Abstract
Hospitalized patients can develop cognitive function decline, the mechanisms of which remain largely to be determined. Sleep disturbance often occurs in hospitalized patients, and neuroinflammation can induce learning and memory impairment. We therefore set out to determine whether sleep disturbance can induce neuroinflammation and impairment of learning and memory in rodents. Five to 6-month-old wild-type C57BL/6J male mice were used in the studies. The mice were placed in rocking cages for 24 hours, and two rolling balls were present in each cage. The mice were tested for learning and memory function using the Fear Conditioning Test one and 7 days post-sleep disturbance. Neuroinflammation in the mouse brain tissues was also determined. Of the Fear Conditioning studies at one day and 7 days after sleep disturbance, twenty-four hours sleep disturbance decreased freezing time in the context test, which assesses hippocampus-dependent learning and memory; but not the tone test, which assesses hippocampus-independent learning and memory. Sleep disturbance increased pro-inflammatory cytokine IL-6 levels and induced microglia activation in the mouse hippocampus, but not the cortex. These results suggest that sleep disturbance induces neuroinflammation in the mouse hippocampus, and impairs hippocampus-dependent learning and memory in mice. Pending further studies, these findings suggest that sleep disturbance-induced neuroinflammation and impairment of learning and memory may contribute to the development of cognitive function decline in hospitalized patients.
Keywords: Sleep disturbance, hippocampus, neuroinflammation, learning and memory
Introduction
Given the increase in the aging population of the U.S. and around the world, it is predicted that cognitive disorder will be one of the most demanding healthcare problems for both patients and their providers, consuming a growing fraction of healthcare resources.
A recent study by Ehlenbach et al. has shown that people who have acute care and critical illness hospitalization can experience cognitive function decline as compared to people who have no hospitalization (Ehlenbach et al., 2010). The underlying mechanisms by which hospitalization is associated with cognitive function decline remain largely to be determined.
Sleep disturbance is one of the common factors associated with hospitalized patients (Aurell and Elmqvist, 1985; Friese et al., 2007; Hardin, 2009). In particular, sleep disturbance often occurs in patients in a critical care unit [(Feeley and Gardner, 2006), reviewed in (Tembo and Parker, 2009)]. Many studies have shown that sleep plays an important role in learning and memory function [(Aleisa et al., 2011; Drummond and Brown, 2001; Graves et al., 2003; Guan et al., 2004; Xu et al., 2010; Zhao et al., 2010), reviewed in (Maquet, 2001; Stickgold et al., 2001)]. In order to further study the role of sleep disturbance in cognitive function decline, we have established a new sleep disturbance model in mice and have assessed the effects of sleep disturbance on learning and memory function in mice.
Neuroinflammation, which includes microglia activation and increases in the levels of pro-inflammatory cytokines in the brain, may lead to cognitive function decline (Kalman et al., 2006; Ramlawi et al., 2006a; Ramlawi et al., 2006b; Rudolph et al., 2008; Wilson et al., 2002). Pro-inflammatory cytokines can be released by the microglia during its activation, fueling neuroinflammation and leading to cognitive function decline (Perry, 2004; Teeling and Perry, 2009; van Gool et al., 2010). Specifically, IL-6 has been shown to be associated with cognitive dysfunction (Patanella et al., 2010) and mild cognitive impairment (MCI) in medical (Schuitemaker et al., 2009) and surgical patients (Hudetz et al., 2010). Thus, we also determined the effects of sleep disturbance on neuroinflammation to test a hypothesis that sleep disturbance can induce both neuroinflammation and impairment of learning and memory in mice.
Materials and Methods
Sleep disturbance in mice
The animal protocol was approved by the Standing Committee on Animals at Massachusetts General Hospital and Beth Israel Deaconess Medical Center, Boston, Massachusetts. Wild type C57BL/6J male mice (five - 6 month-old, The Jackson Laboratory, Bar Harbor, ME) were randomly assigned to a sleep disturbance group or control group. The mice were individually housed in a controlled environment (20–22°C; 12 hour light: dark on a reversed light cycle) for two weeks prior to the sleep disturbance studies. Mice in the sleep disturbance group were placed in cages on moving rockers (rocking cages). Three mice were put in one rocking cage, and two rolling balls were put inside each cage. The balls were made by soft-rubber, each 5 centimeters in diameter. These balls moved with the movement of the rocker. Thus, both the rocking cages and rolling balls inside the cages were used to disturb the mouse sleep cycle. The mice were under this sleep disturbance condition with a rocking cage and rolling balls for 24 hours, 12 hours each in the light-dark cycle. This condition created a clinically relevant sleep disturbance animal model, because patients in the hospital and critical care units often experience sleep disturbance for up to 24 hours. Mice had free access to food and water during the sleep disturbance period. The mice were returned to the home cage (quiet, non-rocking cages and no rolling balls) to rest for 24 hours before the behavioral tests and brain tissue harvest. The 24 hour rest period before the behavioral testing was incorporated to prevent the mice from becoming too tired to perform the learning and memory tests. We used 12 – 15 mice in either the control group or sleep disturbance group in the behavioral studies. For the biochemistry studies, four to six mice were used in the control group or sleep disturbance group. The mice in the control group for both the behavioral and biochemistry studies had the same environment and condition as the mice in the treatment group - 12 hour light: dark on a reversed light cycle and free access to food and water, only without the sleep disturbance by the rocking cages and rolling balls.
Sleep Recordings
Surgery
The mice were implanted with biotelemetry units (TL11M-F20EET, Data Sciences International, St. Paul, MN) for the chronic recording of sleep-wake. Briefly, the biotelemetry units were placed intraperitoneally, and the leads from the transmitter were routed subcutaneously to the skull. Two tiny holes (one in the frontal bone and another one on the parietal bone) were drilled and two EEG leads from the transmitters were secured in such a way that these leads were touching the dura mater. Two EMG leads were secured on to the neck muscles and skin was sutured. After a 14 d recovery period following surgery and a day of habituation to the recording setup, the telemetric recording of EEG/EMG from these animals was performed for 48 h: 24 hours with non-sleep disturbance (baseline) and 24 hours with sleep disturbance.
Analysis
EEG/EMG data signals from each mouse were divided into 4 second epochs and visually scored as wake, non-rapid eye movement (NREM) sleep, or REM sleep. For staging, NREM sleep was identified by a preponderance of high-amplitude, low-frequency (<4 Hz) EEG activity and relatively low and unchanging EMG activity, whereas wakefulness was characterized by a preponderance of low-amplitude, fast EEG activity and highly variable muscle tone on EMG. REM sleep was identified by very low EMG activity and a low-amplitude monotonous EEG containing a predominance of theta range (4–7 Hz) EEG activity. When two states (for example, NREM sleep and wake) occurred within a 4 s epoch, the epoch was scored for the state that predominated (Lu et al., 2002). The percentage of time spent in wake, NREM sleep, and REM sleep, and frequency and the episode durations of each stage were calculated.
