Abstract
AIMS
To develop and empirically validate a mathematical model for identifying new cannabis use in chronic, daily cannabis smokers.
DESIGN
Models were based on urinary creatinine-normalized (CN) cannabinoid excretion in chronic cannabis smokers.
SETTING
For model development, participants resided on a secure research unit for 30 days. For model validation, participants were abstinent with daily observed urine specimens for 28 days.
PARTICIPANTS
48 (model development) and 67 (model validation) daily cannabis smokers were recruited.
MEASUREMENTS
All voided urine was collected and analyzed for 11-nor-9-carboxy-Δ9-tetrahydrocannabinol (THCCOOH) by gas chromatography-mass spectrometry (GCMS, limit of quantification 2.5 ng/mL) and creatinine (mg/mL). Urine THCCOOH was normalized to creatinine, yielding ng/mg CN-THCCOOH concentrations. Urine concentration ratios were determined from 123,513 specimen pairs collected 2–30 days apart.
FINDINGS
A mono-exponential model (with two parameters, initial urine specimen CN-THCCOOH concentration and time between specimens), based on the Marquardt-Levenberg algorithm, provided a reasonable data fit. Prediction intervals with varying probability levels (80, 90, 95, 99%) provide upper ratio limits for each urine specimen pair. Ratios above these limits suggest cannabis re-use. Disproportionate numbers of ratios were higher than expected for some participants, prompting development of two additional rules that avoid misidentification of re-use in participants with unusual CN-THCCOOH excretion patterns.
CONCLUSIONS
For the first time, a validated model is available to aid in the differentiation of new cannabis use from residual CN-THCCOOH excretion in chronic, daily cannabis users. These models are valuable for clinicians, toxicologists, drug treatment staff, and workplace, military and criminal justice drug testing programs.
Keywords: urine cannabinoids, new cannabis use, residual drug excretion, predictive model
Introduction
Cannabis (marijuana, hashish) is the most widely used illegal drug in the world (1). Detection of its use is an important aspect of drug monitoring in many contexts. The primary psychoactive component in cannabis is Δ9-tetrahydrocannabinol (THC), which is rapidly metabolized to equipotent 11-hydroxy-THC (11-OH-THC) and non-psychoactive 11-nor-9-carboxy- Δ9-tetrahydrocannabinol (THCCOOH). THCCOOH glucuronide is the most prevalent urinary cannabis biomarker and the most commonly detected analyte in treatment, workplace, military, and criminal justice drug testing programs. A positive urine cannabinoid test may trigger discharge from drug treatment, loss of employment or child custody, or incarceration. In military courts martial, punishment may be more severe if multiple cannabis uses are established (2). However, because of their high retention in tissues, cannabinoids may be detected in urine for days or weeks after last use, depending upon frequency and chronicity of exposure (3–7). Thus, accurately distinguishing new cannabis use from residual cannabinoid excretion is necessary. We previously published models for predicting new cannabis use after less than daily use (2, 8), but to date, there are no guidelines for identifying new cannabis use following chronic, daily exposure.
In occasional cannabis smokers, mean peak urinary THCCOOH concentrations occurred 10–18 hours after a single 3.55% THC cigarette and remained >15 ng/mL for 80–100 hours (7). Initial concentrations decreased rapidly, with more gradual decreases over several days (7). Normalizing THCCOOH concentrations to urinary creatinine helps control for individual differences in hydration and urine output (9).
After chronic, daily cannabis smoking, THCCOOH detection times in urine may be up to 30 days by gas chromatography mass spectrometry (GCMS) with a limit of quantification (LOQ) of 2.5 ng/mL (4) and up to 67 (10) and 93 days (9) with a 20 ng/mL immunoassay cutoff. THC accumulates in tissues during chronic, daily cannabis smoking due to its high lipophilicity and large volume of distribution. These long detection times are due to slow release of stored THC from tissues back into blood (11), and subsequent metabolism to THCCOOH.
Our previous study of controlled THC administration to less than daily cannabis smokers (8) demonstrated that the ratio of creatinine-normalized (CN) THCCOOH concentrations in urine specimens collected ≥24h apart was useful in distinguishing new cannabis use from residual excretion. A 0.5 ratio of the later to earlier CN-normalized specimen was 85.4% accurate, with 7.4% false negatives (missed relapses) and 5.6% false positives (suggested new cannabis use when actually residual drug excretion) (8). A ratio of 1.5 (12) was only 74.2% accurate, with 0.1% false positives and 24% false negatives (8).
