Abstract
Phosphatidylinositol-4,5-bisphosphate, PI(4,5)P2, is a phospholipid which plays important roles in clathrin-mediated endocytosis. To investigate the possible role of this lipid on viral entry, two viruses important for animal health were selected: the enveloped vesicular stomatitis virus (VSV) − which uses a well characterized clathrin mediated endocytic route − and two different variants of the non-enveloped foot-and-mouth disease virus (FMDV) with distinct receptor specificities. The expression of a dominant negative dynamin, a PI(4,5)P2 effector protein, inhibited the internalization and infection of VSV and both FMDV isolates. Depletion of PI(4,5)P2 from plasma membrane using ionomycin or an inducible system, and inhibition of its de novo synthesis with 1-butanol revealed that VSV as well as FMDV C-S8c1, which uses integrins as receptor, displayed a high dependence on PI(4,5)P2 for internalization. Expression of a kinase dead mutant (KD) of phosphatidylinositol-4-phosphate-5-kinase Iα (PIP5K-Iα), an enzyme responsible for PI(4,5)P2 synthesis that regulates clathrin-dependent endocytosis, also impaired entry and infection of VSV and FMDV C-S8c1. Interestingly FMDV MARLS variant that uses receptors other than integrins for cell entry was less sensitive to PI(4,5)P2 depletion, and was not inhibited by the expression of the KD PIP5K-Iα mutant suggesting the involvement of endocytic routes other than the clathrin-mediated on its entry. These results highlight the role of PI(4,5)P2 and PIP5K-Iα on clathrin-mediated viral entry.
Introduction
Phosphatidylinositols (PIs) and their phosphorylated derivatives are low abundant lipids in cellular membranes (<10% of total phospholipids) that have been revealed as key membrane components, particularly for membrane traffic [1]. One of these lipids, phosphatidyilinositol-4,5-bisphosphate (PI(4,5)P2), which is mostly localized in the internal hemimembrane of the plasma membrane, participates in regulation of a variety of cellular processes such as generation of membrane curvature, fission of endosomes, exocytosis, and binding to different effectors of clathrin-dependent endocytosis as well as at actin regulator proteins [1], [2], [3], [4]. In this way, depletion of PI(4,5)P2 from plasma membrane has been shown to inhibit clathrin-mediated endocytosis [2], [3], [4], [5], [6], [7]. In this endocytic route, clathrin-coated pits (CCPs) are assembled at the plasma membrane from cytosolic coat proteins. Upon capture of transmembrane receptor molecules CCPs invaginate to maturate into clathrin-coated vesicles (CCVs) [8]. Recent reports have shown that synthesis of PI(4,5)P2 is the major determinant of PI(4,5)P2 availability for CCP initiation and nucleation by contributing to progression beyond the endocytosis checkpoint and stabilization of nascent CCPs [2], [3], [4], [5], [6], [9], [10]. Although late states of CCP maturation to CCV do not require the synthesis of PI(4,5)P2, the presence of this lipid is necessary to bind proteins involved in CCV scission [2], 11. As the other PIs, PI(4,5)P2 carries out these regulatory functions by binding to different effector proteins through well characterized domains [1]. It is suggested that PI(4,5)P2 levels regulate CCP assembly, whereas localized turnovers of this phospholipid can control multiple stages in CCV formation [2], [12]. The major route for PI(4,5)P2 synthesis is the phosphorylation of PI4P by type I phosphatidylinositol-4-phosphate-5-kinase (PIP5K-I) [13]. Among the three isoforms reported for this enzyme (α, β and γ) [13], PIP5K-Iα is the major isoform involved in the regulation of clathrin-dependent endocytosis [2].
Proteins interacting with PIs (e.g Rab proteins, dynamin) have been involved in the entry of multiple viruses [14], [15], [16], [17], [18], [19], [20], thus pointing the importance of specific PIs in several steps for viral progression. However, a direct involvement of PIs, and specifically of PI(4,5)P2, in viral entry has been poorly evaluated, and the evidence for this is limited to the Human immunodeficiency virus type-1 (HIV-1) entry [21]. Indeed, HIV-1 binding to the plasma membrane through Env-gp120 actives an specific isoform of PIP5K protein, increasing the production of PI(4,5)P2. In addition, PI(4,5)P2 is required for late steps of HIV-1 and HIV-2 infection to promote the localization of Gag protein on plasma membrane during viral assembly [22], [23].
In the present study, we addressed the role of PI(4,5)P2 on the internalization of non-enveloped as well as of enveloped viruses. For this purpose, two important pathogens for animal health, causing clinically indistinguishable diseases, were selected: foot-and-mouth disease virus (FMDV) and vesicular stomatitis virus (VSV). FMDV is a small, non-enveloped virus responsible for a highly contagious disease affecting cloven-hoofed animals [24]. FMDV initiates infection of cultured cells via different αv integrins [25], [26], [27], [28], although receptors different from integrins can be used by FMDV variants selected upon passages in cultured cells [29], [30]. FMDV isolates that recognize integrins as cellular receptor utilize CCPs to enter cultured cells [31], [32], [33], while FMDV variants using heparan sulphate (HS) proteoglycans as receptor instead of integrins are internalized via caveolae [34]. To compare the PI(4,5)P2 requirements derived from the use of distinct cellular receptors, two different FMDV isolates were included in the study: C-S8c1 that is dependent on integrins for infection [35] and MARLS, a C-S8c1 derivative that has acquired the ability to enter cultured cells using HS and other not well characterized receptor(s) [29], [36]. On the other hand, VSV is an enveloped virus that has become a widely used system for the study of the clathrin-mediated endocytosis of viruses [37], [38], [39], [40].
Our results showed that dynamin − a PI(4,5)P2 effector − was a common requirement for infection of VSV and the two FMDVs analyzed. In contrast, while endocytosis of both FMDV C-S8c1 and VSV was highly dependent on plasma membrane PI(4,5)P2, that of FMDV MARLS showed a lower dependence on this phospholipid.