Fear conditioning test (FCT)
The FCT is a behavioral procedure designed to assess associative learning and memory. First demonstrated by Ivan Pavlov in 1927 (Pavlov, 1927/1960), the FCT is among the most commonly used behavioral tests to detect learning and memory impairment induced by anesthesia alone (Saab et al., 2010; Zhang et al., 2012) and anesthesia with surgery (Terrando et al., 2010). We performed the FCT studies using the methods described in our previous studies (Zhang et al., 2012) and other studies (Saab et al., 2010; Terrando et al., 2010). Specifically, the mice were exposed for training in the FCT (Stoelting Co., Wood Dale, IL) 24 hours after the end of the sleep disturbance. Each mouse was allowed to explore the chamber for 180 seconds before presentation of a 2-Hz pulsating tone (80 dB, 1,500 Hz) that persisted for 60 seconds. The tone was followed immediately by a mild foot shock (0.8 mA for 0.5 seconds). Context (no tone period) and tone (tone period) learning and memory were probed 24 hours after the training in sequence. The same group of mice was tested in the context test first, then in tone test one hour later. For the context test, each mouse (from either the control group or sleep disturbance group) was allowed to stay in the chamber for 180 seconds, followed by another 180-second period without a tone, and finally 30 seconds for recovery. For the tone test, each mouse (from either the control group or sleep disturbance group) was allowed to stay in the chamber for 180 seconds, followed by another 180-second period with a tone, and finally 30 seconds for recovery. Learning and memory were assessed by measuring the amount of time the mouse demonstrated “freezing behavior”, defined as a completely immobile posture except for respiratory efforts, during the test period (the second 180-second period), which was analyzed by Any-Maze (Stoelting). The first 180-second period allowed mice to adjust for the environment before the counting of freezing time in the second 180-second period. A different group of mice was used for the second context and tone tests performed at 7 days post-sleep disturbance. “Freezing behavior” was analyzed by Any-Maze (Stoelting).
Brain tissue harvest and protein level quantification
Different groups of mice in both the control condition and sleep disturbance were used for biochemistry studies. One or 7 days after the end of the sleep disturbance, the mice were killed by decapitation (for Western blot analysis) or transcardial perfusion under anesthesia (for immunohistochemistry studies). Separate groups of mice were used for the Western blot analysis and the immunohistochemistry studies. For the Western blot analysis, the harvested brain tissues were homogenized on ice using immunoprecipitation buffer (10 mM Tris-HCl, pH 7.4, 150 mM NaCl, 2 mM EDTA, 0.5% Nonidet P-40) plus protease inhibitors (1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μg/ml pepstatin A). The lysates were collected, centrifuged at 12,000 rpm for 10 minutes, and quantified for total proteins with BCA protein assay kit (Pierce, Iselin, NJ).
Western blot analysis
IL-6 antibody (1:1,000 dilution, Abcam, Cambridge, MA) was used to recognize IL-6 (24 kDa). Western blot quantification was performed as described by Xie et al. (Xie et al., 2008). Briefly, signal intensity was analyzed using a Bio-Rad (Hercules, CA) image program (Quantity One). We quantified the Western blots in two steps, first using β-Actin levels to normalize (e.g., determine the ratio of IL-6 to β-Actin amount) protein levels and control for loading differences in the total protein amount. Second, we presented protein level changes in mice undergoing sleep disturbance as a percentage of those in the control group. 100% of protein level changes refer to control levels for the purpose of comparison to experimental conditions.
Immunohistochemistry
Mice were anesthetized with isoflurane briefly (1.4% isoflurane for five minutes) and perfused transcardially with heparinized saline followed by 4% paraformaldehyde in 0.1M phosphate buffer at pH 7.4. The anesthesia with 1.4% isoflurane for five minutes in mice provided adequate anesthesia for the perfusion procedure without causing significant changes in blood pressure and blood gas according to our previous studies (Xie et al., 2008). Mouse brain tissues were removed and kept at 4 degrees C in paraformaldehyde. Five μm frozen sections from the mouse brain hemispheres were used for the immunohistochemistry staining. The sections were incubated with Iba-1 antibody (ab5076, 1:100, Abcam) and secondary antibody (Alexa 568, 1:500, Invitrogen, Carlsbad, CA). Finally, the sections were analyzed in mounting medium under a 20 X objective lens confocal microscope and photos of the sections were taken. An investigator who was blind to the experimental design counted the number of Iba-1 positive cells using Image J Version 1.38 (National Institutes of Health, Bethesda, MD).
Statistics
Data were expressed as mean ± standard deviation (SD). The number of samples varied from 4 to 15, and the samples were normally distributed (tested by normality test, data not shown). A two-tailed t test was used to compare the differences in freezing time, IL-6 levels, and numbers of Iba1 positive cells between the sleep disturbance and control groups. A two-tailed t test was also used to compare the differences in time of wake, non-REM (NREM) sleep, or REM sleep between 24 hours non-sleep disturbance period and 24 hours sleep disturbance period of each mouse. P values less than 0.05 (*) and 0.01 (**) were considered statistically significant.
Results
Sleep disturbance induces hippocampus-dependent, but not hippocampus-independent, learning and memory impairment in mice
A recent study has shown that acute disease and critical illness hospitalization are associated with cognitive function decline (Ehlenbach et al., 2010). Sleep disturbance often occurs with hospitalization (Aurell and Elmqvist, 1985; Friese et al., 2007; Hardin, 2009), and we therefore assessed the effects of sleep disturbance on learning and memory function, as well as neuroinflammation.
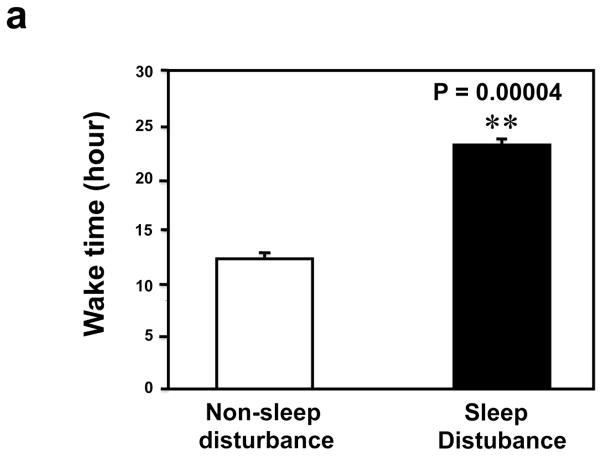
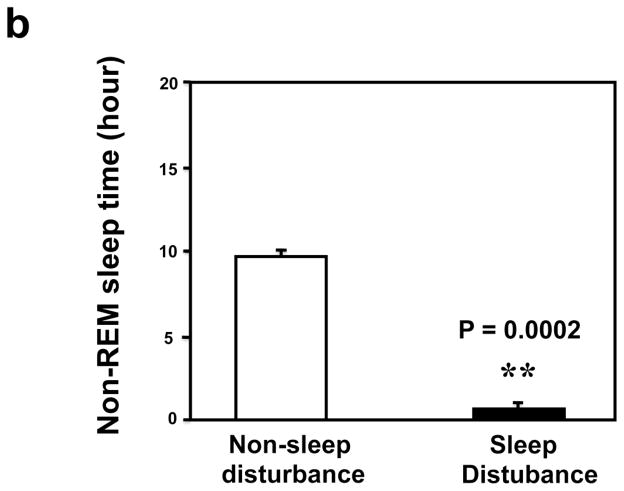
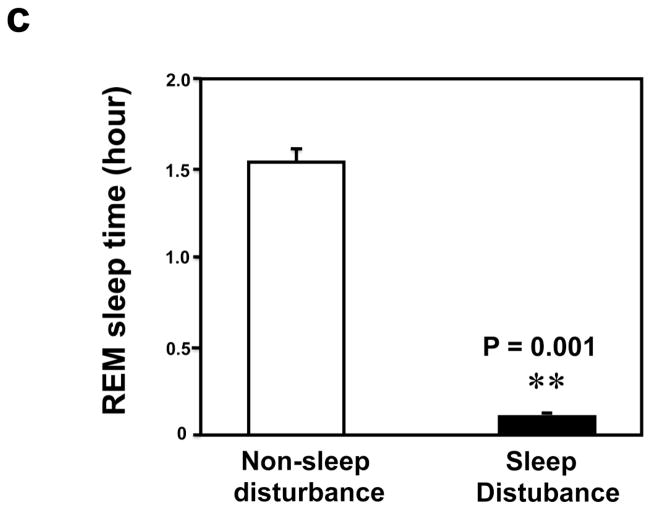
First, we asked whether our newly developed sleep disturbance model in mice indeed reduced the amount of sleep time in mice. Four mice were implanted with EEG/EMG telemetry units to record the extent of sleep loss over 24 hrs. During the 24 hours sleep disturbance period, the mice were awake 22.26 ± 0.46 hours, a significant increase (** P = 0.000004, Figure 1a) as compared to the 24 hours non-sleep disturbance period (12.72 ± 0.49 hours awake). Non-REM sleep was severely impaired, in terms of both the number of bouts (68.5 ± 6.89 sleep disturbance versus 109.5 ± 7.77 non-sleep disturbance, (** P = 0.001), and average bout duration (77.5 ± 18.82 seconds sleep disturbance versus 324.5 ± 22.28 second non-sleep disturbance, ** P = 0.001), leading to a far lower total NREM sleep time over the 24 hours of sleep disturbance (1.65 ± 0.42 hours) compared with the control baseline (24 hours non-sleep disturbance period) (9.71 ± 0.48 hours) (Figure 1b, ** P = 0.001). Meanwhile, REM sleep was completely absent in one mouse and almost nonexistent in the other three mice during the 24 hours sleep disturbance period. The total number of REM bouts was reduced to 4.25 ± 1.49 during the 24 hours sleep disturbance period as compared to 51.00 ± 5.80 during the 24 hours non-sleep disturbance period, and mean bout duration was also cut short (58.50 ± 24.10 seconds sleep disturbance versus 113.25 ± 14.36 seconds non-sleep disturbance), resulting in only 0.09 ± 0.05 hours of REM sleep during the 24 hours sleep disturbance period as compared to 1.56 ± 0.15 hours of REM sleep during the 24 hours non-sleep disturbance period (Figure 1c, ** P = 0.003). These data suggest that the newly developed sleep disturbance model can indeed reduce the amount of sleep time in mice.
