violet and counting of colonies.
Immunoblot Analysis
For immunoblotting, cells were washed once in ice-cold PBS and lysed in nonreducing Laemmli buffer followed by boiling for 5 min. Equal amounts of protein were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA). Membranes were blocked (5% dry milk [WaWa, Inc., WaWa, PA], 0.05% Tween 20 [Sigma Aldrich, St. Louis, MO] in TBS) and incubated with primary antibodies in TBS containing 5% BSA, 0.5% Tween followed by incubation with horseradish peroxidase-labeled secondary antibodies. After washing blots in 0.5% Tween in TBS, signals were visualized by chemiluminescence using reagents from Pierce Chemical Co. (Rockford, IL) according to the manufacturer's instructions. In some cases blots were washed, inactivated with SG substrate (Vector Laboratories), and reused.
Kinase Assays
Akt and MAPK kinase activities were assessed by determining the phosphorylation state of their respective substrates, GSK-3αβ and Elk-1, using nonradioactive assay kits (New England Biolabs). Briefly, attached cells were treated for 48 h and lysed. Equal amounts of protein were immunoprecipitated with immobilized antibodies that bind to either MAPK or Akt. The immobilized precipitated kinases were then used in kinase assays using Elk-1 (for MAPK) and Gsk-3αβ (for Akt) as substrates followed by immunoblot analysis using phosphospecific antibodies.
RESULTS
EGFR Activation by Endogenous Ligands and Exogenous EGF Delays Apoptosis of Human Keratinocytes in Forced Suspension Culture
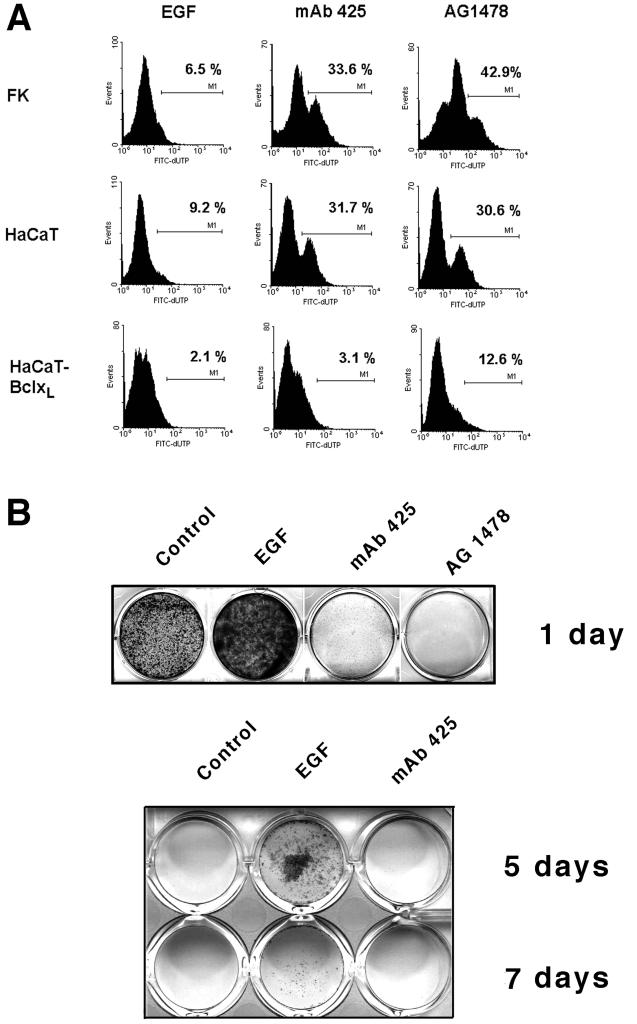
Cultured keratinocytes coexpress the EGFR and several of its ligands, thus establishing EGFR-dependent autocrine loops in this cell type (Coffey et al., 1987; Vardy et al., 1995; Piepkorn et al., 1998). To assess whether EGFR activation by endogenous ligands could protect keratinocytes against anoikis, we determined the effects of an EGFR-antagonistic mAb (mAb 425) (Rodeck et al., 1997a,b) on survival of normal human keratinocytes (FK) and immortalized keratinocytes (HaCaT) in suspension culture. EGFR blockade with mAb 425 used at saturating concentrations (66 nM; 10 μg/ml) was associated with apoptotic changes as exemplified by the appearance of TUNEL-positive cells in both cell types (Figure 1A) within 24 h; a similar and more drastic effect was seen when a pharmacological inhibitor of the EGFR tyrosine kinase moiety (AG1478; 10 μM; 30 μg/ml) was used. By contrast, EGF-treated samples showed little evidence of apoptotic DNA damage. Similar results were obtained using either normal neonatal keratinocytes or immortalized HaCaT cells. Anoikis was markedly alleviated in HaCaT cells overexpressing Bcl-xL. This result is consistent with an earlier study demonstrating protection of HaCaT keratinocytes against anoikis by Bcl-2 (Frisch and Francis, 1994). In contrast to suspension cultures, treatment of either normal or immortalized keratinocytes maintained on tissue culture-treated plastic with mAb 425 for 24 h did not induce spontaneous apoptosis (Rodeck et al., 1997b). Inhibition of the EGFR by either mAb 425 or AG1478 also decreased the fraction of viable cells that could be rescued and regained clonogenic/proliferative potential after 24-h suspension cultures (Figure 1B). Again, AG1478 reduced clonogenicity more effectively than mAb 425. Conversely, addition of EGF (10 ng/ml) to MCDB base medium markedly increased the fraction of cells that were rescued (Figure 1B) and partially antagonized the proapoptotic effect of mAb 425. Even after 7 d of suspension culture, viable and replication-competent cells could be recovered from EGF-treated samples, whereas EGFR blockade caused cell death of the majority (>98%) of suspended HaCaT cells within 2 d (Figure 1B). Beyond 10 d, very few cells with long-term growth potential were recovered in any of the experimental groups.