Results and Discussion
Functional Requirement of Dynamin for FMDV and VSV Infection
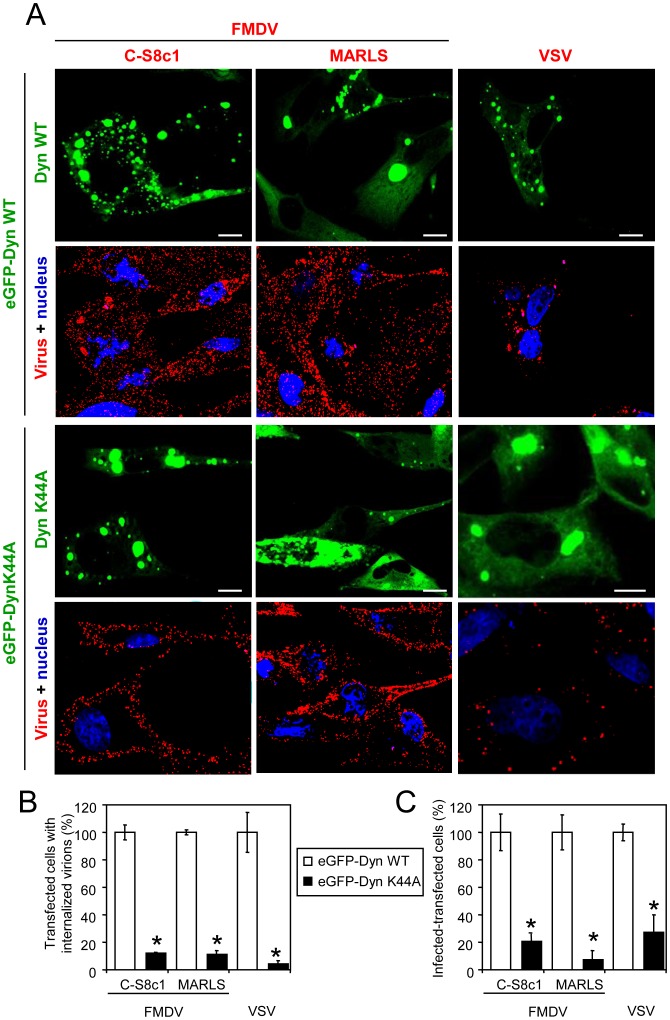
PI(4,5)P2 is involved in several cellular events as a result of its binding to different partners [1], [4], [41], [42], [43], [44], [45]. One of these proteins is the GTPase dynamin that works in endocytosis as a regulatory molecule and as a component of the fission machinery [46]. This prompted us to analyze the effect of the expression of a dominant negative (DN) form of dynamin (Dyn K44A) [47] on the entry of two FMDV isolates with different receptor specificities (C-S8c1 and MARLS). In this study, VSV was included as a positive control since it is well documented that dynamin is necessary for its entry [37], [38], [40]. BHK-21 cells were transfected with the corresponding plasmid, incubated with the viruses, and analysed by confocal microscopy as described [32], [48]. In cells expressing the WT dynamin, the viral particles were located inside the cells. However, the virions were observed at the cellular periphery in cells expressing the DN dynamin (Fig. 1A). When the percentage of cells with internalized viral particles was estimated by confocal microscopy,similar values were observed in control cells infected with VSV, C-S8c1 and MARLS, while the percentages were significantly reduced in cells expressing DN dynamin (12%, 11% and 4%, respectively), indicating that all viruses tested required dynamin for cell entry (Fig. 1B). In addition, expression of DN dynamin significantly reduced the percentage of transfected-infected cells for C-S8c1, MARLS and VSV (Fig. 1C), and no significant differences between the degrees of inhibition of the three viruses were noticed. Overall, these results indicate the functional requirement of this PI(4,5)P2-interacting protein for the entry and infection of the three viruses tested.
Figure 1. Functional requirement for dynamin of FMDV and VSV infection.
(A) BHK-21 cells transfected with eGFP fused to WT or a DN version of dynamin (eGFP-Dyn WT and eGFP-Dyn K44A, respectively) and 24 h later were incubated with the different FMDV variants (C-S8c1 and MARLS) or VSV (MOI of 70 PFU/cell) for 25 min and processed for immunofluorescence. Nuclei were stained using ToPro-3 (blue). GFP and viruses are shown in green and red, respectively. Bar: 10 µm. (B) BHK-21 cells transfected and infected as in (A). The graph represents the percentage of cells that showed internalized virus, determined as described in Materials and Methods. At least 100 transfected cells per coverslip were scored in each assay (3 coverslip). (C) BHK-21 cells were electroporated with a plasmid encoding eGFP-Dyn WT as control, or eGFP-Dyn K44A. At 24 h post-electroporation, monolayers were infected with the corresponding virus (MOI of 1 PFU/cell). Cells were fixed and processed for immunofluorescence at 7 h post-infection. Bars represent the mean percentage of transfected and infected cells ± SD, normalized to the level of infection of cells expressing the eGFP-Dyn WT. Statistically significant differences between cells transfected with eGFP-Dyn WT or K44A are indicated by an asterisk (ANOVA P≤0.05).
Effect of Depletion of PI(4,5)P2 on FMDV and VSV Internalization
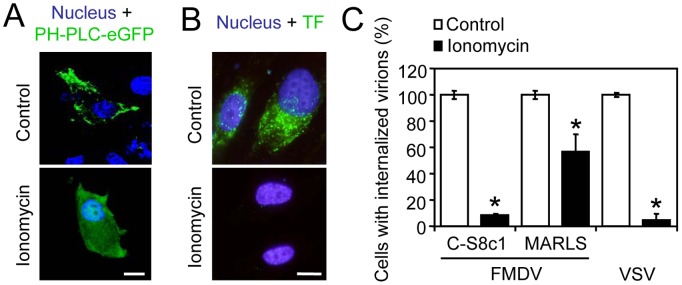
It has been documented that ionomycin treatment reduces the levels of PI(4,5)P2 by activation of phospholipase C [4]. BHK-21 cells were transfected with a reporter plasmid (PH-PLC-eGFP) [49] that expresses a fusion protein that binds and allows detection of this phospholipid by fluorescence microscopy [44]. Under control conditions, fluorescence was concentrated at the plasma membrane. However, in cells treated with 5 µM ionomycin as described [4], a cytoplasmic distribution of the reporter for PI(4,5)P2 was observed (Fig. 2A). This result confirmed that ionomycin treatment induced a depletion of PI(4,5)P2 from plasma membrane, which resulted in a cytoplasmic relocation of the fluorescent reporter protein. The effect of this drug on clathrin-mediated endocytosis was further analyzed by using fluorescent transferrin (TF), a marker of clathrin-mediated endocytosis [50]. In contrast to non treated cells, those treated with ionomycin displayed a reduction in TF internalization (Fig. 2B). As these results supported the conclusion that ionomycin treatment affected clathrin-mediated endocytosis, the effect of this drug on virus internalization was tested. Thus, BHK-21 cells were treated with ionomycin, incubated with the viruses and the proportion of cells that internalized viral particles was determined by immunofluorescence and confocal microscopy. As expected, ionomycin treatment inhibited (reduction by 90%) the internalization of VSV particles (Fig. 2C), whose entry through clathrin-mediated endocytosis is well characterized [37], [38], [39], [40], and a similar reduction was observed for FMDV C-S8c1 that enters into the cells via CCPs [32]. However, the inhibition was lower for FMDV MARLS (only a reduction about 40%), which has the ability to use cellular receptors other than integrins. These results highlight that depletion of PI(4,5)P2 from plasma membrane inhibits endocytosis of viruses that use CCV, and reveal that the different internalization pathway followed by C-S8c1 and MARLS FMDV variants can modulate PI(4,5)P2 requirement. Ionomycin treatment induced a significant reduction (close to 30%) on cell viability (Fig. S1A). To rule out that the inhibitory effect of this drug on viral entry could be related to major metabolic alterations, the effect of modulation of PI(4,5)P2 was further assayed using drugs with lower cytotoxicity.
Figure 2. Effect of PI(4,5)P2 depletion by ionomycin on FMDV and VSV internalization.
