Summary
Hematopoietic stem cells (HSCs) represent one of the first recognized somatic stem cells. As such, nearly 200 genes have been examined for roles in HSC function in knockout mice. In this review, we compile the majority of these reports to provide a broad overview of the functional modules revealed by these genetic analyses and highlight some key regulatory pathways involved, including cell cycle control, TGF-β signaling, Pten/AKT signaling, Wnt signaling, and cytokine signaling. Finally, we propose recommendations for characterization of HSC function in knockout mice to facilitate cross-study comparisons that would generate a more cohesive picture of HSC biology.
In the field of design, the minimalist movement stripped down buildings and objects to their most basic features, a sentiment that architect Ludwig Mies van der Rohe summarized in his motto “less is more”. By depleting HSCs of specific genes, knockout studies transpose the minimalist approach into research biology, providing insights into the essential core of genetic features that is indispensable for a well-functioning hematopoietic system.
Introduction
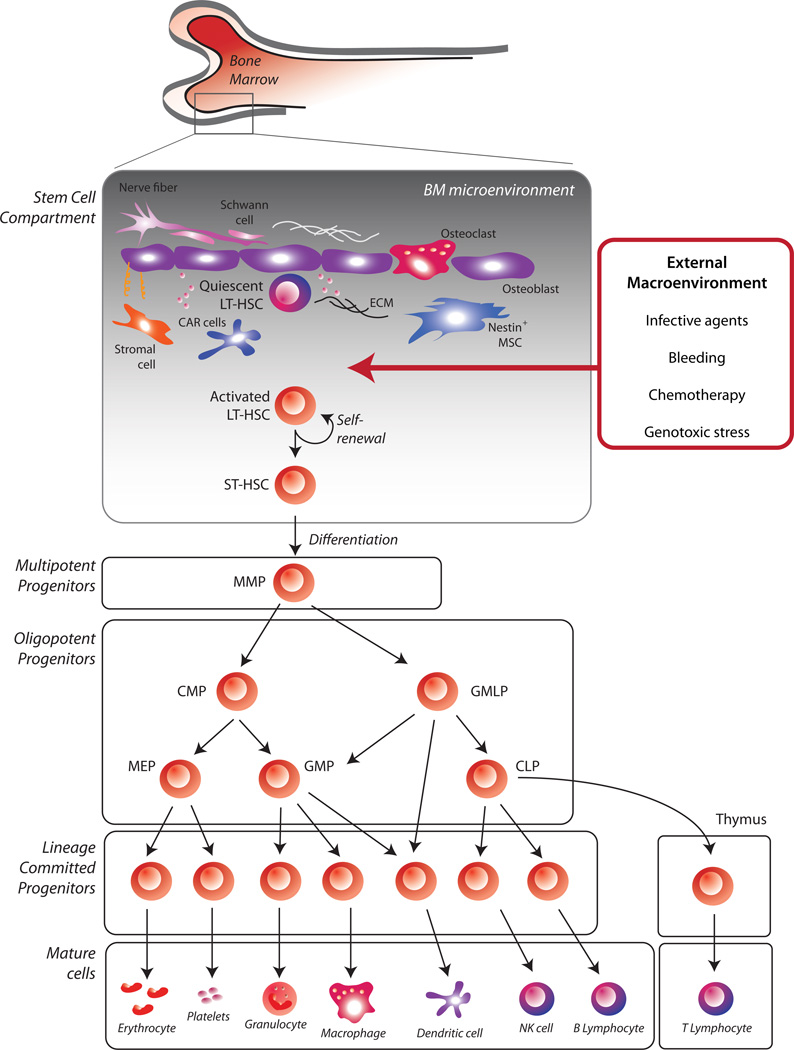
The mammalian blood system is comprised of an array of cell types, including erythrocytes (red blood cells), myeloid cells (granulocytes and monocytes/macrophages), megakaryocytes and platelets, lymphocytes (B and T cells), natural killer cells (NKs), dendritic cells (DCs), and mast cells. As diverse as these cells are, they all originate from hematopoietic stem cells (HSCs), which are a limited pool of immature progenitors residing in the bone marrow (Figure 1). At the very top of the cellular hierarchy lies a reservoir of long-term HSCs (LTHSCs). LT-HSCs guarantee a continuous supply of blood cells throughout an individual’s lifetime due to their potential to self-renew (give rise to identical daughter cells) and differentiate. Downstream of LT-HSCs are pools of stem and progenitor cells with decreasing self-renewal potential: the short-term HSCs (ST-HSCs) and multipotent progenitors (MPPs), that retain full differentiation capacity, and the lineage-restricted progenitors: common lymphoid progenitors (CLPs), common myeloid progenitors (CMPs), megakaryocyte/erythrocyte progenitors (MEPs) and granulocyte/macrophage progenitors (GMPs). These downstream progenitors are the real workhorses of the hematopoietic system, as they divide rapidly and generate a large number of differentiated progeny. In order to guarantee blood homeostasis, the system is tightly regulated but also highly resilient and capable of modulating the production of specific progeny in response to bleeding, infections or environmental insults. This resilience is the result of two opposing forces – one sustaining HSC dormancy, or quiescence, which is critical to maintain a reservoir of stem cells, and the other, activating proliferation and differentiation. Disrupting this balance can have a variety of pathologic consequences, such as bone marrow failure or hematologic malignancy. Thus, it is important to understand the forces that regulate hematopoietic stem and progenitor cell function.
Figure 1. Overview of the hematopoietic hierarchy.
At the top of the mammalian blood system is a restricted pool of multipotent long-term HSCs (LT-HSCs), capable of sustaining a continuous supply of blood cells throughout an individual’s lifetime. Long-term maintenance of hematopoiesis depends on HSC quiescence and, at the same time, on their capability to rapidly respond to proliferative stimuli generated by the BM niche or the external macroenvironment. Downstream of LT-HSCs are pools of stem and progenitor cells with increasingly diminished self-renewal potential: short-term HSCs (STHSCs), multipotent progenitors (MPPs), and lineage-restricted progenitors, such as common lymphoid progenitors (CLPs), common myeloid progenitors (CMPs), Granulocyte/macrophage/lymphocyte progenitors (GMLP), megakaryocyte/erythrocyte progenitors (MEPs) and granulocyte/macrophage progenitors (GMPs). By proliferating rapidly, these progenitors generate a large pool of mature cells that eventually exit the BM and enter the peripheral blood. Notably, some details of this hierarchy are still in flux as evidenced by much discussion in the literature, not covered here.
Over the past ~20 years, a number of genes that influence HSC function have been uncovered by analyses of knock-out (KO) mice, lending insight into some of the key regulators of HSC self-renewal and differentiation. Our goal here is to collate most of these reported KO phenotypes in order to synthesize what the phenotypes collectively tell us about the genetic and functional modules critical to HSC function. First, we review the general phenotypic classes and commonly linked observations, which broadly reveals the types of functional modules critical to HSCs. Second, we take a pathway-specific approach, examining how the phenotypes of KO mice within particular well-studied pathways illuminate the relative importance of each pathway and help to predict additional genes that may be worth investigating for their role in regulating HSCs. Finally, we suggest a set of analyses that could be considered part of a standardized assessment to determine whether a particular gene plays a role in HSC function.
Overview of the major phenotypic categories
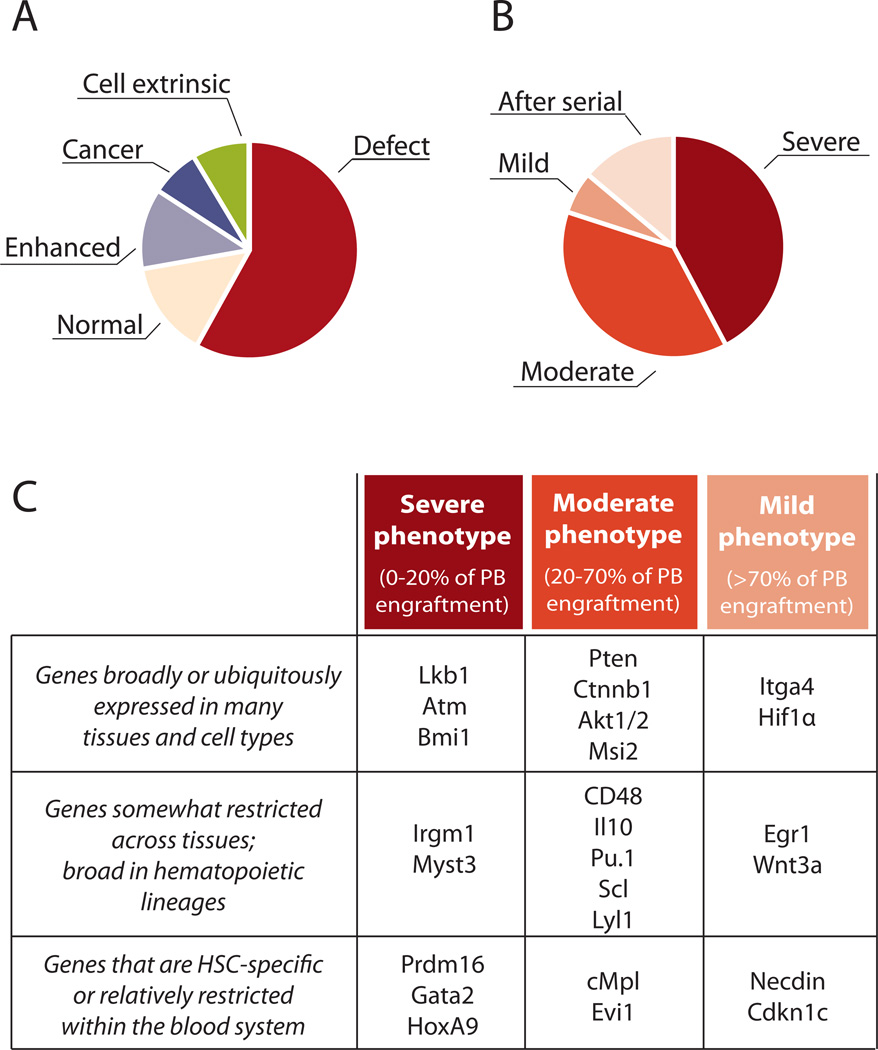
We scanned the literature to identify the majority of instances in which the HSC function of a KO mouse had been studied. We identified around 150 individual KO mice that had been at least partially characterized for an HSC phenotype (Supplemental Table 1). Several of these genes were examined by multiple laboratories, and a number of reports describe concomitant disruption of additional genes (double- or triple-KO mice), resulting in over 200 papers with a rigorous analysis of HSC function after gene disruption. We examined these papers to determine what kind of KO model was used (e.g. null or conditional), and classified the type and severity of the phenotype. In our final analysis, we only included studies in which bone marrow or stem cell transplantation had been performed to assess the capacity of the KO HSCs to regenerate blood and bone marrow (the most rigorous test of stem cell activity). There was large variation in the methodologies used to assess stem cell function, but we attempted to classify reported phenotypes through a single lens, judging their severity based on the degree of peripheral blood regeneration after bone marrow (or HSC) transplant (percent contribution of donor-derived cells in recipients based on our interpretation of data presented in the papers) (Figure 2) (please see Supplemental Table S1 for description of our classification scheme). This means that the collection of these studies in this review share one common methodology – transplantation analysis, which we believe to be the standard of quantifying HSCs in vivo. While some studies inadvertently may have not been included, having this relatively large number of KO mice to examine allows us to make some broad observations based on the frequency of various phenotypes (with the caveat that mild or negligible phenotypes are likely under-reported due to publishing constraints).
Figure 2. Overview of hematopoietic phenotypes.
A, B. Data from Online Table S1 were sorted according to phenotype, duplicate phenotypes of the same gene were removed, and the categories were apportioned as indicated. A few genes are represented in multiple categories (e.g. increased engraftment and cancer – such as Cbl KO), or enhanced engraftment after the primary transplant followed by a total loss of function after a secondary transplant – such as Hif1α)). A. Summary of phenotypes affecting HSCs as per Online Table S1. Phenotypes were ascertained from our interpretation of data in the cited papers. Increased function: Enhanced peripheral blood (PB) production from KO cells after HSC or BM transplantation. B. Summary of different degrees of hematopoietic defects found in KO mice, as per Online Table S1. Categories were arbitrarily defined as: Severe defect: less than 20% of PB production (relative to normal) in both myeloid and lymphoid lineages after deletion or transplantation of KO HSC or bone marrow; moderate defect: reduction in generation of PB to between 20–70% of normal; mild defect: reduction in PB to between 70–100% of normal. After serial: normal hematopoiesis after primary transplant, but defects in PB production emerge after secondary or tertiary transplant; C. Examples of KO phenotypes relative to generalized expression patterns. Breadth of expression gleaned from Unigene EST profiles, Goodell lab data (Chambers 2007); www.bcm.edu/labs/goodell) or the Immunological Genome Project (www.immgen.org). See supplemental Table S1 for details. Readers can add to the database and obtain updated versions of Table S1 at http://www.bonemarrowhsc.com/hscphen/.
First, we find that over two-thirds (116/152) of the analyses reported some degree of defect in peripheral blood regeneration when HSCs or bone marrow cells from KO mice were transplanted in order to test HSC function (Figure 2A). Of these, severe and moderate engraftment defects each comprised about two fifths of analyzed KO mice; a small proportion of defects were only uncovered after serial transplantation (Figure 2B, also likely underreported).