Figure 1.
EGFR-dependent survival and viability of keratinocytes in forced suspension culture. (A) TUNEL staining of normal neonatal keratinocytes (FK), HaCaT keratinocytes, and HaCaT cells overexpressing Bcl-xL–maintained in suspension for 24 h in the presence of EGF (10 ng/ml), mAb 425 (10 μg/ml), or AG1478 (10 μM) as indicated; values represent the cell fractions stained with FITC-dUTP relative to controls, i.e., attached HaCaT cells. (B) Clonal growth of HaCaT cells recovered after 1, 5, and 7 d of suspension culture in different medium conditions as indicated, further propagated on tissue culture-treated plastic for 5 d, and stained with crystal violet.
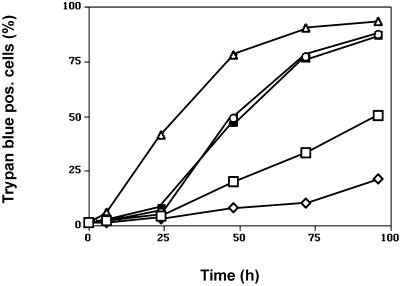
In these experiments, two populations of HaCaT keratinocytes could be distinguished on the basis of different kinetics of cell survival in suspension. Most of the control cells (>90%) maintained in protein-free media were committed to undergo apoptosis within 2 d after being placed in suspension because only few cells could be rescued after 48 h. EGFR blockade by either AG1478 or mAb 425 induced rapid apoptosis of almost all cells (>95%) within the first 24 h of suspension culture because after 24 h only very few (<2%) of mAb 425-treated cells could be rescued (Figure 1B), and viability as determined by trypan blue staining had decreased to <50% as compared with >90% observed in cells cultured in the presence of EGF (Figure 2). Addition of EGF prolonged survival of the bulk population to 3 d after which large-scale apoptosis occurred; however, a subpopulation of EGF-treated HaCaT cells retained long-term viability and clonogenic potential even after 7 d of suspension culture (Figure 1B); at these time points, clonogenicity of cells in all other conditions was <0.1%. In conclusion, EGF treatment delayed anoikis of the bulk population of HaCaT cells by ∼1 d and of a subpopulation for at least up to 7 d when compared with controls maintained in the absence of EGF or in the presence of EGFR inhibitors.
Figure 2.
Time-dependent loss of viability of HaCaT keratinocytes maintained in forced suspension culture. Viability was assessed by trypan blue exclusion after periods of suspension culture ranging from 4 to 96 h. Results shown represent means of triplicate determinations, the SD of which was <5%. Samples were treated with EGF (open rhomboids), mAb425 (open circles), PD98059 (closed squares), or AG1478 (open triangles) or maintained in control medium free of exogenous growth factors (open squares).
Activation of the MEK/MAPK Pathway in Keratinocytes by the EGFR
Next, we determined the effects of EGFR blockade by mAb 425 on signal transduction pathways previously implicated in the survival of epithelial cells. These experiments focused on the PI-3-kinase/Akt and MEK/MAPK pathways, both of which have been described as contributing to the survival of epithelial cells in different settings (Khwaja et al., 1997; Dent et al., 1999; Reardon et al., 1999). We determined the effects of EGFR blockade by mAb 425 on phosphorylation of a known Akt target (GSK-3β) and on phosphorylation of the MAPK target Elk-1. As shown in Figure 3, EGFR inhibition by mAb 425 at 10 μg/ml suppressed Elk-1 phosphorylation but had no detectable effect on GSK-3β phosphorylation in HaCaT cells; at this concentration, mAb 425 effectively blocks autophosphorylation of the EGFR (Murthy et al., 1987). This result indicated that the MEK/MAPK but not the PI-3-kinase/Akt pathway was relevant to the effects of EGFR blockade by mAb 425 on keratinocyte anoikis. Interestingly, AG1478 inhibited both Elk-1 and GSK-3β phosphorylation (Figure 3), consistent with the possibilities that it either inhibits EGFR-dependent signaling more efficiently than mAb 425 or inhibits tyrosine kinases other than the EGFR at the concentration used (10 μM). Because mAb 425 treatment was sufficient to induce keratinocyte death in suspension and affected Elk-1 phosphoryation only, we next considered that inhibition of MAPK activation alone might induce keratinocyte death in suspension.