(A) Visualization of PI(4,5)P2 depletion from plasma membrane. BHK-21 cells transfected (24 h) with PH-PLC-eGFP, encoding a reporter protein for PI(4,5)P2 fused to GFP (green), were treated or not with 5 µM ionomycin 30 min and then fixed and observed by confocal microscopy. Nuclei were stained using ToPro-3 (blue). Bar 10 µm. (B) Treatment with ionomycin inhibits clathrin-mediated endocytosis. BHK-21 cells, treated with ionomycin as in (A), were incubated with Alexa Fluor 488-labelled TF (green) for 5 min in the presence of the drug and extracellular TF was eliminated by acid wash as described [32]. Cells were fixed and nuclei were stained using DAPI (blue). Bar: 10 µm. (C) Inhibition of the ability of cells to internalize FMDV and VSV upon ionomycin treatment. Cells treated with ionomycin as in (A) were incubated with the different FMDV variants (C-S8c1 and MARLS) or with VSV (MOI of 70 PFU/cell) for 25 min in the presence of ionomycin. Cells were fixed and processed for immunofluorescence to stain viral particles as described in Materials and Methods. Bars represent the mean percentage of cells with internalized virions ± SD, normalized to the level of cells with internalized virions in control samples. At least 500 cells per coverslip were scored for each case (3 coverslips). Asterisks denote statistically significant differences (ANOVA P≤0.05).
Effect of Inhibition of PI(4,5)P2 Synthesis on FMDV and VSV Internalization
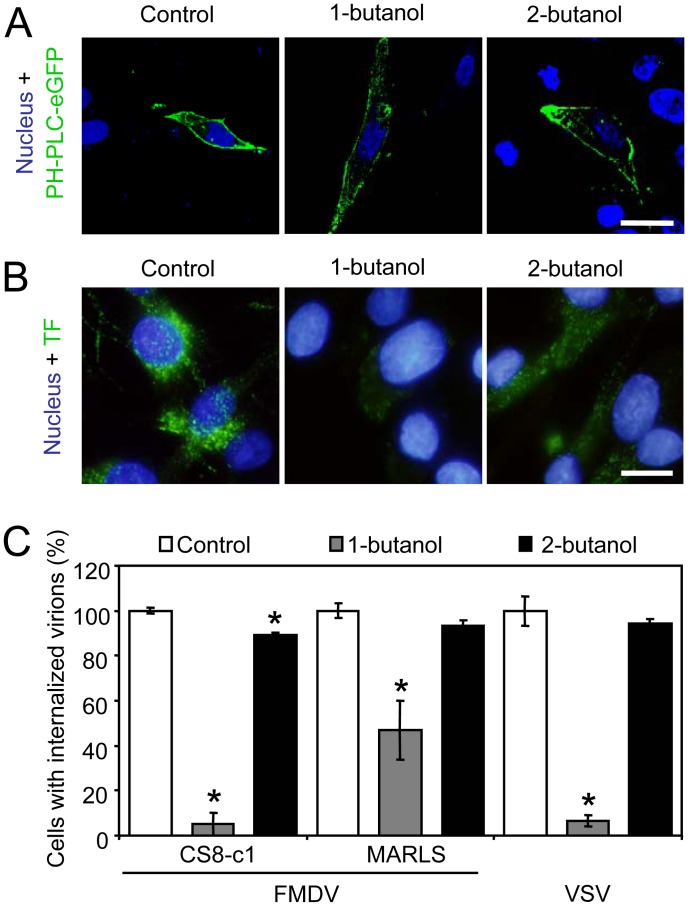
Rather than causing PI(4,5)P2 depletion from plasma membrane, as described for ionomycin, primary alcohols, like 1-butanol, promote phospholipase D (PLD) to generate phosphatidylalcohols instead of phosphatidic acid (PA), leading to a lower activation of PIP5Ks and consequently inhibiting PI(4,5)P2 synthesis and CCP assembly [5]. On the other hand, this effect does not occur in the presence of secondary alcohols such as 2-butanol [5]. Treatment with either 1.5% 1-butanol or 2-butanol did not affect the distribution pattern observed for the PI(4,5)P2 reporter (Fig. 3A), confirming that none of these alcohols produced a significant depletion of PI(4,5)P2 from plasma membrane. However, treatment with 1-butanol reduced the ability of BHK-21 cells to internalize TF, indicating a requirement for de novo synthesis of PI(4,5)P2 of clathrin-mediated endocytosis (Fig. 3B). As expected, treatment with 2-butanol did not result in reduction of TF internalization, confirming the specificity of the inhibition of clathrin-mediated endocytosis by 1-butanol. In contrast to ionomycin, treatment with either 1 or 2-butanol did not significantly affect cell viability (Fig. S1B), confirming that the reduction of clathrin-mediated endocytosis by 1-butanol was not related to major toxic effects of the drug. Regarding viral entry, treatment with 1-butanol reduced by 90% the internalization of C-S8c1 and VSV. Conversely, it only reduced MARLS internalization by 50%, suggesting that the different receptor used by MARLS and C-S8c1 for cell entry can modulate the requirement of PI(4,5)P2 synthesis. The specificity of this inhibition was confirmed as treatment with 2-butanol resulted in a limited reduction by 10% in the internalization of each of the three viruses analyzed (Fig. 3C). These results indicate that FMDV and VSV require synthesis of PI(4,5)P2 at the plasma membrane for internalization, and again suggest differences between MARLS and C-S8c1 internalization pathways.
Figure 3. Effect of blocking de novo synthesis of PI(4,5)P2 with 1-butanol on FMDV and VSV internalization.
(A) BHK-21 cells transfected (24 h) with PH-PLC-eGFP (green) were treated or not with 1.5% 1-butanol or 2-butanol for 5 min and then fixed and observed by confocal microscopy. Nuclei were stained using ToPro-3 (blue). Bar 10 µm. (B) Treatment with 1-butanol inhibits clathrin-dependent endocytosis. BHK-21 cells were treated as in (A) were incubated with fluorescent TF and processed as described in the legend of Fig. 1. Bar: 10 µm. (C) Reduction of the ability of cells to internalize FMDV and VSV upon 1-butanol treatment. Cells treated as in (A) were incubated with the different FMDV variants (C-S8c1 and MARLS) or VSV (MOI of 70 PFU/cell) for 25 min in the presence of the drugs. Bars represent the mean percentage of cells with internalized virions ± SD, normalized to the level of cells with internalized virions in control samples. At least 500 cells per coverslip were scored for each case (3 coverslips). Asterisks denote statistically significant differences (ANOVA P≤0.05).