Figure 1.
a. Mice during the 24 hours sleep disturbance period have more awake time than mice during the 24 hours non-sleep disturbance period. b. Mice during the 24 hours sleep disturbance period have less NREM time than mice during the 24 hours non-sleep disturbance period. c. Mice during the 24 hours sleep disturbance period have less REM time than mice during the 24 hours non-sleep disturbance period.
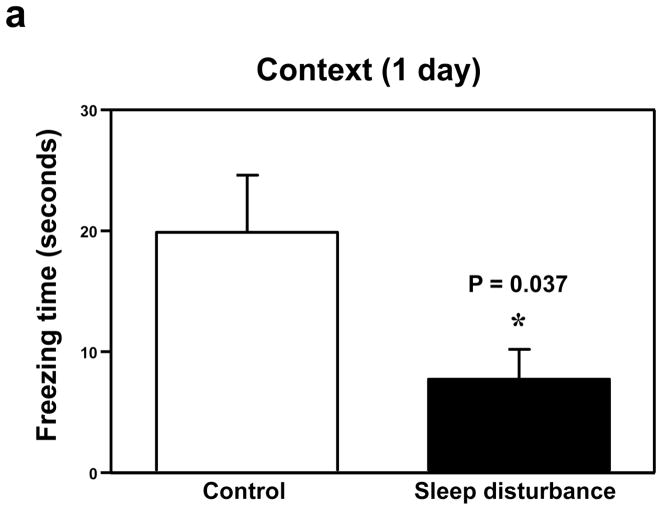
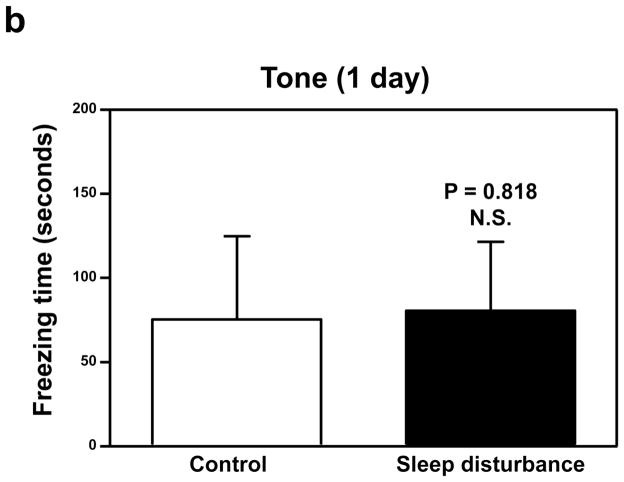
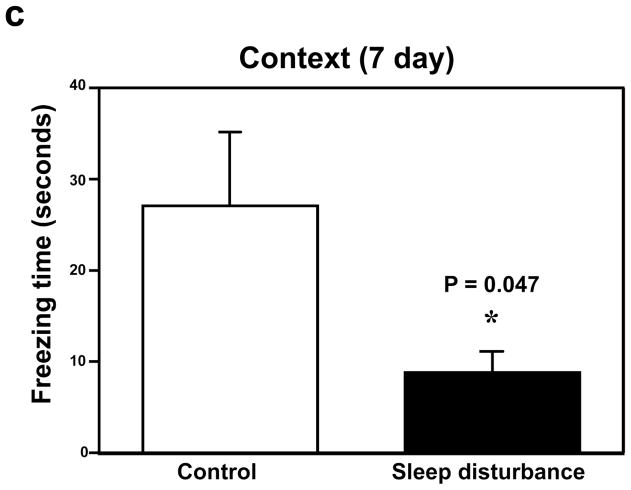
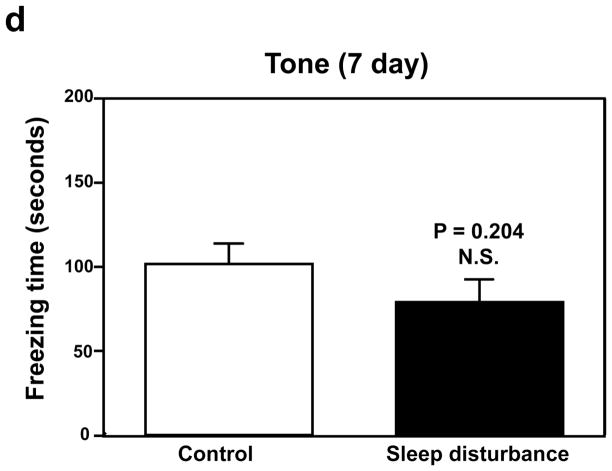
Next, we determined the effects of sleep disturbance on the function of learning and memory in the Fear Conditioning Test (FCT). The FCT studies showed that 24 hours of sleep disturbance (Figure 2a, black bar) led to decreases in the freezing time in the context test of the FCT as compared to the control condition (Figure 2a, white bar) one day post-sleep disturbance: 19.7 versus 7.7, * P = 0.037. Next, we found that 24 hours of sleep disturbance did not decrease the freezing time in the tone test of the FCT as compared to the control condition one day post-sleep disturbance: white bar versus black bar, P = 0.818, N.S. (Figure 2b). 24 hours of sleep disturbance (Figure 2c, black bar) also decreased the freezing time in the context test of the FCT as compared to the control condition (Figure 2c, white bar) 7 days post-sleep disturbance: 26.4 versus 8.1, * P = 0.047. Finally, we found that 24 hours sleep disturbance did not significantly change the freezing time in the tone test of the FCT as compared to the control condition 7 days post-sleep disturbance: white bar versus black bar, P = 0.204, N.S. (Figure 2d). These results suggest that sleep disturbance may impair learning and memory function in mice. Moreover, sleep disturbance may selectively impair hippocampus-dependent learning and memory function.
