Abstract
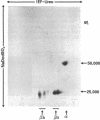
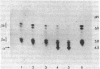
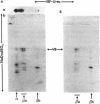
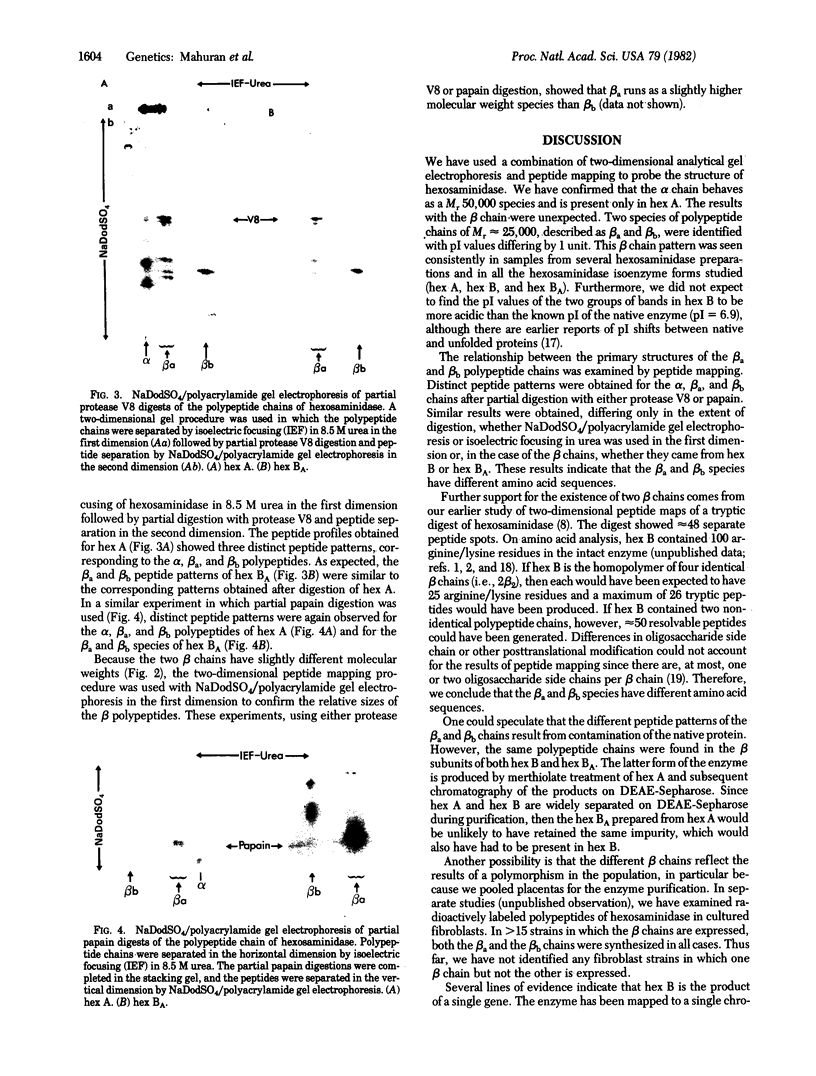
The major isoenzymes of human hexosaminidase have the structures alpha beta 2 (hex A) and 2 beta 2 (hex B). In this study, we present evidence that the beta 2 subunit of hex B and hex BA (the form of hex B derived from hex A) is composed of two nonidentical polypeptide chains. We have called these chains beta a and beta b. They have similar molecular weights (25,000) but have pI values that differ by 1 unit. We have used a two-dimensional analytical gel electrophoresis method in combination with peptide mapping to compare the primary sequence structure of the two beta chains. In this method, the polypeptide chains of hex B or hex BA were first separated by isoelectric focusing in 8.5 M urea. The separated chains were subjected to partial proleolytic digestion in the stacking gel of a second NaDodSO4/polyacrylamide gel with subsequent separation of peptides by electrophoresis into the second gel. Partial digestion by protease V8 or papain showed that the beta a and beta b species have distinct primary structures, neither of which was similar to that of the alpha chain. On the basis of these results, we suggest that the beta 2 subunit of hexosaminidase has the structure of beta a beta b. The possibility that the distinct beta chains are encoded by a single gene is discussed in the light of genetic and other data.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beutler E. The biochemical genetics of the hexosaminidase system in man. Am J Hum Genet. 1979 Mar;31(2):95–105. [PMC free article] [PubMed] [Google Scholar]
- Beutler E., Yoshida A., Kuhl W., Lee J. E. The subunits of human hexosaminidase A. Biochem J. 1976 Dec 1;159(3):541–543. doi: 10.1042/bj1590541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordier C., Crettol-Järvinen A. Peptide mapping of heterogeneous protein samples. J Biol Chem. 1979 Apr 25;254(8):2565–2567. [PubMed] [Google Scholar]
- Chan S. J., Keim P., Steiner D. F. Cell-free synthesis of rat preproinsulins: characterization and partial amino acid sequence determination. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1964–1968. doi: 10.1073/pnas.73.6.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman R. N., Vogt V. M. The biosynthesis of oncovirus proteins. Biochim Biophys Acta. 1978 Apr 6;473(3-4):187–239. doi: 10.1016/0304-419x(78)90014-8. [DOI] [PubMed] [Google Scholar]
- Freeze H., Geiger B., Miller A. L. Carbohydrate composition of human placental N-acetylhexosaminidase A and B. Biochem J. 1979 Feb 1;177(2):749–752. doi: 10.1042/bj1770749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B., Arnon R. Chemical characterization and subunit structure of human N-acetylhexosaminidases A and B. Biochemistry. 1976 Aug 10;15(16):3484–3493. doi: 10.1021/bi00661a014. [DOI] [PubMed] [Google Scholar]