Severe phenotypes
The genes that when ablated resulted in a severe phenotype (defined arbitrarily as less than 20% of blood production from KO cells within 2–3 weeks of gene deletion or after bone marrow or HSC transplantation) fell into a range of functional classes and gene expression patterns (Figure 2C). Ablation of some genes that are highly restricted in their expression to HSCs, such as HoxA9, led to nearly immediate demise of the HSCs (Lawrence et al., 2005). These mutants are of high interest due to their potential to reveal genetic modules that are uniquely important for HSCs (and early progenitors where their expression is often shared). Severe phenotypes are also observed with some broadly expressed genes, which often have central functions for many rapidly dividing cells, such as Lkb1 and Atm (Gan et al., 2010; Gurumurthy et al., 2010; Ito et al., 2004; Nakada et al., 2010). Some KOs may show little impact on baseline blood homeostasis, but exhibit a severe defect after stress or transplantation (e.g Irgm1) (Feng et al., 2008), underscoring the value of transplantation to assay HSC function.
While severe phenotypes have shaped our understanding of the processes essential to HSCs, their analysis and interpretation can present special challenges. First, if the KO HSCs are incapable of repopulating the bone marrow, it can be difficult to discern whether this is due to lack of differentiation capacity, engraftment ability, or simply due to lack of HSC survival. It is also not possible on the basis of the transplantation data to assert that such a gene impacts HSC “self-renewal”, because if loss of the gene prevents initial engraftment, the ability to regenerate HSCs also can not be assayed. Similarly, if loss of a broadly expressed gene results in a severe phenotype, it can be difficult to distinguish between a unique impact on HSC function and an impact on some or all of the downstream progeny (e.g. MPP, CLP, CMP, or differentiated cells). In vitro assays may in some cases provide some insights, but development of specific Cre-deleter strains may eventually be required to more finely dissect more specific roles.
Moderate phenotypes
Within KOs that result in a moderate phenotype (20–70% of blood production from KO cells relative to WT), we also see that the targeted genes display a wide range of gene expression patterns. Some highly HSC-restricted genes such as Evi1 and cMpl result in moderate phenotypes, although in some cases, different KO alleles of these key regulators have different effects (Zhang et al., 2011). Moderate phenotypes are also observed in KOs of some genes that are well known as important hematopoietic regulators, e.g. Pu.1, which is required for development of multiple lineages (Iwasaki et al., 2005). Some genes may have moderate KO phenotypes due to genetic redundancy; for example, Lyl1 and Scl1 have moderate and mild effects respectively when deleted in the adult (Capron et al., 2006; Hall et al., 2003; Mikkola et al., 2003) but the double KO has a severe phenotype due to rapid demise of dKO cells (Souroullas et al., 2009).
Mild phenotypes and effects only seen upon serial transplantation
A relatively mild phenotype (70–100% of blood production from KO cells relative to WT), particularly ones that can only be seen after serial transplantation, can be initially disappointing to investigators, but can also lend critical insights into HSC functions such as “self-renewal.” If HSCs can differentiate and engraft in their niche normally in the initial host, but are defective in generating additional functioning HSCs after further transplantation, the role of a gene may be very specific for mechanisms of self-renewal. For example, deletion of cdkn1a (p21), cd81, prnp, and Tnfrsf1 (p55) resulted in decreased peripheral regeneration only after secondary or tertiary transplantation (Cheng et al., 2000b; Lin et al., 2011; Rebel et al., 1999; Zhang et al., 2006a), demonstrating defective regeneration of otherwise seemingly normal HSCs.
Ablation of some genes may result in mild phenotypes if we are unable to adequately assay the aspect of the HSC lifestyle controlled by that gene. Along these lines, it is interesting that Hif1a KO has a relatively mild impact on HSC function, with a transplantation defect showing up only upon secondary transplantation (after augmented activity in the primary recipients) (Takubo et al., 2010). Although considered a “universal” regulator, Hif1α may have HSC-specific roles due to the specialized hypoxic microenvironment in which HSCs reside. Similarly, Necdin is another highly HSC-specific regulator controlled by p53 (Liu et al., 2009b) that only exhibits a mild KO phenotype of delayed regeneration (e.g. Ndn) (Kubota et al., 2009). Necdin may be redundant with another gene(s), or may control other aspects of the HSC function not tested by conventional assays.
Cell extrinsic effect
A small portion of KO mice exhibited a cell extrinsic defect that affected stem cell function (e.g. Dicer, Opn) (Raaijmakers et al., 2010; Stier et al., 2005). Generally, this is determined by transplanting WT stem cells into a KO recipient, and observing the impact on stem cell engraftment and blood regeneration. We expect this category to be overall underexplored, because most mutants are simply not tested in this manner unless there is a reason to expect a phenotype in the supporting niche. Nevertheless, such experiments can reveal key functional elements of the niche and would be worth performing in a wider variety of mutants. It is notable that although we think of the HSC niche as the cellular non-hematopoietic elements (e.g. stromal, osteoblasts and endothelial cells) that support and instruct the HSCs, there is also feedback to stem cells from other hematopoietic cells. Such feedback would include environmental effects caused by aberrant function of downstream progeny of the mutant transplanted HSC. A prime example is the critical role of thrombopoietin, which is released by platelets, and directly regulates HSC homeostasis (de Graaf et al., 2010). Likewise, loss of Interleukin10 (Il10) resulted in HSC defects because of the imbalance of cytokine production in progeny cells that ultimately affected HSCs (Kang et al., 2007). Similarly, loss of CD48, which is expressed on most HSC progeny but excluded from dormant HSCs, resulted in HSC defects due to deregulated cytokine signaling (Boles et al., 2011).
Increased HSC activity and malignancy development
A moderate proportion (24/150) of KO mice were reported to have an increase in HSC function (Figure 2A). This enhanced function is observed as a greater proportion of peripheral blood generated from KO HSCs compared to WT control HSCs after HSC or bone marrow transplantation. We believe genes with this relatively rare KO phenotype are particularly instructive because their products constrain stem cell number or function in some manner. There were multiple functional categories described in this class. Loss of two different proteins involved in ubiquitin-mediated protein degradation resulted in an increase in HSC activity: Cbl and Itch (Rathinam et al., 2011; Rathinam et al., 2008); their targets, not yet described, may be involved in promoting HSC differentiation or proliferation. Transcription factors whose deletion led to increased HSC function include Hif1α and Egr1 (Min et al., 2008; Takubo et al., 2010). The KO phenotypes of these genes are distinguished by serial transplantation, as the dominance of these “super-HSCs” may fade after secondary (e.g. Hif1 α KO) or tertiary (e.g. Egr1 KO) transplantation. Deletion of the DNA methyltransferase 3a (Dnmt3a) also led to an apparent increase in HSC activity measured by blood production; however, this belied what was actually an alteration of the balance between self-renewal and differentiation that became exposed upon serial transplantation and HSC quantification (Challen et al., 2012).
We might expect that loss of genes that restrain HSCs would inevitably lead to malignancy. Indeed this is the case in some examples, such as loss of Tet2 (Ko et al., 2011; Moran-Crusio et al., 2011; Quivoron et al., 2011) and combined loss of Cbl and Cbl-b (Naramura et al., 2010) (hence, a few genes are included in multiple categories in Figure 2). It is possible that other mutants that expand HSCs would also ultimately lead to transformation if observed over a sufficient period of time.
In general, mutations that increase HSC function (without leading to malignancy development) are of special interest as some of these gene products could be manipulated to expand HSCs ex vivo, a long-sought goal that would have implications for cellular therapies and bone marrow transplantation. However, increased HSC function is a challenging phenotype to dissect. Increased HSC progeny can be a result of increased HSC number in the KO (perhaps of developmental origin) with normal HSC function. Alternatively, the phenotype could reflect higher differentiation capacity (faster generation of downstream cells from normal HSC numbers), or even increased self-renewal. All of these possibilities are informative, but as more members of this phenotypic class are uncovered, it will be important to distinguish the mechanisms, and of course, identify any mutants that truly expand “normal” HSC, from those that simultaneously promote malignant transformation.
Developmental vs. adult phenotypes
Primitive HSCs are first formed in the embryonic yolk sac, and definitive HSCs later in the aortagonad-mesonephros (AGM) region as well as in the placenta (Gekas et al., 2005) from whence they migrate to the fetal liver. In the fetal liver, HSCs undergo tremendous expansion before migrating around the time of birth to the bone marrow, where the majority reside throughout life. The genes required in HSC emergence (e.g. Scl and Runx1) are not covered here. However, the fetal liver (FL) harbors HSCs that have developmental potential highly similar to adultderived HSCs, and their functions can be tested by transplantation into adult recipients. Importantly, their physiology is quite distinct, as they are rapidly expanding in contrast to the quiescent adult HSCs; therefore, we may expect (and observe) different genetic requirements for these distinct stages. However, only a handful of genes have been tested for their roles in both FL and adult hematopoiesis [this often requires both a null allele (for FL analysis) and a conditional allele (for adult analysis)]. Sox17 is the paradigmatic example of a gene required for FL-HSC function that is dispensable in the adult (Kim et al., 2007). In contrast, the CDK inhibitor p57 (Cdkn1c) had minimal impact when the null allele was tested at the FL hematopoiesis stage (Zou et al., 2011), whereas deletion in the adult led to a moderate HSC defect (Matsumoto et al., 2011). Importantly, secondary transplantation from mice originally transplanted with FL-derived HSC showed that the p57-deficient HSCs did indeed display a defect once they had assumed their adult (quiescent) status (Zou et al., 2011). This finding underscores the unique physiology of the fetal versus adult states, and demonstrates the value of a secondary transplantation. In the future, we expect additional genes will be revealed to play a more important role at one or another period in development; these will generate insights into the regulatory modules that are important for the common (self-renewal and differentiation) and distinct (proliferation status and developmental potential) properties of HSCs at different developmental stages.
A Pathway and function view
Cell Cycle Control
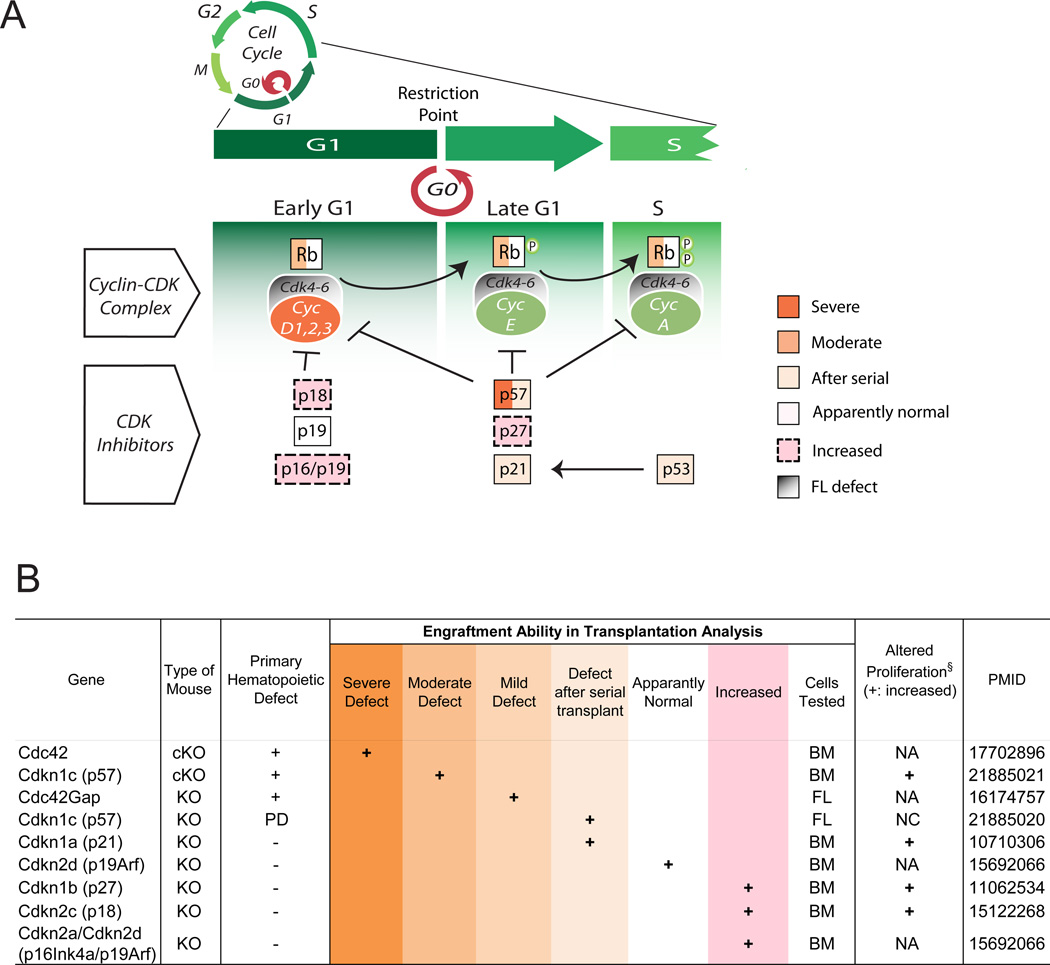
Under homeostatic conditions, adult HSCs that reside in the bone marrow niche are largely quiescent (in the G0 phase of the cell cycle), in a state of relative dormancy that is believed to be critical for their biological role (Wilson et al., 2008). Because passage through the cell cycle offers the HSC an opportunity to change its state, committing to differentiation or returning to self-renewal, the role of cell cycle regulators in HSC function have been thoroughly dissected (Figure 3), sometimes leading to unexpected results.