Figure 3.
EGFR-dependent MEK/MAPK activation in HaCaT keratinocytes. (A) Effects of mAb 425 and AG1478 on Elk-1 phosphorylation. Cells were treated for 48 h with mAb 425 (10 μg/ml) or AG1478 (10 μM) before protein extraction and analysis. As a control, the effect of MEK inhibitor PD98059 at 5 and 50 μM is shown. The lane labeled MAPK shows phosphorylation of the Elk-1 substrate by recombinant MAPK. (B) Effects of mAb 425 and AG1478 on GSK-3β phosphorylation in HaCaT keratinocytes. Samples were treated as described under A. As a control, the effect of PI-3-kinase inhibitor LY294002 at 5 and 20 μM is shown. (C) Effect of the pharmacological inhibitor of MEK1/2 activity PD98059 on clonogenicity of HaCaT keratinocytes after 48 h of suspension culture determined as described in the legend to Figure 1. For comparison, the effects of mAb 425 and AG1478 are shown.
MAPK Phosphorylation Is Required for Enhanced Survival of Keratinocytes in Suspension
To determine whether MAPK phosphorylation was required for keratinocyte survival in suspension culture, we first used PD98059, a pharmacological inhibitor of MEK activity. As shown in Figure 3A, PD98059 at 50 μM down-regulated MAPK phosphorylation in keratinocytes to a level comparable to that in mAb 425-treated cells. At this concentration, PD98059 also reduced viability (Figure 2) and clonogenicity (Figure 3C) of keratinocytes held in suspension culture to levels comparable to those achieved by mAb 425 treatment. In further support of an important role of the MEK/MAPK axis in keratinocyte survival, overexpression of a dominant negative MEK construct under control of a tetracycline-regulated promoter markedly inhibited MAPK phosphorylation and induced apoptosis in HaCaT cells grown under anchorage-dependent conditions (Jost et al., 2001).
EGFR Activation Alters MAPK Phosphorylation in Keratinocytes in Suspension
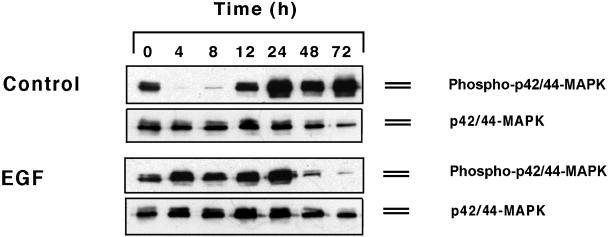
Next, we assessed the levels of MAPK phosphorylation in HaCaT cells maintained in suspension in the presence and absence of EGF (Figure 4). Previous studies indicated that, in fibroblasts, sustained growth factor-dependent MAPK phosphorylation required matrix adhesion (Lin et al., 1997; Renshaw et al., 1997; Bottazzi et al., 1999; Roovers et al., 1999). By contrast, we observed that EGF treatment of HaCaT cells was accompanied by sustained MAPK phosphorylation during the first 24 h of suspension culture. Control cells maintained in MCDB base medium revealed a bimodal pattern of MAPK phosphorylation over time. Within 4–12 h of suspension culture, MAPK phosphorylation was markedly reduced in these cells; however, robust levels of MAPK phosphorylation were observed between 24 and 72 h of control cultures. Thus, EGF treatment obviated the decline in MAPK phosphorylation during the initial 24–48 h of suspension culture.
Figure 4.
MAPK expression and phosphorylation patterns in HaCaT keratinocytes over time in suspension cultures. Time-dependent changes in control cultures in medium free of exogenous growth factors and in cultures maintained in the presence of EGF are shown as indicated.
