Abstract
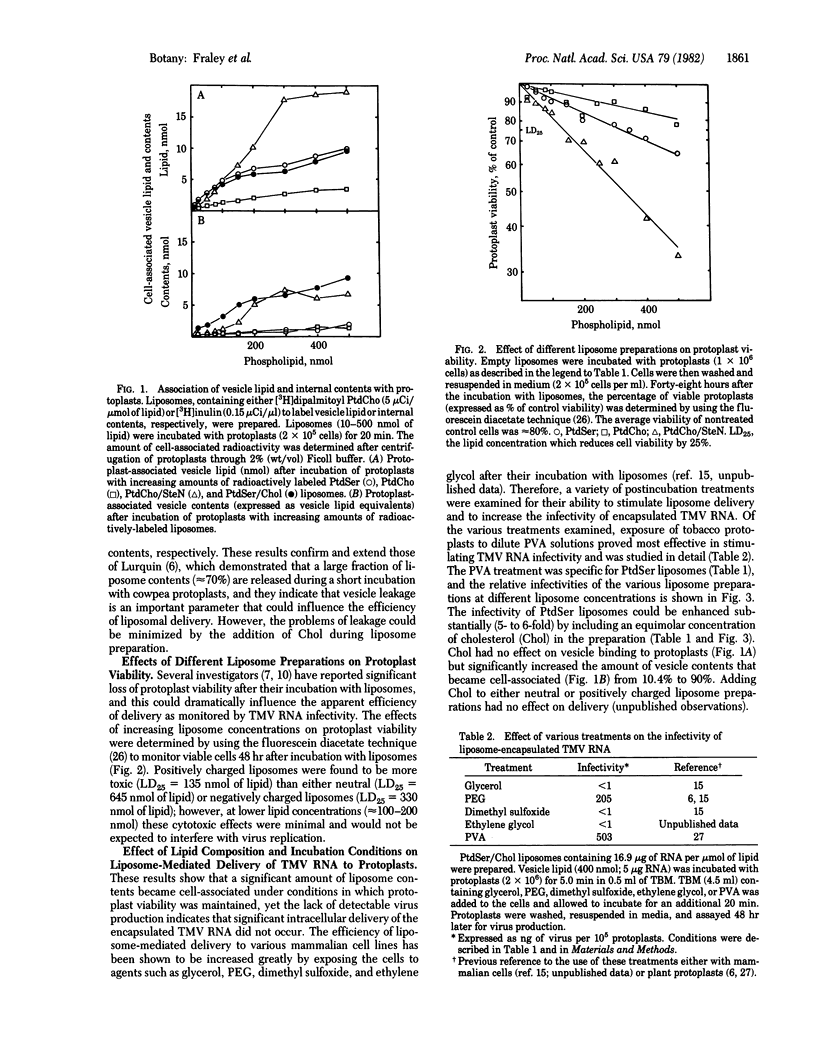
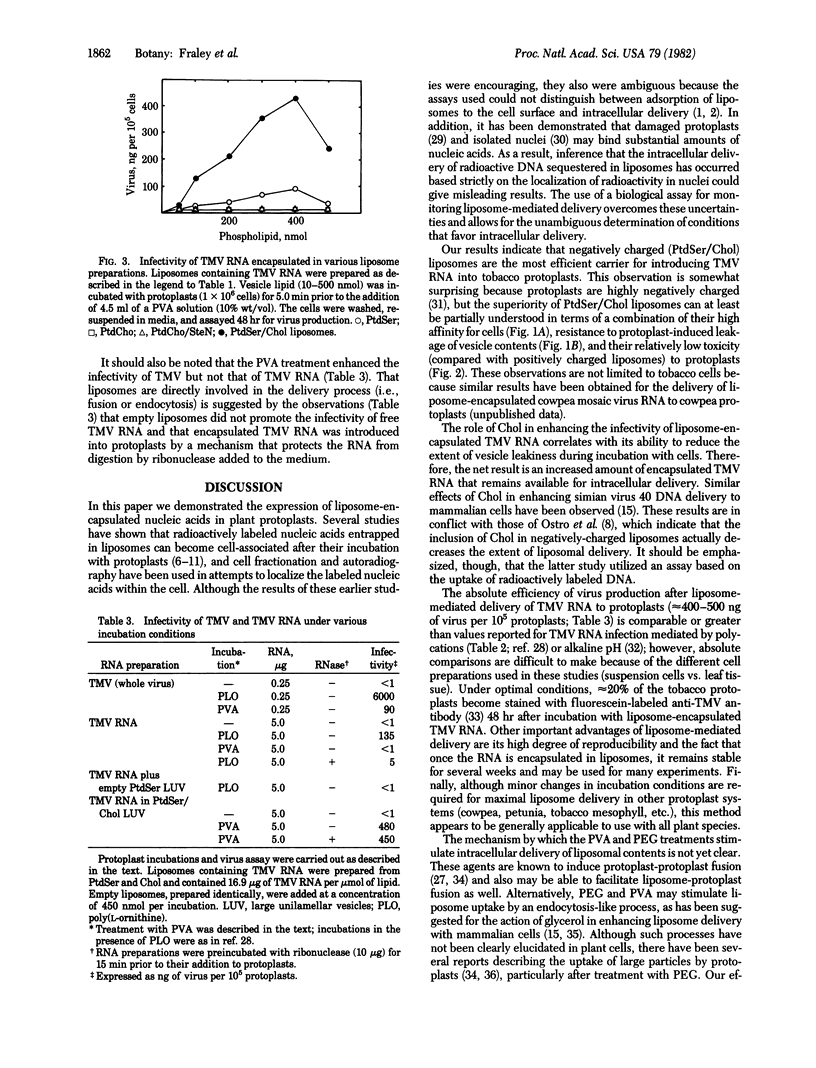
Tobacco mosaic virus (TMV) RNA was encapsulated in large, unilamellar phospholipid vesicles (liposomes), and the encapsulated TMV RNA was shown to be infectious when incubated with tobacco protoplasts under appropriate conditions. Maximal virus production in protoplasts was observed after their incubation with TMV RNA entrapped in phosphatidylserine/cholesterol liposomes. Infection was dependent on the presence of polyalcohols in the incubation mixture. Other parameters, such as the extent of vesicle binding, the cell-induced leakage of vesicle contents, and the degree of liposome toxicity were shown to be important in determining the efficiency of infectivity. Liposome-mediated delivery offers an efficient and reproducible method for introducing RNA into plant protoplasts.
Keywords: phospholipid vesicles, liposomes, intracellular delivery, nucleic acids, plant cells
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Diaz-Ruiz J. R., Kaper J. M. Isolation of viral double-stranded RNAs using a LiCl fractionation procedure. Prep Biochem. 1978;8(1):1–17. doi: 10.1080/00327487808068215. [DOI] [PubMed] [Google Scholar]
- Fraley R., Straubinger R. M., Rule G., Springer E. L., Papahadjopoulos D. Liposome-mediated delivery of deoxyribonucleic acid to cells: enhanced efficiency of delivery related to lipid composition and incubation conditions. Biochemistry. 1981 Nov 24;20(24):6978–6987. doi: 10.1021/bi00527a031. [DOI] [PubMed] [Google Scholar]
- Fraley R., Subramani S., Berg P., Papahadjopoulos D. Introduction of liposome-encapsulated SV40 DNA into cells. J Biol Chem. 1980 Nov 10;255(21):10431–10435. [PubMed] [Google Scholar]