Effect of Induced Depletion of PI(4,5)P2 from the Plasma Membrane on FMDV and VSV Internalization
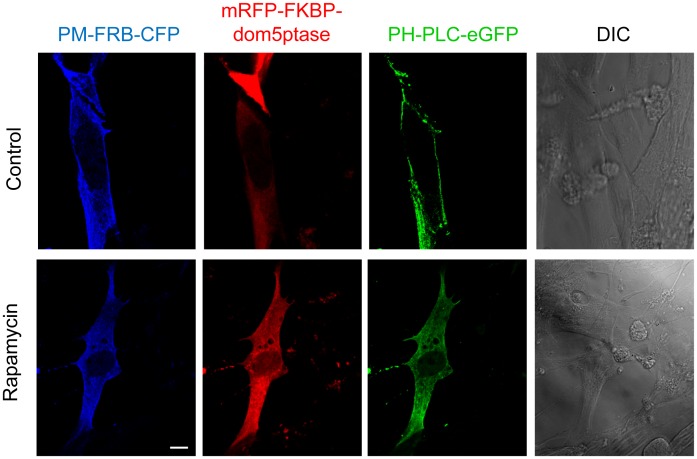
To confirm the results obtained with pharmacological inhibitors, the effect of PI(4,5)P2- targeted depletion with an inducible system [45] was analyzed. The approach used, which has been proven to be highly specific [4], [51], [52], is based on the conditional recruitment to plasma membrane of an inositol 5-phosphatase – fused to red fluorescent protein (RFP)– by its rapamycin-induced heterodimerization with a membrane-targeted, rapamycin-binding domain of mTOR fused to the cyan fluorescent protein (CFP) [45]. In this way, only when rapamycin is added to the culture medium, both fusion proteins interact and the phosphatase is recruited to plasma membrane causing a targeted depletion of PI(4,5)P2. First, we evaluated that this system induced the depletion of PI(4,5)P2 from the plasma membrane when rapamycin was added to BHK-21 cells (Fig. 4). To this end, cells were cotransfected for 24 h with plasmids encoding phosphatase (mRFP-FKBP-dom5ptase), membrane anchored rapamycin-binding domain (PM-FRB-CFP) and PH-PLC-eGFP (to detect PI(4,5)P2). Then, cells were treated with rapamycin for 10 min to induce the depletion of PI(4,5)P2 from plasma membrane. As expected, when rapamycin was added the fluorescence of PI(4,5)P2 reporter protein was relocated from plasma membrane to the cytoplasm (Fig. 4). Next, cells cotransfected with plasmids mRFP-FKBP-dom5ptase and PM-FRB-CFP, were treated with rapamycin to induce the PI(4,5)P2 depletion, and then incubated with the viruses (Fig. S2). About 89% of the cells expressing both plasmids in the absence of rapamycin were able to internalize C-S8c1 (Table 1). However, when rapamycin was added, only 12% of cotransfected cells were shown to internalize C-S8c1 particles. Addition of rapamycin similarly reduced the number of cells internalizing VSV (from 88% in control cells to 15% in rapamycin-treated cells for VSV). On the other hand, targeted depletion of PI(4,5)P2 only slightly reduced the percentage of cells internalizing MARLS (from 83% in control cells to 76% in rapamycin treated cells) (Table 1). Rapamycin alone had no effect on viral internalization of any of the three virus tested, since treatment with rapamycin of untransfected cells or cells only transfected with one plasmid did not reduce the percentages of cells that internalized the viral particles (data not shown). Overall, these results support those previously obtained with pharmacological treatments, indicating that internalization of FMDV C-S8c1 and VSV strongly depends on plasma membrane PI(4,5)P2 phospholipids, while MARLS internalization is less sensitive to PI(4,5)P2 depletion. These differences could be explained by the usage of an alternative dynamin-dependent endocytic pathway, such as caveolae, for MARLS internalization, since caveolae-mediated endocytosis can rely on dynamin function [53] and is less sensitive to PI(4,5)P2 depletion than clathrin-mediated endocytosis [54]. In fact, as commented in the introduction, MARLS could utilize HS binding to gain entry into cells through caveolae.
Figure 4. Depletion of PI(4,5)P2 from plasma membrane after rapamycin-induced membrane targeting of an inositol 5-phosphatase.
BHK-21 cells were cotransfected with PM-FRB-CFP (blue), mRFP-FKBP-dom5ptase (red) and PH-PLC-eGFP (green) plasmids using Lipofectamine Plus. At 24 h post-transfection, cells were treated with 10 nM rapamycin (10 min) to induce the depletion of PI(4,5)P2 from plasma membrane. Then cells were fixed and observed by confocal microscopy. A representative example of a co-transfected cell is shown. See text for details regarding the inducible system for PI(4,5)P2 depletion. Differential interference contrast (DIC) images are also shown. Bar: 10 µm.
Table 1. Inducible depletion of PI(4,5)P2 from plasma membrane inhibits internalization of FMDV and VSV.
| % Cells with internalized virions | % Cells without internalizedvirions | P-value | ||
| CS8-c1 | Control | 89 | 11 | 0.0001* |
| Rapamycin | 12 | 88 | ||
| MARLS | Control | 83 | 17 | 0.2202 |
| Rapamycin | 76 | 24 | ||
| VSV | Control | 88 | 12 | 0.0001* |
| Rapamycin | 15 | 85 |
Number of cotransfected cells scored: 100.
Statistically significant difference.
PIP5K-Iα is Required for Efficient FMDV C-S8c1 and VSV Entry and Infection
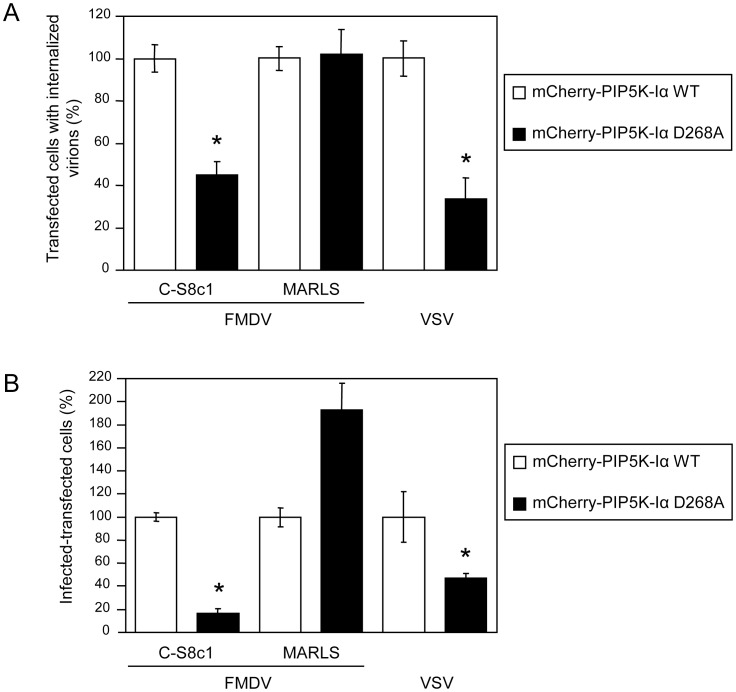
Three distinct isoforms of PIP5K-I (α, β, and γ) are responsible for the synthesis of a major proportion of PI(4,5)P2 in the cell, through the hosphorylation of PI4P [13]. Among these enzymes, PI4P5K-Iα has been identified as the main isoform that regulates clathrin-dependent endocytosis [2], [55]., and expression of kinase dead (KD) or truncated mutants of PIP5K-Iα impairs clathrin mediated endocytosis [55]. In this regard, the role of this kinase on the internalization of FMDV (C-S8c1 and MARLS) and VSV was addressed by studying the effect of the expression of a KD mutant of PIP5K-Iα fused to mCherry protein – mCherry-PIP5KIα D268A [2] – on the entry and infection of these viruses. BHK-21 cells were transfected, incubated with the viruses, processed and analyzed by confocal microscopy as described [32], [48]. The percentages of cells expressing KD PIP5K-Iα that internalized VSV or C-S8c1 were significantly lower than those of control cells, being the reduction values of 66% and 55%, respectively (Fig. 5A). On the other hand, expression of KD PIP5K-Iα did not reduce the percentage of MARLS viral particles internalized by cells when compared with control samples (Fig. 5A). In addition to this, expression of KD PIP5K-Iα significantly reduced the percentage of transfected-infected cells for C-S8c1 (83% reduction) and VSV (53% reduction) (Fig. 5B). Conversely, in the case of MARLS an increase in the percentage of transfected-infected cells was observed upon expression of KD PIP5K-Iα. These results indicate the functional requirement of the PIP5K-Iα for the entry and infection of C-S8c1 and VSV, but not for MARLS. Even when further work is required to understand the infection increase caused by KD PIP5K-Iα on MARLS infection, these differences support the notion that MARLS is internalized using of an alternative dynamin-dependent non-clathrin endocytic pathway less affected by modulation of plasma membrane PI(4,5)P2 and independent on PI4P5K-Iα function.