These ratios are widely employed to predict whether new cannabis use has occurred in the intervening time between two urine collections from less than daily cannabis users. Prediction accuracy improved when the time between urine specimens was taken into account (2). Some investigators also applied the technique to chronic cannabis smokers in an uncontrolled setting when THCCOOH concentrations were greater than 20–50 ng/mL (13–15). However, model applicability to urine specimens from chronic, daily cannabis users has not been rigorously evaluated, and to date, empirically derived ratios from this population while under continuous monitored abstinence have not been determined.
This investigation reports empirically derived statistical models for differentiating residual cannabinoid excretion from new cannabis use in daily cannabis smokers. Urinary CN-THCCOOH excretion in chronic daily cannabis users residing on a closed research unit under continuous medical surveillance for up to 30 days were utilized to develop the models, which were then validated in a separate sample. Formulas were derived from urine CN-THCCOOH concentration ratios associated with specimen pairs collected 2–30 days apart. Models were validated with urine specimens collected during outpatient drug monitoring, and rules were developed to account for unusual cannabinoid excretion profiles.
Methods
Participants and urine specimens
Model development data were obtained from daily cannabis smokers with positive cannabinoid immunoassay results greater than 100 ng/mL THCCOOH equivalents upon entry into inpatient research protocols approved by the National Institute on Drug Abuse (NIDA) Institutional Review Board. Participants resided continuously on the closed clinical research unit for up to 30 days. Participants’ physical and psychological health was verified by medical history and physical examination, psychological tests, ECG, CBC and blood chemistries, and urinalysis. All belongings were searched prior to unit entry and no visitors were allowed. All voided urine was collected ad libitum and analyzed for THCCOOH by GCMS following alkaline hydrolysis (4) with a 2.5 ng/mL LOQ, with concentrations normalized to urine creatinine determined by a modified Jaffe method.
Models were validated with specimens from a separate group of long-term daily cannabis smokers (reporting ≥5000 lifetime episodes of cannabis use) who were engaged in an outpatient research study evaluating neurocognitive performance during extended drug abstinence (16). This study was approved by the McLean Hospital and NIDA IRBs; written informed consent was obtained from all participants after a complete description of the study. These participants agreed to maintain cannabis abstinence for 28 days, with compliance evaluated by urine CN-THCCOOH concentrations in daily urine specimens collected under direct observation. The ratio of Specimen 2/Specimen 1 normalized concentrations was determined; a 50% increase (ratio of 1.5) compared to the preceding concentration indicated new cannabis use. Participants were not currently taking psychoactive medications and had ≤100 lifetime cocaine, stimulant, opioid, sedative-hypnotic, hallucinogen and inhalant uses.
Model Development
CN-THCCOOH ratios (Specimen 2/Specimen 1) were calculated for all specimen pairs collected ≥48h apart. For each participant, all concentrations were compared to every other specimen collected 2–30 days later. CN-THCCOOH concentrations were determined by dividing urinary THCCOOH concentrations (ng/mL) by urinary creatinine concentrations (mg/mL) to yield ng cannabinoids/mg creatinine.
Data were sorted by Specimen 1 concentrations into eight groups: 6–14.9, 15–24.9, 25–49.9, 50–99.9, 100–199.9, 200–399.9, 400–599.9 and ≥600 ng/mg. Specimens containing CN-THCCOOH <6 ng/mg were excluded because 6 ng/mL (40% of the 15 ng/mL positive confirmation cutoff) is the laboratory LOQ required by the Substance Abuse Mental Health Services (SAMHSA) Mandatory Guidelines for federally mandated urine testing (17). Because there are no mandated creatinine-normalized thresholds, we developed models for 15 and 6 ng/mg.
Empirical models were developed to describe the relationship between ratios and time between specimens, providing an expected ratio given a specific time between specimen collections. A unique model was developed for each Specimen 1 concentration group. Details of model development and evaluation can be found in supplemental data. Prediction intervals, with varying levels of certainty (80, 90, 95 and 99%), were calculated for each model, providing upper ratio limits for each urine specimen pair. As these limits were derived from urine excretion data during monitored abstinence, ratios above these limits suggest relapse to cannabis use. For an 80% prediction interval, 10% of ratios would be expected to fall below and 10% above the expected range. Therefore, the expected proportions falling above the upper limit for 80%, 90%, 95% and 99% prediction intervals were 10%, 5%, 2.5% and 0.5%, respectively. The upper prediction interval limits provide probabilities that a single observed ratio would exceed these upper limits and be falsely interpreted as re-use.