Figure 3. Cell cycle regulators in HSCs.
A. Cell cycle regulators play a pivotal role in HSC maintenance, by tuning the balance between quiescence and self-renewal. Gene names are enclosed in colored boxes, based on the degree of hematopoietic defect emerged from KO studies. B. Summary of KO phenotypes related to genes involved in cell cycle regulation. All indicated phenotypes are derived from in vivo transplantation experiments. KO: knock-out; cKO: conditional KO; Defect after serial transplant: normal hematopoiesis after primary transplant, but defects emerge after secondary or tertiary transplant; Increased function: KO mice that showed enhanced PB production after HSC or BM transplantation; PMID: PubMed ID number; PD: Perinatal Death, NA: Not Analyzed, NC: No Change, BM: Bone Marrow, FL: Fetal Liver; § proliferation phenotypes in hematopoietic progenitors (not always homogeneous HSCs).
The cell cycle is regulated by cyclins that, after binding cyclin-dependent kinases (Cdk), phosphorylate target proteins involved in proliferation. The complex formed by D-cyclins and Cdk4/6 controls progression through the G1 phase and, likely, HSC exit from quiescence: if we examine genetic deficiency of regulators of the G1 phase, we see that mice lacking a single Dcyclin or Cdk present with minimal hematopoietic phenotype, suggesting functional redundancy within the system. However, D-type cyclins are collectively required for HSC function, as triple D1−/−D2−/−D3−/− KO mice exhibit decreased absolute numbers of HSCs and progenitors with impaired repopulating ability (Kozar et al., 2004). Within the Cdk family, loss of Cdk2 and Cdk6 does not affect adult hematopoiesis in both single and double mutants, suggesting that their function is dispensable in HSCs (Berthet et al., 2007; Malumbres et al., 2004). However, Cdk4/6−/− double mutants also present with defects in FL hematopoiesis similar to those described in triple D-cyclin knockout mice, highlighting the pivotal role of the cyclin D/Cdk4 complex in HSC cell cycle (Malumbres et al., 2004).
Among the downstream targets of D-cyclins/Cdk4–6 complexes are members of the retinoblastoma (Rb) tumor suppressor gene family, such as Rb, p107, and p130. These proteins have long been known to critically control cell cycle dynamics, and deletion of their corresponding genes was expected to severely destabilize hematopoiesis. Surprisingly, no major defects appeared in Rb-deficient HSCs and cell cycle dysregulation became evident only under stress (Daria et al., 2008). Likewise, loss of p130 produced no hematopoietic phenotypes and only a mild hyperplasia emerged in p107 null mice (Cobrinik et al., 1996; LeCouter et al., 1998). Only when all the three genes were simultaneously deleted did the compartment of early hematopoietic progenitors display a significant increase in proliferation and a hematopoiesis reconstitution defect after transplantation (Viatour et al., 2008), indicating that Rb, p107, and p130 collectively regulate HSC cell cycle.
The Cdk inhibitors, that inactivate Cdk4-Cdk6/D-cyclin heterodimers in the face of antiproliferative signals, exhibit distinct phenotypes after ablation. Loss of p18 (Cdkn2c) results in more potent HSCs that exhibit superior engraftment in multiple rounds of transplantation (Yuan et al., 2004). Interestingly, p16 (Cdkn2a) loss results in an expanded functional HSC pool only in old mice, potentially linking p16 in particular with the hematopoietic decline that emerges with age (Sherr and Roberts, 1999; Stepanova and Sorrentino, 2005; Yuan et al., 2004). These observations also correspond with phenotypes resulting from ablation of genes whose products regulate these regulators, such as Bmi1 (Park et al., 2003).
CDK inhibitors functioning in late G1, such as p21, p27, and p57, also have been examined. Although p21 (Cdkn1a) was initially regarded as a key regulator of the entry of HSCs into the cell-cycle (Cheng et al., 2000b), recent investigation revealed that its role is more restricted to conditions of cellular stress and DNA damage, as observed after exposure to radiation (van Os et al., 2007). Cdkn1b (p27) deletion increases the pool of short-term progenitors and their repopulating power, suggesting that p27 primarily plays an antiproliferative role in progenitor cells, rather than primitive HSCs (Cheng et al., 2000a). In contrast, Cdkn1c (p57) expression is highly enriched in HSCs, relative to their progeny, where it plays a key role (Matsumoto et al., 2011; Zou et al., 2011). Conditional ablation of Cdkn1c (p57) leads to a significant decrease of adult hematopoietic progenitors and HSCs and a loss of HSC quiescence. Notably, p27 (but not p21) compensates for p57 loss, as indicated by Cdkn1b/Cdkn1c double knockouts (Zou et al., 2011).
p53 has long been known as a master regulator of cellular responses to genotoxic stress and DNA damage. Interestingly, recent studies have demonstrated that p53 is highly expressed in the HSC compartment and actively takes part in processes promoting their quiescence under steady-state conditions (Liu et al., 2009a). Ablation of Trp53 (p53) resulted in an increase in long-term progenitors, although their quiescence appeared decreased.
Although cell-cycle pathways are among the most studied features in HSC knockout reports, several genes and molecular cascades still remain to be explored for specific roles in the maintenance of quiescence or activation of self-renewal. The results emerging from this research area will help enlighten how the molecular alterations found in leukemic stem cells contribute to aberrant proliferation.
Perturbation of the Cell Cycle Status
The majority of adult-derived HSCs reside in a quiescent state, so it has been generally accepted that this is an important property of HSCs. If so, we would predict that mutants that promote HSC proliferation would lead to lower HSC function, and those that increase quiescence would lead to higher HSC activity. Broadly, we can see that disruption of other regulators that lead to perturbation of the cell cycle can have a dramatic impact on HSC function (Supplemental Table S2). Many mutants that result in sustained hyperproliferation of HSC are associated with a severe hematopoietic defect (e.g. Adar1, Gfi1, Irgm1 (Feng et al., 2008; Hartner et al., 2009; Hock et al., 2004). There are likely a number of mechanisms behind this, including HSC division events that are associated with rapid differentiation, or intrinsic control of the cell cycle.
Nonetheless, there are a range of alterations to HSC cell cycle kinetics that are associated with different levels of engraftment defects, and a few genetic models even show that increased HSC proliferation does not necessarily lead to HSC exhaustion, suggesting that the link between maintenance of stem cell functions and cell-cycle progression is complex. For instance, inactivation of Cdkn2c (p18) leads to the expansion of both stem and progenitor cells without HSC exhaustion or loss of differentiation. These mutant mice contain more actively cycling HSCs that retain increased stem cell potential even through secondary transplantation (Yuan et al., 2004). Similar findings were reported in specific c-Myb mutants: despite the increased cell-cycle activity, mutant HSCs displayed a remarkable increase in their repopulation potential, up to tertiary transplantation (Sandberg et al., 2005). A number of other models share this behavior (Table S1), in which stem cell functions are preserved – or, in some cases, even enhanced – despite the accelerated HSC proliferation.
Overall, these paradoxical phenotypes associated with increased proliferation--severe HSC defects in some cases versus increased HSC function in others --clearly indicate our poor understanding of the link between HSC proliferation and self-renewal. While quiescence, or dormancy, is a property inextricably linked to the HSC, cell division is obligate for self-renewal and provides an opportunity for differentiation, as the nucleus is remodeled during mitosis. An intriguing explanation of the conundrum is that some genes not only affect the entry of HSCs into the cell cycle, but also determine the susceptibility of HSCs to the effects of other genes or environmental cues. Such genetic factors may either promote HSC regeneration or drive differentiation, with the ultimate outcome reflecting the balance between the forces driving selfrenewal and differentiation. Understanding this puzzle will illuminate the molecular machinery that presides over the maintenance of the HSC compartment, possibly lend insight into malignancies, and may lead to the development of strategies to expand HSCs without differentiation ex vivo, which remains a highly desirable research and therapeutic goal.
TGF-β pathway
TGF-β is a multifunctional protein, involved in a wide variety of processes, including immune responses, tumor development and angiogenesis. In vitro colony formation studies had long hinted at a key role for TGF-β in HSC regulation, and the availability of KO mice finally allowed its importance to be analyzed in vivo. These studies have revealed complex requirements, suggesting that TGF-β activity varies depending on the cell type and stage of differentiation.
In mammals, three TGF-β isoforms have been described (TGF-β1, -β2, and -β3), which bind to two different membrane receptors – known as TβRI and TβRII – and activate specific Smads, organized in two separate nodes (Smad2/3 and Smad1/5/8). The separate branches of TGF-β eventually converge to activate Smad4, which translocates into the nucleus and modulates the transcription of target genes. Although knockout mice lacking TGF-β receptors (either TβRI or TβRII) die early during embryonic development, hematopoiesis remains unaffected (Larsson et al., 2001), which is also confirmed through analyses of conditional TβRI mutant mice (Larsson et al., 2003). Of note, TβRIII appears to modulate R1/RII activity, but has not yet been tested for the control of HSCs (Stenvers et al., 2003).
Smad5 is a major mediator of intracellular signaling activated by bone morphogenetic proteins (which also belong to the TGF-β family) and was expected to produce a significant impairment in HSCs once deleted. However, conditional Smad5−/− HSCs were capable of longterm multilineage reconstitution (Singbrant et al., 2006), suggesting that Smad5 may be redundant with other Smads participating in the same branch. In contrast, deletion of Smad4 resulted in a moderate self-renewal defect (Karlsson et al., 2007). Together, these data suggest redundant activation of convergent signaling pathways downstream of TGFβ.
Among TGF-β knockouts, mice lacking TGF-β1 or TGF-β3 have not been documented to exhibit hematopoietic defects whereas only the deletion of Tgfb2 affected HSCs. Tgfb2+/− BM cells display a moderate decrease in their repopulating ability, while Tgfb2−/− FL cells display a visible defect only following serial transplantations (Langer et al., 2004). These findings suggest that TGF-β2 may act as a positive regulator of HSC functions in vivo, perhaps also dependent on developmental stage (e.g. FL vs adult HSCs). Of note, niche-secreted TGF-β was recently shown to regulate dormancy of HSCs in the bone marrow (Yamazaki et al., 2011).
Additional information on the role of TGF-β in hematopoiesis derives from knockout studies of genes that merge into the same signaling cascade. The product of the gene Pbx1 (a DNA-binding cofactor of several Hox proteins) has been shown to play a critical role in the establishment and long-term maintenance of murine hematopoiesis (Ficara et al., 2008). A remarkable number of genes regulated by Pbx1 take part in the TGF-β signaling pathway, making Pbx1 a potential trait d’union between TGF-β molecules and HSC functions. Pbx1−/− embryos are incapable of multilineage hematopoiesis and display a progressive loss of quiescent HSCs, ultimately leading to severe repopulation defects. In addition, Pbx1−/− HSCs are less responsive to TGF-β stimulation and fail to upregulate the expression of several downstream mediators, including Smad7 and Cdkn1c (p57) (DiMartino et al., 2001; Ficara et al., 2008).
Overall, the role of TGF-β signaling in HSCs appears to be more complex than initially suggested by in vitro studies. Knockout models have contributed to the dissection of TGF-β signaling in the two separate branches, which differently affect HSC function depending on the developmental context. However, other pathway members may need to be investigated before the role of TGF-β in HSCs can be fully appreciated. Alternative pathways may also involve Endoglin (CD105), an ancillary TGF-β receptor and also an HSC marker (Chen et al., 2002a; Yamashita et al., 1994). Endoglin may provide a substitute path for TGF-β signalling, possibly accounting for the lack of a stem cell defect in Tgfbr1 conditional knockouts. In addition, Endoglin has been shown to enhance signaling through Smad2 (Santibanez et al., 2007), which participates with Smad3 in a signaling node leading to the translocation of Smad4 into the nucleus. Due to the defects documented in Smad4−/− HSCs (but not seen in Smad5−/− animals), both Smad2 and Smad3 knockouts are anticipated to harbor defective HSCs. In support of this view, recent work has shown that both Smad2 and Smad3 are highly phosphorylated in quiescent long-term HSCs (but not in proliferative HSCs (Yamazaki et al., 2008), suggesting a fundamental role for the Smad2/3 node in HSC function. Overall, the mild phenotypes of mutants in multiple nodes of the TGF-β pathway imply either marked regulatory redundancy within a critically important pathway, or an inconsequential role. In the future, the concurrent deletion of multiple downstream mediators of the TGF-β signaling (such as Smad1/Smad5 double knockouts) could shed light on this issue providing a clear view of the role of TGF-β in HSCs.
Pten/Akt pathway
Akt signaling, activated by growth factors, triggers a number of cell responses, including proliferation, survival, growth, modulation of metabolic functions and resistance to stress. Phosphatase and Tensin homologue (Pten) is a negative regulator of the phosphatidylinositol-3- OH kinase (PIK3)-Akt pathway. Mechanistically, Pten acts as a negative regulator of this pathway and inhibits Akt activation by dephosphorylating PIP3 (phosphatidylinositol 3,4,5 trisphosphate). Mutations in genes in the Pten/Akt signaling pathway have been shown to influence both HSC number and quiescence.