Figure 2. Sleep disturbance impairs learning and memory in mice.
a. Twenty-four hours of sleep disturbance decreases freezing time in the context test of the Fear Conditioning Test (FCT) as compared to the control condition one day post-sleep disturbance. b. Twenty-four hours of sleep disturbance does not decrease freezing time in the tone test of the FCT as compared to the control condition one day post-sleep disturbance. c. Twenty-four hours of sleep disturbance decreases freezing time in the context test of the FCT as compared to the control condition 7 days post-sleep disturbance. d. Twenty-four hours of sleep disturbance does not decrease freezing time in the tone test of the FCT as compared to the control condition 7 days post-sleep disturbance. N = 15.
Sleep disturbance increases pro-inflammatory cytokine levels in the mouse hippocampus, but not the mouse cortex
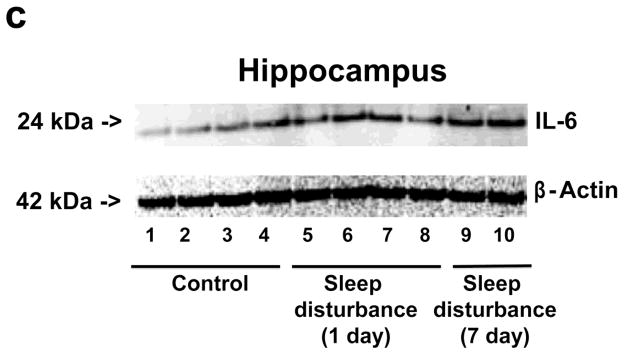
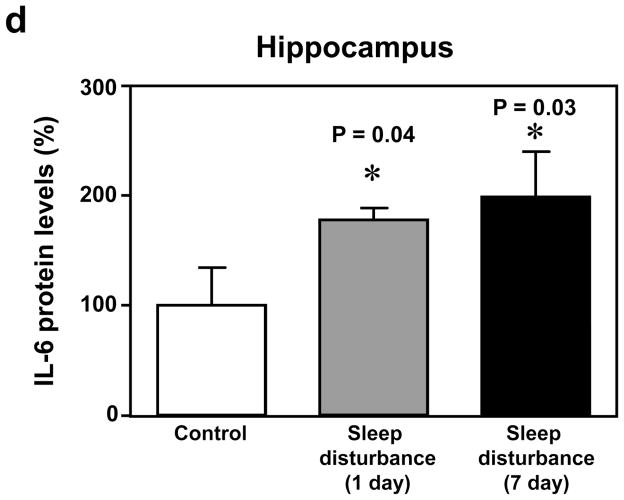
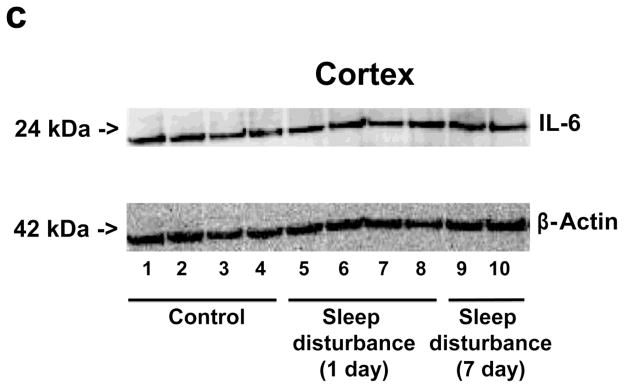
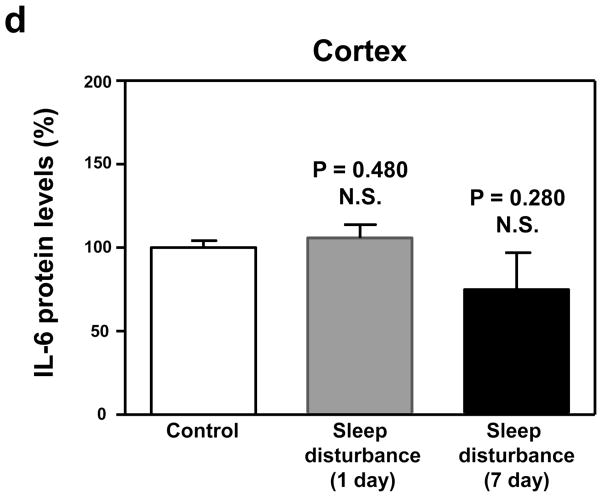
Given that sleep disturbance can impair learning and memory and that pro-inflammatory cytokines, including IL-6, are associated with learning and memory impairment, we asked whether sleep disturbance can increase the levels of IL-6 in mouse brain tissues. Immunoblotting of IL-6 showed that 24 hour sleep disturbance increased IL-6 levels in the mouse hippocampus as compared to the control condition one and 7 days post-sleep disturbance (Figure 3a). There was no significant difference in β-Actin levels between the control condition and sleep disturbance mice (Figure 3a). Quantification of the Western blot, based on the ratio of IL-6 to β-Actin, illustrated that the sleep disturbance increased IL-6 levels in the mouse hippocampus as compared to the control condition at one (177% versus 100%, * P = 0.04) and 7 days (195% versus 100%, * P = 0.03) post-sleep disturbance (Figure 3b). Finally, we found that 24 hours sleep disturbance did not increase levels of IL-6 in the mouse cortex as compared to the control condition one and 7 days post-sleep disturbance (Figure 4). Taken together, these results suggest that sleep disturbance may increase pro-inflammatory cytokine levels in mouse brain tissues. Moreover, sleep disturbance can specifically increase pro-inflammatory cytokine levels in the mouse hippocampus but not in the mouse cortex, which is consistent with the behavioral changes that sleep disturbance selectively impairs hippocampus-dependent learning and memory function.
Figure 3. Sleep disturbance increases IL-6 levels in the mouse hippocampus.
a. Twenty-four hours of sleep disturbance increases IL-6 levels in the mouse hippocampus in Western blot analysis as compared to the control condition one and 7 days post-sleep disturbance. There is no significant difference in the amounts of β-Actin in the mouse hippocampus following sleep disturbance or the control condition. b. Quantification of the Western blot shows that the sleep disturbance increases IL-6 levels in the mouse hippocampus as compared to the control condition one and 7 days post-sleep disturbance. N = 6.
Figure 4. Sleep disturbance does not increase IL-6 levels in the mouse cortex.
a. Twenty-four hours of sleep disturbance does not increase IL-6 levels in the mouse cortex in Western blot analysis as compared to the control condition one and 7 days post-sleep disturbance. There is no significant difference in the amounts of β-Actin in the mouse cortex following the sleep disturbance or control condition. b. Quantification of the Western blot shows that the sleep disturbance does not increase IL-6 levels in the mouse cortex as compared to the control condition one and 7 days post-sleep disturbance. N = 6.
