Abstract
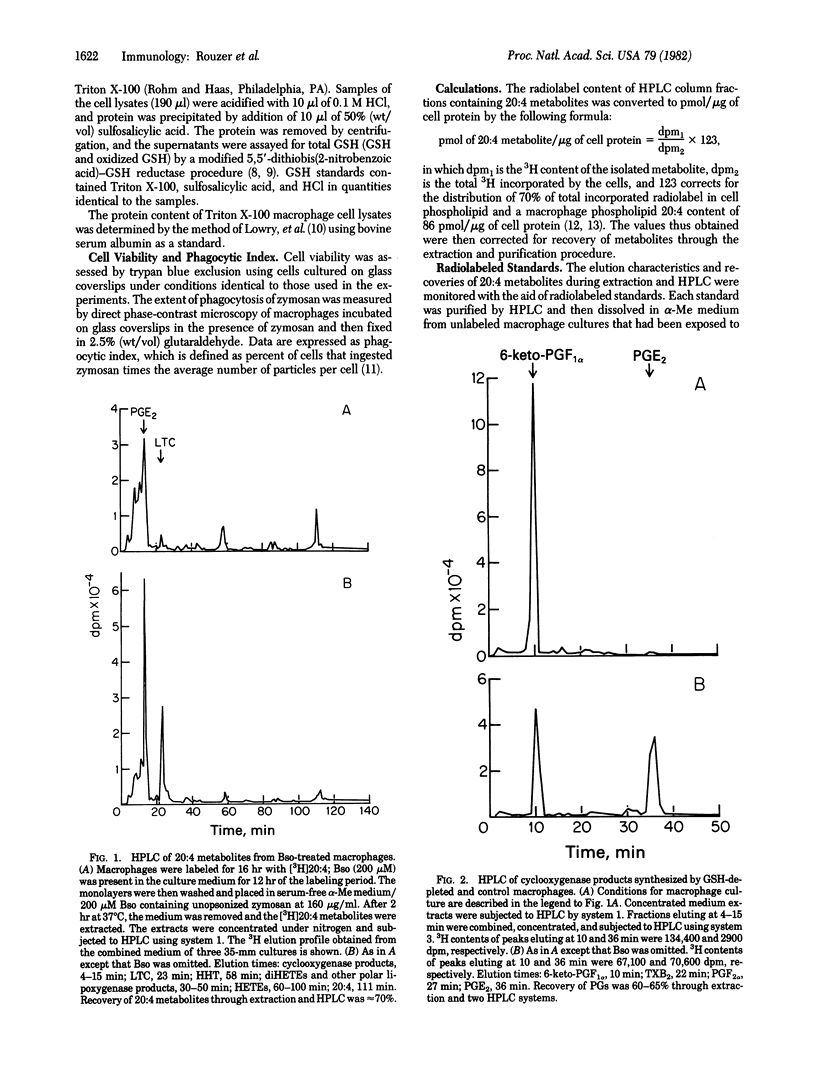
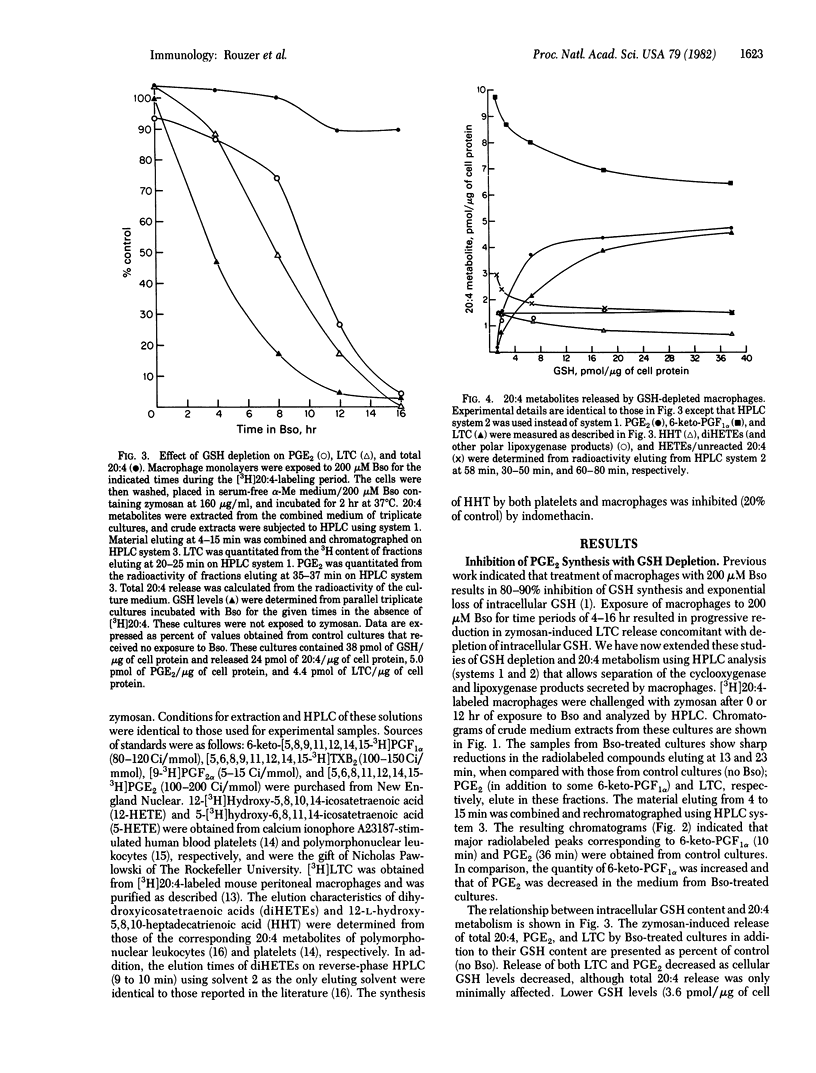
Mouse resident peritoneal macrophages were treated with the glutathione (GSH) synthesis inhibitor buthionine sulfoximine to deplete intracellular GSH. The arachidonic acid metabolites released by the GSH-depleted macrophages in response to a zymosan challenge were analyzed by HPLC. Buthionine sulfoximine treatment resulted in inhibition of both prostaglandin E2 and leukotriene C synthesis that was directly related to the degree of GSH depletion. Macrophages in which GSH levels were reduced to 3% of normal exhibited reductions to 4% and 1%, respectively, in PGE2 and LTC formation. The total quantity of cyclooxygenase metabolites secreted by GSH-deficient macrophages was identical to that of control cells as a result of increased synthesis of prostacyclin and, to a lesser extent, 12-L-hydroxy-5,8,10-heptadecatrienoic acid. Total lipoxygenase products were decreased, however; increased formation of hydroxyicosatetraenoic acids only partially compensated for the deficit in leukotriene C production. These findings extent our earlier observations on the inhibition of leukotriene C synthesis in GSH-depleted macrophages and confirm with intact cells the previously suggested role of GSH in prostaglandin E2 formation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonney R. J., Wightman P. D., Davies P., Sadowski S. J., Kuehl F. A., Jr, Humes J. L. Regulation of prostaglandin synthesis and of the selective release of lysosomal hydrolases by mouse peritoneal macrophages. Biochem J. 1978 Nov 15;176(2):433–442. doi: 10.1042/bj1760433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgeat P., Samuelsson B. Arachidonic acid metabolism in polymorphonuclear leukocytes: effects of ionophore A23187. Proc Natl Acad Sci U S A. 1979 May;76(5):2148–2152. doi: 10.1073/pnas.76.5.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgeat P., Samuelsson B. Metabolism of arachidonic acid in polymorphonuclear leukocytes. Structural analysis of novel hydroxylated compounds. J Biol Chem. 1979 Aug 25;254(16):7865–7869. [PubMed] [Google Scholar]
- COHN Z. A., BENSON B. THE DIFFERENTIATION OF MONONUCLEAR PHAGOCYTES. MORPHOLOGY, CYTOCHEMISTRY, AND BIOCHEMISTRY. J Exp Med. 1965 Jan 1;121:153–170. doi: 10.1084/jem.121.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin F. M., Jr, Bianco C., Silverstein S. C. Characterization of the macrophage receptro for complement and demonstration of its functional independence from the receptor for the Fc portion of immunoglobulin G. J Exp Med. 1975 Jun 1;141(6):1269–1277. doi: 10.1084/jem.141.6.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. W. Depletion of glutathione by inhibition of biosynthesis. Methods Enzymol. 1981;77:59–63. doi: 10.1016/s0076-6879(81)77011-3. [DOI] [PubMed] [Google Scholar]