The Pten tumor suppressor gene is often mutated in tumors and several investigations highlight the role of Pten/Akt pathway in both normal hematopoiesis and leukemia. Mice with a conditional loss of Pten in the hematopoietic system present with a significant depletion of the stem cell pool (Table 1, Table 2.2, and Table S1); mutant mice have decreased numbers of LTHSCs, while the ST-HSCs remain unaffected. Strikingly, loss of Pten has been linked to leukemogenesis; mutant mice develop a myeloproliferative disorder that eventually evolves into acute leukemia (Guo et al., 2008; Yilmaz et al., 2006; Zhang et al., 2006b).
Table 1. Summary of phenotype of KO mice.
This is an excerpt of online table S1. All indicated phenotypes are derived from in vivo transplantation experiments. KO= knock out; cKO= conditional KO; TP=transplant; 1°TP= primary TP; 2°TP= secondary TP; 3°TP= tertiary TP; Severe defect= less than 20% multi-lineage peripheral blood (PB) production from KO cells after bone marrow or HSC transplantation; moderate defect= reduction in generation of PB to between 20–70% of normal; mild defect= reduction in PB to between 70–100% of normal. Defect after serial transplant= normal hematopoiesis in primary transplant, but phenotype after secondary or tertiary transplant. Increased function = KO mice that showed enhanced PB production after HSC or BM transplantation. Cell intrinsic effect generally is shown by demonstrating an impact on PB production from WT HSCs after transplantation into KO recipients. PMID= PubMed ID number.
| Gene | Type of Mouse (deleter) |
Altered FL hemo |
Severe defect |
Moderate defect |
Mild defect |
Defect after serial txt |
Normal | Increase d function |
Cell extrinsic effect |
Cancer | Comments | PMID |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gfi1 | KO | + | Gfi1−/− HSCs cannot sustain competition with WT HSCs in 2month old chimera mice |
15457180 | ||||||||
| Sox17 | KO | + | + | + | Decreased fetal HSC pool and hematopoietic cells; no impact of deletion in adult HSC |
17655922 | ||||||
| Lkb1 | cKO (Mx1Cre) |
+ | HSCs profoundly depleted by 18 days after deletion; Pancytopenia, decline in all blood lineages |
21124450; 21124456; 21124451 |
||||||||
| Irgm | KO | + | Hyper interferon signalling; severely decreased repopulating ability of HSC; increased proliferation |
18371424 | ||||||||
| Atm | KO | + | Increased intracellular ROS in the KO; NAC rescue the KLS engraftment defect |
15496926 | ||||||||
| Cdkn1c (p57) |
cKO (Mx1Cre) |
+ | BM and BM KSL transplant | 21885021 | ||||||||
| CD48 | KO | + | + | + | Increased HSC quiescence; dysregulated cytokine signaling ; Pak1 hyperactivation; lymphomas |
21576698 | ||||||
| Pten | cKO (Mx1Cre) |
+ | + | Decreased HSC pool and repopulating ability; Myeloproliferative disorder/AML |
16633340 | |||||||
| Hoxa9 | KO | + | + | FL: Defective engraftment of Hoxa9−/− FL HSCs |
17761289; 16091451 |
|||||||
| PU.1 | cKO (Mx1Cre) |
+ | + | Decrease in FL HSC by transplantation; block myelomonocytic differentiation in CMP/GMP; |
15914556 | |||||||
| IL10 | KO | + | Decreased repopulating ability; IL10 expressed in endosteal osteoblasts; Hypcellular Pre-TP |
17464085 | ||||||||
| Rac1 | cKO (Mx1Cre) |
+ | Decreased homing leading to decreased repopulating ability |
16025125 | ||||||||
| Smad4 | cKO (Mx1Cre) |
+ | Decreased repopulating ability; Pre- TP: Reduction in RBC and hemoglobin |
17353364 | ||||||||
| Hif1alph a |
cKO (Mx1Cre) |
+ | + | Significantly increased engraftment after 1°TP, and almost total loss after 2°TP. Increased cycling. Phenotype normalized after cross to Vhl KO. |
20804974 | |||||||
| CD81 | KO | + | CD81 cluster induced quiescence (returning from proliferation); Severe defect after 2°TP |
21931533 | ||||||||
| Opn (Spp1) |
KO | + | + | + | KO BM txt showed higher engraftment, lost by 2°TP. Host effect |
15928197 | ||||||
| Egr1 | KO | + | + | Increased engraftment in limiting dilution Increased circulating HSCs;defect after 3°TP |
18397757 | |||||||
| Necdin | KO | + | Slightly reduced stress recovery | 19770359 | ||||||||
| Dicer | cKO (Osx- Cre) |
+ | + | + | Increased HSC proliferation and apoptosis; development of MDS and leukemia. Host effect |
20305640 | ||||||
| Ifnar | KO | + | Proliferation defect in response to IFNa- induced stress |
19212321 | ||||||||
| Gfi1b | cKO (Mx1Cre) |
+ | + | Increased engraftment may reflect higher HSC frequency; increased ROS in HSCs |
20826720 | |||||||
| Cebpa | cKO (Mx1Cre) |
+ | + | + | Increased repopulating ability in 1°TP and 2°TP; myeloid diff. block in BM |
15589173 | ||||||
| Cbl | KO | + | + | Increased HSC number and repopulating ability; Myeloproliferative disease- see PMID 20951944 |
18413713 | |||||||
| Tet2 | KO, cKO (Mx1- and Vav-Cre) |
+ | + | By ~20 weeks, massive increase in progentiors. Mice eventually develop CMML-like disease. Het phenotype. |
21723200; 21873190; 21723201 |
|||||||
| Itch | KO | + | Itch involved in negative regulation of Notch signaling 20–50% incr after competitiveTP. |
21478879 | ||||||||
| Trp53 | KO | + | Increased HSC number and repopulating ability |
12829028 |
Table 2. Summary of KO Phenotypes Related to Genes Involved in TGF-βand AKT/PTEN pathways.
All indicated phenotypes are derived from in vivo transplantation experiments. KO = knock-out; cKO = conditional KO; Defect after serial transplant = normal hematopoiesis after primary transplant, but phenotype after secondary or tertiary transplant; Increased function = KO mice that showed enhanced PB production after BM (bone marrow) or FL (fetal liver) transplantation; Some KOs have been investigated for proliferation phenotypes: NC = no changes in proliferation; NA = not analyzed; PMID = PubMed ID number
| 2.1 Tgf-βpathway | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | Type of Mouse | Primary Hematopoietic Defect |
Engraftment Ability in Transplantation Analysis |
Altered Proliferation (+:increased; –: decreased) |
PMID | ||||||
| Severe Defect |
Moderate Defect |
Mild Defect | Defect after serial txt |
Apparantly Increased Normal |
Increased | Cells Tested | |||||
| Pbx1 | KO | + | + | FL | − | 11468159 | |||||
| Pbx1 | cKO (Mx1-Cre and Tie2-Cre) |
+ | + | BM | + | 18462698 | |||||
| Tgfb2 | Het | − | + | BM | + | 14707111 | |||||
| Smad4 | cKO (Mx1-Cre) | + | + | BM | NA | 17353364 | |||||
| Tgfb2 | KO | − | + | FL | + | 14707111 | |||||
| Tgfbr1 | KO | − | + | BM | NC | 12842983 | |||||
| Smad5 | cKO (Mx1-Cre) | − | + | BM | NC | 16896158 | |||||
| 2.2 Pten/Akt pathway | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | Type of Mouse | Primary Hematopoietic Defect |
Engraftment Ability in Transplantation Analysis |
Altered Proliferation§ (+: increased; –: decreased) |
PMID | ||||||
| Severe Defect |
Moderate Defect |
Mild Defect | Defect after serial txt |
Apparantly Increased Normal |
Increased | Cells Tested | |||||
| FoxO3a | KO | − | + | BM | NC | 18371339 | |||||
| AKT1/AKT2 | dKO | + | + | FL | − | 20354168 | |||||
| FoxO1/FoxO3/FoxO4 | triple cKO (Mx1-Cre) | − | + | BM | + | 17254970 | |||||
| Pten | cKO (Mx1-Cre) | + | + | BM | + | 16633340 | |||||
| Akt1 | KO | − | + | BM | NA | 20354168 | |||||
| Akt2 | KO | − | + | BM | NA | 20354168 | |||||
Genes downstream of Pten have also been examined. Loss of either Akt1 or Akt2 has only subtle consequences on hematopoiesis. Conversely, HSC function in Akt1−/−/Akt2−/− double mutants is significantly impaired, as displayed by competitive transplants (Juntilla et al., 2010), suggesting a functional overlap between the two members of the family. Several studies also focused on Forkhead O (FoxO) proteins, a family of transcription factors involved in cell proliferation, differentiation, apoptosis, and resistance to stress agents. FoxO proteins are negatively regulated by the PI3K/Akt pathway and, similar to Pten inactivation, Akt activation results in FoxO phosphorylation and inhibition. As a consequence, the hematopoietic phenotype of FoxO-deficient mice is strikingly similar to that of Pten-deficient mice, with the exception that FoxO knockouts do not develop leukemia (Tothova et al., 2007). The conditional deletion of all three FoxO genes (FoxO1, -3a, and 4) has been reported to reduce LT-HSCs numbers and their ability for long-term multilineage reconstitution (Tothova et al., 2007). Incidentally, the deletion of FoxO3a alone resulted in loss of hematopoietic reconstitution ability, and this defect in hematopoietic engraftment manifested in secondary and tertiary BM-transplants, supporting a regulatory role for FoxOs in HSC self-renewal (Miyamoto et al., 2007).
Besides controlling the size of the HSC pool, FoxO proteins promote HSC quiescence as well. Several proteins involved in cell cycle regulation (including Rb/p130, Cyclin D2, Cyclin G2, p21 and p27) represent transcriptional targets of FoxOs. The concomitant loss of FoxO 1, 3, and 4 was shown to increase the number of actively cycling LSK HSCs (Tothova et al., 2007). Likewise, HSCs from FoxO3a−/− mice proliferate more and present decreased levels of both p27 and p57 (Miyamoto et al., 2007). HSC functional defects found in FoxO-mutant mice are likely the consequence of a more intense cycling activity that drives HSCs out of quiescence and progressively depletes the stem cell compartment.
The Pten/Akt/FoxO pathway offers a rich vein for other potential studies. By looking at the Pten/Akt/FoxO pathway, we may make additional predictions on how perturbations of this pathway will affect the stem cell pool. For example, removing inhibitors of Akt activation is expected to decrease FoxO transcription, which will likely lead to an increase in HSC proliferation and, possibly, to the premature exhaustion of the stem cell pool. An additional avenue would be to explore the roles of particular targets of the pathway by the generation of animals with multiple null alleles. For instance, Akt can activate the NF-kB pathway through the activation of the inhibitory kB kinase IKK. On the other hand, Akt-driven phosphorylation has been shown to play a role in regulating apoptosis through the activation of BAD. A double mutant lacking Bad and Ikk could be used to separate the effects of Akt through BAD or IKKdependent cell survival pathways from its effects through FoxO transcription. Such studies would likely provide further mechanistic insights into this pathway in HSC biology.
The Wnt Pathway
Wnt ligands are a family of secreted glycoproteins that interact with membrane-associated receptors to activate signaling cascades. Wnt-dependent pathways (classically distinguished in canonical and non-canonical branches) are involved in diverse processes, such as embryonic development, cell differentiation, proliferation and polarity. Of note, many Wnt proteins are expressed in blood cells and aberrant activation of Wnt-dependent signaling has been reported in several hematologic malignancies, suggesting a pivotal role of Wnt-signaling in blood cell production. However, over the past decade, investigation of the role of the Wnt signaling pathway in hematopoiesis has lead to controversial results. Varied experimental approaches can partly account for the difficulty in reaching a consensus. However, recent reports suggest that different variables such as developmental stage, Wnt protein dosage, and microenvironmental factors contribute to tuning the impact of Wnt signaling, making the role of Wnts in HSCs difficult to generalize.
The so-called canonical Wnt signaling cascade, in which β-catenin is stabilized and translocated into the nucleus, is arguably the best studied. However, Ctnnb1 (β-catenin) KO studies have generated results that are difficult to reconcile: conditional Mx1-Cre-mediated inactivation of Ctnnb1 in adult animals resulted in normal HSC repopulation and self-renewal (Cobas et al., 2004). However, Vav-Cre-mediated deletion of Ctnnb1 showed decreased longterm repopulation efficiency of β-catenin-deficient HSCs (Zhao et al., 2007). Because Vav-Cre exerts its effect during fetal hematopoiesis, the apparent discrepancy may indicate a developmental requirement for β-catenin. β-catenin may be less critical in adult HSCs, or compensated by other proteins. Indeed, the continued influence of β-catenin in adult HSCs is supported by over-expression of constitutively active β-catenin, which caused severe disruptions in HSC function and led to a differentiation block (Kirstetter et al., 2006; Scheller et al., 2006).
Wnt3a, a ligand in the Wnt signaling pathway, has been shown to play a role in FL HSC function, as Wnt3a-null mice exhibit a significant reduction in FL HSC numbers, the function of which is also severely impaired in serial transplantation (Luis et al., 2009). Similar findings derive from Sox17 knockout mice. The Sox17 transcription factor is an inhibitor of the canonical Wnt pathway which is highly expressed in the fetal liver. Germline deletion of Sox17 resulted in a striking decrease in the FL-derived HSCs, but conditional adult loss of Sox17 had minimal impact (Kim et al., 2007). These data illustrate the importance of inhibition of Wnt signaling during development and support its distinct role in the adult.