Figure 5. PIP5K-Iα is involved on entry and infection of FMDV C-S8c1 and VSV.
(A) BHK-21 cells transfected with mCherry fused to WT or a KD version of PIP5K-Iα (mCherry-PIP5K-Iα WT and mCherry-PIP5K-Iα D268A, respectively) and 24 h later were incubated with the different FMDV variants (C-S8c1 and MARLS) or VSV (MOI of 70 PFU/cell) for 25 min and processed for immunofluorescence. The graph represents the percentage of cells that showed internalized virus determined as described in Materials and Methods. At least 100 transfected cells per coverslip were scored for each assay (3 coverslip). (B) BHK-21 cells were electroporated with a plasmid encoding mCherry-PIP5K-Iα WT as control, or mCherry-PIP5K-Iα D268A. At 24 h post-electroporation, monolayers were infected with the corresponding virus (MOI of 1 PFU/cell) and cells were fixed and processed for immunofluorescence at 7 h post-infection. Bars represent the mean percentage of transfected and infected cells ± SD, normalized to the level of infection of cells expressing the mCherry-PIP5K-Iα WT. Statistically significant differences between cells transfected with mCherry-PIP5K-Iα WT or D268A are indicated by an asterisk (ANOVA P≤0.05).
Considering these results, modulation of PI(4,5)P2 metabolism, and of PIP5K function (especially PIP5K-Iα) could potentially constitute a new antiviral approach to fight viral diseases, a concept which has been already proposed for HIV-1 [21]. However, acute depletion of this lipid from plasma membrane has different effects on cellular functions [45], thus constituting a major concern for its potential application in vivo. Nevertheless, the experience with a variety of PI kinases that regulate synthesis of different PI species has revealed that these enzymes, and the lipids they synthesize, constitute good druggable targets to treat diverse diseases [56], including viral infections [57], [58], [59], [60], cancer [61], [62] or diabetes [63]. In particular, specific isoforms of PI 4-kinases can be chemically inhibited resulting in a blockage of viral replication without having a significant impact on cell viability [64]. As PI(4,5)P2 requirement is expected to be shared by a wide variety of viruses that are internalized using clathrin-mediated endocytosis, successful intervention on this pathway could lead to the development of broad spectrum antivirals. This concept, already proposed for PI 4-kinases, could offer therapeutic advantages since inhibition of host components instead of viral components could circumvent the problem of rapid selection of drug-resistant viruses – for a discussion see [64] –. Indeed, modulation of the metabolism of specific lipids is currently raising as a feasible antiviral approach [65], [66], [67]. Overall, the results presented in this study highlight the involvement of PI(4,5)P2, on viral entry of either enveloped and non-enveloped viruses. Further work has to be performed to evaluate the feasibility of the depletion of PI(4,5)P2 from plasma membrane as a novel antiviral strategy.
Materials and Methods
Cells and Viruses
BHK-21 cells (ATCC) were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 5% fetal calf serum (FCS), L-glutamine (2 mM), penicillin (100 U/ml), and streptomycin (100 µg/ml). FMDV isolate C-S8c1 is a derivative of a type C field virus isolated in Santa Pau (Spain, 1970) by triple plaque purification [68]. MARLS virus is a monoclonal antibody (MAb)-resistant mutant isolated with MAb SD6, which recognizes the G-H loop of capsid protein VP1 [69], from C-S8c1 virus after 213 passages on BHK-21 cells [36]. Mutations in MARLS virus compared to parental C-S8c1 have described previously [29]. VSV Indiana [70] was also used. The sequence of the capsid proteins of C-S8c1 and MARLS stocks used in this work was confirmed by RT-PCR amplification of viral RNA and sequencing of the amplicons obtained as described [71].
Antibodies and Reagents
FMDV VP1 and VSV glycoprotein (G) protein were detected using MAb 5C4 [72] and I1 [73], respectively. Goat anti-mouse IgG labelled with Alexa Fluor (AF) 555 or 647 were from Molecular Probes. Transferrin (TF) conjugated to AF 488 was from Invitrogen. Ionomycin (Sigma) and rapamicyn (Calbiochem) were prepared in DMSO as 1.4 mM and 1.1 mM stock solutions, respectively. 1-butanol and 2-butanol were from Merck.
Drug Treatments
BHK-21 cells grown on coverslips were washed twice with DMEM and incubated with ionomycin (5 µM) for 30 min, or with 1.5% 1-butanol or 2-butanol for 5 min. Control cells were incubated in the same conditions in DMEM containing the solvent concentration used for each drug. The drug was maintained during the virus internalization time. Cell viability upon drug treatments was determined by propidium iodide staining and flow cytometry [74], [75] using a FACScalibur flow cytometer (Becton Dickinson).
Plasmids and Transfections
The following plasmids were used in this study: eGFP-Dyn WT eGFP-Dyn K44A [47], PH-PLC-eGFP [44], PM-FRB-CFP, mRFP-FKBP-dom5ptase [45], mCherry-PIP5K-Iα WT and KD mCherry-PIP5K-Iα D268A [2] (all the PIP5K isoform designations in the text refer to the human nomenclature for these genes). BHK-21 cells were transfected using Lipofectamine Plus (Invitrogen) as described by the manufacturer or electroparated with the corresponding plasmid using Gene Pulser XCell™ (Bio Rad).
Immunofluorescence
Immunofluorescence was performed as described previously [76]. For confocal microscopy; a LSM510 META Inverted (Zeiss) confocal laser scanning microscope coupled to an Axiovert200 (Zeiss) inverted microscope (objective Plan-Apochromat 63x/AN 1.4) was used. Images were acquired using Zeiss LSM510 4.2 Sp2 software. The percentage of cells which internalized viral particles was determined by observation of Z-stacks (scan zoom 1×, step size 0.4 µm) (n ≥100) of cells using confocal microscopy [77]. For conventional fluorescence microscopy an Axioskop (Zeiss) fluorescence microscope coupled to a Coolsnap FX monochrome camera Roper Scientific was used and were acquired using RS Image software (Roper Scientific). To determine the number of infected cells, more than 150 cells expressing GFP were analyzed and the experiment was carried out three independent times. The images were processed using Adobe Photoshop 7.0 (Adobe System Inc.).
