Abstract
The distribution and dynamic changes of CD4+ CD25+ FoxP3+ and CD4+ CD25− FoxP3+ regulatory T (Treg) cells induced by human rotavirus (HRV) infection and vaccination were examined in neonatal gnotobiotic pigs infected with virulent HRV (VirHRV) or vaccinated with attenuated HRV (AttHRV). Subsets of gnotobiotic pigs in the AttHRV and control groups were challenged with VirHRV at post-inoculation day (PID) 28. We demonstrated that VirHRV infection or AttHRV vaccination reduced frequencies and numbers of tissue-residing Treg cells, and decreased the frequencies of interleukin-10 (IL-10) and transforming growth factor-β (TGF-β) producing CD4+ CD25− Treg cells in ileum, spleen and blood at PID 28. The frequencies of IL-10 and TGF-β producing CD4+ CD25− Treg cells in all sites at PID 28 were significantly inversely correlated with the protection rate against VirHRV-caused diarrhoea (r = −1, P < 0·0001). Hence, higher frequencies of functional CD4+ CD25− Treg cells can be an indicator for poorer protective immunity against rotavirus. Our results highlighted the importance of CD4+ CD25− Treg cells over CD4+ CD25+ Treg cells in rotavirus infection and immunity. AttHRV vaccination (induction of immune effector responses) reduced the expansion of CD4+ CD25− Treg cells in ileum seen in the challenged naive pigs during the acute phase of VirHRV infection and preserved normal levels of intestinal TGF-β producing Treg cells post-challenge. The reduced suppressive effect of Treg cells in AttHRV-vaccinated pigs would unleash effector/memory T-cell activation upon challenge. Preserving TGF-β producing CD4+ CD25− Treg cells is important in maintaining homeostasis. Based on our findings, a model is proposed to depict the dynamic equilibrium course of Treg and effector T-cell responses after primary rotavirus infection/vaccination and challenge.
Keywords: gnotobiotic pigs, regulatory T cells, rotavirus infection, vaccination
Introduction
Rotavirus is the commonest cause of severe viral gastroenteritis in infants and young children. Rotavirus infection is estimated to cause 527 000 deaths in children under 5 years of age annually worldwide, 85% of whom live in developing countries.1 Symptoms associated with rotavirus disease typically are diarrhoea and vomiting accompanied by fever, nausea, anorexia, cramping and malaise. Rotavirus-induced symptoms typically appear in 1–2 days and rotavirus diarrhoea ceases 4–7 days after infection.2 The primary site of rotavirus replication is the mature enterocytes at the tips of villi in the small intestine where virus replication causes mononuclear cell infiltration and villous atrophy during the acute phase of infection in neonatal pigs and humans.3 Rotavirus also spreads beyond the gastrointestinal tract and causes an acute phase of viraemia.4,5
Viral infections often lead to a short-term, generalized immunosuppression characterized by the down-regulation of virus-specific CD4+ and CD8+ T-cell responses and non-specific inflammation.6 One of the mechanisms for the virus-induced immunosuppression is the induction of regulatory T (Treg) cell responses.7–11 Treg-cell-mediated suppression can be either beneficial (e.g. control of immune pathology10,12) or detrimental (e.g. subverting protective immunity13) to the host defence depending on the context,14 but early studies only examined CD4+ CD25+ Treg cells or did not distinguish between CD4+ CD25+ and CD4+ CD25− Treg cells.
Previous studies showed that murine rotavirus infection induced an expansion of the Treg-cell population (CD4+ CD25+ FoxP3+) in mesenteric lymph nodes (MLN) and spleen in mice.9 Depletion of CD25+ Treg cells by using anti-CD25 monoclonal antibodies before rotavirus inoculation enhanced virus-specific interferon-γ (IFN-γ) producing T-cell responses after infection. The study also demonstrated that CD4+ CD25+ Treg-cell depletion had no effect on either the degree of viral replication and shedding or the severity of diseases after primary infection, suggesting that the immunosuppressive effects of CD4+ CD25+ Treg cells are not sufficient to modify primary rotavirus infection.9 Another study using peripheral blood mononuclear cells (PBMC) from adult human volunteers showed that depletion of CD25+ cells significantly increased frequencies of rotavirus-specific IFN-γ producing T cells, suggesting that the IFN-γ+ memory T-cell response against rotavirus is modulated by CD25+ cells.8 Hence, the role of Treg cells in rotavirus infection and immunity is still an area of immunological interest because there remain many unaddressed questions.
Previous studies on Treg-cell responses to rotavirus infections did not distinguish CD4+ CD25− FoxP3+ Treg cells from CD4+ CD25+ FoxP3+ Treg cells.8,9 Natural Treg cells develop in the thymus under the influence of self-antigens15 and mainly function to prevent auto-immune and exaggerated immune responses16 even though they also down-regulate the immune responses to some pathogen infections.17,18 Natural Treg cells enter the peripheral sites as CD4+ CD25+ T cells19 and are defined as CD4+ CD25+ FoxP3+ cells by the expression of the forkhead box P3 (FoxP3), a transcription factor essential for programming and developing Treg cells.20 Inducible Treg cells are developed from naive CD4+ CD25− T cells under specific antigen exposure or cytokine stimulation in peripheral sites,20 and are important in regulating inflammatory responses to pathogens.16 Inducible Treg cells include a major subset of CD4+ CD25− FoxP3+ cells (with the stimulation of highly antigenic epitopes21) and a relatively small subset of CD4+ CD25+ FoxP3+ cells (with the stimulation of weak antigenic epitopes). Hence, the inducible Treg cells function differentially from natural Treg cells when facing invading pathogens. Our recent study demonstrated that after rotavirus infection and lactobacillus colonization, the frequencies of CD4+ CD25− FoxP3+ cells were substantially higher than of CD4+ CD25+ FoxP3+ cells in both intestinal and systemic lymphoid tissues in gnotobiotic (Gn) pigs.22
Treg cells are considered to play an important role in regulating protective immunity. However, the extent to which they limit optimal host defences against infection is not well understood. Our hypothesis is that the number of functioning Treg cells present at the time of challenge – mostly inducible Treg cells, which will be down-regulated by the immune effector responses established after vaccination – is highly influential to the efficacy of rotavirus vaccines. A new model was recently suggested by Rowe et al.14 that during immune homeostasis, Treg cells actively suppress effector cell activation. Following immune stimulation, Treg-cell suppression is dampened, allowing effector T-cell activation through previously described cell intrinsic stimulation signals [T-cell receptor (signal 1), co-stimulation (signal 2) and inflammatory cytokines (signal 3)]. Over-riding Treg-cell-mediated suppression represents a prerequisite ‘signal zero’ that, together with other stimulation signals and inflammatory cytokines, is essential for T-cell activation in vivo.14
The goal of the present study is to address some of these questions using the well-established Gn pig model of human rotavirus (HRV) infection and disease,23 including (i) whether CD4+ CD25+ and CD4+ CD25− Treg cells react differently to rotavirus infection/vaccination; (ii) whether the number of Treg cells present at the time of challenge has an effect on the resistance to rotavirus re-infection; and (iii) how the primary rotavirus infection or vaccination modulates Treg-cell responses in different lymphoid tissues? It is known that Treg-cell phenotype and function in swine are similar to those in humans and mice,24 making Gn pigs an excellent model to address these questions.