- Gilbert F., Kucherlapati R., Creagan R. P., Murnane M. J., Darlington G. J., Ruddle F. H. Tay-Sachs' and Sandhoff's diseases: the assignment of genes for hexosaminidase A and B to individual human chromosomes. Proc Natl Acad Sci U S A. 1975 Jan;72(1):263–267. doi: 10.1073/pnas.72.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasilik A., Neufeld E. F. Biosynthesis of lysosomal enzymes in fibroblasts. Synthesis as precursors of higher molecular weight. J Biol Chem. 1980 May 25;255(10):4937–4945. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lalley P. A., Rattazzi M. C., Shows T. B. Human beta-D-N-acetylhexosaminidases A and B: expression and linkage relationships in somatic cell hybrids. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1569–1573. doi: 10.1073/pnas.71.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. E., Yoshida A. Purification and chemical characterization of human hexosaminidases A and B. Biochem J. 1976 Dec 1;159(3):535–539. doi: 10.1042/bj1590535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahuran D., Lowden J. A. A structural difference between the beta-chains in hexosaminidase B and hexosaminidase A. Can J Biochem. 1981 Apr;59(4):237–241. doi: 10.1139/o81-032. [DOI] [PubMed] [Google Scholar]
- Mahuran D., Lowden J. A. The subunit and polypeptide structure of hexosaminidases from human placenta. Can J Biochem. 1980 Apr;58(4):287–294. doi: 10.1139/o80-038. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Rattazzi M. C., Brown J. A., Davidson R. G., Shows T. B. Studies on complementation of beta hexosaminidase deficiency in human GM2 gangliosidosis. Am J Hum Genet. 1976 Mar;28(2):143–154. [PMC free article] [PubMed] [Google Scholar]
- Srivastava S. K., Ansari N. H. Altered alpha subunits in Tay-Sachs disease. Nature. 1978 May 18;273(5659):245–246. doi: 10.1038/273245a0. [DOI] [PubMed] [Google Scholar]
- Srivastava S. K., Beutler E. Studies on human beta-D-N-acetylhexosaminidases. 3. Biochemical genetics of Tay-Sachs and Sandhoff's diseases. J Biol Chem. 1974 Apr 10;249(7):2054–2057. [PubMed] [Google Scholar]
- Srivastava S. K., Yoshida A., Awasthi Y. C., Beutler E. Studies on human beta-D-N-acetylhexosaminidases. II. Kinetic and structural properties. J Biol Chem. 1974 Apr 10;249(7):2049–2053. [PubMed] [Google Scholar]
- Tuszynski G. P., Buck C. A., Warren L. A two-dimensional polyacrylamide gel electrophoresis (PAGE) system using sodium dodecyl sulfate-PAGE in the first dimension. Anal Biochem. 1979 Mar;93(2):329–338. doi: 10.1016/s0003-2697(79)80159-1. [DOI] [PubMed] [Google Scholar]
- Wood S. Juvenile Sandhoff Disease: complementation tests with Sandhoff and Tay-Sachs disease using polyethylene glycol-induced cell fusion. Hum Genet. 1978 Apr 24;41(3):325–329. doi: 10.1007/BF00284766. [DOI] [PubMed] [Google Scholar]