MAPK Phosphorylation during Apoptotic Death
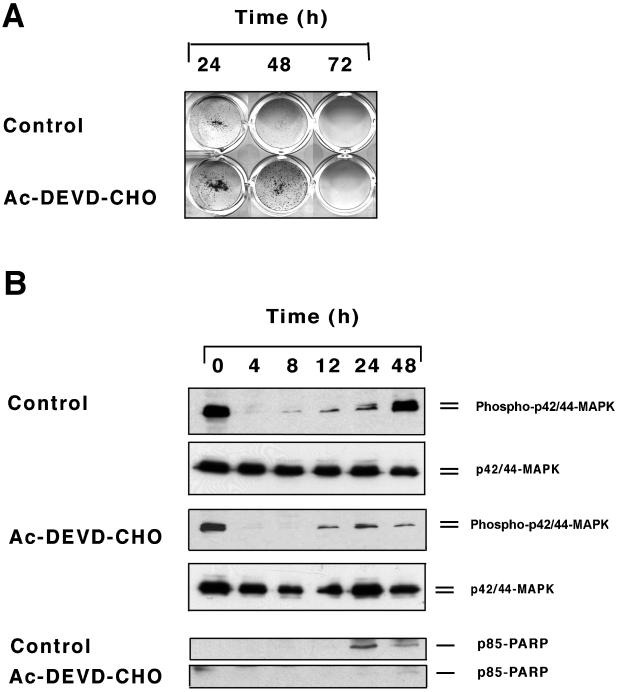
At later time points of suspension culture (24–72 h), most of the cells were undergoing apoptosis, as determined by TUNEL staining (Figure 1), but had not yet lost membrane permeability, as determined by trypan blue staining (Figure 2). Therefore, we tested whether late-stage MAPK phosphorylation was a consequence of the apoptotic process. To this end, we used the caspase 3 inhibitor Ac-DEVD-CHO, which partially inhibited anoikis (Figure 5A) and PARP cleavage in HaCaT keratinocytes in suspension (Figure 5B). Consistent with a role of caspases in late-stage MAPK phosphorylation, inhibition of caspase activity using Ac-DEVD-CHO attenuated this effect in control cultures (Figure 5) but had little effect on MAPK phosphorylation patterns observed in EGF-treated samples.
Figure 5.
Effects of caspase inhibitor Ac-DEVD-CHO on clonogenicity (A) and MAPK phosphorylation (B) of HaCaT keratinocytes in suspension culture. Control HaCaT cells were kept in forced suspension in base medium free of exogenous growth factors for the time periods indicated. Parallel cultures were incubated in the presence of Ac-DEVD-CHO at 120 μM. P42/44 MAPK expression and phosphorylation thereof were determined by Western blot analysis as described in the legend to Figure 5. PARP cleavage was assessed by reprobing the blots using an antibody to the p85 PARP cleavage product.
Modulation of Bcl-xL Expression by EGFR Activation
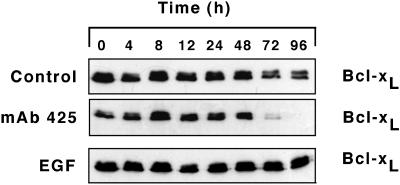
Previously we (Rodeck et al., 1997a) and others (Stoll et al., 1998) determined that inhibition of the EGFR tyrosine kinase activity in attached keratinocytes is accompanied by down-regulation of the anti-apoptotic Bcl-2 family member, Bcl-xL, at the mRNA and protein levels. Because Bcl-xL expression affects susceptibility to apoptosis, we determined Bcl-xL expression over time in HaCaT suspension cultures (Figure 6). This was done in protein-free medium and in medium supplemented with either EGF or mAb 425. As in attached keratinocytes (Rodeck et al., 1997a), EGF treatment was associated with robust and sustained Bcl-xL expression throughout the observation period of 96 h. By contrast and as expected, mAb 425 treatment was associated with marked down-regulation of Bcl-xL expression between 48 and 72 h of suspension culture. These results indicate that EGFR activation contributed to Bcl-xL expression in suspended as well as in attached keratinocytes; however, it is important to recognize that anoikis occurred on a large scale in all experimental conditions before and independent of down-regulation of Bcl-xL expression. For example, the majority of mAb 425-treated HaCaT cells initiated apoptosis during the first 36 h of suspension, without attendant reduction of Bcl-xL expression levels. Thus, sustained expression of endogenous Bcl-xL alone did not effectively prevent apoptosis in suspension culture; however, high levels of Bcl-xL expression achieved by forced expression attenuated apoptotic death of HaCaT cells in suspension (Figure 1).
Figure 6.
Bcl-xL expression in HaCaT keratinocytes in forced suspension culture. Bcl-xL expression over time was determined by immunoblot analysis in control cultures maintained in the absence of exogenous growth factors and in parallel cultures treated with mAb 425 and EGF as indicated.