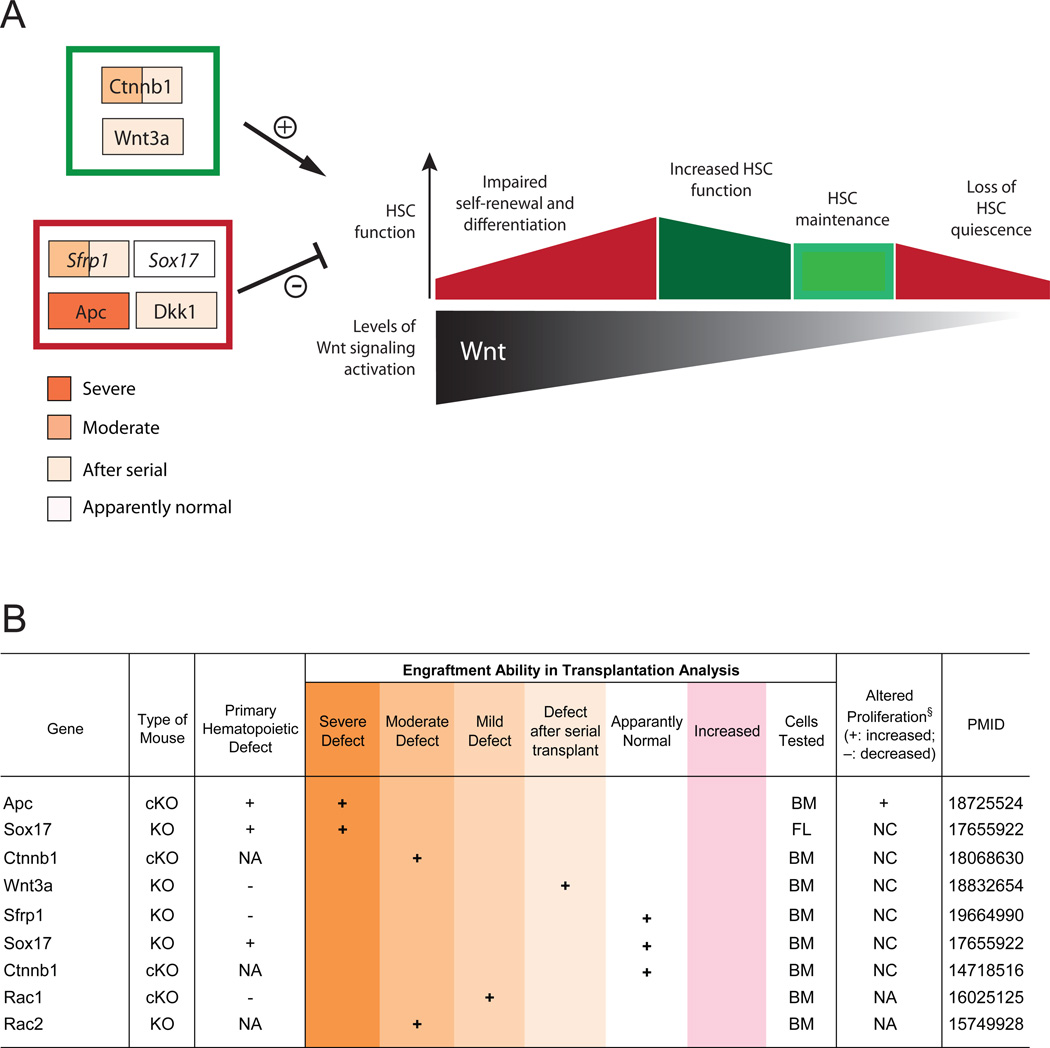
Other inhibitors of the Wnt canonical pathway have provided further insights. The product of the tumor suppressor gene Apc (Adenomatous polyposis coli) negatively regulates the Wnt/β-catenin signaling pathway. Conditional loss of Apc drastically increases HSC cycling and ultimately leads to severe defects in multilineage hematopoiesis (Qian et al., 2008). The high levels of β-catenin found in Apc-mutant cells bolster the idea that abnormal activation of Wnt signaling is detrimental to HSC function. Along these lines, differences in Wnt dosages and activation levels of its pathway may be a factor in the discrepancies previously observed by different investigators. By combining different Apc conditional knockouts each permitting different levels of Wnt signaling, Luis et al. recently created five different levels of Wnt signaling in vivo (Luis et al., 2011). Different levels of Wnt signaling appear to affect the balance of HSC self-renewal versus differentiation. Mild levels of Wnt-pathway activation contribute to HSC maintenance and result in increased hematopoietic reconstitution. In contrast, abnormal activation of the Wnt pathway hinders HSC self-renewal and differentiation (Figure 4).
Figure 4. Wnt signaling in HSCs.
A. Maintenance of normal HSC function requires a tightly regulated Wnt dosage. While a decrease in Wnt signaling leads to loss of HSC quiescence and self-renewal, mild increases in Wnt pathway activation contribute to HSC maintenance and result in enhanced hematopoietic reconstitution. However – much as its inhibition – the excessive activation of Wnt pathway hinders HSC differentiation, leading to the loss of hematopoietic functions. Both positive and negative regulators contribute to maintaining Wnt-signaling activation within physiological ranges: these include both intrinsic (cell-autonomous) and extrinsic (microenvironmental) factors (the latter indicated in italics in the figure). Gene names are enclosed in colored boxes, based on the degree of hematopoietic defect emerged from KO studies. B. Summary of KO phenotypes related to genes involved in Wnt-signaling. All indicated phenotypes are derived from in vivo transplantation experiments. KO: knock-out; cKO: conditional KO; Defect after serial transplant: normal hematopoiesis after primary transplant, but defects emerge after secondary or tertiary transplant; Increased function: KO mice that showed enhanced PB production after HSC or BM transplantation; PMID: PubMed ID number; PD: Perinatal Death, NA: Not Analyzed, NC: No Change, BM: Bone Marrow, FL: Fetal Liver; § proliferation phenotypes in hematopoietic progenitors (not always homogeneous HSCs).
In addition to the intrinsic modulation of HSC functions, Wnt-signaling in the hematopoietic niche is also crucial. Recent studies have demonstrated stroma-mediated Wnt/β-catenin effects that may eventually resolve some of the controversies raised by previous investigations. Transgenic mice overexpressing the Wnt inhibitor Dickkopf1 (Dkk1) in the osteoblastic lineage display increased HSC proliferation and decreased regeneration after transplantation (Fleming et al., 2008). Similarly, the enforced expression of the Wnt inhibitory factor 1 (Wif1) in osteoblasts produced loss of HSC quiescence and decreased self-renewal ability (Schaniel et al., 2011). Secreted Frizzled-related proteins (sFRPs) can also antagonize Wnt signaling by directly binding Wnt ligands and preventing interaction with their cognate receptors. Although Sfrp1 deletion has no intrinsic consequences on the stem cell compartment, loss of Sfrp1 in BM stromal cells leads to severe defects in HSC maintenance (Renstrom et al., 2009). The hematopoietic phenotypes described here validate a requirement for canonical Wnt signaling in the BM niche and highlight its function in containing proliferation and maintaining HSC quiescence (Fleming et al., 2008).
As compared to the canonical pathway, the role of non-canonical Wnt signaling in HSCs is not as well defined and therefore offers an opportunity for further research. KO of two downstream targets of the non-canonical pathway, the small Rho GTPase Rac1 and Rac2 resulted in defective fetal-liver and adult hematopoiesis (Cancelas et al., 2005; Ghiaur et al., 2008; Jansen et al., 2005). However, these genes have broad roles in cell migration, and thus their phenotype may reflect features other than Wnt signaling. Recently, non-canonical Wnt signaling mediated by Frizzled (Fz8) was shown to antagonize canonical Wnt signaling, contributing to quiescence of HSCs through suppression of the NFAT-IFNg pathway (Sugimura Cell 2012).
On the whole, although Wnt signaling is now regarded as a key regulator of HSCs (and several other types of somatic stem cells), its inherent complexity is far from being fully resolved. More details are expected to emerge from knockout studies of downstream Wntsignaling effectors, such as Tcf3 and Tcf4, which have already been shown to be critical in skin stem cells (Nguyen et al., 2006). In addition, the role of the disheveled genes (scaffolding proteins involved in both canonical and non-canonical signaling) in HSCs has yet to be examined. Finally, while both canonical and non-canonical pathways need to be better elucidated for their role in HSCs, the greatest advances will lie in discovering how the two branches of Wnt signaling are functionally intertwined.
Cytokine Signaling in the stem cell niche
Since the 1960s, the hematopoietic organs such as bone marrow and spleen have been known to be sources of soluble factors that can stimulate differentiation of progenitor cells; these cytokines and chemokines are natural candidates for regulating HSCs. Niche factors that contribute to HSC maintenance are more extensively summarized in a recent review (Mercier et al., 2012). While many of these soluble factors have been studied by exogenous addition in in vitro culture or overexpression in vivo, only a handful have been investigated by gene disruption for their role in stem cell regulation.
Steel factor, also known as SCF or cKit ligand, was one of the earliest soluble factors found to significantly affect HSC function. Scf-deficient animals do not maintain long-term repopulating activity (McCarthy et al., 1977). Thrombopoietin (TPO) has been reported to promote HSC proliferation in combination with SCF or IL3 (Sitnicka et al., 1996); and disruption of the TPO receptor cMPL appeared to lead to a cell autonomous defect in HSC engraftment (Kimura et al., 1998). Indeed, thrombopoietin (Thpo−/−) knock-out mice have a 150-fold reduction in HSC number (Qian et al., 2007). Similarly, angiopoietin-1 (Ang-1) signaling through the receptor Tie2 is required for adult hematopoiesis (Puri and Bernstein, 2003; Takakura et al., 1998) and HSC quiescence and reconstitution ability is severely impaired in the absence of Tie2/Ang-1 (Arai et al., 2004). By contrast, effects of mutations in the TGFβ pathway are relatively mild, perhaps because of redundancy in the system (see section on TGFβ above). Finally, bone marrow of mice in which the receptor for G-CSF, Csf3r, was ablated gave rise to a decreased number of hematopoietic colonies than their WT counterparts, suggesting a smaller hematopoietic progenitor pool (Liu et al., 1996). Thus, a wide variety of soluble growth cytokines have been implicated in the maintenance of the HSC population. Use of knock-out mice as recipients in bone marrow transplant experiments is particularly informative to address the environmental role of such secreted factors.
Perhaps unsurprisingly given the close interaction between the hematologic and immune systems, a number of inflammatory cytokines have also been found to influence HSC biology. Mice mutant for the chemokine Cxcl12 show defects in HSC homing to the bone marrow during the fetal liver to bone marrow transition in late gestation (Rebel et al., 2002); similarly, mice lacking the Cxcl12 receptor Cxcr4 are hyperproliferative (Nie et al., 2008), and disruption of Cxcr4 signaling leads to HSC mobilization (Christopher et al., 2009). Interleukin-10 silencing has been shown to decrease HSC ability to repopulate recipients (Kang et al., 2007). Likewise, transplantation of IL-2 knockout HSCs results in decreased engraftment (Chen et al., 2002b), suggesting that IL-2 - typically thought of as a T-cell cytokine – plays a role in HSC maintenance as well.
Recently, work on Type 1 and Type II interferons (Ifns) has illuminated their central role in controlling HSCs, as they can drive dormant stem cells into an active and proliferative state by acting directly on the stem cells rather than by altering their niche (Baldridge et al., 2010; Essers et al., 2009). Furthermore, loss of negative regulators of Ifn signaling, including Interferon regulatory factor 2 (Irf2), Adar1, and Irgm1 (Feng et al., 2008; Hartner et al., 2009; King et al., 2011; Sato et al., 2009) all lead to rapid loss of HSC function through hyperproliferation in the absence of tightly regulated Ifn signaling. It is likely that Ifn-stimulated HSC proliferation leads to HSC deficits due to differentiation-associated HSC proliferation. Regulation of HSCs by these classic immune-system modulators may contribute to coordination of the entire immune response during infectious stress (King and Goodell, 2011).
The Ifn response has also been used extensively to generate conditional KO mice: Mx1-cre (Kuhn et al., 1995), the most commonly used Cre-deleter strain in HSC research, is inducible due to its Ifn responsiveness. This has raised the possibility that the pro-proliferative effects of Ifn induction may also impact HSCs during conditional KO, an effect that must be taken into account for data interpretation. Tamoxifen-inducible Cre deletion (Hayashi and McMahon, 2002) may circumvent this concern, but thus far, has not proven to be as efficient in the hematopoietic system. It would be useful to develop additional inducible lineage-restricted Cre-deleter strains in order to ascertain HSC-specific effects that are independent of Ifn and other cytokine signaling.
Conclusions and Future Perspectives
The well-defined hematopoietic stem and progenitor cell hierarchy, as well as its tractability as an experimental system has led to an impressive collection of studies that have probed the impact of gene ablation on HSC function. The studies encompassed in this review provide a framework in which to consider the key functional modules of HSCs, and to consider the major questions that still need illumination.