Data Analysis
Analysis of variance (ANOVA) using F Fischer-Snedecor distribution was performed with statistical package SPSS v.17.0 (SPSS Inc) for Windows. Data are presented as means ± standard deviations (SD). Chi-square test was performed with statistical package Graph Pad Prism. Statistically significant differences are denoted in the figures by one asterisk for a P value of <0.05.
Supporting Information
Analysis of cellular viability upon drug treatments. Cellular viability upon treatment with ionomycin (A) or 1 and 2-butanol (B) was determined by propidium iodide staining and flow cytometry. Control cells were treated in parallel with drug vehicles.
(TIF)
Inducible depletion of PI(4,5)P2 from plasma membrane inhibits internalization of FMDV and VSV. BHK-21 cells were cotransfected with PM-FRB-CFP – indicated as CFP (blue) – and mRFP-FKBP-dom5ptase – indicated as RFP (red) – plasmids [45]. At 24 h post-transfection, cells were treated (right panels) or not (left panels) with 10 nM rapamycin (10 min) to induce the depletion of PI(4,5)P2 from plasma membrane. Then, cells were incubated with the different viruses (green) (MOI of 70 PFU/cell, 25 min) in the presence of rapamycin and cells were processed for immunofluorescence. The percentage of cells that showed internalized virions, determined as described in Materials and Methods, is indicated. White dashed lines indicate the cell periphery of cotransfected cells; white solid lines indicate the cell periphery of untransfected cells. Insets show the lasser lines corresponding to the fluorochromes expressed by each of the transfecting plasmids, as well as a DIC image depicting the shape of the cells present in each field. Bar: 10 µm.
(TIF)
Acknowledgments
We wish to thank, E. Domingo for MARLS and VSV, and J.A Esteban, M. A. Alonso, C. Aragón, T. Balla, C. N. Antonescu, and S. L. Schmid for plasmid constructs.
Funding Statement
This work was supported by Spanish grants BIO2008-0447-C03-01 and BIO2011-24351, and by an institutional grant from Fundación Ramón Areces. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. De Matteis MA, Godi A (2004) PI-loting membrane traffic. Nat Cell Biol 6: 487–492. [DOI] [PubMed] [Google Scholar]
- 2. Antonescu CN, Aguet F, Danuser G, Schmid SL (2011) Phosphatidylinositol-(4,5)-bisphosphate regulates clathrin-coated pit initiation, stabilization, and size. Mol Biol Cell 22: 2588–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. James DJ, Khodthong C, Kowalchyk JA, Martin TF (2008) Phosphatidylinositol 4,5-bisphosphate regulates SNARE-dependent membrane fusion. J Cell Biol 182: 355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zoncu R, Perera RM, Sebastian R, Nakatsu F, Chen H, et al. (2007) Loss of endocytic clathrin-coated pits upon acute depletion of phosphatidylinositol 4,5-bisphosphate. Proc Natl Acad Sci U S A 104: 3793–3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boucrot E, Saffarian S, Massol R, Kirchhausen T, Ehrlich M (2006) Role of lipids and actin in the formation of clathrin-coated pits. Exp Cell Res 312: 4036–4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haucke V (2005) Phosphoinositide regulation of clathrin-mediated endocytosis. Biochem Soc Trans 33: 1285–1289. [DOI] [PubMed] [Google Scholar]
- 7.Richard JP, Leikina E, Langen R, Henne WM, Popova M, et al.. (2011) Intracellular curvature generating proteins in cell-to-cell fusion. Biochem J. [DOI] [PMC free article] [PubMed]
- 8. McMahon HT, Boucrot E (2011) Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol 12: 517–533. [DOI] [PubMed] [Google Scholar]
- 9. Henne WM, Boucrot E, Meinecke M, Evergren E, Vallis Y, et al. (2010) FCHo proteins are nucleators of clathrin-mediated endocytosis. Science 328: 1281–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jackson LP, Kelly BT, McCoy AJ, Gaffry T, James LC, et al. (2010) A large-scale conformational change couples membrane recruitment to cargo binding in the AP2 clathrin adaptor complex. Cell 141: 1220–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bethoney KA, King MC, Hinshaw JE, Ostap EM, Lemmon MA (2009) A possible effector role for the pleckstrin homology (PH) domain of dynamin. Proc Natl Acad Sci U S A 106: 13359–13364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wenk MR, Pellegrini L, Klenchin VA, Di Paolo G, Chang S, et al. (2001) PIP kinase Igamma is the major PI(4,5)P(2) synthesizing enzyme at the synapse. Neuron 32: 79–88. [DOI] [PubMed] [Google Scholar]
- 13. van den Bout I, Divecha N (2009) PIP5K-driven PtdIns(4,5)P2 synthesis: regulation and cellular functions. J Cell Sci 122: 3837–3850. [DOI] [PubMed] [Google Scholar]
- 14. Chaudhry A, Das SR, Jameel S, George A, Bal V, et al. (2008) HIV-1 Nef induces a Rab11-dependent routing of endocytosed immune costimulatory proteins CD80 and CD86 to the Golgi. Traffic 9: 1925–1935. [DOI] [PubMed] [Google Scholar]
- 15. Chu H, Wang JJ, Spearman P (2009) Human immunodeficiency virus type-1 gag and host vesicular trafficking pathways. Curr Top Microbiol Immunol 339: 67–84. [DOI] [PubMed] [Google Scholar]
- 16. Eisfeld AJ, Kawakami E, Watanabe T, Neumann G, Kawaoka Y (2011) RAB11A is essential for transport of the influenza virus genome to the plasma membrane. J Virol 85: 6117–6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Johns HL, Berryman S, Monaghan P, Belsham GJ, Jackson T (2009) A dominant-negative mutant of rab5 inhibits infection of cells by foot-and-mouth disease virus: implications for virus entry. J Virol 83: 6247–6256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Krishnan MN, Sukumaran B, Pal U, Agaisse H, Murray JL, et al. (2007) Rab 5 is required for the cellular entry of dengue and West Nile viruses. J Virol 81: 4881–4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vidricaire G, Tremblay MJ (2005) Rab5 and Rab7, but not ARF6, govern the early events of HIV-1 infection in polarized human placental cells. J Immunol 175: 6517–6530. [DOI] [PubMed] [Google Scholar]
- 20. Vonderheit A, Helenius A (2005) Rab7 associates with early endosomes to mediate sorting and transport of Semliki forest virus to late endosomes. PLoS Biol 3: e233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barrero-Villar M, Barroso-Gonzalez J, Cabrero JR, Gordon-Alonso M, Alvarez-Losada S, et al. (2008) PI4P5-kinase Ialpha is required for efficient HIV-1 entry and infection of T cells. J Immunol 181: 6882–6888. [DOI] [PubMed] [Google Scholar]
- 22. Chukkapalli V, Hogue IB, Boyko V, Hu WS, Ono A (2008) Interaction between the human immunodeficiency virus type 1 Gag matrix domain and phosphatidylinositol-(4,5)-bisphosphate is essential for efficient gag membrane binding. J Virol 82: 2405–2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Legendre-Guillemin V, Wasiak S, Hussain NK, Angers A, McPherson PS (2004) ENTH/ANTH proteins and clathrin-mediated membrane budding. J Cell Sci 117: 9–18. [DOI] [PubMed] [Google Scholar]