DISCUSSION
This study demonstrates that the EGFR conveys survival signals to human keratinocytes, which delay apoptotic death triggered by loss of matrix interaction. This observation is reminiscent of a very recent report that described protection of lung fibroblasts against anoikis by addition of serum (Le Gall et al., 2000); however, our results assign a critical role to a specific growth factor receptor in anchorage-independent survival of epithelial cells. It should be noted that limited protection by EGFR activation against anoikis was observed in normal keratinocytes and thus constitutes a physiological mechanism; however, this phenomenon may have particular relevance to disease states in which deregulated expression of the EGFR or its ligands, or both, is commonly observed, including wound healing (Marikovsky et al., 1993), hyperproliferative skin diseases such as psoriasis (Gottlieb et al., 1988; Elder et al., 1989; Finzi et al., 1991; Cook et al., 1992), and advanced epithelial malignancies including squamous cell carcinomas (Derynck et al., 1987; Ozawa et al., 1989; Reiss et al., 1991). All of these diseases are characterized by survival of epithelial cells in conditions of suboptimal matrix interaction and by increased expression or activation of the EGFR. A role of EGFR activation in matrix-independent growth and survival of NIH3T3 cells was implied by early findings that forced expression of the EGFR in NIH3T3 cells enables anchorage-independent proliferation and in vivo tumorigenicity of these cells in a ligand-dependent manner (Di Fiore et al., 1987; Di Marco et al., 1989). Subsequently it was shown that forced expression of the EGFR in rat mammary carcinoma cells promotes the metastatic potential of these cells in vivo (Lichtner et al., 1995). Functional contributions of the EGFR to the invasive phenotype are potentially manifold and include the modulation of the expression levels of adhesion molecules or proteolytic enzymes and the induction of cell migration; however, it may be argued that EGFR-dependent cell survival as described here is critical to and rate limiting for the successful establishment of metastatic lesions.
Sustained MEK activity during the first 24 h of suspension culture was associated with and required for EGFR-dependent survival of keratinocytes in suspension culture. This conclusion is supported by the following observations: 1) EGF treatment sustained robust MAPK phosphorylation during the first 24 h of suspension culture, 2) the MEK1/2 inhibitor PD98059 accelerated keratinocyte death in suspension similar to the EGFR antagonistic mAb 425, and 3) overexpression of the dominant negative MEK construct MKK1–8E induced apoptosis even in attached HaCaT keratinocytes (Jost et al., 2001). These results assign an important role to the MEK/MAPK signaling module in preventing anoikis. They are consistent with and corroborate previous studies implying the Ras/Raf/MEK/MAPK pathway in support of anchorage-independent survival and growth of epithelial cells (Valverius et al., 1989; Walker et al., 1998). It remains to be investigated how MEK/MAPK activation protects keratinocytes against death in suspension. It is possible that phosphorylation and functional inactivation of the proapoptotic Bcl2 homologue Bad plays a role in this process. Bad initially has been identified as a target for the PI-3-K/AKT pathway, which leads to phosphorylation of serine 136 of Bad followed by inactivation of Bad through sequestration (Datta et al., 1997; del Peso et al., 1997; Dudek et al., 1997). Very recently, several lines of evidence highlighted that, in hemopoietic cells, Bad is also phosphorylated on serine 112 and possibly serine 155 through a MAPK-dependent mechanism (Scheid and Duronio, 1998; Scheid et al., 1999). These authors also showed that serine 112 phosphorylation was required for dissociation of Bad from Bcl-xL, enabling Bcl-xL to serve its anti-apoptotic role. Bad phosphorylation at serine 112 also protects neurons against apoptosis and is accomplished through MAPK-dependent kinases (Rsks) in these cells (Bonni et al., 1999). Collectively, these results suggest that posttranslational modification and functional inactivation of Bad is an anti-apoptotic mechanism shared by both the PI-3-kinase/Akt and MEK/MAPK pathways. Other potential mechanisms include regulation by MAP kinases of anti-apoptotic Bcl2 family members, including Bcl-xL (Jost et al., 2001), and of FLIP/FLICE, an inhibitor of death receptors of the CD95/Fas family (Yeh et al., 1998).
An important aspect of the present study is the observation that EGFR activation alone can sustain MAPK phosphorylation in keratinocytes for the first 24 h of suspension culture. By contrast, previous studies in fibroblasts demonstrated that cell adhesion and integrin engagement are indispensable for sustained growth factor receptor signaling to MEK/MAPK (Renshaw et al., 1997; Roovers et al., 1999). Differences in the experimental design may account for this apparent discrepancy. Specifically, in the present study, steady-state MAPK phosphorylation was measured in the continuous presence of EGF and without previous starvation. By contrast, both Renshaw et al. (1997) and Roovers et al. (1999) used serum-starved fibroblasts restimulated with serum or defined growth factors, including EGF. Alternatively, epithelial cells may differ in their matrix requirements for MEK/MAPK activation. Experiments are under way to distinguish between these two possibilities.
Unexpectedly, at later time points of suspension culture (24–72 h), robust MAPK phosphorylation was restored to keratinocytes maintained in the absence of exogenous EGF. At these time points, most of the cells were undergoing apoptosis, as determined by TUNEL staining, but had not yet lost membrane permeability, as determined by trypan blue staining. Our results are similar to those reported very recently for CCL39 lung fibroblasts, which undergo apoptosis during suspension culture (Le Gall et al., 2000). These cells down-regulate MAPK phosphorylation within the first 10 h of suspension culture, followed by a gradual increase at later time points (12–24 h) at which cells undergo large scale apoptosis. On the basis of these earlier results and our own observations, we consider late-stage MAPK phosphorylation to be a consequence of the apoptotic process. In support of this idea, we observed generalized keratinocyte apoptosis when high levels of MAPK phosphorylation recurred in control cultures. Furthermore, we observed that late-stage MAPK phosphorylation was markedly attenuated by caspase inhibition.