Collectively, these studies underscore the view that the unique lifestyle of adult HSCs, characterized by periods of dormancy followed by activation and return to quiescence, represents a fine balance that is critical for long-term stem cell viability. There is a relatively narrow window of tolerance within which the cell cycle activity can be perturbed, either directly or indirectly, while maintaining essentially normal or improved HSC function. This is substantiated by the number of KOs in which the cell cycle activity is perturbed with concomitant changes in HSC function. However, we still do not understand the mechanistic link between HSC cycling and (the general) loss of long-term activity: does rapid HSC division normally favor symmetric differentiation? In this context, the highly proliferative state of FL-derived HSCs without compromising long-term HSC function is particularly noteworthy; insights into the regulatory modes that permit this distinction, implied by the different FL vs adult phenotypes of some of the mutants collected here, could have implications for ex vivo expansion of HSCs, as well as cancer. Similarly, the role of genes that normally restrain HSC number or function, the behavior of these KOs with regard to HSC cycling, and the implications of these genes for malignancy development warrants further study.
Broadly, these studies reinforce the notion that key pathways such as Wnt and TGF-β - signaling strongly influence HSC cell fate. The inability to generalize the specific roles of these pathways in terms of an absolute requirement of specific HSC functions probably reflects context-dependence (i.e. fetal vs adult requirements) of the specific pathways, as well as gaps in the analysis of specific pathway members. The roles of these and other pathways can be further refined with new mutants, combination of mutants, and rigorous analyses of stem cell function.
We also observe from this collection that the HSC “niche” could be much more broadly considered, capturing the larger stem cell “environment” that includes cellular and non-cellular elements such as soluble growth factors and extracellular matrix. Considering that we have little insight into how appropriate HSC numbers are maintained despite their wide but sparse dispersion through the vast bone marrow, understanding such long-range feedback mechanisms that may work at an organismal scale is important. This would promote the development of a more holistic view of the way in which HSCs are regulated in the context of normal and disease physiology.
What can we do to bring this analysis from a list of interesting mutants to a sophisticated understanding of the mechanisms, including all their subtleties, that regulate HSC function? We expect that at any given time, there are extrinsic forces pushing HSCs to differentiate, as well as others restraining them in the niche. Elucidation of how these opposing signals are integrated and interpreted is needed.
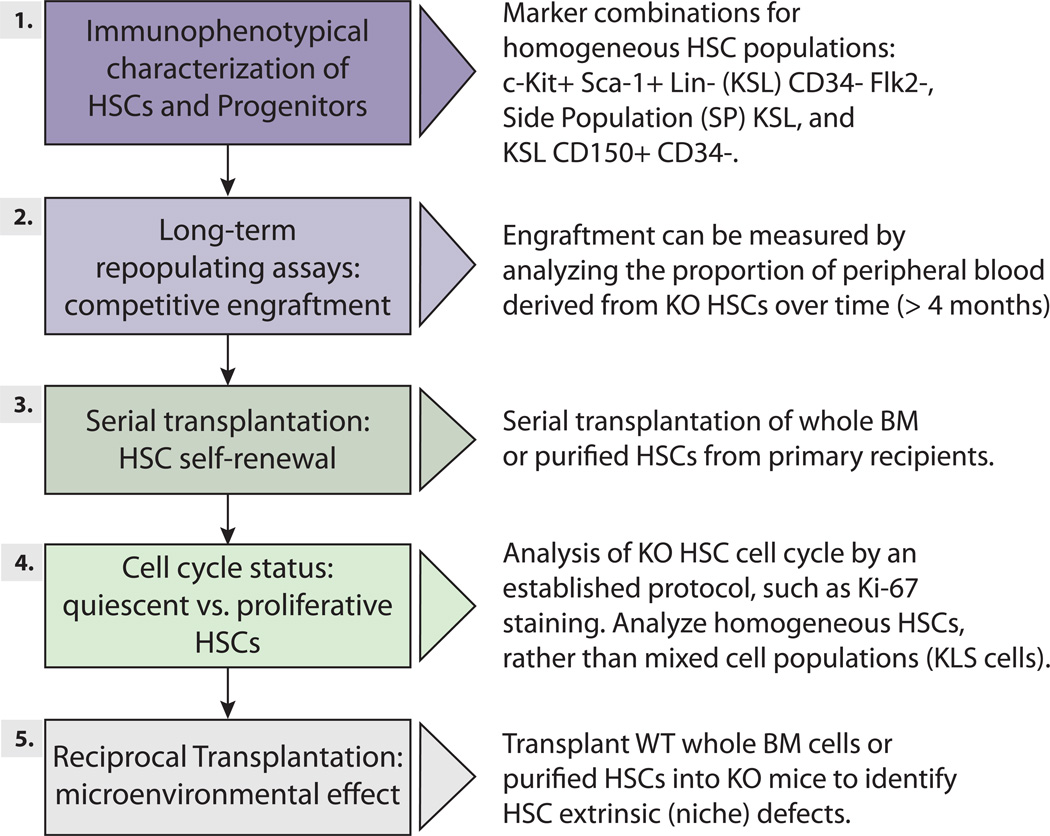
Importantly, in order to better integrate our understanding of these different mutants, we need to facilitate cross comparison of emerging studies. After reviewing the ~200 papers we cover here, the challenge of comparing studies using widely differing methodologies was starkly evident. Here, we suggest a set of experiments to test whether a particular gene plays a role in HSCs, not all of which need to be applied in every situation (Figure 5, Supplemental Table S3). The gold standard assay is testing the function of the mutant HSCs by bone marrow or stem cell transplantation, but ancillary studies will assist interpretation (also see (Purton and Scadden, 2007) for a comprehensive review of some of these assays). Also, the increasing availability of high-throughput sequencing may allow molecular signatures of stem cell status to be defined by gene expression consequences and/or transcription factor binding behavior after gene perturbations; if performed in a standardized manner, such data when accumulated might be comparable from lab to lab, and thus enable relationships between different genetic modules of HSCs to be inferred.
Figure 5. Overview of recommended steps for determining whether a gene has an impact on HSC function.
See Supplemental Table S3 for details.
Finally, we think it will be important in the long-run to distinguish between functions that are critical to many/all cells that have the potential to rapidly divide (e.g. DNA repair genes, metabolic regulators), and those that have particular functions for HSCs. Some of this is inferred by HSC-specific gene expression patterns (e.g. Nurr1 (Sirin et al., 2010), but in other cases, there may be HSC-specific post-transcriptional regulatory mechanisms, such as protein modifications, microRNA regulation, or substrate availability, that are not immediately obvious.
Ultimately, the next big leap forward in our understanding will derive from in vivo assessments of how graded changes in signaling pathways affect HSC function. i.e. combinatorial and realtime assessments of gene expression using new technologies like RNA seq. As we identify individual players, we need to begin to understand how they work together in a dynamic physiologic setting. This new level of understanding will lead to improved application of gene-specific therapeutics and the development of more integrative approaches to regenerative medicine.
Supplementary Material
Acknowledgements
We apologize to those authors whose work could not be referenced or discussed owing to space limitations. Please email us at HSCs@bcm.edu to add your favourite genes to our online table, which also be found at http://www.bcm.edu/labs/goodell/ after review publication. We thank all members of the Goodell lab for helping to collate table S1, particularly, Aysegul Ergen, Min Luo, Maria Imperato, Sean Cullen, Xiaotian Zhang, and Grant Challen. We acknowledge support from DK092883, DK060445, AG036562, CA126752, and CA125123, AI007495, NSC100-2811-B-002-163 (Taiwan) and the Italian Leukemia and Lymphoma Association, section of Bologna (BolognaAIL). Readers can add to the HSC phenotype database and obtain updated versions of Table S1 at http://www.bonemarrowhsc.com/hsc-phen/. We thank Chad Shaw and lab (BCM) for constructing the HSC phenotype database and for many insightful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolfsson J, Borge OJ, Bryder D, Theilgaard-Monch K, Astrand-Grundstrom I, Sitnicka E, Sasaki Y, Jacobsen SE. Upregulation of Flt3 expression within the bone marrow Lin(-)Sca1(+)c-kit(+) stem cell compartment is accompanied by loss of self-renewal capacity. Immunity. 2001;15:659–669. doi: 10.1016/s1074-7613(01)00220-5. [DOI] [PubMed] [Google Scholar]
- Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Baldridge MT, King KY, Boles NC, Weksberg DC, Goodell MA. Quiescent haematopoietic stem cells are activated by IFN-gamma in response to chronic infection. Nature. 2010;465:793–797. doi: 10.1038/nature09135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz C, Copley MR, Kent DG, Wohrer S, Cortes A, Aghaeepour N, Ma E, Mader H, Rowe K, Day C, et al. Hematopoietic stem cell subtypes expand differentially during development and display distinct lymphopoietic programs. Cell Stem Cell. 2012;10:273–283. doi: 10.1016/j.stem.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Berthet C, Rodriguez-Galan MC, Hodge DL, Gooya J, Pascal V, Young HA, Keller J, Bosselut R, Kaldis P. Hematopoiesis and thymic apoptosis are not affected by the loss of Cdk2. Mol Cell Biol. 2007;27:5079–5089. doi: 10.1128/MCB.00029-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boles NC, Lin KK, Lukov GL, Bowman TV, Baldridge MT, Goodell MA. CD48 on hematopoietic progenitors regulates stem cells and suppresses tumor formation. Blood. 2011;118:80–87. doi: 10.1182/blood-2010-12-322339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancelas JA, Lee AW, Prabhakar R, Stringer KF, Zheng Y, Williams DA. Rac GTPases differentially integrate signals regulating hematopoietic stem cell localization. Nat Med. 2005;11:886–891. doi: 10.1038/nm1274. [DOI] [PubMed] [Google Scholar]
- Capron C, Lecluse Y, Kaushik AL, Foudi A, Lacout C, Sekkai D, Godin I, Albagli O, Poullion I, Svinartchouk F, et al. The SCL relative LYL-1 is required for fetal and adult hematopoietic stem cell function and B-cell differentiation. Blood. 2006;107:4678–4686. doi: 10.1182/blood-2005-08-3145. [DOI] [PubMed] [Google Scholar]
- Challen GA, Sun D, Jeong M, Luo M, Jelinek J, Berg JS, Bock C, Vasanthakumar A, Gu H, Xi Y, et al. Dnmt3a is essential for hematopoietic stem cell differentiation. Nat Genet. 2012;44:23–31. doi: 10.1038/ng.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CZ, Li M, de Graaf D, Monti S, Gottgens B, Sanchez MJ, Lander ES, Golub TR, Green AR, Lodish HF. Identification of endoglin as a functional marker that defines long-term repopulating hematopoietic stem cells. Proc Natl Acad Sci U S A. 2002a;99:15468–15473. doi: 10.1073/pnas.202614899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Astle CM, Harrison DE. Hematopoietic stem cell functional failure in interleukin-2-deficient mice. J Hematother Stem Cell Res. 2002b;11:905–912. doi: 10.1089/152581602321080565. [DOI] [PubMed] [Google Scholar]
- Cheng T, Rodrigues N, Dombkowski D, Stier S, Scadden DT. Stem cell repopulation efficiency but not pool size is governed by p27(kip1) Nat Med. 2000a;6:1235–1240. doi: 10.1038/81335. [DOI] [PubMed] [Google Scholar]
- Cheng T, Rodrigues N, Shen H, Yang Y, Dombkowski D, Sykes M, Scadden DT. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000b;287:1804–1808. doi: 10.1126/science.287.5459.1804. [DOI] [PubMed] [Google Scholar]
- Christopher MJ, Liu F, Hilton MJ, Long F, Link DC. Suppression of CXCL12 production by bone marrow osteoblasts is a common and critical pathway for cytokine-induced mobilization. Blood. 2009;114:1331–1339. doi: 10.1182/blood-2008-10-184754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobas M, Wilson A, Ernst B, Mancini SJ, MacDonald HR, Kemler R, Radtke F. Beta-catenin is dispensable for hematopoiesis and lymphopoiesis. J Exp Med. 2004;199:221–229. doi: 10.1084/jem.20031615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobrinik D, Lee MH, Hannon G, Mulligan G, Bronson RT, Dyson N, Harlow E, Beach D, Weinberg RA, Jacks T. Shared role of the pRB-related p130 and p107 proteins in limb development. Genes & development. 1996;10:1633–1644. doi: 10.1101/gad.10.13.1633. [DOI] [PubMed] [Google Scholar]
- Daria D, Filippi MD, Knudsen ES, Faccio R, Li Z, Kalfa T, Geiger H. The retinoblastoma tumor suppressor is a critical intrinsic regulator for hematopoietic stem and progenitor cells under stress. Blood. 2008;111:1894–1902. doi: 10.1182/blood-2007-02-071746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf CA, Kauppi M, Baldwin T, Hyland CD, Metcalf D, Willson TA, Carpinelli MR, Smyth GK, Alexander WS, Hilton DJ. Regulation of hematopoietic stem cells by their mature progeny. Proc Natl Acad Sci U S A. 2010;107:21689–21694. doi: 10.1073/pnas.1016166108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMartino JF, Selleri L, Traver D, Firpo MT, Rhee J, Warnke R, O'Gorman S, Weissman IL, Cleary ML. The Hox cofactor and proto-oncogene Pbx1 is required for maintenance of definitive hematopoiesis in the fetal liver. Blood. 2001;98:618–626. doi: 10.1182/blood.v98.3.618. [DOI] [PubMed] [Google Scholar]
- Dykstra B, Kent D, Bowie M, McCaffrey L, Hamilton M, Lyons K, Lee SJ, Brinkman R, Eaves C. Long-term propagation of distinct hematopoietic differentiation programs in vivo. Cell Stem Cell. 2007;1:218–229. doi: 10.1016/j.stem.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Essers MA, Offner S, Blanco-Bose WE, Waibler Z, Kalinke U, Duchosal MA, Trumpp A. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature. 2009;458:904–908. doi: 10.1038/nature07815. [DOI] [PubMed] [Google Scholar]
