Abstract
Synchronized C3H/10T1/2 clone 8 cells were treated in vitro with a nontoxic dose of N-methyl-N-nitrosourea during their S phase. Chromatographic isolation of the deoxyribonucleotide DNA precursor pool and measurement of the precursor content per cell showed that a nucleic acid residue in the precursor pool is 190-13,000 times more susceptible to methylation than a residue in the DNA duplex, depending on the site of methylation. This conclusion comes from measurements indicating that, for example, the N-1 position of adenine in dATP is 6.3 times more methylated than the same position in the DNA, even though the adenine content of the pool is only a fraction (0.0005) of the adenine content of the DNA helix. The comparative susceptibility between pool and DNA was found to vary with the site of methylation in the order the N-1 position of adenine greater than phosphate greater than the N-3 position of adenine greater than the O6 position of guanine greater than the N-7 position of guanine. The significance of these results for chemical mutagenesis and carcinogenesis is discussed.
Full text
PDF

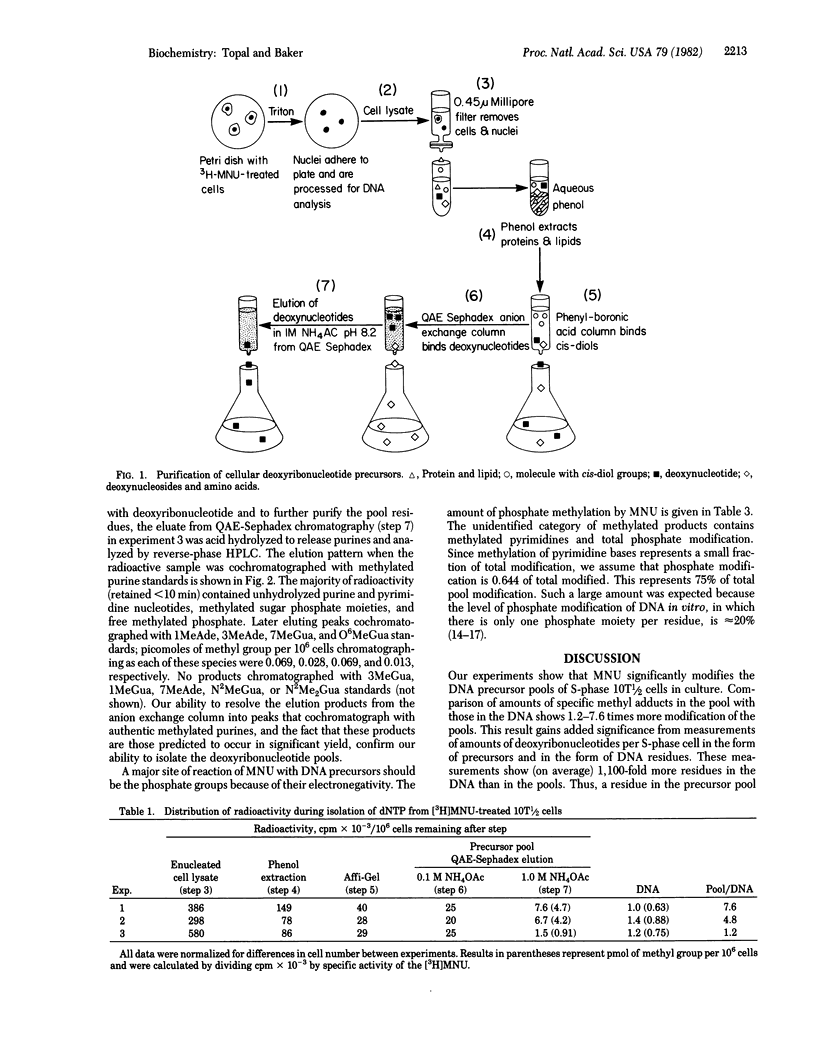
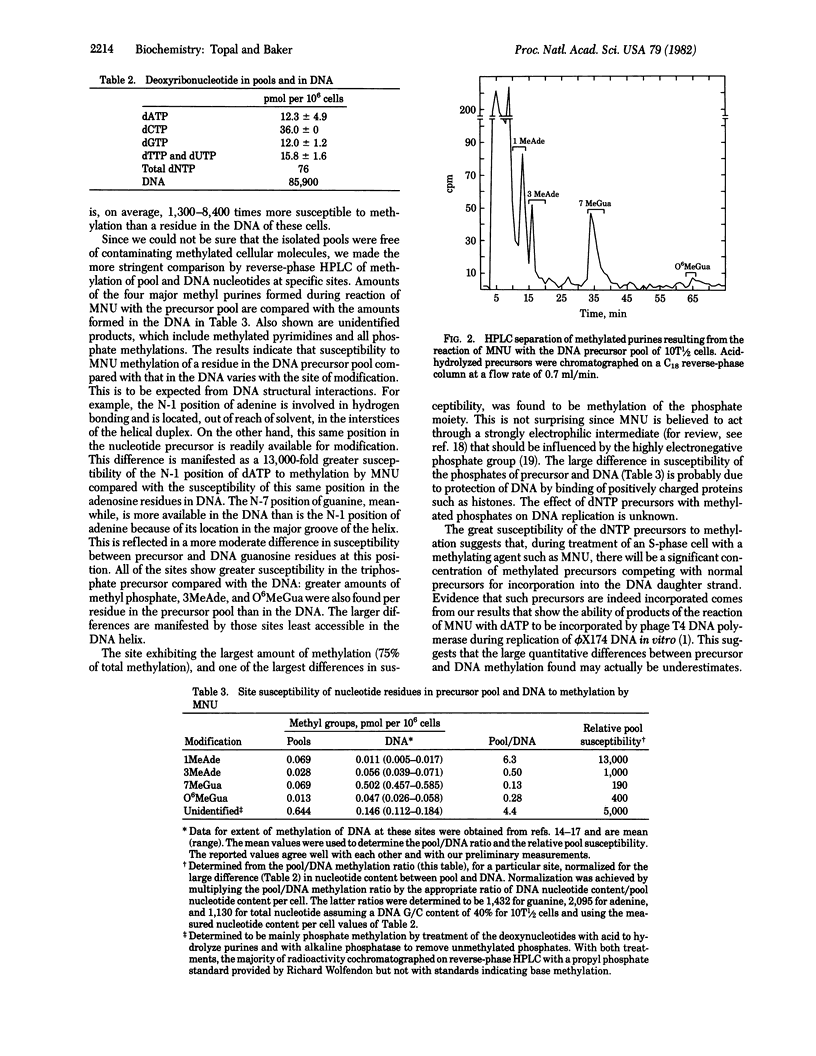

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cerdá-Olmedo E., Hanawalt P. C., Guerola N. Mutagenesis of the replication point by nitrosoguanidine: map and pattern of replication of the Escherichia coli chromosome. J Mol Biol. 1968 May 14;33(3):705–719. doi: 10.1016/0022-2836(68)90315-x. [DOI] [PubMed] [Google Scholar]
- DasGupta U. B., Summers W. C. Ultraviolet reactivation of herpes simplex virus is mutagenic and inducible in mammlian cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2378–2381. doi: 10.1073/pnas.75.5.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisham J. W., Greenberg D. S., Kaufman D. G., Smith G. J. Cycle-related toxicity and transformation in 10T1/2 cells treated with N-methyl-N'-nitro-N-nitrosoguanidine. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4813–4817. doi: 10.1073/pnas.77.8.4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauschka P. V. Analysis of nucleotide pools in animal cells. Methods Cell Biol. 1973;7:361–462. doi: 10.1016/s0091-679x(08)61787-2. [DOI] [PubMed] [Google Scholar]
- Hince T. A., Neale S. A comparison of the mutagenic action of the methyl and ethyl derivatives of nitrosamides and nitrosamidines on Escherichia coli. Mutat Res. 1974 Sep;24(3):383–387. doi: 10.1016/0027-5107(74)90183-3. [DOI] [PubMed] [Google Scholar]