- 24. Sobrino F, Saiz M, Jimenez-Clavero MA, Nunez JI, Rosas MF, et al. (2001) Foot-and-mouth disease virus: a long known virus, but a current threat. Vet Res 32: 1–30. [DOI] [PubMed] [Google Scholar]
- 25. Berinstein A, Roivainen M, Hovi T, Mason PW, Baxt B (1995) Antibodies to the vitronectin receptor (integrin alpha V beta 3) inhibit binding and infection of foot-and-mouth disease virus to cultured cells. J Virol 69: 2664–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jackson T, Blakemore W, Newman JW, Knowles NJ, Mould AP, et al. (2000) Foot-and-mouth disease virus is a ligand for the high-affinity binding conformation of integrin alpha5beta1: influence of the leucine residue within the RGDL motif on selectivity of integrin binding. J Gen Virol 81: 1383–1391. [DOI] [PubMed] [Google Scholar]
- 27. Jackson T, Clark S, Berryman S, Burman A, Cambier S, et al. (2004) Integrin alphavbeta8 functions as a receptor for foot-and-mouth disease virus: role of the beta-chain cytodomain in integrin-mediated infection. J Virol 78: 4533–4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jackson T, Mould AP, Sheppard D, King AM (2002) Integrin alphavbeta1 is a receptor for foot-and-mouth disease virus. J Virol 76: 935–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baranowski E, Ruiz-Jarabo CM, Sevilla N, Andreu D, Beck E, et al. (2000) Cell recognition by foot-and-mouth disease virus that lacks the RGD integrin-binding motif: flexibility in aphthovirus receptor usage. J Virol 74: 1641–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jackson T, Ellard FM, Ghazaleh RA, Brookes SM, Blakemore WE, et al. (1996) Efficient infection of cells in culture by type O foot-and-mouth disease virus requires binding to cell surface heparan sulfate. J Virol 70: 5282–5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Berryman S, Clark S, Monaghan P, Jackson T (2005) Early events in integrin alphavbeta6-mediated cell entry of foot-and-mouth disease virus. J Virol 79: 8519–8534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Martin-Acebes MA, Gonzalez-Magaldi M, Sandvig K, Sobrino F, Armas-Portela R (2007) Productive entry of type C foot-and-mouth disease virus into susceptible cultured cells requires clathrin and is dependent on the presence of plasma membrane cholesterol. Virology 369: 105–118. [DOI] [PubMed] [Google Scholar]
- 33. O’Donnell V, LaRocco M, Duque H, Baxt B (2005) Analysis of foot-and-mouth disease virus internalization events in cultured cells. J Virol 79: 8506–8518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. O’Donnell V, Larocco M, Baxt B (2008) Heparan sulfate-binding foot-and-mouth disease virus enters cells via caveola-mediated endocytosis. J Virol 82: 9075–9085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nunez JI, Molina N, Baranowski E, Domingo E, Clark S, et al. (2007) Guinea pig-adapted foot-and-mouth disease virus with altered receptor recognition can productively infect a natural host. J Virol 81: 8497–8506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Baranowski E, Sevilla N, Verdaguer N, Ruiz-Jarabo CM, Beck E, et al. (1998) Multiple virulence determinants of foot-and-mouth disease virus in cell culture. J Virol 72: 6362–6372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cureton DK, Massol RH, Saffarian S, Kirchhausen TL, Whelan SP (2009) Vesicular stomatitis virus enters cells through vesicles incompletely coated with clathrin that depend upon actin for internalization. PLoS Pathog 5: e1000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Johannsdottir HK, Mancini R, Kartenbeck J, Amato L, Helenius A (2009) Host cell factors and functions involved in vesicular stomatitis virus entry. J Virol 83: 440–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Matlin KS, Reggio H, Helenius A, Simons K (1982) Pathway of vesicular stomatitis virus entry leading to infection. J Mol Biol 156: 609–631. [DOI] [PubMed] [Google Scholar]
- 40. Sun X, Yau VK, Briggs BJ, Whittaker GR (2005) Role of clathrin-mediated endocytosis during vesicular stomatitis virus entry into host cells. Virology 338: 53–60. [DOI] [PubMed] [Google Scholar]
- 41. Abe N, Inoue T, Galvez T, Klein L, Meyer T (2008) Dissecting the role of PtdIns(4,5)P2 in endocytosis and recycling of the transferrin receptor. J Cell Sci 121: 1488–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Erlmann P, Schmid S, Horenkamp FA, Geyer M, Pomorski TG, et al.. (2009) DLC1 Activation Requires Lipid Interaction through a Polybasic Region Preceding the RhoGAP Domain. Mol Biol Cell. [DOI] [PMC free article] [PubMed]
- 43. Johnson CM, Chichili GR, Rodgers W (2008) Compartmentalization of phosphatidylinositol 4,5-bisphosphate signaling evidenced using targeted phosphatases. J Biol Chem 283: 29920–29928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Szentpetery Z, Balla A, Kim YJ, Lemmon MA, Balla T (2009) Live cell imaging with protein domains capable of recognizing phosphatidylinositol 4,5-bisphosphate; a comparative study. BMC Cell Biol 10: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Varnai P, Thyagarajan B, Rohacs T, Balla T (2006) Rapidly inducible changes in phosphatidylinositol 4,5-bisphosphate levels influence multiple regulatory functions of the lipid in intact living cells. J Cell Biol 175: 377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Loerke D, Mettlen M, Yarar D, Jaqaman K, Jaqaman H, et al. (2009) Cargo and dynamin regulate clathrin-coated pit maturation. PLoS Biol 7: e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Martin-Belmonte F, Martinez-Menarguez JA, Aranda JF, Ballesta J, de Marco MC, et al. (2003) MAL regulates clathrin-mediated endocytosis at the apical surface of Madin-Darby canine kidney cells. J Cell Biol 163: 155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Martin-Acebes MA, Herrera M, Armas-Portela R, Domingo E, Sobrino F (2010) Cell density-dependent expression of viral antigens during persistence of foot-and-mouth disease virus in cell culture. Virology 403: 47–55. [DOI] [PubMed] [Google Scholar]
- 49. Arendt KL, Royo M, Fernandez-Monreal M, Knafo S, Petrok CN, et al. (2010) PIP3 controls synaptic function by maintaining AMPA receptor clustering at the postsynaptic membrane. Nat Neurosci 13: 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sandvig K, Olsnes S, Petersen OW, van Deurs B (1987) Acidification of the cytosol inhibits endocytosis from coated pits. J Cell Biol 105: 679–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Adjobo-Hermans MJ, Goedhart J, Gadella TW Jr (2008) Regulation of PLCbeta1a membrane anchoring by its substrate phosphatidylinositol (4,5)-bisphosphate. J Cell Sci 121: 3770–3777. [DOI] [PubMed] [Google Scholar]
- 52. Suh BC, Inoue T, Meyer T, Hille B (2006) Rapid chemically induced changes of PtdIns(4,5)P2 gate KCNQ ion channels. Science 314: 1454–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mercer J, Schelhaas M, Helenius A (2010) Virus entry by endocytosis. Annu Rev Biochem 79: 803–833. [DOI] [PubMed] [Google Scholar]