Upper 80%, 90%, 95% and 99% prediction intervals were developed with non- linear least squares regression employing the Marquardt-Levenberg algorithm (18).
| Eq.1 |
Where:
Upper PI Limit = maximum ratio, above which new cannabis use is predicted
A = linear model parameter
k = non-linear model parameter
t = time between samples
Z1-α/2 = standard normal value (1.28, 1.64, 1.96, 2.57) corresponding to the selected prediction interval (80, 90, 95 or 99%)
S2Model = the variance associated with the fitted ratio estimates
RMS = the residual mean square (variance) of the actual data used for the best fit line
First, we randomly divided each Specimen 1 group data set in half. One half was used for model development and the other half for model evaluation (see supplemental data). From the full data set for each group, mono-exponential models were developed for the same four prediction interval probabilities. This provided a higher N from which model parameters could be estimated. Final model parameters based on all the urine pairs from participants residing on the closed clinical research unit are reported in Table 1. Ratios from the validation group (outpatients) were then compared to these limits to determine the percentage of ratios that exceeded the upper prediction interval limit (new cannabis use). Model parameters associated with the appropriate Specimen 1 concentration group and times between specimens were entered into equation 1. If the observed concentration ratio was greater than the predicted ratio, there is a strong likelihood (80, 90, 95 or 99% probability) that cannabis re-use has occurred. To address unusual urinary excretion patterns, we also developed two decision rules to improve model prediction accuracy that are included in the results (pages 12–13).
Table 1.
New cannabis use model parameters developed with all urine creatinine normalized (CN)-11-nor-9-carboxy-Δ9-tetrahydrocannabinol (THCCOOH) excretion data from the model development group of 48 chronic heavy cannabis users during up to 30 days abstinence on a closed research unit. Specimen 1 groups are based on CN-THCCOOH urine concentrations.
| Specimen 1 CN-THCCOOH concentration (ng/mg) | N | Model Parameter | ||
|---|---|---|---|---|
| A | k | RMS | ||
| 6–14.9 | 3,317 | 1.244 | 0.00160 | 0.513 |
| 15–24.9 | 7,150 | 0.994 | 0.00087 | 0.282 |
| 25–49.9 | 21,790 | 1.018 | 0.00131 | 0.264 |
| 50–99.9 | 49,052 | 0.891 | 0.00086 | 0.174 |
| 100–199.9 | 29,695 | 0.664 | 0.00129 | 0.064 |
| 200–399.9 | 10,488 | 0.384 | 0.00144 | 0.014 |
| 400–599.9 | 1,066 | 0.213 | 0.00188 | 0.003 |
| 600–1166 | 955 | 0.212 | 0.00342 | 0.001 |
N = number of paired urine CN-THCCOOH concentration ratios
RMS = Residual mean square
A = Linear parameter in exponential model
k = Nonlinear parameter in exponential model
Results
Participants
Forty-eight daily cannabis users (ages 20–38, 75% male, 83% African-American) provided data for model development: 37 reported median (range) cannabis use of 8 (1–40) joints or blunts/day, seven of $20 ($10–150)/day, and three of 1 oz/day (0.5–1). Drug use data was not available for one participant. These participants provided 2,377 urine specimens from which 123,513 ratios were derived. The median number of urine specimens and ratios per participant were 27 (4–243) and 151 (3–32,260), respectively. The number of ratios for model development varied by Specimen 1 concentration group (supplemental Table 1). Initial specimen CN-THCCOOH concentrations were 16–1166 ng/mg.