- Gamborg O. L., Miller R. A., Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968 Apr;50(1):151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- Locker J. Analytical and preparative electrophoresis of RNA in agarose-urea. Anal Biochem. 1979 Oct 1;98(2):358–367. doi: 10.1016/0003-2697(79)90154-4. [DOI] [PubMed] [Google Scholar]
- Lurquin P. F. Entrapment of plasmid DNA by liposomes and their interactions with plant protoplasts. Nucleic Acids Res. 1979 Aug 24;6(12):3773–3784. doi: 10.1093/nar/6.12.3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhew E., Rustum Y. M., Szoka F., Papahadjopoulos D. Role of cholesterol in enhancing the antitumor activity of cytosine arabinoside entrapped in liposomes. Cancer Treat Rep. 1979 Nov-Dec;63(11-12):1923–1928. [PubMed] [Google Scholar]
- Ohyama K., Pelcher L. E., Horn D. DNA binding and uptake by nuclei isolated from plant protoplasts: factors affecting DNA binding and uptake. Plant Physiol. 1977 Jul;60(1):98–101. doi: 10.1104/pp.60.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostro M. J., Lavelle D., Paxton W., Matthews B., Giacomoni D. Parameters affecting the liposome-mediated insertion of RNA into eucaryotic cells in vitro. Arch Biochem Biophys. 1980 May;201(2):392–402. doi: 10.1016/0003-9861(80)90527-5. [DOI] [PubMed] [Google Scholar]
- Otsuki Y., Takebe I. Fluorescent antibody staining of tobacco mosaic virus antigen in tobacco mesophyll protoplasts. Virology. 1969 Jul;38(3):497–499. doi: 10.1016/0042-6822(69)90167-6. [DOI] [PubMed] [Google Scholar]
- Pagano R. E., Weinstein J. N. Interactions of liposomes with mammalian cells. Annu Rev Biophys Bioeng. 1978;7:435–468. doi: 10.1146/annurev.bb.07.060178.002251. [DOI] [PubMed] [Google Scholar]