- Feng CG, Weksberg DC, Taylor GA, Sher A, Goodell MA. The p47 GTPase Lrg-47 (Irgm1) links host defense and hematopoietic stem cell proliferation. Cell Stem Cell. 2008;2:83–89. doi: 10.1016/j.stem.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficara F, Murphy MJ, Lin M, Cleary ML. Pbx1 regulates self-renewal of long-term hematopoietic stem cells by maintaining their quiescence. Cell Stem Cell. 2008;2:484–496. doi: 10.1016/j.stem.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming HE, Janzen V, Lo Celso C, Guo J, Leahy KM, Kronenberg HM, Scadden DT. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell. 2008;2:274–283. doi: 10.1016/j.stem.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan B, Hu J, Jiang S, Liu Y, Sahin E, Zhuang L, Fletcher-Sananikone E, Colla S, Wang YA, Chin L, et al. Lkb1 regulates quiescence and metabolic homeostasis of haematopoietic stem cells. Nature. 2010;468:701–704. doi: 10.1038/nature09595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gekas C, Dieterlen-Lievre F, Orkin SH, Mikkola HK. The placenta is a niche for hematopoietic stem cells. Dev Cell. 2005;8:365–375. doi: 10.1016/j.devcel.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Ghiaur G, Ferkowicz MJ, Milsom MD, Bailey J, Witte D, Cancelas JA, Yoder MC, Williams DA. Rac1 is essential for intraembryonic hematopoiesis and for the initial seeding of fetal liver with definitive hematopoietic progenitor cells. Blood. 2008;111:3313–3321. doi: 10.1182/blood-2007-08-110114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Lasky JL, Chang CJ, Mosessian S, Lewis X, Xiao Y, Yeh JE, Chen JY, Iruela-Arispe ML, Varella-Garcia M, et al. Multi-genetic events collaboratively contribute to Pten-null leukaemia stem-cell formation. Nature. 2008;453:529–533. doi: 10.1038/nature06933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurumurthy S, Xie SZ, Alagesan B, Kim J, Yusuf RZ, Saez B, Tzatsos A, Ozsolak F, Milos P, Ferrari F, et al. The Lkb1 metabolic sensor maintains haematopoietic stem cell survival. Nature. 2010;468:659–663. doi: 10.1038/nature09572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MA, Curtis DJ, Metcalf D, Elefanty AG, Sourris K, Robb L, Gothert JR, Jane SM, Begley CG. The critical regulator of embryonic hematopoiesis, SCL, is vital in the adult for megakaryopoiesis, erythropoiesis, and lineage choice in CFU-S12. Proc Natl Acad Sci U S A. 2003;100:992–997. doi: 10.1073/pnas.0237324100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartner JC, Walkley CR, Lu J, Orkin SH. ADAR1 is essential for the maintenance of hematopoiesis and suppression of interferon signaling. Nat Immunol. 2009;10:109–115. doi: 10.1038/ni.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol. 2002;244:305–318. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- Hock H, Hamblen MJ, Rooke HM, Schindler JW, Saleque S, Fujiwara Y, Orkin SH. Gfi-1 restricts proliferation and preserves functional integrity of haematopoietic stem cells. Nature. 2004;431:1002–1007. doi: 10.1038/nature02994. [DOI] [PubMed] [Google Scholar]
- Ito K, Hirao A, Arai F, Matsuoka S, Takubo K, Hamaguchi I, Nomiyama K, Hosokawa K, Sakurada K, Nakagata N, et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- Iwasaki H, Somoza C, Shigematsu H, Duprez EA, Iwasaki-Arai J, Mizuno S, Arinobu Y, Geary K, Zhang P, Dayaram T, et al. Distinctive and indispensable roles of PU.1 in maintenance of hematopoietic stem cells and their differentiation. Blood. 2005;106:1590–1600. doi: 10.1182/blood-2005-03-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen M, Yang FC, Cancelas JA, Bailey JR, Williams DA. Rac2-deficient hematopoietic stem cells show defective interaction with the hematopoietic microenvironment and long-term engraftment failure. Stem Cells. 2005;23:335–346. doi: 10.1634/stemcells.2004-0216. [DOI] [PubMed] [Google Scholar]
- Juntilla MM, Patil VD, Calamito M, Joshi RP, Birnbaum MJ, Koretzky GA. AKT1 and AKT2 maintain hematopoietic stem cell function by regulating reactive oxygen species. Blood. 2010;115:4030–4038. doi: 10.1182/blood-2009-09-241000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang YJ, Yang SJ, Park G, Cho B, Min CK, Kim TY, Lee JS, Oh IH. A novel function of interleukin-10 promoting self-renewal of hematopoietic stem cells. Stem Cells. 2007;25:1814–1822. doi: 10.1634/stemcells.2007-0002. [DOI] [PubMed] [Google Scholar]
- Karlsson G, Blank U, Moody JL, Ehinger M, Singbrant S, Deng CX, Karlsson S. Smad4 is critical for self-renewal of hematopoietic stem cells. J Exp Med. 2007;204:467–474. doi: 10.1084/jem.20060465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, Saunders TL, Morrison SJ. Sox17 dependence distinguishes the transcriptional regulation of fetal from adult hematopoietic stem cells. Cell. 2007;130:470–483. doi: 10.1016/j.cell.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S, Roberts AW, Metcalf D, Alexander WS. Hematopoietic stem cell deficiencies in mice lacking c-Mpl, the receptor for thrombopoietin. Proc Natl Acad Sci U S A. 1998;95:1195–1200. doi: 10.1073/pnas.95.3.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King KY, Baldridge MT, Weksberg DC, Chambers SM, Lukov GL, Wu S, Boles NC, Jung SY, Qin J, Liu D, et al. Irgm1 protects hematopoietic stem cells by negative regulation of IFN signaling. Blood. 2011;118:1525–1533. doi: 10.1182/blood-2011-01-328682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King KY, Goodell MA. Inflammatory modulation of HSCs: viewing the HSC as a foundation for the immune response. Nature reviews Immunology. 2011;11:685–692. doi: 10.1038/nri3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirstetter P, Anderson K, Porse BT, Jacobsen SE, Nerlov C. Activation of the canonical Wnt pathway leads to loss of hematopoietic stem cell repopulation and multilineage differentiation block. Nat Immunol. 2006;7:1048–1056. doi: 10.1038/ni1381. [DOI] [PubMed] [Google Scholar]
- Ko M, Bandukwala HS, An J, Lamperti ED, Thompson EC, Hastie R, Tsangaratou A, Rajewsky K, Koralov SB, Rao A. Ten-Eleven-Translocation 2 (TET2) negatively regulates homeostasis and differentiation of hematopoietic stem cells in mice. Proc Natl Acad Sci U S A. 2011;108:14566–14571. doi: 10.1073/pnas.1112317108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozar K, Ciemerych MA, Rebel VI, Shigematsu H, Zagozdzon A, Sicinska E, Geng Y, Yu Q, Bhattacharya S, Bronson RT, et al. Mouse development and cell proliferation in the absence of D-cyclins. Cell. 2004;118:477–491. doi: 10.1016/j.cell.2004.07.025. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Osawa M, Jakt LM, Yoshikawa K, Nishikawa S. Necdin restricts proliferation of hematopoietic stem cells during hematopoietic regeneration. Blood. 2009;114:4383–4392. doi: 10.1182/blood-2009-07-230292. [DOI] [PubMed] [Google Scholar]
- Kuhn R, Schwenk F, Aguet M, Rajewsky K. Inducible gene targeting in mice. Science. 1995;269:1427–1429. doi: 10.1126/science.7660125. [DOI] [PubMed] [Google Scholar]
- Langer JC, Henckaerts E, Orenstein J, Snoeck HW. Quantitative trait analysis reveals transforming growth factor-beta2 as a positive regulator of early hematopoietic progenitor and stem cell function. J Exp Med. 2004;199:5–14. doi: 10.1084/jem.20030980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson J, Blank U, Helgadottir H, Bjornsson JM, Ehinger M, Goumans MJ, Fan X, Leveen P, Karlsson S. TGF-beta signaling-deficient hematopoietic stem cells have normal self-renewal and regenerative ability in vivo despite increased proliferative capacity in vitro. Blood. 2003;102:3129–3135. doi: 10.1182/blood-2003-04-1300. [DOI] [PubMed] [Google Scholar]
- Larsson J, Goumans MJ, Sjostrand LJ, van Rooijen MA, Ward D, Leveen P, Xu X, ten Dijke P, Mummery CL, Karlsson S. Abnormal angiogenesis but intact hematopoietic potential in TGF-beta type I receptor-deficient mice. The EMBO journal. 2001;20:1663–1673. doi: 10.1093/emboj/20.7.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence HJ, Christensen J, Fong S, Hu YL, Weissman I, Sauvageau G, Humphries RK, Largman C. Loss of expression of the Hoxa-9 homeobox gene impairs the proliferation and repopulating ability of hematopoietic stem cells. Blood. 2005;106:3988–3994. doi: 10.1182/blood-2005-05-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeCouter JE, Kablar B, Whyte PF, Ying C, Rudnicki MA. Strain-dependent embryonic lethality in mice lacking the retinoblastoma-related p130 gene. Development. 1998;125:4669–4679. doi: 10.1242/dev.125.23.4669. [DOI] [PubMed] [Google Scholar]
- Lin KK, Rossi L, Boles NC, Hall BE, George TC, Goodell MA. CD81 is essential for the re-entry of hematopoietic stem cells to quiescence following stress-induced proliferation via deactivation of the Akt pathway. PLoS Biol. 2011;9:e1001148. doi: 10.1371/journal.pbio.1001148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Wu HY, Wesselschmidt R, Kornaga T, Link DC. Impaired production and increased apoptosis of neutrophils in granulocyte colony-stimulating factor receptor-deficient mice. Immunity. 1996;5:491–501. doi: 10.1016/s1074-7613(00)80504-x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Elf SE, Asai T, Miyata Y, Sashida G, Huang G, Di Giandomenico S, Koff A, Nimer SD. The p53 tumor suppressor protein is a critical regulator of hematopoietic stem cell behavior. Cell Cycle. 2009a;8:3120–3124. doi: 10.4161/cc.8.19.9627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Elf SE, Miyata Y, Sashida G, Huang G, Di Giandomenico S, Lee JM, Deblasio A, Menendez S, Antipin J, et al. p53 regulates hematopoietic stem cell quiescence. Cell Stem Cell. 2009b;4:37–48. doi: 10.1016/j.stem.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luis TC, Naber BA, Roozen PP, Brugman MH, de Haas EF, Ghazvini M, Fibbe WE, van Dongen JJ, Fodde R, Staal FJ. Canonical wnt signaling regulates hematopoiesis in a dosage-dependent fashion. Cell Stem Cell. 2011;9:345–356. doi: 10.1016/j.stem.2011.07.017. [DOI] [PubMed] [Google Scholar]
- Luis TC, Weerkamp F, Naber BA, Baert MR, de Haas EF, Nikolic T, Heuvelmans S, De Krijger RR, van Dongen JJ, Staal FJ. Wnt3a deficiency irreversibly impairs hematopoietic stem cell self-renewal and leads to defects in progenitor cell differentiation. Blood. 2009;113:546–554. doi: 10.1182/blood-2008-06-163774. [DOI] [PubMed] [Google Scholar]
- Malumbres M, Sotillo R, Santamaria D, Galan J, Cerezo A, Ortega S, Dubus P, Barbacid M. Mammalian cells cycle without the D-type cyclin-dependent kinases Cdk4 and Cdk6. Cell. 2004;118:493–504. doi: 10.1016/j.cell.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Takeishi S, Kanie T, Susaki E, Onoyama I, Tateishi Y, Nakayama K, Nakayama KI. p57 is required for quiescence and maintenance of adult hematopoietic stem cells. Cell Stem Cell. 2011;9:262–271. doi: 10.1016/j.stem.2011.06.014. [DOI] [PubMed] [Google Scholar]
- Mayle A, Luo M, Jeong M, Goodell MA. Flow cytometry analysis of murine hematopoietic stem cells. Cytometry Part A : the journal of the International Society for Analytical Cytology. 2012 doi: 10.1002/cyto.a.22093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy KF, Ledney GD, Mitchell R. A deficiency of hematopoietic stem cells in steel mice. Cell and tissue kinetics. 1977;10:121–126. doi: 10.1111/j.1365-2184.1977.tb00137.x. [DOI] [PubMed] [Google Scholar]
- Mercier FE, Ragu C, Scadden DT. The bone marrow at the crossroads of blood and immunity. Nature reviews Immunology. 2012;12:49–60. doi: 10.1038/nri3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkola HK, Klintman J, Yang H, Hock H, Schlaeger TM, Fujiwara Y, Orkin SH. Haematopoietic stem cells retain long-term repopulating activity and multipotency in the absence of stem-cell leukaemia SCL/tal-1 gene. Nature. 2003;421:547–551. doi: 10.1038/nature01345. [DOI] [PubMed] [Google Scholar]