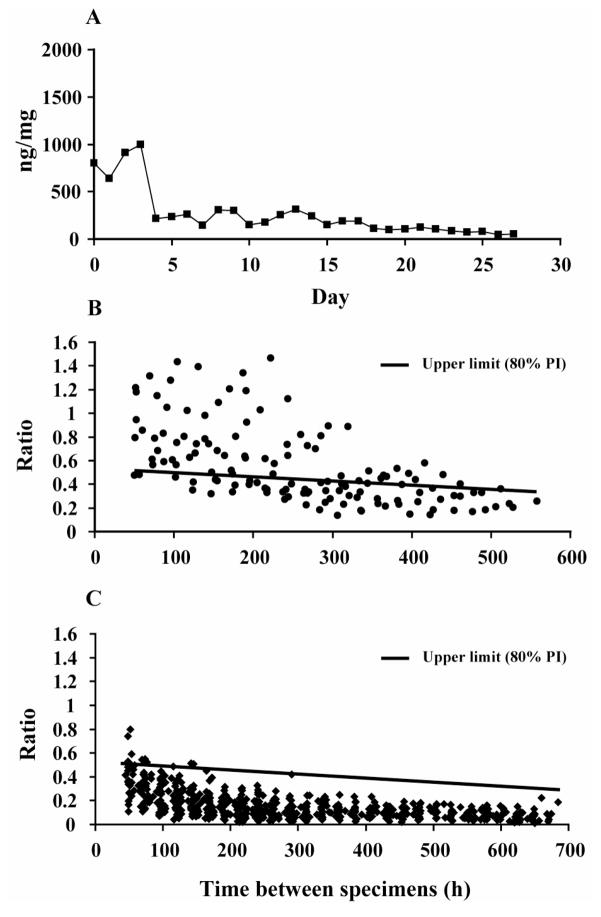
Sixty-eight participants (aged 32–41, 87% male, 86% Caucasian) provided 1,100 urine specimens (9,315 ratios) for model validation. One participant in this outpatient validation group had an initial CN-THCCOOH concentration of 803ng/mg and was suspected of cannabis use during study participation because of significant concentration increases observed on multiple days (Figure 1). Calculations do not include data (339 ratios total) from this participant.
Figure 1.
Model development
The mono-exponential model was selected primarily as an empirically convenient non-linear model based on its simplicity (only two parameters), general familiarity, and applicability to obviously non-linear data. There was no theoretical basis or biological significance assumed to be associated with the parameters. The models did not provide ideal fit for all data sets. There was considerable variability associated with modeling ratios with time between specimens, most of which is probably of biological rather than analytical origin. Nevertheless, for the validation half of the split data set, Specimen 1 concentration groups closely approximated their expected ratio percentages above the upper prediction interval limit (supplemental data, Table 1).
Model Validation
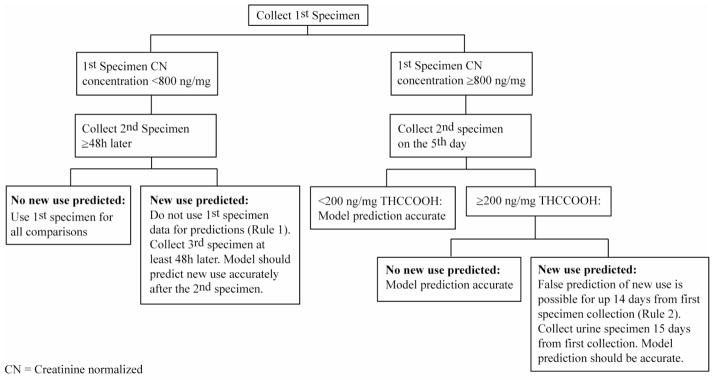
Models based on all urine pairs from participants residing on the closed clinical research unit associated with Specimen 1 concentrations 15–199.9 ng/mg performed well with urine pairs from the validation group, with re-use predictions closely approximating the a priori probability rates (Table 2). Higher than expected %re-use rates did occur in two infrequent circumstances. In 6 (9%) participants from the validation group, the first specimen CN THCCOOH concentration was lower than in specimens collected up to 40h later, due to last cannabis smoking close to the time of first urine specimen collection. Median CN–THCCOOH concentrations increased 83 (9–226) ng/mg and times to peak were 23–40h after admission. Such an increase (rather than decrease) in CN-THCCOOH concentration after initial Specimen 1 collection may result in a false prediction of new cannabis use. To account for this situation, we developed Rule 1 (Figure 2): If cannabis re-use is predicted by the first and second specimens collected, it is possible that last cannabis use was recent and peak urine THCCOOH excretion may not yet have occurred. In this case, do not use the first specimen for prediction, but use another specimen collected at least 48 hours later.
Table 2.
