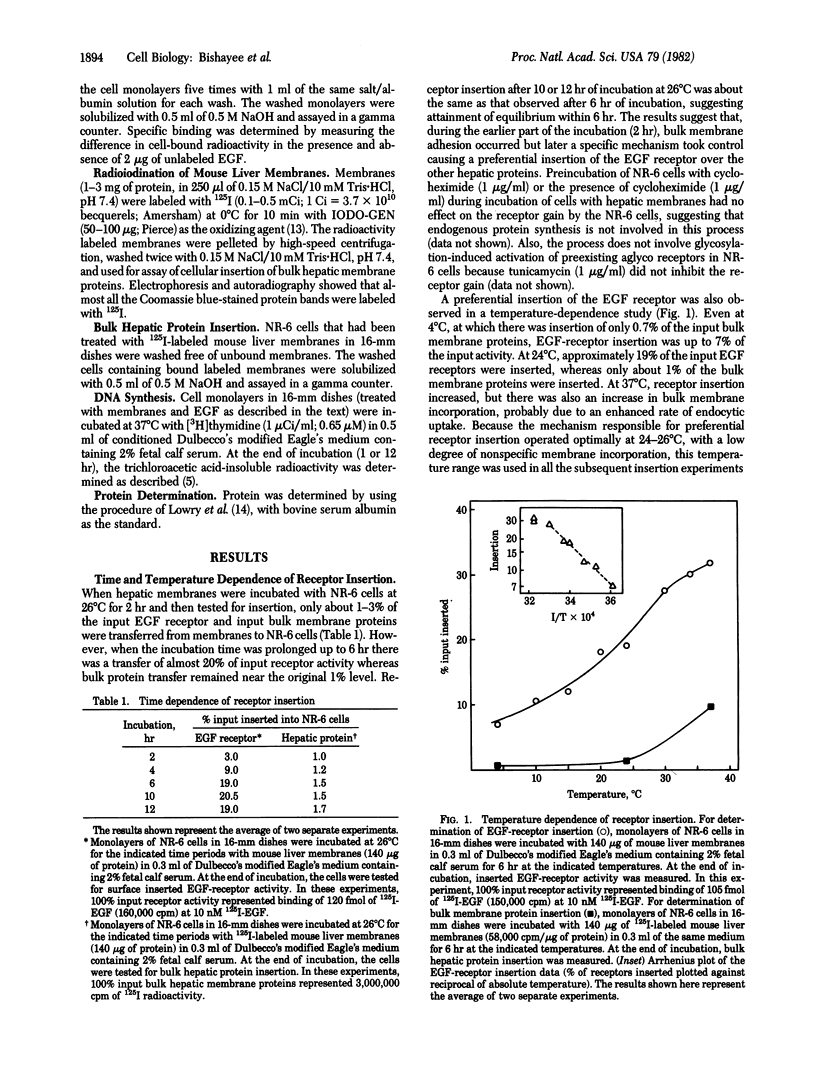
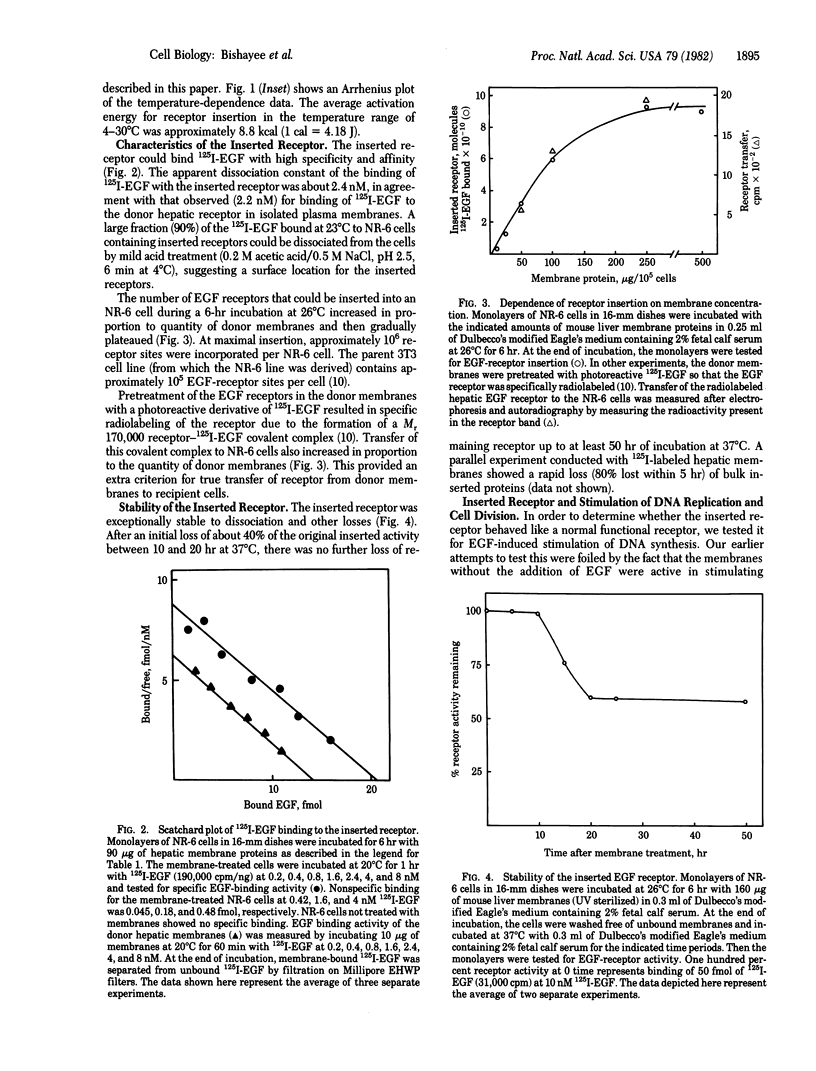
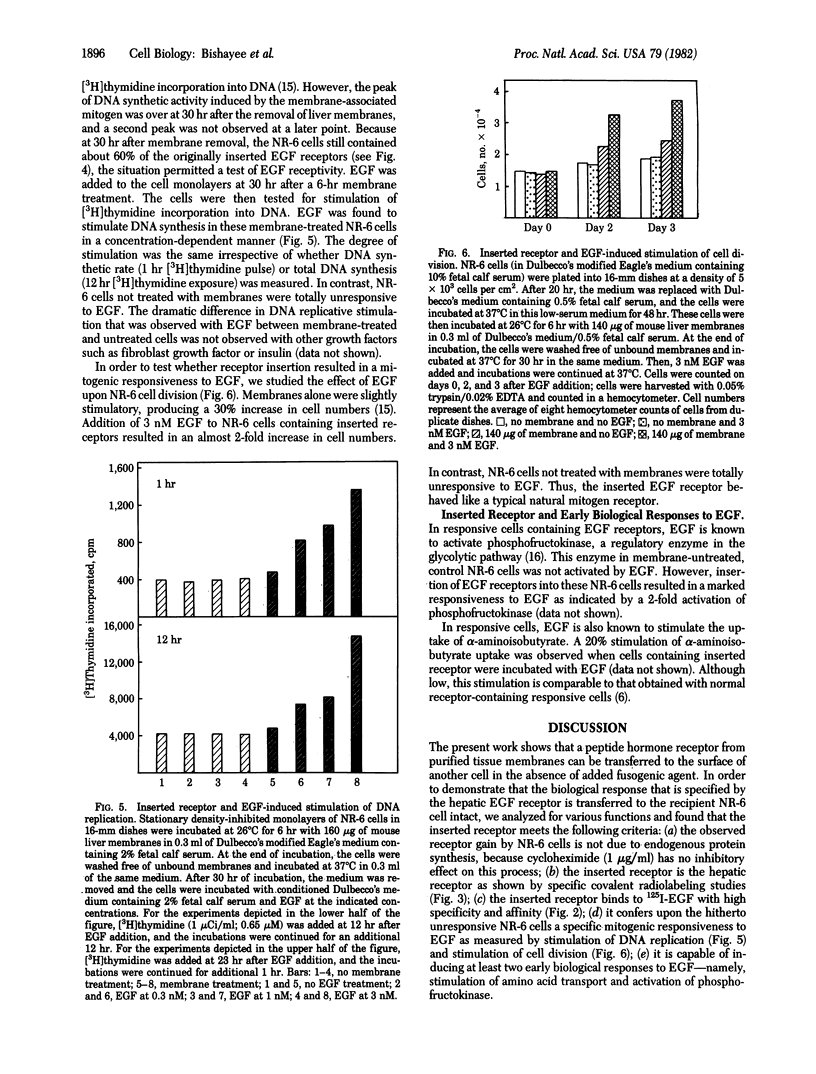
Abstract
We show that epidermal growth factor (EGF) receptor can be transferred in a biologically active orientation from donor hepatic membranes to recipient receptorless fibroblast cells. The recipient cells (NR-6) normally lack EGF receptors and are biologically unresponsive to EGF. The transfer of receptors from donor plasma membranes to recipient NR-6 surface membranes occurs in the absence of any added fusogenic agent. Studies on time and temperature dependence of this transfer indicate that it is due to preferential insertion of the EGF receptor over the other hepatic proteins. The inserted receptor is exceptionally stable to dissociation or damage, and this facilitated studies on its biological properties. The inserted receptor confers upon the hitherto unresponsive variant NR-6 cells a specific biological responsiveness to EGF as measured by EGF-induced stimulation of DNA replication and cell division. These findings suggest the existence of an affinity-mediated mechanism for the biologically active insertion of exogenous EGF receptors into receptorless variant cells. This insertion approach may be of use in the identification of receptor-associated membrane proteins that play a role in the transmission of EGF biological message.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carpenter G., Cohen S. Epidermal growth factor. Annu Rev Biochem. 1979;48:193–216. doi: 10.1146/annurev.bi.48.070179.001205. [DOI] [PubMed] [Google Scholar]