One of the functional mechanisms of Treg cells is to secret the immunosuppressive cytokines interleukin-10 (IL-10) and transforming growth factor-β (TGF-β).20 Blocking TGF-β or inhibiting the TGF-β receptor 1 signalling pathway increased the frequencies of rotavirus-specific IFN-γ producing T cells in the blood of adult humans,8 suggesting that TGF-β was involved in the immunosuppressive functions of Treg cells. Kim et al.9 found that the levels of IL-10 decreased significantly in the MLN of CD25-depleted mice compared with the controls at post-inoculation day (PID) 7, indicating that Treg cells contribute significantly to IL-10 production in the MLN. In this study, we evaluated the distribution and magnitude of total CD4+ FoxP3+ Treg cells and CD4+ CD25+ FoxP3+ and CD4+ CD25− FoxP3+ Treg-cell subsets in ileum, intraepithelial lymphocytes (IEL), spleen and blood of Gn pigs and the frequencies of IL-10 and TGF-β production by the Treg-cell subsets in each anatomic site after vaccination with the attenuated human rotavirus (AttHRV) or infection with the virulent HRV (VirHRV) compared with the mock-inoculated control pigs.
Materials and methods
Virus
The cell culture-adapted Wa strain (G1P1A[8]) AttHRV, derived from the 35th passage in African green monkey kidney cells (MA104), was used as an oral vaccine for inoculation of Gn pigs at a dose of 5 × 107 fluorescent focus-forming units (FFU).25 The AttHRV was also used as stimulating antigen in the intracellular IFN-γ staining assay as described previously.26
The Wa strain VirHRV was passaged through Gn pigs and the pooled intestinal contents from the 27th passage were collected and used for primary infection and challenge of Gn pigs at a dose of 1 × 105 FFU. The 50% infectious dose (ID50) and 50% diarrhoea dose (DD50) of the VirHRV in Gn pigs were determined as approximately 1 FFU.27
Inoculation of Gn pigs
Near-term pigs were derived by hysterectomy and maintained in germ-free isolator units as described previously.28 All piglets were confirmed seronegative for rotavirus antibodies and germ-free before HRV exposure. The Gn pigs (both males and females) were randomly assigned to three treatment groups: (1) VirHRV-infected, (2) AttHRV-vaccinated and (3) mock-inoculated controls (Control). Pigs in the VirHRV group were orally inoculated with 1 × 105 FFU VirHRV diluted in 5 ml Dulbecco's modified Eagle's medium (DMEM) at 5 days of age (PID 0). Pigs in the AttHRV group were orally inoculated with 5 × 107 FFU AttHRV in 5 ml DMEM at PID 0 and again at PID 10. Control pigs were given an equal volume of DMEM. At PID 28, subsets of pigs from the AttHRV-vaccinated and Control groups were orally challenged with 1 × 105 FFU VirHRV (AttHRV/VirHRV and Mock/VirHRV). Pigs were given 8 ml of 100 mm sodium bicarbonate to reduce gastric acidity 20 min before all inoculations. Pigs were killed on PID 28 or PID 35 [post-challenge day (PCD) 7]. Mononuclear cells (MNC) from ileum, spleen and peripheral blood were isolated as previously described25 and IEL were collected by incubating ileum with EDTA buffer before being processed for MNC isolation. All animal experimental procedures were conducted in accordance with protocols approved by the Institutional Animal Care and Use Committees of Virginia Polytechnic Institute and State University.
Control pigs killed on PID 28 were used in the comparisons among treatment groups for both PID 28 and PCD 7. Studies have shown that the number and function of Treg cells are remarkably stable throughout life,29 especially without the stimulation of external antigens. As the control pigs were not stimulated with any external antigens, the frequency, number and cytokine expression of Treg cells in the normal naive Gn pigs between the age of 33 days [mock-inoculated Gn pigs, PID 28 (PCD 0)] and 40 days [mock-inoculated/mock-challenged Gn pigs, PID 35 (PCD 7)] should be similar. The HRV-specific IFN-γ producing CD4+ T cells in both mock-inoculated control pigs and mock-challenged control pigs are expected to be non-detectable or at very low levels (non-specific background). Therefore the mock-challenged control pig group at PCD 7 was not included to save animal resources and costs. The Gn pig treatment groups and timeline of treatments are listed in the Supplementary material, Table S1.
Clinical signs and protection
Pigs were observed daily for diarrhoea and virus shedding for 7 days post-challenge and virus shedding was determined from faecal swab samples as described elsewhere.25 Faecal consistency was scored as follows: 0: normal; 1: pasty; 2: semi-liquid; 3: liquid. Pigs with daily faecal scores ≥ 2 were considered diarrhoeic. Protection rate against diarrhoea upon challenge was calculated as [1 − (percentage of AttHRV-inoculated pigs with diarrhoea/percentage of mock-inoculated control pigs with diarrhoea)] × 100.
Intracellular cytokine staining and flow cytometry analysis of IL-10 and TGF-β producing CD4+ CD25+ and CD4+ CD25− Treg cells and IFN-γ producing CD4+ T cells (Th1 cell)
Flow cytometry was used to determine frequencies of FoxP3+ Treg cells among CD4+ T cells, frequencies of CD4+ CD25+ FoxP3+ and CD4+ CD25− FoxP3+ Treg-cell subsets and IL-10 and TGF-β producing CD25+ and CD25− Treg cells in ileum, IEL, spleen and blood of Gn pigs as previously described.22 Briefly, the MNC (2 × 106 cells/tube) were first stained at 4° for 15 min with FITC-conjugated mouse anti-porcine CD4 (IgG2b, clone 74-12-4, BD Pharmingen, San Jose, CA) and mouse anti-porcine CD25 (IgG1, clone K231.3B2, AbDSerotec, Raleigh, NC), followed by allophycocyanin (APC) conjugated rat anti-mouse IgG1 (IgG1, clone X56, BD Pharmingen). The MNC were then permeabilized with FoxP3 Staining Buffer Set (eBioscience, San Diego, CA) at 4° for 30 min and stained with the phycoerythrin-cyanine tandem fluorochrome (PE-Cy7) -conjugated rat anti-mouse/rat FoxP3 (IgG2a, clone FJK-16s, eBioscience), biotin-conjugated mouse anti-porcine IL-10 (IgG1, clone 945A1A926C2, Cell Sciences, Sharon, MA), and PE-conjugated mouse anti-human TGF-β1 (IgG1, clone 27232, R&D Systems, Minneapolis, MN), followed by staining with streptavidin conjugated Pacific blue (Invitrogen, Carlsbad, CA) at 4° for 30 min. The appropriate isotype-matched irrelevant control antibody PE-conjugated mouse IgG1 isotype control (IgG1, clone P3.6.2.8.2, eBioscience, San Diego, CA) and the secondary antibody streptavidin-conjugated Pacific blue (Invitrogen) were used for staining as negative controls for TGF-β1 and IL-10, respectively. At least 100 000 cells were acquired on a FACSAria flow cytometer (BD Biosciences, San Diego, CA). Frequencies of HRV-specific IFN-γ producing CD3+ CD4+ T cells in ileum, IEL, spleen and blood of Gn pigs were determined by using flow cytometry as previously described.26 Data were analysed using FlowJo 7.2.2 software (Tree Star, Ashland, OR). The absolute numbers of Treg cells per tissue were calculated based on the frequencies of Treg cells and the total number of MNC isolated from each tissue. The frequencies of FoxP3+ cells were expressed as percentages among CD4+ cells. The frequencies of CD4+ CD25+ FoxP3+ and CD4+ CD25− FoxP3+ cells were expressed as the percentages among gated MNC. The frequencies of IL-10+ or TGF-β+ CD4+CD25+ FoxP3+ and CD4+ CD25− FoxP3+ Treg cells were expressed as the percentages of IL-10+ or TGF-β+ cells among the Treg cells. The frequencies of IFN-γ+ CD4+ T cells were expressed as percentages among total CD3+ T cells. All mean frequencies are reported after subtraction of the background frequencies.