Sleep disturbance induces microglia activation in the mouse hippocampus, but not the mouse cortex
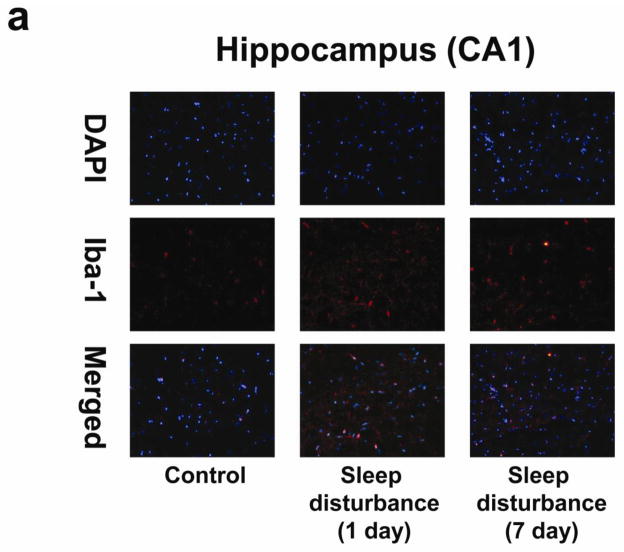
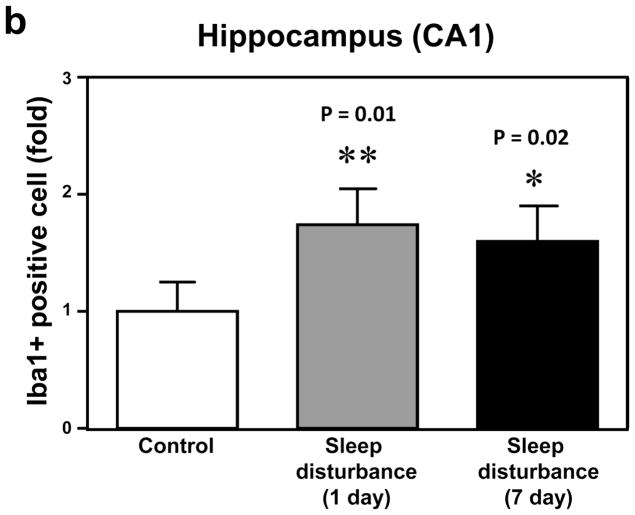
Neuroinflammation, including microglia activation, may induce learning and memory impairment (Cibelli et al., 2010; Terrando et al., 2010; Wan et al., 2007; Wan et al., 2010). We therefore set out to determine whether sleep disturbance can induce microglia activation in mice. Immunohistochemistry staining with Iba-1 (Figure 5a) was used to determine the effects of sleep disturbance on microglia activation in the mouse hippocampus. Row 1 is an image of DAPI staining, row 2 is Iba-1 staining, and row 3 is a merged staining image. The immunohistochemistry staining with Iba-1 showed that 24 hours of sleep disturbance induced microglia activation as evidenced by the increased number of Iba-1 positive cells in the mouse hippocampus CA1 area as compared to the control condition (column 1) at one day (column 2) and 7 days (column 3) post-sleep disturbance (Figure 5a). Quantification of the staining images showed that 24 hours of sleep disturbance increased the number of Iba-1 positive cells in the mouse hippocampus CA1 area as compared to the control condition at one day (1.83 fold versus 1 fold, ** P = 0.01) and 7 days (1.77 fold versus 1 fold, * P = 0.02) post-sleep disturbance (Figure 5b).
Figure 5. Sleep disturbance induces microglia activation in the mouse hippocampus.
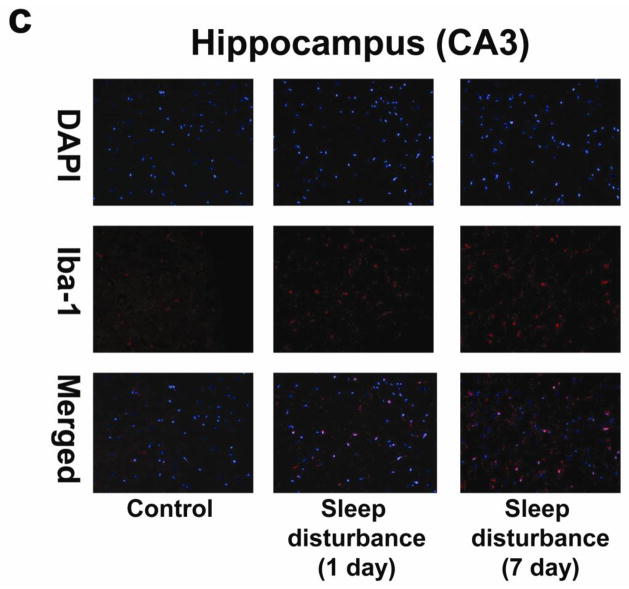
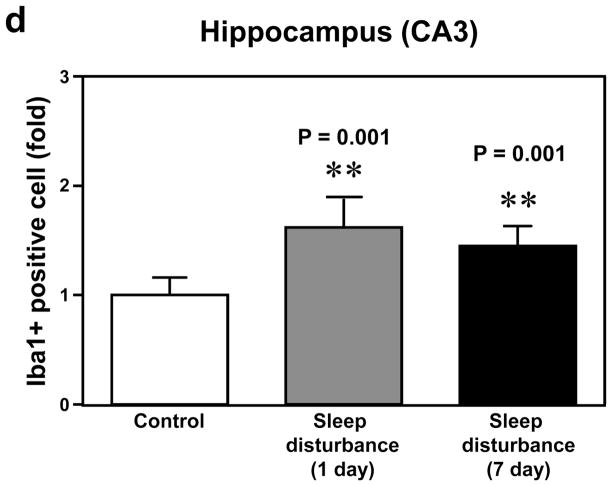
a. Twenty-four hours of sleep disturbance increases the number of Iba-1 positive cells in the mouse hippocampus (CA1) in immunohistochemistry staining as compared to the control condition one and 7 days post-sleep disturbance. b. Quantification of the immunohistochemistry staining shows that the sleep disturbance increases the number of Iba-1 positive cells in the mouse hippocampus (CA1) as compared to the control condition one and 7 days post-sleep disturbance. c. Twenty-four hours of sleep disturbance increases the number of Iba-1 positive cells in the mouse hippocampus (CA3) in immunohistochemistry staining as compared to the control condition one and 7 days post-sleep disturbance. d. Quantification of the immunohistochemistry staining shows that the sleep disturbance increases the number of Iba-1 positive cells in the mouse hippocampus (CA3) as compared to the control condition one and 7 days post-sleep disturbance. N = 6.
Next, we determined the effects of sleep disturbance on microglia activation in the mouse hippocampus CA3 area. Immunohistochemistry staining with Iba-1 (Figure 5c) showed that 24 hours of sleep disturbance induced microglia activation as evidenced by the increased number of Iba-1 positive cells in the mouse hippocampus CA3 area as compared to the control condition (column 1) one day (column 2) and 7 days (column 3) post-sleep disturbance (Figure 5c). Quantification of the immunohistochemistry staining images illustrated that 24 hours of sleep disturbance increased the number of Iba-1 positive cells in the mouse hippocampus CA3 area as compared to the control condition one day (1.68 fold versus 1 fold, ** P = 0.001) and 7 days (1.49 fold versus 1 fold, ** P = 0.001) post-sleep disturbance (Figure 5d).
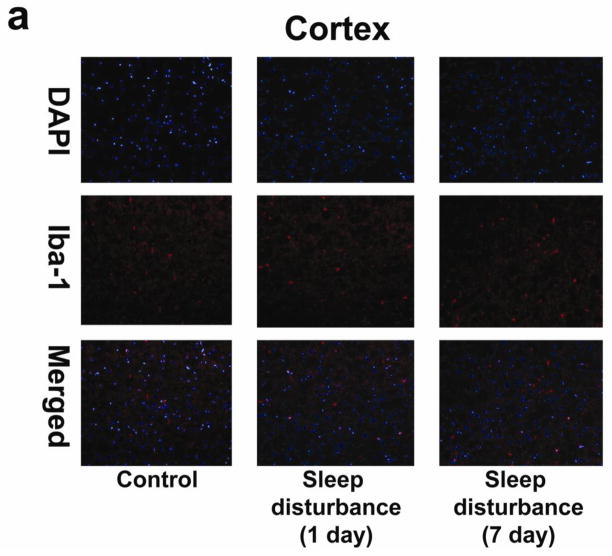
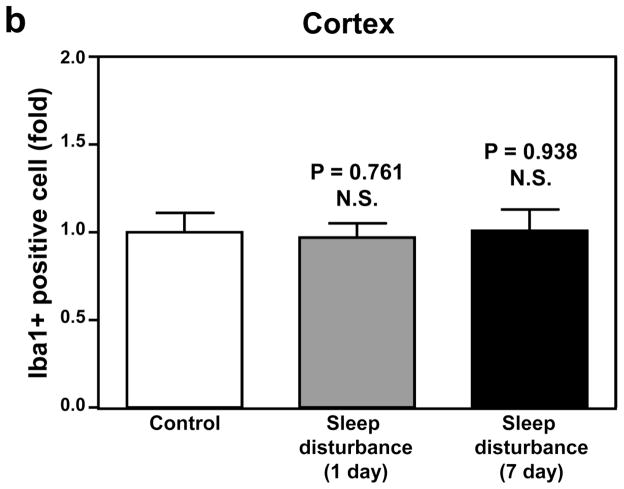
Finally, immunohistochemistry staining with Iba-1 showed that 24 hours of sleep disturbance did not induce microglia activation in the mouse cortex as compared to the control condition one and 7 days post-sleep disturbance (Figure 6). Taken together, these results suggest that sleep disturbance can induce microglia activation in mouse brain tissues. Moreover, these findings suggest that sleep disturbance can specifically induce microglia activation in the mouse hippocampus but not the mouse cortex, which is consistent with the findings that sleep disturbance selectively increases pro-inflammatory cytokine levels in the hippocampus and impairs hippocampus-dependent learning and memory function. Collectively, these findings suggest that sleep disturbance can induce neuroinflammation in the hippocampus and impair hippocampus-dependent learning and memory, and moreover that sleep disturbance may induce neither neuroinflammation in the cortex nor hippocampus-independent learning and memory impairment (Figure 7).