In attached keratinocytes, EGFR activation is known to contribute to expression of the anti-apoptotic Bcl-2 family member Bcl-xL, and this effect enhances their ability to withstand cellular stress (Rodeck et al., 1997a; Stoll et al., 1998; Jost et al., 1999). Here we demonstrate that EGF treatment was similarly associated with robust Bcl-xL expression during suspension culture of HaCaT keratinocytes. Consistent with an earlier report (Frisch and Francis, 1994), we also observed that forced expression of Bcl-xL protected keratinocytes against anoikis; however, Bcl-xL expression levels achieved by EGFR activation alone were not sufficient to prevent large-scale anoikis of HaCaT keratinocytes. This apparent discrepancy may be due to the fact that the levels of Bcl-xL expression in transfected cells are 20- to 50-fold higher than those observed in EGF-treated HaCaT cells (Jost et al., 1999).
In summary, we have identified EGFR activation as a potential mechanism to alleviate the requirement of matrix engagement for epithelial cell survival. Protection through EGFR activation was associated with and required sustained MEK/MAPK signaling during the early phase of suspension culture. In addition, high levels of MAPK phosphorylation accompanied apoptotic death in suspension culture in a caspase-dependent manner.
ACKNOWLEDGMENTS
We thank Drs. N. Ahn and T. F. Franke for expression constructs, Dr. P.J. Jensen for primary keratinocyte cultures, Dr R. Class for help with FACS analysis, and Dr. N. Fusenig for HaCaT keratinocytes. This work was supported in part by the National Institutes of Health (CA81008).
REFERENCES
- Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286:1358–1362. doi: 10.1126/science.286.5443.1358. [DOI] [PubMed] [Google Scholar]
- Bottazzi ME, Zhu X, Bohmer RM, Assoian RK. Regulation of p21(cip1) expression by growth factors and the extracellular matrix reveals a role for transient ERK activity in G1 phase. J Cell Biol. 1999;146:1255–1264. doi: 10.1083/jcb.146.6.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey RJ, Jr, Derynck R, Wilcox JN, Bringman TS, Goustin AS, Moses HL, Pittelkow MR. Production and auto-induction of transforming growth factor-alpha in human keratinocytes. Nature. 1987;328:817–820. doi: 10.1038/328817a0. [DOI] [PubMed] [Google Scholar]
- Cook PW, Pittelkow MR, Keeble WW, Graves-Deal R, Coffey RJ, Jr, Shipley GD. Amphiregulin messenger RNA is elevated in psoriatic epidermis and gastrointestinal carcinomas. Cancer Res. 1992;52:3224–3227. [PubMed] [Google Scholar]
- Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- Dent P, Reardon DB, Park JS, Bowers G, Logsdon C, Valerie K, Schmidt-Ullrich R. Radiation-induced release of transforming growth factor alpha activates the epidermal growth factor receptor and mitogen-activated protein kinase pathway in carcinoma cells, leading to increased proliferation and protection from radiation-induced cell death. Mol Biol Cell. 1999;10:2493–2506. doi: 10.1091/mbc.10.8.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R, Goeddel DV, Ullrich A, Gutterman JU, Williams RD, Bringman TS, Berger WH. Synthesis of messenger RNAs for transforming growth factors alpha and beta and the epidermal growth factor receptor by human tumors. Cancer Res. 1987;47:707–712. [PubMed] [Google Scholar]
- Di Fiore PP, Pierce JH, Fleming TP, Hazan R, Ullrich A, King CR, Schlessinger J, Aaronson SA. Overexpression of the human EGF receptor confers an EGF-dependent transformed phenotype to NIH 3T3 cells. Cell. 1987;51:1063–1070. doi: 10.1016/0092-8674(87)90592-7. [DOI] [PubMed] [Google Scholar]
- Di Marco E, Pierce JH, Fleming TP, Kraus MH, Molloy CJ, Aaronson SA, Di Fiore PP. Autocrine interaction between TGF alpha and the EGF-receptor: quantitative requirements for induction of the malignant phenotype. Oncogene. 1989;4:831–838. [PubMed] [Google Scholar]
- Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, Segal RA, Kaplan DR, Greenberg ME. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–664. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- Elder JT, Fisher GJ, Lindquist PB, Bennett GL, Pittelkow MR, Coffey RJ, Jr, Ellingsworth L, Derynck R, Voorhees JJ. Overexpression of transforming growth factor alpha in psoriatic epidermis. Science. 1989;243:811–814. doi: 10.1126/science.2916128. [DOI] [PubMed] [Google Scholar]