Statistical analysis
Non-parametric Kruskal–Wallis rank sum test was performed to compare frequencies of FoxP3+ Treg cells, frequencies and the absolute number of CD25+ and CD25− Treg cells, frequencies of cytokine producing Treg cells, and frequencies of IFN-γ producing CD4+ T cells in ileum, IEL, spleen and blood among the VirHRV, AttHRV and Control groups at PID 28, and Mock/VirHRV, AttHRV/VirHRV and Control group at PCD 7. When differences among these groups were detected, the same test was used in a pairwise fashion to clarify the nature of the differences. Correlations between protection rate against diarrhoea and frequencies of cytokine producing Treg cells were analysed using Spearman's correlation coefficient. All statistical analyses were performed using SAS program 9·2 (SAS Institute, Cary, NC). All statistical significance was assessed at P < 0·05.
Results
VirHRV infection or AttHRV vaccination reduced tissue-residing Treg cells but increased Treg cells in the circulation at PID 28
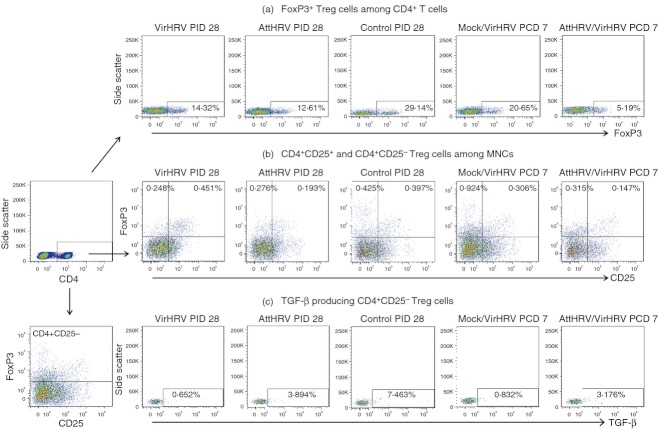
The representative flow cytometry dot plots for detection of FoxP3+ Treg cells among CD4+ T cells, the CD25+ and CD25− Treg-cell subsets and the TGF-β producing CD25− Treg cells among the five treatment groups are depicted in Fig. 1.
Figure 1.
Representative dot plots of frequencies of FoxP3+ regulatory T (Treg) cells among CD4+ T cells (a), CD4+ CD25+ and CD4+ CD25− Treg cells (b), and transforming growth factor-β (TGF-β) producing CD4+ CD25− Treg cells (c) in ileum of gnotobiotic (Gn) pigs from the five treatment groups. Mononuclear cells (MNC) were stained freshly without in vitro stimulation (same for MNC in all Treg-cell figures). In panels a and c, the numbers in the rectangles in dot plots are the frequencies of FoxP3+ Treg cells among CD4+ T cells and TGF-β+ cells among CD4+ CD25− Treg cells, respectively. In panel b, the numbers at the upper left and right corners of dot plots are the frequencies of CD4+ CD25− Treg cells (CD4+ CD25− FoxP3+) and CD4+ CD25+ Treg cells (CD4+ CD25− FoxP3+) respectively, among gated MNC.
Frequencies of FoxP3+ Treg cells, and frequencies and absolute numbers of CD4+ CD25+ and CD4+ CD25− Treg cells in the intestinal lymphoid tissues (ileum and IEL) are depicted in Fig. 2, and in the systemic lymphoid tissues (spleen and blood) in Fig. 3. In all the figures (Figs 2–5), different lowercase letters above bars indicate significant differences among VirHRV infection, AttHRV inoculation and Control groups at PID 28; whereas different capital letters above bars indicate significant differences among Mock/VirHRV, AttHRV/VirHRV groups at PCD 7 and the Control group at PID 28 for the same cell type and tissue (Kruskal–Wallis test, P < 0·05).
Figure 2.
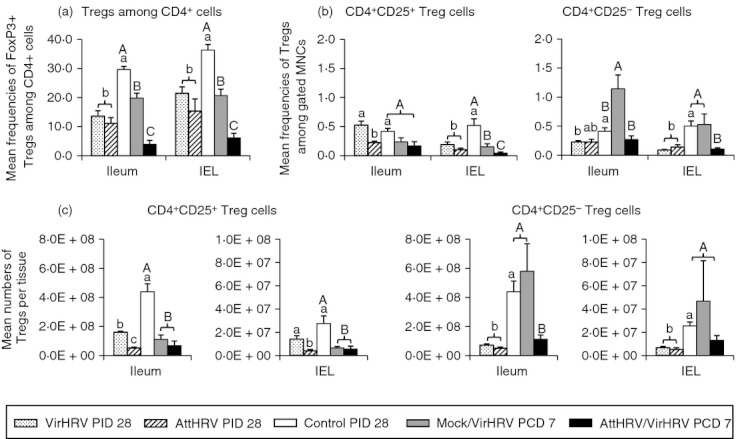
Frequencies of FoxP3+ regulatory T (Treg) cells among CD4+ cells (a), CD4+ CD25+ and CD4+ CD25− Treg cells among mononuclear cells (MNC) (b), and numbers of CD4+ CD25+ and CD4+ CD25− Treg cells per tissue (c) in intestinal lymphoid tissues of gnotobiotic (Gn) pigs. The graph in panel a shows the frequencies of FoxP3+ cells among CD4+ cells. The two graphs in panel b show the frequencies of CD4+ CD25+ and CD4+ CD25− Treg cells, respectively. The two graphs in panel c show the absolute numbers of CD4+ CD25+ and CD4+ CD25− Treg cells per tissue, respectively. Data are presented as mean frequency or number ± SEM (n = 3 to n = 6). Different lowercase letters above bars indicate significant differences in frequencies compared among virulent human rotavirus (VirHRV) infection, attenuated human rotavirus (AttHRV) inoculation and control groups at PID 28 for the same cell type and tissue (Kruskal–Wallis test, P < 0·05); different capital letters above bars indicate significant differences in frequencies compared among Mock/VirHRV, AttHRV/VirHRV groups at post-challenge day (PCD) 7 and the control group at post-inoculation day (PID) 28 for the same cell type and tissue (Kruskal–Wallis test, P < 0·05), whereas shared letters indicate no significant difference.
Figure 3.
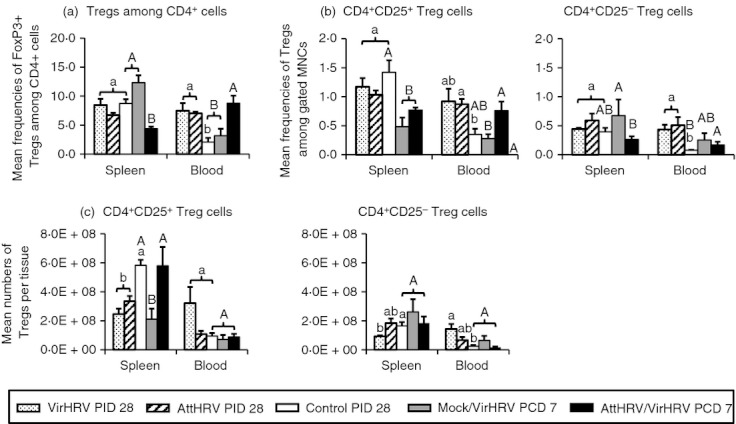
Frequencies of FoxP3+ regulatory T (Treg) cells among CD4+ cells (a), CD4+ CD25+ and CD4+ CD25− Treg cells among mononuclear cells (MNC) (b), and numbers of CD4+ CD25+ and CD4+ CD25− Treg cells per tissue (c) in spleen and blood of gnotobiotic (Gn) pigs. See Fig. 2 legend for panel description and statistical analysis.