- Das M., Fox C. F. Molecular mechanism of mitogen action: processing of receptor induced by epidermal growth factor. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2644–2648. doi: 10.1073/pnas.75.6.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M. Initiation of nuclear DNA replication: evidence for formation of committed prereplicative cellular state. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5677–5681. doi: 10.1073/pnas.78.9.5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M. Mitogenic hormone-induced intracellular message: assay and partial characterization of an activator of DNA replication induced by epidermal growth factor. Proc Natl Acad Sci U S A. 1980 Jan;77(1):112–116. doi: 10.1073/pnas.77.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M., Miyakawa T., Fox C. F., Pruss R. M., Aharonov A., Herschman H. R. Specific radiolabeling of a cell surface receptor for epidermal growth factor. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2790–2794. doi: 10.1073/pnas.74.7.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle D., Hou E., Warren R. Transfer of the hepatocyte receptor for serum asialo-glycoproteins to the plasma membrane of a fibroblast. Acquisition of the hepatocyte receptor functions by mouse L-cells. J Biol Chem. 1979 Aug 10;254(15):6853–6856. [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Michael H. J., Bishayee S., Das M. Effect of methylamine on internalization, processing and biological activation of epidermal growth factor receptor. FEBS Lett. 1980 Aug 11;117(1):125–130. doi: 10.1016/0014-5793(80)80927-6. [DOI] [PubMed] [Google Scholar]
- Pruss R. M., Herschman H. R. Variants of 3T3 cells lacking mitogenic response to epidermal growth factor. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3918–3921. doi: 10.1073/pnas.74.9.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage C. R., Jr, Cohen S. Epidermal growth factor and a new derivative. Rapid isolation procedures and biological and chemical characterization. J Biol Chem. 1972 Dec 10;247(23):7609–7611. [PubMed] [Google Scholar]
- Schneider J. A., Diamond I., Rozengurt E. Glycolysis of quiescent cultures of 3T3 cells. Addition of serum, epidermal growth factor, and insulin increases the activity of phosphofructokinase in a protein synthesis-independent manner. J Biol Chem. 1978 Feb 10;253(3):872–877. [PubMed] [Google Scholar]
- Schramm M., Orly J., Eimerl S., Korner M. Coupling of hormone receptors to adenylate cyclase of different cells by cell fusion. Nature. 1977 Jul 28;268(5618):310–313. doi: 10.1038/268310a0. [DOI] [PubMed] [Google Scholar]
- Schramm M. Transfer of glucagon receptor from liver membranes to a foreign adenylate cyclase by a membrane fusion procedure. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1174–1178. doi: 10.1073/pnas.76.3.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]