Figure 6. Sleep disturbance does not induce microglia activation in the mouse cortex.
a. Twenty-four hours of sleep disturbance does not increase the number of Iba-1 positive cells in the mouse cortex in immunohistochemistry staining as compared to the control condition one and 7 days post-sleep disturbance. b. Quantification of the immunohistochemistry staining shows that the sleep disturbance does not increase the number of Iba-1 positive cells in the mouse cortex as compared to the control condition one and 7 days post-sleep disturbance. N = 6.
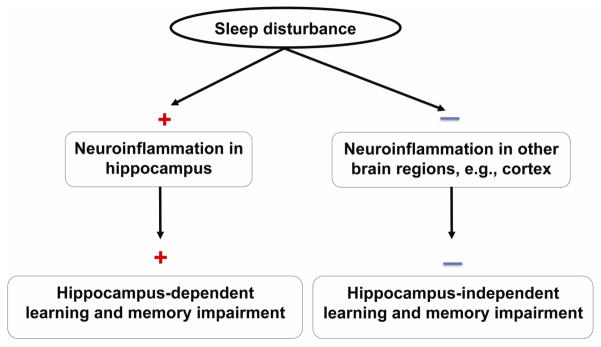
Figure 7. Hypothesized model of sleep disturbance on learning and memory.
Sleep disturbance induces neuroinflammation in the mouse hippocampus, but not other brain regions, e.g., cortex, and hippocampus-dependent, but not hippocampus-independent, learning and memory impairment in mice.
Discussion
Patients in the hospital, and especially the critical care unit, often experience sleep disturbance [(Feeley and Gardner, 2006), reviewed in (Tembo and Parker, 2009)] and develop cognitive function decline [reviewed in (Gordon et al., 2004)]. We therefore established a sleep disturbance model with mice to assess the effects of sleep disturbance on mouse learning and memory. We used rocking cages and rolling balls inside these cages to set up an animal model of sleep disturbance in mice. The mice may have a mixture of sleep and sleep deprivation during the disturbance period, but this sleep disturbance setting resembles clinical conditions in which patients usually have the combination of sleep and sleep deprivation.
First, we found that 24 hours of sleep disturbance reduced the amount of sleep time (both REM and NREM sleep) in mice (Figure 1). The, we were able to show that the sleep disturbance decreased the freezing time in the context test, but not tone test, of the FCT. Although none of the animal behavior studies can be directly applied to human learning and memory, the FCT is among the most commonly used behavioral tests to detect learning and memory impairment induced by anesthesia (Saab et al., 2010; Satomoto et al., 2009; Zhang et al., 2012) and surgery (Cibelli et al., 2010; Terrando et al., 2010). The context and tone test of the FCT can be used to assess hippocampus-dependent and hippocampus-independent learning and memory, respectively (Anagnostaras et al., 1999; Kim and Fanselow, 1992; Kitamura et al., 2009; Wiltgen et al., 2010). Therefore, the findings that sleep disturbance reduces freezing time in the context, but not tone, test of the FCT suggest that sleep disturbance may selectively induce hippocampus-dependent learning and memory impairment.
Other behavior tasks and procedures, including the Radial Maze (Etchamendy et al., 2003) and specific paradigms of contextual fear conditioning (Calandreau et al., 2006), have been suggested as traditional learning tasks known to be critically dependent on hippocampus integrity. We will either develop these tasks in our own laboratory or seek collaborations to use these tasks in our future studies to further investigate whether sleep disturbance can specifically impair hippocampus-dependent learning and memory.
The findings in our studies that sleep disturbance impaired learning and memory in mice are supported by the results from another study that sleep deprivation impaired memory consolidation (Graves et al., 2003). In this study, Graves et al. showed that sleep deprivation from 0–5 h after training for contextual and cued fear conditioning induced memory consolidation impairment for contextual fear conditioning (Graves et al., 2003). The studies by Graves et al., however, did not investigate the mechanisms underlying the sleep-deprivation induced learning and memory impairment, whereas the finding from our studies suggested for the first time that the sleep disturbance-induced neuroinflammation could be responsible for the learning and memory impairment associated with sleep disturbance, pending on further studies. In addition, the studies by Graves et al. used a traditional method (hand touch on animals) to cause sleep deprivation in mice, whereas we developed a new method of sleep disturbance in mice, rocking cages with rolling balls, in the current studies.
Consistent with the results that the sleep disturbance induced hippocampus-dependent, but not hippocampus-independent, learning and memory impairment, we were able to show that the sleep disturbance increased levels of pro-inflammatory cytokines (IL-6) and induced microglia activation in the mouse hippocampus, but not in the mouse cortex. Neuroinflammation may induce learning and memory impairment in animals (Cibelli et al., 2010; Nguyen et al., 1998; O’Connor et al., 2003; Terrando et al., 2010; Wan et al., 2007; Wan et al., 2010). Microglia activation is pivotal for the mediation of these behavioral abnormalities (van Gool et al., 2010). Pro-inflammatory cytokines induce microglia activation in discrete brain regions, leading to the production of the same pro-inflammatory cytokines (Buttini and Boddeke, 1995; Maier et al., 1998; Quan et al., 1998; Rivest, 2003). These pro-inflammatory cytokines can induce neurobehavioral deficits (Barrientos et al., 2006; Liu et al., 2007; Schmidt et al., 2006; Sparkman et al., 2006; Sparkman et al., 2005; Wan et al., 2007; Weaver et al., 2002; Yirmiya et al., 2002). Findings from the current studies that sleep disturbance increases IL-6 levels and induces microglia activation in the hippocampus, but not in the cortex, suggest that sleep disturbance may selectively impair hippocampus-dependent learning and memory through neuroinflammation in the hippocampus.
The mechanisms by which sleep disturbance selectively induces neuroinflammation in the hippocampus remain to be determined. Other studies have shown that high-cholesterol diets can induce neuroinflammation in the hippocampus, but not in the cortex (Xue et al., 2007), and that Paraquat, a herbicide which can cause neurotoxicty, can induce different brain region expression of pro-inflammatory cytokines and neuroinflammation in the hippocampus (Mitra et al., 2011). These findings, together with the data from our current study, suggest that the hippocampus could be more susceptible to inflammation insult. We therefore postulate that the hippocampus blood brain barrier is more permeable, which allows easier entrance of blood pro-inflammatory cytokines to the hippocampus. Future studies to test this hypothesis are warranted.