Figure 5.
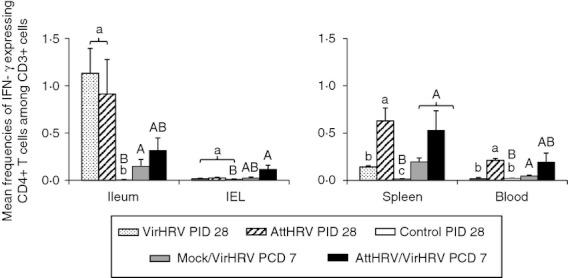
Human rotavirus (HRV)-specific interferon-γ (IFN-γ) producing CD4+ T-cell responses in intestinal and systemic lymphoid tissues of gnotobiotic (Gn) pigs. Mononuclear cells (MNC) were stimulated with semi-purified attenuated HRV (AttHRV) antigen in vitro for 17 hr. Brefeldin A was added for the last 5 hr to block secretion of cytokines produced by the T cells. IFN-γ production was detected by intracellular staining and flow cytometry and presented as IFN-γ+ CD4+ cells among CD3+ cells. Data are presented as mean frequency ± SEM (n = 3 to n = 6). See Fig. 2 legend for statistical analysis.
In the intestinal lymphoid tissues, both VirHRV and AttHRV pigs had significantly reduced frequencies of FoxP3+ Treg cells in ileum and IEL (Fig. 2a) and significantly reduced frequencies of CD25+ and CD25− Treg cells in IEL compared with the control pigs at PID 28 (Fig. 2b). Corroborating with the reduced frequencies, absolute numbers of CD25+ and CD25− Treg cells in ileum were significantly reduced in the VirHRV (2·7-fold and 6·1-fold, respectively) and AttHRV (8·5-fold and 8·8-fold, respectively) pigs compared with the control pigs (Fig. 2c). The same trends of reduction, but at a lower scale in the numbers of CD25+ and CD25− Treg cells were also observed in IEL (Fig. 2c).
In the systemic lymphoid tissue (spleen), VirHRV pigs had significantly reduced absolute numbers (but not frequencies) of CD25+ and CD25− Treg cells; AttHRV pigs had signif-icantly reduced numbers of CD25+ Treg cells compared with the control pigs (Fig. 3c). In contrast, in blood, the frequencies of FoxP3+ Treg cells increased significantly in both VirHRV and AttHRV pigs compared with the control pigs (Fig. 3a). VirHRV pigs had higher frequencies of CD25+ (2·6-fold) and significantly higher frequencies of CD25− (5·4-fold) Treg cells in blood; AttHRV pigs had significantly higher frequencies of both CD25+ (2·5-fold higher) and CD25− (6·4-fold higher) Treg cells in blood compared with the control pigs at PID 28 (Fig. 3b). The numbers of CD25− Treg cells in blood also increased in the AttHRV pigs and significantly increased in the VirHRV pigs (Fig. 3c). The numbers of CD25+ Treg cells in the blood of theVirHRV pigs showed the same increasing trend, but did not differ significantly compared with the control pigs beca-use of high variability (Fig. 3c). Hence, circulating Treg cells showed different dynamics from tissue-residing Treg cells. VirHRV infection and AttHRV vaccination increased circula-ting Treg cells but reduced tissue-residing Treg cells at PID 28.
VirHRV induced an overall decrease of CD4+ CD25+ Treg cells in all tissues but an expansion of CD4+ CD25− Treg cells in ileum during the acute phase of infection
We compared the frequencies of total FoxP3+ Treg cells, the frequencies and numbers of CD25+ and CD25− Treg cells in normal naive Gn pigs (control pigs at PID 28, pre-infection) to those in the VirHRV-infected pigs at PCD 7 (Mock/VirHRV) (Figs 2 and 3). Mock/VirHRV pigs had significantly reduced frequencies of FoxP3+ Treg cells in ileum (1·5-fold) and IEL (1·7-fold), of CD25+ Treg cells in IEL (3·4-fold) and spleen (2·9-fold), and of CD25+ Treg cells in ileum (3·97-fold), IEL (4·3-fold) and spleen (2·8-fold) compared with the control pigs (Figs 2 and 3). On the other hand, Mock/VirHRV pigs had significantly increased frequencies of CD25− Treg cells in ileum (2·8-fold) compared with the control pigs (Fig. 2b). Frequencies and numbers of CD25+ Treg cells showed an overall trend of decrease whereas CD25− Treg cells showed an overall trend of increase in all tissues in Mock/VirHRV pigs compared with the controls (Figs 2 and 3). Hence, during the acute phase of rotavirus infection, the frequencies of total FoxP3+ Treg cells and numbers of CD25+ Treg cells were reduced, but there was an expansion of CD25− Treg cells, especially in the ileum of the Gn pigs.
AttHRV vaccination prevented the expansion of CD4+ CD25− Treg cells in Gn pigs post-challenge
To determine whether vaccination prevents the expansion of Treg cells post-challenge, frequencies of FoxP3+ cells, and frequencies and numbers of CD25+ and CD25− Treg cells in AttHRV/VirHRV pigs were compared with those in Mock/VirHRV pigs at PCD 7 (Figs 2 and 3). AttHRV/VirHRV pigs had significantly lower frequencies of FoxP3+ Treg cells in ileum, IEL and spleen, of CD25− Treg cells in ileum, IEL and spleen, and of CD25− Treg cells in ileum, compared with the Mock/VirHRV pigs (Figs 2 and 3). Hence, AttHRV vaccination (induction of immune effector responses) further reduced the total FoxP3+ Treg cells and CD25− Treg cells, and prevented the expansion of CD25− Treg cells seen in the challenged naive pigs during the acute phase of VirHRV infection.
AttHRV/VirHRV pigs also had significantly lower frequencies of FoxP3+ Treg cells in ileum, IEL and spleen, lower frequencies of CD25+ Treg cells in IEL and spleen, and lower numbers of CD25+ Treg cells in ileum and IEL compared with the control pigs (Figs 2 and 3).
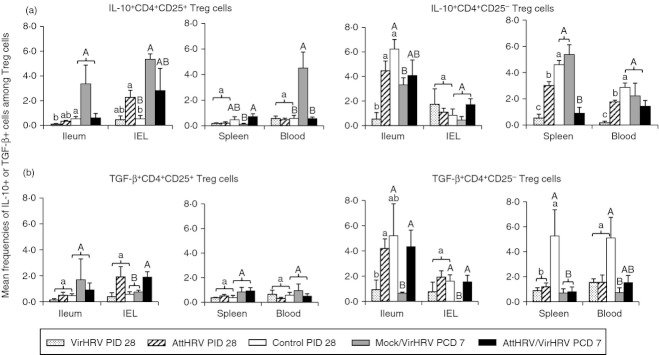
VirHRV infection or AttHRV vaccination decreased the frequencies of IL-10 and TGF-β producing CD4+ CD25− Treg cells at PID 28
To further examine the influence of rotavirus infection or vaccination on the functionality of the Treg-cell subsets, we determined the frequencies of IL-10 and TGF-β intracellular production by the CD25+ and CD25− Treg-cell subsets. VirHRV pigs had significantly lower frequencies of IL-10+ CD4+ CD25− Treg cells in ileum, spleen and blood and significantly lower frequencies of TGF-β+ CD4+ CD25− Treg cells in ileum, compared with the control pigs at PID 28 (Fig. 4). AttHRV pigs had significantly lower frequencies of IL-10+ CD4+ CD25− Treg cells in spleen and blood (Fig. 4a) and TGF-β+ CD4+ CD25− Treg cells in spleen compared with the control pigs (Fig. 4b). Frequencies of IL-10+ and TGF-β+ cells among CD4+ CD25+ Treg cells were substantially lower than CD4+CD25− Treg cells in all tissues; and they did not differ significantly among groups except that the frequencies of IL-10+ CD4+ CD25+ Treg cells in ileum of the VirHRV pigs were significantly lower than in the control pigs whereas IL-10+ CD4+ CD25+ Treg cells in IEL of the AttHRV pigs were significantly higher than in the control pigs at PID 28 (Fig. 4a).