- Griffith O. W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980 Jul 15;106(1):207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J Biol Chem. 1979 Aug 25;254(16):7558–7560. [PubMed] [Google Scholar]
- Hamberg M., Samuelsson B. Prostaglandin endoperoxides. Novel transformations of arachidonic acid in human platelets. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3400–3404. doi: 10.1073/pnas.71.9.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarström S., Murphy R. C., Samuelsson B., Clark D. A., Mioskowski C., Corey E. J. Structure of leukotriene C. Identification of the amino acid part. Biochem Biophys Res Commun. 1979 Dec 28;91(4):1266–1272. doi: 10.1016/0006-291x(79)91203-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Rouzer C. A., Scott W. A., Cohn Z. A., Blackburn P., Manning J. M. Mouse peritoneal macrophages release leukotriene C in response to a phagocytic stimulus. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4928–4932. doi: 10.1073/pnas.77.8.4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouzer C. A., Scott W. A., Griffith O. W., Hamill A. L., Cohn Z. A. Depletion of glutathione selectively inhibits synthesis of leukotriene C by macrophages. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2532–2536. doi: 10.1073/pnas.78.4.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouzer C. A., Scott W. A., Kempe J., Cohn Z. A. Prostaglandin synthesis by macrophages requires a specific receptor-ligand interaction. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4279–4282. doi: 10.1073/pnas.77.7.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott W. A., Zrike J. M., Hamill A. L., Kempe J., Cohn Z. A. Regulation of arachidonic acid metabolites in macrophages. J Exp Med. 1980 Aug 1;152(2):324–335. doi: 10.1084/jem.152.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenson W. F., Parker C. W. Metabolism of arachidonic acid in ionophore-stimulated neutrophils. Esterification of a hydroxylated metabolite into phospholipids. J Clin Invest. 1979 Nov;64(5):1457–1465. doi: 10.1172/JCI109604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Unger W. G., Stamford I. F., Bennett A. Extraction of prostaglandins from human blood. Nature. 1971 Oct 1;233(5318):336–337. doi: 10.1038/233336b0. [DOI] [PubMed] [Google Scholar]