Model validation with urine collected from 67 outpatient cannabis smokers initiating cannabis abstinence. Specimen 1 groups are based on urinary creatinine-normalized (CN)-11-nor-9-carboxy-Δ9-tetrahydrocannabinol (THCCOOH) concentrations. Data are %reuse predictions exceeding the upper prediction limits (80, 90, 95 and 99% probabilities) and therefore, are suggestive of new cannabis use. Expected %re-use prediction rates are 10, 5, 2.5 and 0.5%, respectively. Abstinence was closely monitored with daily observed urine specimens, A includes all data; B includes all data after application of Rules 1 and 2 (see text).
| Specimen 1 CN-THCCOOH concentration (ng/mg) | A | B | ||||||
|---|---|---|---|---|---|---|---|---|
| 80% | 90% | 95% | 99% | 80% | 90% | 95% | 99% | |
| 6–14.9 | 13.7 | 11.7 | 7.8 | 2.9 | 6.9 | 5.3 | 3.7 | 1.6 |
| 15–24.9 | 4.7 | 2.9 | 2.2 | 1.0 | 4.7 | 2.9 | 2.2 | 1.0 |
| 25–49.9 | 2.4 | 1.0 | 0.6 | 0.3 | 2.4 | 0.6 | 0.4 | 0.1 |
| 50–99.9 | 1.5 | 1.0 | 0.6 | 0.2 | 1.3 | 0.8 | 0.5 | 0.2 |
| 100–199.9 | 6.7 | 4.2 | 2.9 | 1.8 | 2.6 | 0.9 | 0.3 | 0.1 |
| 200–399.9 | 30.9 | 25.6 | 19.4 | 12.4 | 2.3 | 1.0 | 0.3 | 0.3 |
| 400–599.9 | 26.3 | 21.5 | 19.7 | 15.4 | 8.6 | 4.9 | 3.7 | 2.2 |
| 600–1166 | 36.1 | 32.7 | 29.5 | 24.3 | 5.4 | 3.8 | 3.3 | 1.1 |
Figure 2.
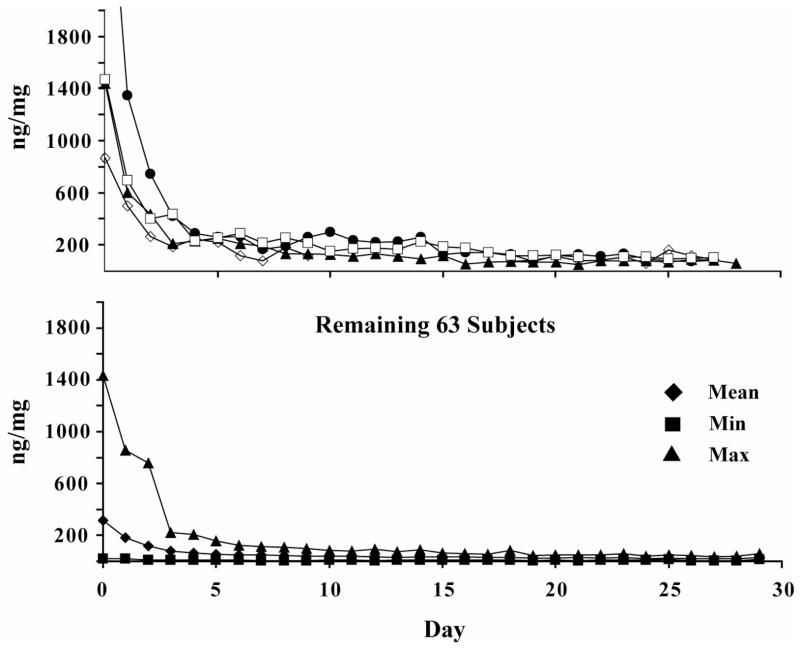
Another source of higher than expected %re-use rates was prolonged elevation of urinary CN-THCCOOH concentrations in 4 (6.0%) participants from the validation group with initial CN-THCCOOH concentrations >800 ng/mg, and remaining >200 ng/mg on day 5. Concentrations were elevated and variable (sometimes <200 ng/mg) for up to 14 days. To account for this unusual urinary excretion pattern, we devised Rule 2 (Figure 2): If urinary CN-THCCOOH concentration is ≥800 ng/mg in initial Specimen 1 (first day of monitoring) and is still ≥200 ng/mg on the 5th day, false predictions of new use may occur for up to 14 days. If concentrations exceed this level for more than 14 days and the model predicts new use, re-use is likely. Figure 3 illustrates differences in urinary CN-THCCOOH excretion patterns in these four individuals from the validation group compared to the remaining participants in the validation study.