Figure 4.
Frequencies of interleukin-10 (IL-10) (a) or transforming growth factor-β (TGF-β) (b) producing CD4+ CD25+ and CD4+ CD25− regulatory T (Treg) cells in intestinal and systemic lymphoid tissues of gnotobiotic (Gn) pigs. The two graphs in panel a depict the frequencies of IL-10 producing CD4+ CD25+ and CD4+ CD25− Treg cells and the two graphs in panel b depict the frequencies of TGF-β producing CD4+ CD25+ and CD4+ CD25− Treg cells. Data are presented as mean frequency ± SEM (n = 3 to n = 6). See Fig. 2 legend for statistical analysis.
A strong negative correlation (r = −1, P < 0·0001) was found between protection rates against rotavirus diarrhoea and the mean frequencies of IL-10 and TGF-β producing CD4+ CD25− Treg cells in ileum, spleen and blood (but not IEL) at challenge [PID 28 (PCD 0)] (Table 1). The mean frequencies of IL-10+ CD4+ CD25+ Treg cells in ileum and spleen (but not blood and IEL) were also significantly inversely correlated with protection rates against diarrhoea. On the other hand, the mean frequencies of TGF-β+ CD4+ CD25+ Treg cells, total FoxP3+ Treg cells, and CD4+ CD25+ and CD4+ CD25− Treg-cell subsets in ileum, IEL, spleen and blood did not correlate with protection against diarrhoea (data not shown). Hence, dynamics of cytokine-producing CD25− Treg cells (inducible Treg cells) are more closely associated with immune activation status after VirHRV infection or AttHRV vaccination than the CD25+ Treg cells.
Table 1.
Correlation between mean frequencies of cytokine producing CD4+ CD25+ and CD4+ CD25− Treg cells in AttHRV, VirHRV or mock inoculated pigs and protection rates against rotavirus diarrhoea upon VirHRV challenge
| CD4+ CD25+ Treg cells | CD4+ CD25− Treg cells | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| IL-10 | IL-10 | TGF-β | |||||||
| Inoculation groups | Protection rate against diarrhoea1 | Ileum | Spleen | Ileum | Spleen | Blood | Ileum | Spleen | Blood |
| Mock | 0 | 0·532 | 0·47 | 6·23 | 4·60 | 2·87 | 5·20 | 5·27 | 5·10 |
| AttHRV2x | 33% | 0·34 | 0·20 | 4·47 | 3·01 | 1·77 | 4·19 | 1·19 | 1·55 |
| VirHRV | 87% | 0·10 | 0·18 | 0·53 | 0·53 | 0·18 | 0·93 | 0·89 | 1·54 |
| Spearman correlation efficiency | |||||||||
| R= | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | |
| P value | <0·0001 | <0·0001 | <0·0001 | <0·0001 | <0·0001 | <0·0001 | <0·0001 | <0·0001 | |
AttHRV, attenuated human rotavirus; HRV, human rotavirus; IL-10, interleukin-10; TGF-β, transforming growth factor-β; VirHRV, virulent human rotavirus.
VirHRV inoculated pigs were not challenged in this study. Protection rate against rotavirus diarrhoea conferred by the VirHRV is from historical data.26
Mean frequency of cytokine producing cells among Treg cells.
AttHRV-vaccinated pigs maintained normal levels of TGF-β producing Treg cells in ileum and IEL after challenge
Corresponding to the overall lower frequencies and absolute numbers of CD25− Treg cells, AttHRV/VirHRV pigs had significantly lower (sixfold) frequencies of IL-10+ CD4+ CD25− Treg cells in spleen and also IL-10+ CD4+ CD25+ Treg cells in blood (eightfold) compared with the Mock/VirHRV pigs (Fig. 4a). However, AttHRV/VirHRV pigs had significantly higher frequencies of TGF-β+ CD4+ CD25− Treg cells in ileum (6·7-fold) and IEL than the Mock/VirHRV pigs and were similar to the control pigs (Fig. 4b). Hence, vaccination maintained the normal levels of TGF-β+ CD4+ CD25− Treg cells in the intestine post challenge. Maintaining the level of TGF-β producing Treg cells in ileum post challenge is an indicator of protective immunity induced by the AttHRV vaccine, thereby conserving intestinal immunological homeostasis in the pigs.
There was discordance in the changes between the function (cytokine production) and frequencies or numbers of Treg cells in the acute phase after rotavirus infection
Mock/VirHRV pigs had significantly reduced frequencies of IL-10+ CD4+ CD25− Treg cells in ileum and significantly reduced TGF-β+ CD4+ CD25− Treg cells in ileum and IEL, at PCD 7 compared with the control pigs (Fig. 4). On the other hand, those pigs had increased or significantly increased frequencies of IL-10+ CD4+ CD25+ Treg cells in ileum (6·4-fold), IEL (16-fold) and blood (7·9-fold) compared with the control pigs. These reductions or increases in the frequencies of IL-10+ or TGF-β+ Treg cells were not reflected in the changes in the frequencies or the absolute numbers of the Treg cells in the same tissues at the same time-point as shown in Figs 2 and 3. Because frequencies of IL-10 and TGF-β producing CD4+ CD25− Treg cells, but not the frequencies/numbers of total Treg cells, are significantly inversely correlated with protection rate against rotavirus diarrhoea, it is important to measure the functionality, not just the magnitude of Treg-cell responses when studying the role of Treg cells in infection/vaccine-induced protective immunity.
HRV-specific IFN-γ producing CD4+ T-cell responses to VirHRV infection or AttHRV vaccination
Frequencies of IFN-γ producing CD4+ cells [T helper type 1 (Th1) cells] in the intestinal and systemic lymphoid tissues of Gn pigs from the five treatment groups are depicted in Fig. 5. Mock/VirHRV pigs had significantly higher frequencies of IFN-γ+ CD4+ T cells in ileum, spleen and blood compared with the control pigs (Fig. 5). However, the frequencies in all tissues were low. Hence, during the early phase after rotavirus infection (7 days), IFN-γ producing T cells were detectable, but at low frequencies. At PID 28, VirHRV and AttHRV pigs had significantly higher frequencies of IFN-γ+ CD4+ T cells in ileum and spleen. Additionally, AttHRV pigs had significantly higher frequencies of IFN-γ+ CD4+ T cells in blood compared with the control pigs (Fig. 5). The magnitudes of IFN-γ producing CD4+ T-cell responses are substantially higher in the VirHRV and AttHRV groups in ileum, and in the AttHRV group in spleen and blood, compared with the Mock/VirHRV group, indicating the gradual establishment of the Th1 type immune response at the convalescent phase after rotavirus infection/vaccination. AttHRV/VirHRV pigs had significantly higher frequencies of IFN-γ+ CD4+ T cells in IEL and spleen and higher frequencies in ileum and blood compared with the control pigs (Fig. 5). The high variability in frequencies of IFN-γ+ CD4+ T cells in the AttHRV/VirHRV group was as expected because some pigs in this group would be fully protected by the AttHRV vaccination whereas some were partially protected post-challenge (Table 1). The fully protected pigs, unlike the partially protected ones, would not have an anamnestic T-cell response.