- Finzi E, Harkins R, Horn T. TGF-alpha is widely expressed in differentiated as well as hyperproliferative skin epithelium. J Invest Dermatol. 1991;96:328–332. doi: 10.1111/1523-1747.ep12465223. [DOI] [PubMed] [Google Scholar]
- Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb AB, Chang CK, Posnett DN, Fanelli B, Tam JP. Detection of transforming growth factor alpha in normal, malignant, and hyperproliferative human keratinocytes. J Exp Med. 1988;167:670–675. doi: 10.1084/jem.167.2.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmstrom TH, Tran SE, Johnson VL, Ahn NG, Chow SC, Eriksson JE. Inhibition of mitogen-activated kinase signaling sensitizes HeLa cells to Fas receptor-mediated apoptosis. Mol Cell Biol. 1999;19:5991–6002. doi: 10.1128/mcb.19.9.5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost M, Class R, Kari C, Jensen PJ, Rodeck U. A central role of Bcl-X(L) in the regulation of keratinocyte survival by autocrine EGFR ligands. J Invest Dermatol. 1999;112:443–449. doi: 10.1046/j.1523-1747.1999.00543.x. [DOI] [PubMed] [Google Scholar]
- Jost M, Hugett TM, Kari C, Boise LH, Rodeck U. EGFR-dependent control of keratinocyte survival and Bcl-xL expression through a MEK-dependent pathway. J Biol Chem. 2001;276:6320–6326. doi: 10.1074/jbc.M008210200. [DOI] [PubMed] [Google Scholar]
- Jost M, Kari C, Rodeck U. An episomal vector for stable tetracycline-regulated gene expression. Nucleic Acids Res. 1997;25:3131–3134. doi: 10.1093/nar/25.15.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khwaja A, Rodriguez-Viciana P, Wennstrom S, Warne PH, Downward J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 1997;16:2783–2793. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gall M, Chambard JC, Breittmayer JP, Grall D, Pouyssegur J, Van Obberghen-Schilling E. The p42/p44 MAP kinase pathway prevents apoptosis induced by anchorage and serum removal. Mol Biol Cell. 2000;11:1103–1112. doi: 10.1091/mbc.11.3.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtner RB, Kaufmann AM, Kittmann A, Rohde-Schulz B, Walter J, Williams L, Ullrich A, Schirrmacher V, Khazaie K. Ligand mediated activation of ectopic EGF receptor promotes matrix protein adhesion and lung colonization of rat mammary adenocarcinoma cells. Oncogene. 1995;10:1823–1832. [PubMed] [Google Scholar]
- Lin TH, Chen Q, Howe A, Juliano RL. Cell anchorage permits efficient signal transduction between ras and its downstream kinases. J Biol Chem. 1997;272:8849–8852. [PubMed] [Google Scholar]
- Luttrell DK, Lee A, Lansing TJ, Crosby RM, Jung KD, Willard D, Luther M, Rodriguez M, Berman J, Gilmer TM. Involvement of pp60c-src with two major signaling pathways in human breast cancer. Proc Natl Acad Sci USA. 1994;91:83–87. doi: 10.1073/pnas.91.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marikovsky M, Breuing K, Liu PY, Eriksson E, Higashiyama S, Farber P, Abraham J, Klagsbrun M. Appearance of heparin-binding EGF-like growth factor in wound fluid as a response to injury. Proc Natl Acad Sci USA. 1993;90:3889–3893. doi: 10.1073/pnas.90.9.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill H, Jensen PJ. A high-affinity receptor for urokinase plasminogen activator on human keratinocytes: characterization and potential modulation during migration. Cell Regul. 1990;1:843–852. doi: 10.1091/mbc.1.11.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith JE, Jr, Fazeli B, Schwartz MA. The extracellular matrix as a cell survival factor. Mol Biol Cell. 1993;4:953–961. doi: 10.1091/mbc.4.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy U, Basu A, Rodeck U, Herlyn M, Ross AH, Das M. Binding of an antagonistic monoclonal antibody to an intact and fragmented EGF-receptor polypeptide. Arch Biochem Biophys. 1987;252:549–560. doi: 10.1016/0003-9861(87)90062-2. [DOI] [PubMed] [Google Scholar]
- Murthy U, Rieman DJ, Rodeck U. Inhibition of TGF alpha-induced second messengers by anti-EGF receptor antibody-425. Biochem Biophys Res Commun. 1990;172:471–476. doi: 10.1016/0006-291x(90)90696-k. [DOI] [PubMed] [Google Scholar]
- Ozawa S, Ueda M, Ando N, Shimizu N, Abe O. Prognostic significance of epidermal growth factor receptor in esophageal squamous cell carcinomas. Cancer. 1989;63:2169–2173. doi: 10.1002/1097-0142(19890601)63:11<2169::aid-cncr2820631117>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Piepkorn M, Pittelkow MR, Cook PW. Autocrine regulation of keratinocytes: the emerging role of heparin-binding, epidermal growth factor-related growth factors. J Invest Dermatol. 1998;111:715–721. doi: 10.1046/j.1523-1747.1998.00390.x. [DOI] [PubMed] [Google Scholar]