Figure 3.
Table 2 includes the %ratios exceeding the prediction rates before (A) and after (B) application of rules 1 and 2 for specimens from the validation group. For rule 1, if the initial Specimen 1 CN-THCCOOH concentration was lower than the subsequent specimen, only ratios associated with the entry specimen were deleted from the data set. The proportions of ratios removed from Specimen 1 CN-THCCOOH concentration groups 6–14.9, 15–24.9, 25–49.9, 50–99.9, 100–199.9, 200–399.9, 400–599.9 and 600–1156 ng/mg were 8.3, 0, 0, 1.2, 2.1, 0, 3.3 and 5.9%, respectively. For rule 2, ratios associated with CN-THCCOOH concentration >800 ng/mg for specimen 1 and >200 ng/mg on day 5 from four participants for the first 14 days were deleted, while ratios associated with later specimens were included. The proportions of ratios removed from Specimen 1 CN-THCCOOH concentration groups 6–14.9, 15–24.9, 25–49.9, 50–99.9, 100–199.9, 200–399.9, 400–599.9 and 600–1156 ng/mg after applying rule 2, were 0, 0, 0, 8.5, 18.9, 41.1, 28.6 and 45.2%, respectively. Application of rules 1 and 2 improved the accuracy of model predictions for the validation group (Table 2B).
Discussion
This is the first investigation of urinary CN-THCCOOH excretion in daily, long-term cannabis smokers under controlled monitoring conditions, allowing probability estimates of new cannabis use between two specimens collected 48–720h apart. Time between specimen collections and Specimen 1 concentrations are two variables included in the models.
An example of how to use the model to identify new cannabis use in a chronic daily cannabis smoker follows. Two urine specimens collected from an individual 48–720h apart are analyzed for creatinine and THCCOOH to obtain CN-THCCOOH concentrations (ng/mg). The first specimen concentration determines the Specimen 1 group (and thus the appropriate model; see Table 1 for model parameters). Review of Specimen 1 and 2 CN-THCCOOH concentrations determines whether either decision rule applies. Rule #1 requires that the first specimen collected (<800 ng/mg) cannot be used for any prediction if new use is predicted from the first and second specimens. Rule #2 requires that a specimen be collected 5 days after the first specimen if Specimen 1 CN-THCCOOH concentration is ≥800 ng/mg. If the CN-THCCOOH concentration is greater than 200 ng/mg on day 5, no prediction of new use can be used until after 14 days. Decision rules must be followed for accurate predictions. If the decision rules are not applicable, the appropriate model parameters and time between specimens (h) are entered into the appropriate model. The resulting ratio is compared to the actual CN-THCCOOH ratio of the two specimens. If the actual ratio is > the model’s upper prediction limit, new cannabis use is predicted at a specific probability, depending upon the selected stringency level (80, 90, 95 or 99%).
Our new model differs in two important respects from previous models proposed for less than daily cannabis users (8, 12). First, those models recommended a single ratio for all specimen pairs, based on optimum sensitivity and specificity, regardless of Specimen 1 concentration or actual time between specimen collections. These approaches did not account for the persistence of low urinary CN-THCCOOH concentrations (<100 ng/mg) for several days, perhaps weeks, in some chronic daily cannabis users (2, 4, 5, 9, 10). Our model takes into account time between specimens and the CN-THCCOOH concentration in Specimen 1, and so more accurately predicts re-use in chronic, heavy cannabis users. Second, previous methods did not account for two infrequent (6–9% of validation sample) urinary CN-THCCOOH excretion patterns that may generate false new use identification. One pattern occurred when last cannabis use was close to collection of the first monitoring specimen and CN-THCCOOH concentrations had not yet peaked. A second pattern was high initial CN-THCCOOH concentrations (>800 ng/mg) followed by persistent high concentrations (>200 ng/mg) 5 days later, presumably due to high body stores of cannabinoids in chronic daily users. Two decision rules applied to these situations improved prediction accuracy in this population.