The reductions in the numbers/frequencies of CD4+ CD25+ and CD4+ CD25− Treg cells and especially IL-10/TGF-β production in CD4+ CD25− Treg cells in the intestinal and systemic lymphoid tissues after VirHRV infection or AttHRV vaccination at PID 28 (Figs 2–4) corresponded to the increase in HRV-specific IFN-γ producing CD4+ T cells in Gn pigs at the same time point (PID 28, 4 weeks after primary inoculation) observed in this study (Fig. 5) and our previous studies,26 indicating that establishment of Th1-cell immune responses after rotavirus infection or vaccination of the Gn pigs down-regulated numbers and functions of Treg cells.
Discussion
In this study, we demonstrated strong negative correlations between the magnitudes of IL-10 and TGF-β producing CD4+ CD25− Treg-cell responses in ileum, spleen and blood and protective immunity against rotavirus diarrhoea. Our findings suggest that effector T cells and inducible Treg cells are present at a certain equilibrium.30 Primary VirHRV infection and AttHRV vaccination provide different levels of protective immunity by tuning the equilibrium between effector T cells and Treg cells to different levels from the immune homeostasis of naive hosts. During the acute phase of rotavirus infection, a significant expansion of CD4+ CD25− Treg cells occurs in ileum whereas a virus-specific IFN-γ producing effector T-cell response is initiated. The number of effector T cells starts low and increases over time.31 At the convalescent phase, the host resumes immune homeostasis and establishes the equilibrium between effector T cells and Treg cells, but at a different level, with increased numbers of effector/memory T cells and decreased numbers of Treg cells. After challenge, vaccinated and partially protected hosts develop anamnestic effector T-cell responses that are stronger and faster than the primary responses, but the Treg cells remain at levels similar to those pre-challenge. This course is clearly indicated by the dynamics of Treg-cell responses and effector Th1-cell responses in Gn pigs after VirHRV infection and AttHRV vaccination demonstrated in this study in combination with our previous studies.26,31,32 Here we propose a simplistic model to depict this dynamic course (Fig. 6). The Treg-cell responses to rotavirus infection observed in mice and humans also support this model.8,9 The significant negative correlation between frequencies of IL-10/TGF-β producing Treg cells, and the significant positive correlation between frequencies of IFN-γ producing T cells and protection rate are each a component of the equation, reflecting the presence or absence of immunity against rotavirus reinfection and disease. Hence, similar to IFN-γ producing T cells, IL-10/TGF-β producing CD4+ CD25− Treg cells may also be used as an indicator for evaluating vaccine-induced protective immunity. A Yin-Yang reciprocal relationship between IFN-γ and TGF-β responses has long been demonstrated in various experimental models of intestinal inflammation.33
Figure 6.
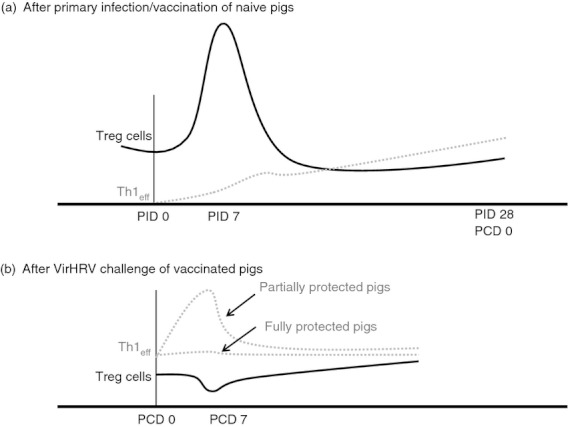
Model for the dynamics of CD4+ CD25− regulatory T (Treg) cell responses after primary infection/vaccination and challenge. During the acute phase of rotavirus infection/vaccination [post-inoculation days (PID) 1–7], human rotavirus-specific interferon-γ (IFN-γ) producing effector T helper type 1 cell (Th1eff) responses are induced in the intestinal lymphoid tissue; meanwhile there is an expansion of CD25− Treg cells in the same site. At the convalescent phase after rotavirus infection/vaccination, the host resumes immune homeostasis and establishes the equilibrium between Th1eff cells and Treg cells but at a different level, with increased numbers of Th1eff cells and decreased numbers of Treg cells (a). After challenge, vaccinated and partially protected pigs develop anamnestic Th1eff cell responses that are stronger and faster than those after primary infection but the Treg cell levels remain similar to pre-challenge levels (b). Fully protected pigs do not develop an anamnestic Th1eff cell immune response.
Only the AttHRV/VirHRV group (not the VirHRV/VirHRV group) was compared with the Mock/VirHRV group to examine whether previous immune activation alters the Treg-cell responses upon rotavirus challenge. Our previous study showed that pigs that had recovered from primary VirHRV infection were 100% protected against VirHRV reinfection25; hence, the VirHRV/VirHRV pig group was not included in this study. Noticeably, the trend of Treg-cell responses after VirHRV infection and AttHRV vaccination at PID 28 was similar in reducing frequencies of tissue-residing Treg cells in ileum and IEL and IL-10 and TGF-β producing CD4+ CD25− Treg cells in ileum, IEL, spleen and blood. Therefore, it should be safe to presume that VirHRV primary infection has a similar modulating effect (possibly at a slightly different magnitude) as AttHRV vaccination on Treg-cell responses post-challenge.
It is important to note that tissue-residing Treg cells have different dynamics after rotavirus infection/vaccination from circulating Treg cells. Study of porcine Treg cells reported that CD25− cells contain only 0·6% of FoxP3+ cells whereas CD25+ cells contain 85·1% of FoxP3+ cells in PBMC.24 Our study also found higher frequencies and numbers (fourfold) of CD25+ Treg cells than CD25− Treg cells in blood. In contrast, in tissues (ileum and spleen), the frequencies and numbers of FoxP3+ Treg cells and CD25− Treg cells are substantially higher than those in blood and the CD25− Treg-cell subset is the more responsive subset in terms of cytokine production. VirHRV infection or AttHRV vaccination reduced the frequencies and numbers of tissue-residing Treg cells, yet increased these populations in the blood at PID 28. It is not clear whether the Treg cells in blood are the tissue Treg cells released into circulation at PID 28. Nonetheless, when studying Treg-cell responses using PBMC only, precautions need to be taken in interpreting results and making extrapolations.
The CD25+ and CD25− Treg cells reacted differently in Gn pigs after rotavirus infection and vaccination. The dynamics and functions of CD25− Treg cells (inducible Treg cells) are more closely associated with immune activation status after VirHRV infection or AttHRV vaccination than CD25+ Treg cells. VirHRV infection or AttHRV vaccination decreased frequencies of IL-10 and TGF-β producing CD25− Treg cells in ileum, spleen and blood at PID 28; whereas cytokine production by CD25+ Treg cells was low and infection/vaccination had little or no influence on the frequencies of those Treg cells. These findings highlight the difference between CD25+ and CD25− Treg cells, and support the notion that initial reports regarding the role of Treg cells in host defence for each specific pathogen using strategies that manipulate these cells based on CD25 expression should be interpreted with caution, and re-investigated using FoxP3-specific reagents for experimentally manipulating Treg cells, especially for CD25− Treg cells.14
The IEL of the small intestine are one of the first immune cell types to encounter invading pathogens that have entered the body via the local epithelial surface.34 They are monitored and controlled properly to rapidly and effectively respond to foreign antigens in the beginning of pathogen invasion and then to restore the local homeostasis and prevent excessive inflammatory responses after pathogen clearance.35 Our study suggested that Treg cells in IEL and ileum (ileal MNC were isolated without distinguishing between Peyer's patches and lamina propria) are equally important during this process in rotavirus infection. Although IEL are mostly CD8αα T cells,34 there are still an average of 2·75 × 107 CD25+ Treg cells and 2·54 × 107 CD25− Treg cells present among IEL in normal control pigs at PID 28. Our data suggest that Treg cells in IEL and ileum may play an important role in the maintenance of immune homeostasis upon rotavirus challenge in immunized hosts and that the CD25− Treg cells may be more important than CD25+ Treg cells and TGF-β production may be more important than IL-10 production.