- Reardon DB, Contessa JN, Mikkelsen RB, Valerie K, Amir C, Dent P, Schmidt-Ullrich RK. Dominant negative EGFR-CD533 and inhibition of MAPK modify JNK1 activation and enhance radiation toxicity of human mammary carcinoma cells. Oncogene. 1999;18:4756–4766. doi: 10.1038/sj.onc.1202849. [DOI] [PubMed] [Google Scholar]
- Reiss M, Stash EB, Vellucci VF, Zhou ZL. Activation of the autocrine transforming growth factor alpha pathway in human squamous carcinoma cells. Cancer Res. 1991;51:6254–6262. [PubMed] [Google Scholar]
- Renshaw MW, Ren XD, Schwartz MA. Growth factor activation of MAP kinase requires cell adhesion. EMBO J. 1997;16:5592–5599. doi: 10.1093/emboj/16.18.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodeck U, Herlyn M, Menssen HD, Furlanetto RW, Koprowsk H. Metastatic but not primary melanoma cell lines grow in vitro independently of exogenous growth factors. Int J Cancer. 1987;40:687–690. doi: 10.1002/ijc.2910400520. [DOI] [PubMed] [Google Scholar]
- Rodeck U, Jost M, DuHadaway J, Kari C, Jensen PJ, Risse B, Ewert DL. Regulation of Bcl-xL expression in human keratinocytes by cell-substratum adhesion and the epidermal growth factor receptor. Proc Natl Acad Sci USA. 1997a;94:5067–5072. doi: 10.1073/pnas.94.10.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodeck U, Jost M, Kari C, Shih DT, Lavker RM, Ewert DL, Jensen PJ. EGF-R dependent regulation of keratinocyte survival. J Cell Sci. 1997b;110:113–121. doi: 10.1242/jcs.110.2.113. [DOI] [PubMed] [Google Scholar]
- Roovers K, Davey G, Zhu X, Bottazzi ME, Assoian RK. Alpha5beta1 integrin controls cyclin D1 expression by sustaining mitogen-activated protein kinase activity in growth factor-treated cells. Mol Biol Cell. 1999;10:3197–3204. doi: 10.1091/mbc.10.10.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen K, Rak J, Leung T, Dean NM, Kerbel RS, Filmus J. Activated ras prevents downregulation of Bcl-X-L triggered by detachment from the extracellular matrix: a mechanism of ras-induced resistance to anoikis in intestinal epithelial cells. J Cell Biol. 2000;149:447–455. doi: 10.1083/jcb.149.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid MP, Duronio V. Dissociation of cytokine-induced phosphorylation of Bad and activation of PKB/akt: involvement of MEK upstream of Bad phosphorylation. Proc Natl Acad Sci USA. 1998;95:7439–7444. doi: 10.1073/pnas.95.13.7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid MP, Schubert KM, Duronio V. Regulation of bad phosphorylation and association with Bcl-x(L) by the MAPK/Erk kinase. J Biol Chem. 1999;274:31108–31113. doi: 10.1074/jbc.274.43.31108. [DOI] [PubMed] [Google Scholar]
- Stoll SW, Benedict M, Mitra R, Hiniker A, Elder JT, Nunez G. EGF receptor signaling inhibits keratinocyte apoptosis: evidence for mediation by Bcl-XL. Oncogene. 1998;16:1493–1499. doi: 10.1038/sj.onc.1201657. [DOI] [PubMed] [Google Scholar]
- Valverius EM, Bates SE, Stampfer MR, Clark R, McCormick F, Salomon DS, Lippman ME, Dickson RB. Transforming growth factor alpha production and epidermal growth factor receptor expression in normal and oncogene transformed human mammary epithelial cells. Mol Endocrinol. 1989;3:203–214. doi: 10.1210/mend-3-1-203. [DOI] [PubMed] [Google Scholar]
- Vardy DA, Kari C, Lazarus GS, Jensen PJ, Zilberstein A, Plowman GD, Rodeck U. Induction of autocrine epidermal growth factor receptor ligands in human keratinocytes by insulin/insulin-like growth factor-1. J Cell Physiol. 1995;163:257–265. doi: 10.1002/jcp.1041630206. [DOI] [PubMed] [Google Scholar]
- Walker F, Kato A, Gonez LJ, Hibbs ML, Pouliot N, Levitzki A, Burgess AW. Activation of the Ras/mitogen-activated protein kinase pathway by kinase-defective epidermal growth factor receptors results in cell survival but not proliferation. Mol Cell Biol. 1998;18:7192–7204. doi: 10.1128/mcb.18.12.7192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh JH, Hsu SC, Han SH, Lai MZ. Mitogen-activated protein kinase kinase antagonized fas-associated death domain protein-mediated apoptosis by induced FLICE-inhibitory protein expression. J Exp Med. 1998;188:1795–1802. doi: 10.1084/jem.188.10.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]