These new models substantially improve upon previous methods for identifying new cannabis use in daily cannabis smokers (8, 12). Applying a single 0.5 ratio to the data from our validation sample generated unrealistically high re-use rates when Specimen 1 concentrations were low (90.2, 83.9, 72.9, 45.9 and 33.2% for Specimen 1 groups 6–15, 15–25, 25–50, 50–100 and 100–200 ng/mg, respectively) and unrealistically low rates when Specimen 1 concentrations were high (20.7% and <4% for Specimen 1 groups 200–400 ng/mg and >400 ng/mg, respectively). Conversely, applying a single 1.5 ratio to these data generated unrealistically low new use rates when Specimen 1 concentrations were >50 ng/mg (e.g., <1% for Specimen 1 concentration groups >50 ng/mg and 0% for Specimen 1 concentration groups >200 ng/mg). Overall, these new models provide more accurate and uniform cannabis re-use predictions at all Specimen 1 concentrations and should be applied to participants self-reporting daily cannabis use. Previously reported models including the time between urine specimen collections (2) are available for less than daily cannabis smokers.
One limitation of this research is that the time of last cannabis use, prior to initiation of urine monitoring, is unknown. Rule 1 was developed to account for the possibility of peak urinary CN-THCCOOH excretion occurring after collection of the first urine monitoring specimen. Another limitation is that new cannabis use cannot be completely eliminated in the validation data, as participants’ access to cannabis as outpatients was not controlled. Urine specimens were collected under observation each day and urinary CN-THCCOOH excretion data evaluated. A continuously decreasing trend was observed in these individuals and the criterion for new cannabis use (greater than 1.5 times the earlier concentration) was never met. This was the best criterion available at the time. There are two possible explanations for the higher than predicted %re-use rates in Specimen 1 groups’ ≥200 ng/mg (Table 2A). Either participants occasionally smoked cannabis during outpatient monitoring, or they represent a different subgroup of cannabis users in whom CN-THCCOOH concentrations are elevated for prolonged periods. Of 48 inpatient participants in our model development study, 14 had initial urine specimens >200 ng/mg. The interval needed for these participants to produce a urine specimen <200 ng/mg was 2–76h. Only 6% of outpatient validation study participants had concentrations ≥200 ng/mg on day five. Application of rule 2 identifies this pattern of persistently elevated CN-THCCOOH urinary excretion. Other limitations associated with model development are reported in supplementary data.
Conclusions
These models provide clinicians, toxicologists, probation and parole officers, and medical review officers with the first validated method for differentiating new cannabis use from residual cannabinoid excretion in chronic daily cannabis users. Four different probability levels with varying degrees of stringency are provided to offer flexibility for the practitioner to optimize model applicability in specific settings. Drug monitoring programs with more severe penalties, e.g., criminal justice system and forensic cases, and lower tolerance for false-positive identification, might adopt the highest level of stringency (99%) to maximize model specificity (i.e., minimize false predictions of new cannabis use). Monitoring programs desiring detection of all cannabis relapses, e.g., drug abuse treatment programs, with a greater tolerance for false predictions, might adopt lower (80%, 90%) stringency levels to increase model sensitivity (i.e., minimize false negative predictions). These new prediction models provide practitioners a valuable new tool to assess cannabis relapse and should be helpful in the treatment of cannabis dependence.
Supplementary Material
Acknowledgments
Disclosures and Acknowledgements
We thank the participants and clinical research staff and Ms. An-Ting Wei for providing statistical support.
Footnotes
Conflict of Interest:
This research was supported by the Intramural Research Program, National Institutes of Health, National Institute on Drug Abuse, Contract N01DA-1–8817 to BRCI and by NIDA grants RO1–06522 and R01- DA10346 to Dr. Pope. All coauthors report no competing interests.