It has been reported that sleep deprivation can impair learning and memory by decreasing extracellular signal regulated kinase phosphorylation in the hippocampus (Guan et al., 2004) and by activating cAMP-response element binding protein phosphorylation in the hippocampus (Zhao et al., 2010). Future studies should include the assessment of the potential association of neuroinflammation in the hippocampus and phosphorylation of kinase and cAMP-response element binding protein to further demonstrate the underlying mechanisms by which sleep disturbance impairs learning and memory. These studies should also include determining whether sleep disturbance-induced hippocampus inflammation can impair learning and memory function by negatively affecting synapse function in the hippocampus.
The studies have several limitations. First, we did not assess whether sleep disturbance can induce a long term cognitive function decline, e.g., 60 days post-sleep disturbance. However, even short-term cognitive function decline can still negatively affect the functioning of patients after leaving the hospital, such as taking medications, providing self-care, and so forth, which could ultimately cause other adverse health outcomes (Leung and Sands, 2009). Second, we only assessed the effects of sleep disturbance on inflammation in the hippocampus and cortex. Future studies should determine whether sleep disturbance could also induce inflammation in other brain regions, e.g., the amygdala. Nevertheless, the current findings suggest that sleep disturbance can induce hippocampus inflammation and hippocampus-dependent learning and memory impairment (Figure 7).
Conclusion
In conclusion, we have established an animal model of sleep disturbance in mice and have found that 24 hours of sleep disturbance increases pro-inflammatory cytokines IL-6 levels in the mouse hippocampus, but not in the mouse cortex. Sleep disturbance also induces microglia activation in the mouse hippocampus, but not in the mouse cortex. Finally, sleep disturbance impairs hippocampus-dependent learning and memory. These findings would promote more studies to further determine the role of sleep disturbance in cognitive function decline in hospitalized patients, especially those in critical care units. These studies may include investigation of the underlying mechanism by which sleep disturbance induces neuroinflammation, and whether anti-inflammatory treatments, e.g., non-steroidal anti-inflammatory drugs, may attenuate the sleep disturbance-induced neuroinflammation and impairment of learning and memory in animals, and sleep disturbance-associated cognitive function decline in humans.
Highlights.
Sleep disturbance causes impairment in performance of hippocampus-dependent tasks in mice.
Sleep disturbance increases levels of pro-inflammatory cytokine IL-6 in the mouse hippocampus.
Sleep disturbance induces microglia activation in the mouse hippocampus.
Acknowledgments
This research was supported by R21AG029856, R21AG038994 and R01 GM088801 (National Institutes of Health), Cure Alzheimer’s Fund, USA (to Z.X.).
Abbreviation
- AD
Alzheimer’s disease
- IL
Interleukin
- MCI
Mild cognitive impairment
- WT
Wild type
- FCT
Fear conditioning test
- EEG
Electroencephalogram
- EMG
Electromyography
- REM
rapid eye movement
Footnotes
Conflict of interests: The authors deny any conflict of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aleisa AM, et al. Post-learning REM sleep deprivation impairs long-term memory: reversal by acute nicotine treatment. Neurosci Lett. 2011;499:28–31. doi: 10.1016/j.neulet.2011.05.025. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, et al. Temporally graded retrograde amnesia of contextual fear after hippocampal damage in rats: within-subjects examination. J Neurosci. 1999;19:1106–14. doi: 10.1523/JNEUROSCI.19-03-01106.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurell J, Elmqvist D. Sleep in the surgical intensive care unit: continuous polygraphic recording of sleep in nine patients receiving postoperative care. Br Med J (Clin Res Ed) 1985;290:1029–32. doi: 10.1136/bmj.290.6474.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, et al. Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiol Aging. 2006;27:723–32. doi: 10.1016/j.neurobiolaging.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Buttini M, Boddeke H. Peripheral lipopolysaccharide stimulation induces interleukin-1 beta messenger RNA in rat brain microglial cells. Neuroscience. 1995;65:523–30. doi: 10.1016/0306-4522(94)00525-a. [DOI] [PubMed] [Google Scholar]
- Calandreau L, et al. Extracellular hippocampal acetylcholine level controls amygdala function and promotes adaptive conditioned emotional response. J Neurosci. 2006;26:13556–66. doi: 10.1523/JNEUROSCI.3713-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cibelli M, et al. Role of interleukin-1beta in postoperative cognitive dysfunction. Ann Neurol. 2010;68:360–8. doi: 10.1002/ana.22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond SP, Brown GG. The effects of total sleep deprivation on cerebral responses to cognitive performance. Neuropsychopharmacology. 2001;25:S68–73. doi: 10.1016/S0893-133X(01)00325-6. [DOI] [PubMed] [Google Scholar]
- Ehlenbach WJ, et al. Association between acute care and critical illness hospitalization and cognitive function in older adults. JAMA. 2010;303:763–70. doi: 10.1001/jama.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchamendy N, et al. Hippocampal lesions and discrimination performance of mice in the radial maze: sparing or impairment depending on the representational demands of the task. Hippocampus. 2003;13:197–211. doi: 10.1002/hipo.10055. [DOI] [PubMed] [Google Scholar]
- Feeley K, Gardner A. Sedation and analgesia management for mechanically ventilated adults: literature review, case study and recommendations for practice. Aust Crit Care. 2006;19:73–7. doi: 10.1016/s1036-7314(06)80012-3. [DOI] [PubMed] [Google Scholar]
- Friese RS, et al. Quantity and quality of sleep in the surgical intensive care unit: are our patients sleeping? J Trauma. 2007;63:1210–4. doi: 10.1097/TA.0b013e31815b83d7. [DOI] [PubMed] [Google Scholar]
- Gordon SM, et al. Clinical identification of cognitive impairment in ICU survivors: insights for intensivists. Intensive Care Med. 2004;30:1997–2008. doi: 10.1007/s00134-004-2418-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves LA, et al. Sleep deprivation selectively impairs memory consolidation for contextual fear conditioning. Learn Mem. 2003;10:168–76. doi: 10.1101/lm.48803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Z, et al. Sleep deprivation impairs spatial memory and decreases extracellular signal-regulated kinase phosphorylation in the hippocampus. Brain Res. 2004;1018:38–47. doi: 10.1016/j.brainres.2004.05.032. [DOI] [PubMed] [Google Scholar]
- Hardin KA. Sleep in the ICU: potential mechanisms and clinical implications. Chest. 2009;136:284–94. doi: 10.1378/chest.08-1546. [DOI] [PubMed] [Google Scholar]
- Hudetz JA, et al. Elevated postoperative inflammatory biomarkers are associated with short- and medium-term cognitive dysfunction after coronary artery surgery. J Anesth. 2010 doi: 10.1007/s00540-010-1042-y. [DOI] [PubMed] [Google Scholar]
- Kalman J, et al. Elevated levels of inflammatory biomarkers in the cerebrospinal fluid after coronary artery bypass surgery are predictors of cognitive decline. Neurochem Int. 2006;48:177–80. doi: 10.1016/j.neuint.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–7. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Kitamura T, et al. Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory. Cell. 2009;139:814–27. doi: 10.1016/j.cell.2009.10.020. [DOI] [PubMed] [Google Scholar]
- Leung JM, Sands LP. Long-term cognitive decline: is there a link to surgery and anesthesia? Anesthesiology. 2009;111:931–2. doi: 10.1097/ALN.0b013e3181bc988f. [DOI] [PubMed] [Google Scholar]