Murine rotavirus infection in neonatal mice induced an expansion of Treg cells in spleen and MLN during the acute phase of infection and the frequencies of Treg cells returned to pre-infection levels at PID 18 in mice.9 We observed in our study that VirHRV infection induced an expansion of CD25− Treg-cell frequencies and numbers in ileum at 7 days after VirHRV infection and then a decline in the convalescent phase in Gn pigs. The Treg-cell expansion may explain the relatively weak intestinal inflammation during rotavirus acute infection in contrast to the strong intestinal inflammation during Toxoplasma gondii acute infection, which causes a collapse of Treg-cell frequencies and numbers.36 It has been postulated that at an early stage after infection, activation of effector cells is associated with heightened IL-2 production. Because a primary function of IL-2 is to promote the expansion and survival of Treg cells,37 an increased IL-2 level is expected to increase the numbers of suppressive Treg cells in the gut.30 The expanded Treg cells in turn limit effector T-cell responses, restraining inflammation and controlling immunopathology during viral infection.
Infection with VirHRV decreased the frequencies of FoxP3+ Treg cells and frequencies and numbers of CD25+ Treg cells in ileum, IEL and spleen at the acute phase of rotavirus infection compared with the controls. However, among the CD25+ Treg cells in pigs, frequencies of IL-10 and TGF-β production showed an overall increasing trend (except for IL-10+ CD25+ Treg cells in spleen). These observations are in contrast to the findings in mice where there were increased frequencies and numbers of CD25+ Treg cells in MLN and spleen, and decreased IL-10 and TGF-β levels in MLN after infection of mice with wild-type murine rotavirus EC strain.9 Possible reasons for this discrepancy include differences in species, the age of animals (8–9 days old for mice versus 33 days old for Gn pigs) when infected with rotavirus, and virulence of the rotavirus strains in the hosts (homologous murine rotavirus in mice versus heterologous HRV in pigs).
Whether the Treg-cell responses observed in the Gn pigs can be generalized to normal animals/humans receiving commensal antigen stimulation is unknown. Whereas there is a lack of detailed studies on the development and function of Treg cells in germ-free or Gn animals, particularly the induced Treg cells, a few studies have demonstrated that commensal microbiota are required for the development of fully functional CD4+ CD25+ Treg cells38 and colonization of Gn animals by commensal bacteria induces an expansion of peripheral Treg cells.22,39 In addition, it is widely recognized that the immune tolerance for the commensal microbiota in the intestine is maintained by the induction of the Treg cells.40 However, it is currently unknown whether peripheral Treg cells, both CD25+ and CD25−, induced by the gut microbiota or environmental antigens in the conventional animals versus Treg cells induced by viral infection or vaccination in the Gn animals, such as in this study, possess distinct T-cell receptor repertoires or if their T-cell receptor repertoires significantly overlap. Further comparative studies using both Gn and conventional animals are required to address these questions.
In conclusion, we demonstrated that VirHRV infection or AttHRV vaccination reduced frequencies and absolute numbers of tissue-residing Treg cells and down-regulated frequencies of IL-10 and TGF-β producing CD25− Treg cells in ileum, spleen and blood at PID 28. This study, for the first time to our knowledge, established the negative correlation between frequencies of functional Treg cells and protective immunity induced by infection/vaccination. The protection rate against rotavirus diarrhoea post-challenge is negatively correlated with IL-10 and TGF-β producing CD25− Treg cells in ileum, spleen and blood whereas it is positively correlated with virus-specific IFN-γ producing CD4+ T cells in ileum.26 The mechanism for the conversion from Treg cells to effector Th1 cells requires further investigation. Studies have shown that FoxP3+ Treg cells can start expressing T-bet and producing IFN-γ during intestinal inflammation.36 Our finding furthered the understanding of the role of Treg cells in the immunity induced by rotavirus infection and vaccination against rotavirus disease and has significant clinical, translational and basic implications. Inhibition of CD25− Treg cells may be one of the mechanisms for adjuvanticity, and so deserves further investigation. Inhibitors of CD25− Treg cells may be potentially used as vaccine adjuvants. The advances in the knowledge of Treg-cell responses in HRV infection and vaccination will be helpful in developing strategies to improve the efficacy of current rotavirus vaccines and the development of new rotavirus vaccines.
Acknowledgments
We thank Dr Marlice Vonck, Dr Kevin Pelzer, Pete Jobst, Andrea Aman and Shannon Viers for animal care. We thank Tian Hong for reviewing this manuscript and for his insightful suggestions. We thank Melissa Makris for assistance in flow cytometry. We acknowledge that the virulent and attenuated Wa human rotavirus strains were provided by Dr Linda Saif, The Ohio State University. This work was supported by a grant (R01AT004789) from the National Center of Complementary and Alternative Medicine (NCCAM), National Institutes of Health, Bethesda, MD.
Disclosures
The authors have no financial conflicts of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table S1. Gnotobiotic pig groups and timeline of treatments. Black arrow indicates time of virus inoculation
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Parashar UD, Burton A, Lanata C, Boschi-Pinto C, Shibuya K, Steele D, Birmingham M, Glass RI. Global mortality associated with rotavirus disease among children in 2004. J Infect Dis. 2009;200(Suppl. 1):S9–15. doi: 10.1086/605025. [DOI] [PubMed] [Google Scholar]
- 2.Gray J, Vesikari T, Van Damme P, et al. Rotavirus. J Pediatr Gastroenterol Nutr. 2008;46(Suppl. 2):S24–31. doi: 10.1097/MPG.0b013e31816f78ee. [DOI] [PubMed] [Google Scholar]
- 3.Yuan L, Stevenson G, Saif L. Rotavirus and reovirus. In: Straw BE, Zimmerman JJ, D'Allaire S, Taylor DJ, editors. Diseases of Swine. 9th edn. Ames, IA: Blackwell Publishing; 2006. pp. 435–54. [Google Scholar]
- 4.Azevedo MS, Yuan L, Jeong KI, et al. Viremia and nasal and rectal shedding of rotavirus in gnotobiotic pigs inoculated with Wa human rotavirus. J Virol. 2005;79:5428–36. doi: 10.1128/JVI.79.9.5428-5436.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blutt SE, Matson DO, Crawford SE, Staat MA, Azimi P, Bennett BL, Piedra PA, Conner ME. Rotavirus antigenemia in children is associated with viremia. PLoS Med. 2007;4:e121. doi: 10.1371/journal.pmed.0040121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naniche D, Oldstone MB. Generalized immunosuppression: how viruses undermine the immune response. Cell Mol Life Sci. 2000;57:1399–407. doi: 10.1007/PL00000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Granelli-Piperno A, Golebiowska A, Trumpfheller C, Siegal FP, Steinman RM. HIV-1-infected monocyte-derived dendritic cells do not undergo maturation but can elicit IL-10 production and T cell regulation. Proc Natl Acad Sci U S A. 2004;101:7669–74. doi: 10.1073/pnas.0402431101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mesa MC, Gutierrez L, Duarte-Rey C, Angel J, Franco MA. A TGF-β mediated regulatory mechanism modulates the T cell immune response to rotavirus in adults but not in children. Virology. 2010;399:77–86. doi: 10.1016/j.virol.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 9.Kim B, Feng N, Narvaez CF, He XS, Eo SK, Lim CW, Greenberg HB. The influence of CD4+ CD25+ Foxp3+ regulatory T cells on the immune response to rotavirus infection. Vaccine. 2008;26:5601–11. doi: 10.1016/j.vaccine.2008.07.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suvas S, Azkur AK, Kim BS, Kumaraguru U, Rouse BT. CD4+CD25+ regulatory T cells control the severity of viral immunoinflammatory lesions. J Immunol. 2004;172:4123–32. doi: 10.4049/jimmunol.172.7.4123. [DOI] [PubMed] [Google Scholar]