References
- 1.UNODC. World Drug Report. 2009. pp. 1–314. [Google Scholar]
- 2.Smith ML, Barnes AJ, Huestis MA. Identifying New Cannabis Use with Urine Creatinine-Normalized THCCOOH Concentrations and Time Intervals Between Specimen Collections. J Anal Toxicol. 2009;33:185–9. doi: 10.1093/jat/33.4.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith-Kielland A, Skuterud B, Morland J. Urinary Excretion of 11-nor-9-carboxy-delta-9-tetrahydrocannabinol and cannabinoids in frequent and infrequent drug users. J Anal Toxicol. 1999;23:323–332. doi: 10.1093/jat/23.5.323. [DOI] [PubMed] [Google Scholar]
- 4.Goodwin RS, Darwin WD, Chiang CN, et al. Urinary Elimination of 11-Nor-9-Carboxy-Δ9-tetrahydrocannabinol in Cannabis Users During Continuously Monitored Abstinence. J Anal Toxicol. 2008;32:562–6. doi: 10.1093/jat/32.8.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lowe R, Abraham T, Darwin W, et al. Extended Urinary delta 9 Tetrahydrocannabinol Excretion in Chronic Cannabis Users Precludes Use as a Biomarker of New Drug Exposure. Drug Alcohol Depend. 2009;105:24–32. doi: 10.1016/j.drugalcdep.2009.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huestis MA, Cone EJ. Urinary excretion half-life of 11-nor-9-carboxy-delta-9-tetrahydrocannabinol in humans. Ther Drug Monit. 1998;20:570–576. doi: 10.1097/00007691-199810000-00021. [DOI] [PubMed] [Google Scholar]
- 7.Huestis MA, Mitchell JM, Cone EJ. Urinary excretion profiles of 11-nor-9-carboxy-9-tetrahydrocannabinol in humans after single smoked doses of marijuana. J Anal Toxicol. 1996;20:441–452. doi: 10.1093/jat/20.6.441. [DOI] [PubMed] [Google Scholar]
- 8.Huestis MA, Cone EJ. Differentiating new marijuana use from residual drug excretion in occasional marijuana users. J Anal Toxicol. 1998;22:445–454. doi: 10.1093/jat/22.6.445. [DOI] [PubMed] [Google Scholar]
- 9.Lafolie P, Beck O, Blennow G, et al. Importance of creatinine analyses of urine when screening for abused drugs. Clin Chem. 1991;37:1927–31. [PubMed] [Google Scholar]
- 10.Ellis GM, Mann MA, Judson BA, Schramm NT, Tashchian A. Excretion patterns of cannabinoid metabolites after last use in a group of chronic users. Clin Pharmacol Ther. 1985;38:572–578. doi: 10.1038/clpt.1985.226. [DOI] [PubMed] [Google Scholar]
- 11.Hunt CA, Jones RT. Tolerance and disposition of tetrahydrocannabinol in man. J Pharmacol Exp Ther. 1980;215:35–44. [PubMed] [Google Scholar]
- 12.Manno JE, Ferslew KE, Manno BR. Urine excretion patterns of cannabinoids and the clinical application of the EMIT-d.a.u. cannabinoid urine assay for substance abuse treatment. In: Agurell S, Dewey WL, Willette RE, editors. The Cannabinoids: Chemical, Pharmacologic, and Therapeutic Aspects. Orlando: Harcourt Brace Jonanovich; 1984. pp. 281–290. [Google Scholar]
- 13.Fraser AD, Worth D. Monitoring urinary excretion of cannabinoids by fluorescence-polarization immunoassay: a cannabinoid-to-creatinine ratio study. Ther Drug Monit. 2002;24:746–750. doi: 10.1097/00007691-200212000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Fraser AD, Worth D. Urinary excretion profiles of 11-nor-9-corboxy-delta-9-tetrahydrocannabinol: a delta-9-THC-COOH to creatinine ratio study #2. Forensic Sci Int. 2003;133:26–31. doi: 10.1016/s0379-0738(03)00046-x. [DOI] [PubMed] [Google Scholar]
- 15.Fraser AD, Worth D. Urinary excretion profiles of 11-nor-9-carboxy-Delta9-tetrahydrocannabinol. Study III. A Delta9-THC-COOH to creatinine ratio study. Forensic Sci Int. 2003;137:196–202. doi: 10.1016/j.forsciint.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Pope H, Gruber A, Hudson J, Huestis M, Yurgelun-Todd D. Neuropsychological performance in long-term cannabis users. Arch Gen Psychiatry. 2001;58:909–915. doi: 10.1001/archpsyc.58.10.909. [DOI] [PubMed] [Google Scholar]
- 17.SAMHSA. Mandatory Guidelines for Federal Workplace Drug Testing Programs; Notice. 228. Vol. 73. Rockville, MD: Substance Abuse and Mental Health Services Administration, HHS; 2008. [Google Scholar]
- 18.Marquardt DW. An algorithm for least-squares estimation of nonlinear parameters. J Soc Indust Appl Math. 1963;11:431–441. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.