- 54. Fujita A, Cheng J, Tauchi-Sato K, Takenawa T, Fujimoto T (2009) A distinct pool of phosphatidylinositol 4,5-bisphosphate in caveolae revealed by a nanoscale labeling technique. Proc Natl Acad Sci U S A 106: 9256–9261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Barbieri MA, Heath CM, Peters EM, Wells A, Davis JN, et al. (2001) Phosphatidylinositol-4-phosphate 5-kinase-1beta is essential for epidermal growth factor receptor-mediated endocytosis. J Biol Chem 276: 47212–47216. [DOI] [PubMed] [Google Scholar]
- 56. Prestwich GD (2004) Phosphoinositide signaling; from affinity probes to pharmaceutical targets. Chem Biol 11: 619–637. [DOI] [PubMed] [Google Scholar]
- 57. Arita M, Kojima H, Nagano T, Okabe T, Wakita T, et al. (2011) Phosphatidylinositol-4 kinase III beta is a target of enviroxime-like compounds for anti-poliovirus activity. J Virol 85: 2364–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hsu NY, Ilnytska O, Belov G, Santiana M, Chen YH, et al. (2010) Viral reorganization of the secretory pathway generates distinct organelles for RNA replication. Cell 141: 799–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bianco A, Reghellin V, Donnici L, Fenu S, Alvarez R, et al. (2012) Metabolism of phosphatidylinositol 4-kinase IIIalpha-dependent PI4P Is subverted by HCV and is targeted by a 4-anilino quinazoline with antiviral activity. PLoS Pathog 8: e1002576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jefferies HB, Cooke FT, Jat P, Boucheron C, Koizumi T, et al. (2008) A selective PIKfyve inhibitor blocks PtdIns(3,5)P(2) production and disrupts endomembrane transport and retroviral budding. EMBO Rep 9: 164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Liu P, Cheng H, Roberts TM, Zhao JJ (2009) Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov 8: 627–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bartholomeusz C, Gonzalez-Angulo AM (2012) Targeting the PI3K signaling pathway in cancer therapy. Expert Opin Ther Targets 16: 121–130. [DOI] [PubMed] [Google Scholar]
- 63. Knight ZA, Gonzalez B, Feldman ME, Zunder ER, Goldenberg DD, et al. (2006) A pharmacological map of the PI3-K family defines a role for p110alpha in insulin signaling. Cell 125: 733–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Altan-Bonnet N, Balla T (2012) Phosphatidylinositol 4-kinases: hostages harnessed to build panviral replication platforms. Trends Biochem Sci 37: 293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martin-Acebes MA, Vazquez-Calvo A, Caridi F, Saiz JC, Sobrino F (2012) Lipid involvement in viral infections: present and future perspectives for the design of antiviral strategies. In: Valenzuela R, editor. Lipid metabolism: InTech. pp. In press.
- 66. Munger J, Bennett BD, Parikh A, Feng XJ, McArdle J, et al. (2008) Systems-level metabolic flux profiling identifies fatty acid synthesis as a target for antiviral therapy. Nat Biotechnol 26: 1179–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chukkapalli V, Heaton NS, Randall G (2012) Lipids at the interface of virus-host interactions. Curr Opin Microbiol 15: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sobrino F, Davila M, Ortin J, Domingo E (1983) Multiple genetic variants arise in the course of replication of foot-and-mouth disease virus in cell culture. Virology 128: 310–318. [DOI] [PubMed] [Google Scholar]
- 69. Mateu MG, Martinez MA, Capucci L, Andreu D, Giralt E, et al. (1990) A single amino acid substitution affects multiple overlapping epitopes in the major antigenic site of foot-and-mouth disease virus of serotype C. J Gen Virol 71 (Pt. 3): 629–637. [DOI] [PubMed] [Google Scholar]
- 70. Novella IS, Cilnis M, Elena SF, Kohn J, Moya A, et al. (1996) Large-population passages of vesicular stomatitis virus in interferon-treated cells select variants of only limited resistance. J Virol 70: 6414–6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Nunez JI, Baranowski E, Molina N, Ruiz-Jarabo CM, Sanchez C, et al. (2001) A single amino acid substitution in nonstructural protein 3A can mediate adaptation of foot-and-mouth disease virus to the guinea pig. J Virol 75: 3977–3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mateu MG, Rocha E, Vicente O, Vayreda F, Navalpotro C, et al. (1987) Reactivity with monoclonal antibodies of viruses from an episode of foot-and-mouth disease. Virus Res 8: 261–274. [DOI] [PubMed] [Google Scholar]
- 73. Lefrancois L, Lyles DS (1982) The interaction of antibody with the major surface glycoprotein of vesicular stomatitis virus. II. Monoclonal antibodies of nonneutralizing and cross-reactive epitopes of Indiana and New Jersey serotypes. Virology 121: 168–174. [DOI] [PubMed] [Google Scholar]
- 74. King MA (2000) Detection of dead cells and measurement of cell killing by flow cytometry. J Immunol Methods 243: 155–166. [DOI] [PubMed] [Google Scholar]
- 75. Lamm GM, Steinlein P, Cotten M, Christofori G (1997) A rapid, quantitative and inexpensive method for detecting apoptosis by flow cytometry in transiently transfected cells. Nucleic Acids Res 25: 4855–4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Martin-Acebes MA, Gonzalez-Magaldi M, Vazquez-Calvo A, Armas-Portela R, Sobrino F (2009) Internalization of swine vesicular disease virus into cultured cells: a comparative study with foot-and-mouth disease virus. J Virol 83: 4216–4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Gastaldelli M, Imelli N, Boucke K, Amstutz B, Meier O, et al. (2008) Infectious adenovirus type 2 transport through early but not late endosomes. Traffic 9: 2265–2278. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Analysis of cellular viability upon drug treatments. Cellular viability upon treatment with ionomycin (A) or 1 and 2-butanol (B) was determined by propidium iodide staining and flow cytometry. Control cells were treated in parallel with drug vehicles.
(TIF)
Inducible depletion of PI(4,5)P2 from plasma membrane inhibits internalization of FMDV and VSV. BHK-21 cells were cotransfected with PM-FRB-CFP – indicated as CFP (blue) – and mRFP-FKBP-dom5ptase – indicated as RFP (red) – plasmids [45]. At 24 h post-transfection, cells were treated (right panels) or not (left panels) with 10 nM rapamycin (10 min) to induce the depletion of PI(4,5)P2 from plasma membrane. Then, cells were incubated with the different viruses (green) (MOI of 70 PFU/cell, 25 min) in the presence of rapamycin and cells were processed for immunofluorescence. The percentage of cells that showed internalized virions, determined as described in Materials and Methods, is indicated. White dashed lines indicate the cell periphery of cotransfected cells; white solid lines indicate the cell periphery of untransfected cells. Insets show the lasser lines corresponding to the fluorochromes expressed by each of the transfecting plasmids, as well as a DIC image depicting the shape of the cells present in each field. Bar: 10 µm.
(TIF)