- 11.Suvas S, Kumaraguru U, Pack CD, Lee S, Rouse BT. CD4+CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. J Exp Med. 2003;198:889–901. doi: 10.1084/jem.20030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maloy KJ, Powrie F. Regulatory T cells in the control of immune pathology. Nat Immunol. 2001;2:816–22. doi: 10.1038/ni0901-816. [DOI] [PubMed] [Google Scholar]
- 13.Johanns TM, Ertelt JM, Rowe JH, Way SS. Regulatory T cell suppressive potency dictates the balance between bacterial proliferation and clearance during persistent Salmonella infection. PLoS Pathog. 2010;6:e1001043. doi: 10.1371/journal.ppat.1001043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rowe J, Ertelt J, Way S. Foxp3+ regulatory T cells, immune stimulation and host defense against infection. Immunology. 2012;136:1–10. doi: 10.1111/j.1365-2567.2011.03551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Picca CC, Larkin J, III, Boesteanu A, Lerman MA, Rankin AL, Caton AJ. Role of TCR specificity in CD4+ CD25+ regulatory T-cell selection. Immunol Rev. 2006;212:74–85. doi: 10.1111/j.0105-2896.2006.00416.x. [DOI] [PubMed] [Google Scholar]
- 16.Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive Foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009;30:626–35. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Ojeda G, Pini E, Eguiluz C, Montes-Casado M, Broere F, van Eden W, Rojo JM, Portoles P. Complement regulatory protein Crry/p65 costimulation expands natural Treg cells with enhanced suppressive properties in proteoglycan-induced arthritis. Arthritis Rheum. 2011;63:1562–72. doi: 10.1002/art.30328. [DOI] [PubMed] [Google Scholar]
- 18.Taylor MD, van der Werf N, Harris A, Graham AL, Bain O, Allen JE, Maizels RM. Early recruitment of natural CD4+ Foxp3+ Treg cells by infective larvae determines the outcome of filarial infection. Eur J Immunol. 2009;39:192–206. doi: 10.1002/eji.200838727. [DOI] [PubMed] [Google Scholar]
- 19.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 20.Nizar S, Meyer B, Galustian C, Kumar D, Dalgleish A. T regulatory cells, the evolution of targeted immunotherapy. Biochim Biophys Acta. 2010;1806:7–17. doi: 10.1016/j.bbcan.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Geng S, Yu Y, Kang Y, et al. Efficient induction of CD25– iTreg by co-immunization requires strongly antigenic epitopes for T cells. BMC Immunol. 2011;12:27. doi: 10.1186/1471-2172-12-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wen K, Li G, Bui T, et al. High dose and low dose Lactobacillus acidophilus exerted differential immune modulating effects on T cell immune responses induced by an oral human rotavirus vaccine in gnotobiotic pigs. Vaccine. 2012;30:1198–207. doi: 10.1016/j.vaccine.2011.11.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuan L, Saif LJ. Induction of mucosal immune responses and protection against enteric viruses: rotavirus infection of gnotobiotic pigs as a model. Vet Immunol Immunopathol. 2002;87:147–60. doi: 10.1016/S0165-2427(02)00046-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaser T, Gerner W, Saalmuller A. Porcine regulatory T cells: mechanisms and T-cell targets of suppression. Dev Comp Immunol. 2011;35:1166–72. doi: 10.1016/j.dci.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Yuan L, Ward LA, Rosen BI, To TL, Saif LJ. Systematic and intestinal antibody-secreting cell responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. J Virol. 1996;70:3075–83. doi: 10.1128/jvi.70.5.3075-3083.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuan L, Wen K, Azevedo MS, Gonzalez AM, Zhang W, Saif LJ. Virus-specific intestinal IFN-γ producing T cell responses induced by human rotavirus infection and vaccines are correlated with protection against rotavirus diarrhea in gnotobiotic pigs. Vaccine. 2008;26:3322–31. doi: 10.1016/j.vaccine.2008.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ward LA, Rosen BI, Yuan L, Saif LJ. Pathogenesis of an attenuated and a virulent strain of group A human rotavirus in neonatal gnotobiotic pigs. J Gen Virol. 1996;7:1431–41. doi: 10.1099/0022-1317-77-7-1431. [DOI] [PubMed] [Google Scholar]
- 28.Meyer RC, Bohl EH, Kohler EM. Procurement and maintenance of germ-free seine for microbiological investigations. Appl Microbiol. 1964;12:295–300. doi: 10.1128/am.12.4.295-300.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santner-Nanan B, Seddiki N, Zhu E, Quent V, Kelleher A, Fazekas de St Groth B, Nanan R. Accelerated age-dependent transition of human regulatory T cells to effector memory phenotype. Int Immunol. 2008;20:375–83. doi: 10.1093/intimm/dxm151. [DOI] [PubMed] [Google Scholar]
- 30.Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845–58. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 31.Azevedo MS, Yuan L, Pouly S, Gonzales AM, Jeong KI, Nguyen TV, Saif LJ. Cytokine responses in gnotobiotic pigs after infection with virulent or attenuated human rotavirus. J Virol. 2006;80:372–82. doi: 10.1128/JVI.80.1.372-382.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Azevedo M, Zhang W, Wen K, Gonzalez M, Saif L, Yousef A, Yuan L. Lactobacillus acidophilus and L. reuteri modulate cytokine responses in gnotobiotic pigs infected with human rotavirus. Benef Microbes. 2012;3:33–42. doi: 10.3920/BM2011.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strober W, Kelsall B, Fuss I, Marth T, Ludviksson B, Ehrhardt R, Neurath M. Reciprocal IFN-γ and TGF-β responses regulate the occurrence of mucosal inflammation. Immunol Today. 1997;18:61–4. doi: 10.1016/s0167-5699(97)01000-1. [DOI] [PubMed] [Google Scholar]
- 34.Cheroutre H. IELs: enforcing law and order in the court of the intestinal epithelium. Immunol Rev. 2005;206:114–31. doi: 10.1111/j.0105-2896.2005.00284.x. [DOI] [PubMed] [Google Scholar]
- 35.Montufar-Solis D, Garza T, Klein JR. T-cell activation in the intestinal mucosa. Immunol Rev. 2007;215:189–201. doi: 10.1111/j.1600-065X.2006.00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oldenhove G, Bouladoux N, Wohlfert EA, et al. Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity. 2009;31:772–86. doi: 10.1016/j.immuni.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malek TR, Bayer AL. Tolerance, not immunity, crucially depends on IL-2. Nat Rev Immunol. 2004;4:665–74. doi: 10.1038/nri1435. [DOI] [PubMed] [Google Scholar]
- 38.Ostman S, Rask C, Wold AE, Hultkrantz S, Telemo E. Impaired regulatory T cell function in germ-free mice. Eur J Immunol. 2006;36:2336–46. doi: 10.1002/eji.200535244. [DOI] [PubMed] [Google Scholar]
- 39.Atarashi K, Tanoue T, Shima T, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–41. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci U S A. 2010;107:12204–9. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.