- Sarkar S., Upadhya M. D., Melchers G. A highly efficient method of inoculation of tobacco mesophyll protoplasts with ribonucleic acid of tobacco mosaic virus. Mol Gen Genet. 1974;135(1):1–9. doi: 10.1007/BF00433895. [DOI] [PubMed] [Google Scholar]
- Suzuki M., Takebe I., Kajita S., Honda Y., Matsup C. Endocytosis of polystyrene spheres by tobacco leaf protoplasts. Exp Cell Res. 1977 Mar 1;105(1):127–135. doi: 10.1016/0014-4827(77)90158-6. [DOI] [PubMed] [Google Scholar]
- Szoka F. C., Jr, Jacobson K., Papahadjopoulos D. The use of aqueous space markers to determine the mechanism of interaction between phospholipid vesicles and cells. Biochim Biophys Acta. 1979 Mar 8;551(2):295–303. doi: 10.1016/0005-2736(89)90007-2. [DOI] [PubMed] [Google Scholar]
- Szoka F., Jacobson K., Derzko Z., Papahadjopoulos D. Fluorescence studies on the mechanism of liposome-cell interactions in vitro. Biochim Biophys Acta. 1980 Jul 16;600(1):1–18. doi: 10.1016/0005-2736(80)90406-x. [DOI] [PubMed] [Google Scholar]
- Szoka F., Jr, Papahadjopoulos D. Comparative properties and methods of preparation of lipid vesicles (liposomes). Annu Rev Biophys Bioeng. 1980;9:467–508. doi: 10.1146/annurev.bb.09.060180.002343. [DOI] [PubMed] [Google Scholar]
- Szoka F., Jr, Papahadjopoulos D. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4194–4198. doi: 10.1073/pnas.75.9.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchimiya H., Murashige T. Evaluation of parameters in the isolation of viable protoplasts from cultured tobacco cells. Plant Physiol. 1974 Dec;54(6):936–944. doi: 10.1104/pp.54.6.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widholm J. M. The use of fluorescein diacetate and phenosafranine for determining viability of cultured plant cells. Stain Technol. 1972 Jul;47(4):189–194. doi: 10.3109/10520297209116483. [DOI] [PubMed] [Google Scholar]