- Min IM, Pietramaggiori G, Kim FS, Passegue E, Stevenson KE, Wagers AJ. The transcription factor EGR1 controls both the proliferation and localization of hematopoietic stem cells. Cell Stem Cell. 2008;2:380–391. doi: 10.1016/j.stem.2008.01.015. [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Araki KY, Naka K, Arai F, Takubo K, Yamazaki S, Matsuoka S, Miyamoto T, Ito K, Ohmura M, et al. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell. 2007;1:101–112. doi: 10.1016/j.stem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Moran-Crusio K, Reavie L, Shih A, Abdel-Wahab O, Ndiaye-Lobry D, Lobry C, Figueroa ME, Vasanthakumar A, Patel J, Zhao X, et al. Tet2 loss leads to increased hematopoietic stem cell self-renewal and myeloid transformation. Cancer Cell. 2011;20:11–24. doi: 10.1016/j.ccr.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Sieburg CE, Sieburg HB, Bernitz JM, Cattarossi G. Stem cell heterogeneity: implications for aging and regenerative medicine. Blood. 2012;119:3900–3907. doi: 10.1182/blood-2011-12-376749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakada D, Saunders TL, Morrison SJ. Lkb1 regulates cell cycle and energy metabolism in haematopoietic stem cells. Nature. 2010;468:653–658. doi: 10.1038/nature09571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naramura M, Nandwani N, Gu H, Band V, Band H. Rapidly fatal myeloproliferative disorders in mice with deletion of Casitas B-cell lymphoma (Cbl) and Cbl-b in hematopoietic stem cells. Proc Natl Acad Sci U S A. 2010;107:16274–16279. doi: 10.1073/pnas.1007575107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen H, Rendl M, Fuchs E. Tcf3 governs stem cell features and represses cell fate determination in skin. Cell. 2006;127:171–183. doi: 10.1016/j.cell.2006.07.036. [DOI] [PubMed] [Google Scholar]
- Nie Y, Han YC, Zou YR. CXCR4 is required for the quiescence of primitive hematopoietic cells. J Exp Med. 2008;205:777–783. doi: 10.1084/jem.20072513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park IK, Qian D, Kiel M, Becker MW, Pihalja M, Weissman IL, Morrison SJ, Clarke MF. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature. 2003;423:302–305. doi: 10.1038/nature01587. [DOI] [PubMed] [Google Scholar]
- Puri MC, Bernstein A. Requirement for the TIE family of receptor tyrosine kinases in adult but not fetal hematopoiesis. Proc Natl Acad Sci U S A. 2003;100:12753–12758. doi: 10.1073/pnas.2133552100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purton LE, Scadden DT. Limiting factors in murine hematopoietic stem cell assays. Cell Stem Cell. 2007;1:263–270. doi: 10.1016/j.stem.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Qian H, Buza-Vidas N, Hyland CD, Jensen CT, Antonchuk J, Mansson R, Thoren LA, Ekblom M, Alexander WS, Jacobsen SE. Critical role of thrombopoietin in maintaining adult quiescent hematopoietic stem cells. Cell Stem Cell. 2007;1:671–684. doi: 10.1016/j.stem.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Qian Z, Chen L, Fernald AA, Williams BO, Le Beau MM. A critical role for Apc in hematopoietic stem and progenitor cell survival. J Exp Med. 2008;205:2163–2175. doi: 10.1084/jem.20080578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quivoron C, Couronne L, Della Valle V, Lopez CK, Plo I, Wagner-Ballon O, Do Cruzeiro M, Delhommeau F, Arnulf B, Stern MH, et al. TET2 inactivation results in pleiotropic hematopoietic abnormalities in mouse and is a recurrent event during human lymphomagenesis. Cancer Cell. 2011;20:25–38. doi: 10.1016/j.ccr.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Raaijmakers MH, Mukherjee S, Guo S, Zhang S, Kobayashi T, Schoonmaker JA, Ebert BL, Al-Shahrour F, Hasserjian RP, Scadden EO, et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature. 2010;464:852–857. doi: 10.1038/nature08851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathinam C, Matesic LE, Flavell RA. The E3 ligase Itch is a negative regulator of the homeostasis and function of hematopoietic stem cells. Nat Immunol. 2011;12:399–407. doi: 10.1038/ni.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathinam C, Thien CB, Langdon WY, Gu H, Flavell RA. The E3 ubiquitin ligase c-Cbl restricts development and functions of hematopoietic stem cells. Genes Dev. 2008;22:992–997. doi: 10.1101/gad.1651408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebel VI, Hartnett S, Hill GR, Lazo-Kallanian SB, Ferrara JL, Sieff CA. Essential role for the p55 tumor necrosis factor receptor in regulating hematopoiesis at a stem cell level. J Exp Med. 1999;190:1493–1504. doi: 10.1084/jem.190.10.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebel VI, Kung AL, Tanner EA, Yang H, Bronson RT, Livingston DM. Distinct roles for CREB-binding protein and p300 in hematopoietic stem cell self-renewal. Proc Natl Acad Sci U S A. 2002;99:14789–14794. doi: 10.1073/pnas.232568499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renstrom J, Istvanffy R, Gauthier K, Shimono A, Mages J, Jardon-Alvarez A, Kroger M, Schiemann M, Busch DH, Esposito I, et al. Secreted frizzled-related protein 1 extrinsically regulates cycling activity and maintenance of hematopoietic stem cells. Cell Stem Cell. 2009;5:157–167. doi: 10.1016/j.stem.2009.05.020. [DOI] [PubMed] [Google Scholar]
- Sandberg ML, Sutton SE, Pletcher MT, Wiltshire T, Tarantino LM, Hogenesch JB, Cooke MP. c-Myb and p300 regulate hematopoietic stem cell proliferation and differentiation. Dev Cell. 2005;8:153–166. doi: 10.1016/j.devcel.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Santibanez JF, Letamendia A, Perez-Barriocanal F, Silvestri C, Saura M, Vary CP, Lopez-Novoa JM, Attisano L, Bernabeu C. Endoglin increases eNOS expression by modulating Smad2 protein levels and Smad2-dependent TGF-beta signaling. J Cell Physiol. 2007;210:456–468. doi: 10.1002/jcp.20878. [DOI] [PubMed] [Google Scholar]
- Sato T, Onai N, Yoshihara H, Arai F, Suda T, Ohteki T. Interferon regulatory factor-2 protects quiescent hematopoietic stem cells from type I interferon-dependent exhaustion. Nat Med. 2009;15:696–700. doi: 10.1038/nm.1973. [DOI] [PubMed] [Google Scholar]
- Schaniel C, Sirabella D, Qiu J, Niu X, Lemischka IR, Moore KA. Wnt-inhibitory factor 1 dysregulation of the bone marrow niche exhausts hematopoietic stem cells. Blood. 2011;118:2420–2429. doi: 10.1182/blood-2010-09-305664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller M, Huelsken J, Rosenbauer F, Taketo MM, Birchmeier W, Tenen DG, Leutz A. Hematopoietic stem cell and multilineage defects generated by constitutive beta-catenin activation. Nat Immunol. 2006;7:1037–1047. doi: 10.1038/ni1387. [DOI] [PubMed] [Google Scholar]
- Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- Singbrant S, Moody JL, Blank U, Karlsson G, Umans L, Zwijsen A, Karlsson S. Smad5 is dispensable for adult murine hematopoiesis. Blood. 2006;108:3707–3712. doi: 10.1182/blood-2006-02-003384. [DOI] [PubMed] [Google Scholar]
- Sirin O, Lukov GL, Mao R, Conneely OM, Goodell MA. The orphan nuclear receptor Nurr1 restricts the proliferation of haematopoietic stem cells. Nat Cell Biol. 2010;12:1213–1219. doi: 10.1038/ncb2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitnicka E, Lin N, Priestley GV, Fox N, Broudy VC, Wolf NS, Kaushansky K. The effect of thrombopoietin on the proliferation and differentiation of murine hematopoietic stem cells. Blood. 1996;87:4998–5005. [PubMed] [Google Scholar]
- Souroullas GP, Salmon JM, Sablitzky F, Curtis DJ, Goodell MA. Adult hematopoietic stem and progenitor cells require either Lyl1 or Scl for survival. Cell Stem Cell. 2009;4:180–186. doi: 10.1016/j.stem.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenvers KL, Tursky ML, Harder KW, Kountouri N, Amatayakul-Chantler S, Grail D, Small C, Weinberg RA, Sizeland AM, Zhu HJ. Heart and liver defects and reduced transforming growth factor beta2 sensitivity in transforming growth factor beta type III receptor-deficient embryos. Mol Cell Biol. 2003;23:4371–4385. doi: 10.1128/MCB.23.12.4371-4385.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova L, Sorrentino BP. A limited role for p16Ink4a and p19Arf in the loss of hematopoietic stem cells during proliferative stress. Blood. 2005;106:827–832. doi: 10.1182/blood-2004-06-2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stier S, Ko Y, Forkert R, Lutz C, Neuhaus T, Grunewald E, Cheng T, Dombkowski D, Calvi LM, Rittling SR, et al. Osteopontin is a hematopoietic stem cell niche component that negatively regulates stem cell pool size. J Exp Med. 2005;201:1781–1791. doi: 10.1084/jem.20041992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura N, Huang XL, Naruse T, Hamaguchi I, Dumont DJ, Yancopoulos GD, Suda T. Critical role of the TIE2 endothelial cell receptor in the development of definitive hematopoiesis. Immunity. 1998;9:677–686. doi: 10.1016/s1074-7613(00)80665-2. [DOI] [PubMed] [Google Scholar]
- Takubo K, Goda N, Yamada W, Iriuchishima H, Ikeda E, Kubota Y, Shima H, Johnson RS, Hirao A, Suematsu M, et al. Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell. 2010;7:391–402. doi: 10.1016/j.stem.2010.06.020. [DOI] [PubMed] [Google Scholar]
- Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, McDowell EP, Lazo-Kallanian S, Williams IR, Sears C, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- van Os R, Kamminga LM, Ausema A, Bystrykh LV, Draijer DP, van Pelt K, Dontje B, de Haan G. A Limited role for p21Cip1/Waf1 in maintaining normal hematopoietic stem cell functioning. Stem Cells. 2007;25:836–843. doi: 10.1634/stemcells.2006-0631. [DOI] [PubMed] [Google Scholar]
- Viatour P, Somervaille TC, Venkatasubrahmanyam S, Kogan S, McLaughlin ME, Weissman IL, Butte AJ, Passegue E, Sage J. Hematopoietic stem cell quiescence is maintained by compound contributions of the retinoblastoma gene family. Cell Stem Cell. 2008;3:416–428. doi: 10.1016/j.stem.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, Jaworski M, Offner S, Dunant CF, Eshkind L, Bockamp E, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135:1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- Yamashita H, Ichijo H, Grimsby S, Moren A, ten Dijke P, Miyazono K. Endoglin forms a heteromeric complex with the signaling receptors for transforming growth factor-beta. J Biol Chem. 1994;269:1995–2001. [PubMed] [Google Scholar]
- Yamazaki S, Ema H, Karlsson G, Yamaguchi T, Miyoshi H, Shioda S, Taketo MM, Karlsson S, Iwama A, Nakauchi H. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell. 2011;147:1146–1158. doi: 10.1016/j.cell.2011.09.053. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Iwama A, Takayanagi SI, Eto K, Ema H, Nakauchi H. TGF-{beta} as a candidate bone marrow niche signal to induce hematopoietic stem cell hibernation. Blood. 2008 doi: 10.1182/blood-2008-04-146480. [DOI] [PubMed] [Google Scholar]
- Yilmaz OH, Valdez R, Theisen BK, Guo W, Ferguson DO, Wu H, Morrison SJ. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Shen H, Franklin DS, Scadden DT, Cheng T. In vivo self-renewing divisions of haematopoietic stem cells are increased in the absence of the early G1-phase inhibitor, p18INK4C. Nat Cell Biol. 2004;6:436–442. doi: 10.1038/ncb1126. [DOI] [PubMed] [Google Scholar]
- Zhang CC, Steele AD, Lindquist S, Lodish HF. Prion protein is expressed on long-term repopulating hematopoietic stem cells and is important for their self-renewal. Proc Natl Acad Sci U S A. 2006a;103:2184–2189. doi: 10.1073/pnas.0510577103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Grindley JC, Yin T, Jayasinghe S, He XC, Ross JT, Haug JS, Rupp D, Porter-Westpfahl KS, Wiedemann LM, et al. PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature. 2006b;441:518–522. doi: 10.1038/nature04747. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Stehling-Sun S, Lezon-Geyda K, Juneja SC, Coillard L, Chatterjee G, Wuertzer CA, Camargo F, Perkins AS. PR-domain-containing Mds1-Evi1 is critical for long-term hematopoietic stem cell function. Blood. 2011;118:3853–3861. doi: 10.1182/blood-2011-02-334680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Blum J, Chen A, Kwon HY, Jung SH, Cook JM, Lagoo A, Reya T. Loss of beta-catenin impairs the renewal of normal and CML stem cells in vivo. Cancer Cell. 2007;12:528–541. doi: 10.1016/j.ccr.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou P, Yoshihara H, Hosokawa K, Tai I, Shinmyozu K, Tsukahara F, Maru Y, Nakayama K, Nakayama KI, Suda T. p57(Kip2) and p27(Kip1) cooperate to maintain hematopoietic stem cell quiescence through interactions with Hsc70. Cell Stem Cell. 2011;9:247–261. doi: 10.1016/j.stem.2011.07.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.