- Liu Z, et al. Chronic treatment with minocycline preserves adult new neurons and reduces functional impairment after focal cerebral ischemia. Stroke. 2007;38:146–52. doi: 10.1161/01.STR.0000251791.64910.cd. [DOI] [PubMed] [Google Scholar]
- Lu J, et al. Selective activation of the extended ventrolateral preoptic nucleus during rapid eye movement sleep. J Neurosci. 2002;22:4568–76. doi: 10.1523/JNEUROSCI.22-11-04568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF, et al. The role of the vagus nerve in cytokine-to-brain communication. Ann N Y Acad Sci. 1998;840:289–300. doi: 10.1111/j.1749-6632.1998.tb09569.x. [DOI] [PubMed] [Google Scholar]
- Maquet P. The role of sleep in learning and memory. Science. 2001;294:1048–52. doi: 10.1126/science.1062856. [DOI] [PubMed] [Google Scholar]
- Mitra S, et al. Differential regional expression patterns of alpha-synuclein, TNF-alpha, and IL-1beta; and variable status of dopaminergic neurotoxicity in mouse brain after Paraquat treatment. J Neuroinflammation. 2011;8:163. doi: 10.1186/1742-2094-8-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KT, et al. Exposure to acute stress induces brain interleukin-1beta protein in the rat. J Neurosci. 1998;18:2239–46. doi: 10.1523/JNEUROSCI.18-06-02239.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor KA, et al. Peripheral and central proinflammatory cytokine response to a severe acute stressor. Brain Res. 2003;991:123–32. doi: 10.1016/j.brainres.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Patanella AK, et al. Correlations between peripheral blood mononuclear cell production of BDNF, TNF-alpha, IL-6, IL-10 and cognitive performances in multiple sclerosis patients. J Neurosci Res. 2010;88:1106–12. doi: 10.1002/jnr.22276. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. Conditional Reflexes. New York: Dover Publications; 1927/1960. [Google Scholar]
- Perry VH. The influence of systemic inflammation on inflammation in the brain: implications for chronic neurodegenerative disease. Brain Behav Immun. 2004;18:407–13. doi: 10.1016/j.bbi.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Quan N, et al. Time course and localization patterns of interleukin-1beta messenger RNA expression in brain and pituitary after peripheral administration of lipopolysaccharide. Neuroscience. 1998;83:281–93. doi: 10.1016/s0306-4522(97)00350-3. [DOI] [PubMed] [Google Scholar]
- Ramlawi B, et al. C-Reactive protein and inflammatory response associated to neurocognitive decline following cardiac surgery. Surgery. 2006a;140:221–6. doi: 10.1016/j.surg.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Ramlawi B, et al. Serologic markers of brain injury and cognitive function after cardiopulmonary bypass. Ann Surg. 2006b;244:593–601. doi: 10.1097/01.sla.0000239087.00826.b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivest S. Molecular insights on the cerebral innate immune system. Brain Behav Immun. 2003;17:13–9. doi: 10.1016/s0889-1591(02)00055-7. [DOI] [PubMed] [Google Scholar]
- Rudolph JL, et al. Chemokines are associated with delirium after cardiac surgery. J Gerontol A Biol Sci Med Sci. 2008;63:184–9. doi: 10.1093/gerona/63.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saab BJ, et al. Short-term memory impairment after isoflurane in mice is prevented by the alpha5 gamma-aminobutyric acid type A receptor inverse agonist L-655,708. Anesthesiology. 2010;113:1061–71. doi: 10.1097/ALN.0b013e3181f56228. [DOI] [PubMed] [Google Scholar]
- Satomoto M, et al. Neonatal exposure to sevoflurane induces abnormal social behaviors and deficits in fear conditioning in mice. Anesthesiology. 2009;110:628–37. doi: 10.1097/ALN.0b013e3181974fa2. [DOI] [PubMed] [Google Scholar]
- Schmidt H, et al. Neuropsychological sequelae of bacterial and viral meningitis. Brain. 2006;129:333–45. doi: 10.1093/brain/awh711. [DOI] [PubMed] [Google Scholar]
- Schuitemaker A, et al. Inflammatory markers in AD and MCI patients with different biomarker profiles. Neurobiol Aging. 2009;30:1885–9. doi: 10.1016/j.neurobiolaging.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Sparkman NL, et al. Interleukin-6 facilitates lipopolysaccharide-induced disruption in working memory and expression of other proinflammatory cytokines in hippocampal neuronal cell layers. J Neurosci. 2006;26:10709–16. doi: 10.1523/JNEUROSCI.3376-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkman NL, et al. Bacterial endotoxin-induced behavioral alterations in two variations of the Morris water maze. Physiol Behav. 2005;86:244–51. doi: 10.1016/j.physbeh.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Stickgold R, et al. Sleep, learning, and dreams: off-line memory reprocessing. Science. 2001;294:1052–7. doi: 10.1126/science.1063530. [DOI] [PubMed] [Google Scholar]
- Teeling JL, Perry VH. Systemic infection and inflammation in acute CNS injury and chronic neurodegeneration: underlying mechanisms. Neuroscience. 2009;158:1062–73. doi: 10.1016/j.neuroscience.2008.07.031. [DOI] [PubMed] [Google Scholar]
- Tembo AC, Parker V. Factors that impact on sleep in intensive care patients. Intensive Crit Care Nurs. 2009;25:314–22. doi: 10.1016/j.iccn.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Terrando N, et al. Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline. Proc Natl Acad Sci U S A. 2010;107:20518–22. doi: 10.1073/pnas.1014557107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gool WA, et al. Systemic infection and delirium: when cytokines and acetylcholine collide. Lancet. 2010;375:773–5. doi: 10.1016/S0140-6736(09)61158-2. [DOI] [PubMed] [Google Scholar]
- Wan Y, et al. Postoperative impairment of cognitive function in rats: a possible role for cytokine-mediated inflammation in the hippocampus. Anesthesiology. 2007;106:436–43. doi: 10.1097/00000542-200703000-00007. [DOI] [PubMed] [Google Scholar]
- Wan Y, et al. Cognitive decline following major surgery is associated with gliosis, beta-amyloid accumulation, and tau phosphorylation in old mice. Crit Care Med. 2010 doi: 10.1097/CCM.0b013e3181f17bcb. [DOI] [PubMed] [Google Scholar]
- Weaver JD, et al. Interleukin-6 and risk of cognitive decline: MacArthur studies of successful aging. Neurology. 2002;59:371–8. doi: 10.1212/wnl.59.3.371. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, et al. Cytokines and cognition--the case for a head-to-toe inflammatory paradigm. J Am Geriatr Soc. 2002;50:2041–56. doi: 10.1046/j.1532-5415.2002.50619.x. [DOI] [PubMed] [Google Scholar]
- Wiltgen BJ, et al. The hippocampus plays a selective role in the retrieval of detailed contextual memories. Curr Biol. 2010;20:1336–44. doi: 10.1016/j.cub.2010.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, et al. The common inhalation anesthetic isoflurane induces caspase activation and increases amyloid beta-protein level in vivo. Ann Neurol. 2008;64:618–27. doi: 10.1002/ana.21548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu ZQ, et al. The mechanism and characterization of learning and memory impairment in sleep-deprived mice. Cell Biochem Biophys. 2010;58:137–40. doi: 10.1007/s12013-010-9098-8. [DOI] [PubMed] [Google Scholar]
- Xue QS, et al. Microglial activation in the hippocampus of hypercholesterolemic rabbits occurs independent of increased amyloid production. J Neuroinflammation. 2007;4:20. doi: 10.1186/1742-2094-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya R, et al. Brain interleukin-1 is involved in spatial memory and passive avoidance conditioning. Neurobiol Learn Mem. 2002;78:379–89. doi: 10.1006/nlme.2002.4072. [DOI] [PubMed] [Google Scholar]
- Zhang Y, et al. Anesthetics isoflurane and desflurane differently affect mitochondrial function, learning, and memory. Ann Neurol. 2012;71:687–98. doi: 10.1002/ana.23536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, et al. Neuropeptide S mitigates spatial memory impairment induced by rapid eye movement sleep deprivation in rats. Neuroreport. 2010;21:623–8. doi: 10.1097/WNR.0b013e328339b5f9. [DOI] [PubMed] [Google Scholar]