- Holliday R., Pugh J. E. DNA modification mechanisms and gene activity during development. Science. 1975 Jan 24;187(4173):226–232. [PubMed] [Google Scholar]
- Kirtikar D. M., Goldthwait D. A. The enzymatic release of O6-methylguanine and 3-methyladenine from DNA reacted with the carcinogen N-methyl-N-nitrosourea. Proc Natl Acad Sci U S A. 1974 May;71(5):2022–2026. doi: 10.1073/pnas.71.5.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kröger M., Singer B. Ambiguity and transcriptional errors as a result of methylation of N-1 of purines and N-3 of pyrimidines. Biochemistry. 1979 Aug 7;18(16):3493–3500. doi: 10.1021/bi00583a009. [DOI] [PubMed] [Google Scholar]
- Lawley P. D., Orr D. J., Shah S. A., Farmer P. B., Jarman M. Reaction products from N-methyl-N-nitrosourea and deoxyribonucleic acid containing thymidine residues. Synthesis and identification of a new methylation product, O4-methylthymidine. Biochem J. 1973 Sep;135(1):193–201. doi: 10.1042/bj1350193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawley P. D., Thatcher C. J. Methylation of deoxyribonucleic acid in cultured mammalian cells by N-methyl-N'-nitro-N-nitrosoguanidine. The influence of cellular thiol concentrations on the extent of methylation and the 6-oxygen atom of guanine as a site of methylation. Biochem J. 1970 Feb;116(4):693–707. doi: 10.1042/bj1160693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt H. Cell cycle dependence of chemically induced malignant transformation in vitro. Cancer Res. 1974 Jul;34(7):1612–1615. [PubMed] [Google Scholar]
- Meselson M., Yuan R., Heywood J. Restriction and modification of DNA. Annu Rev Biochem. 1972;41:447–466. doi: 10.1146/annurev.bi.41.070172.002311. [DOI] [PubMed] [Google Scholar]
- Newbold R. F., Warren W., Medcalf A. S., Amos J. Mutagenicity of carcinogenic methylating agents is associated with a specific DNA modification. Nature. 1980 Feb 7;283(5747):596–599. doi: 10.1038/283596a0. [DOI] [PubMed] [Google Scholar]
- Pullman B., Perahia D., Cauchy D. The molecular electrostatic potential of the B-DNA helix. VI. The regions of the base pairs in poly (dG.dC) and poly (dA.dT). Nucleic Acids Res. 1979 Aug 24;6(12):3821–3829. doi: 10.1093/nar/6.12.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznikoff C. A., Brankow D. W., Heidelberger C. Establishment and characterization of a cloned line of C3H mouse embryo cells sensitive to postconfluence inhibition of division. Cancer Res. 1973 Dec;33(12):3231–3238. [PubMed] [Google Scholar]
- Sager R., Kitchin R. Selective silencing of eukaryotic DNA. Science. 1975 Aug 8;189(4201):426–433. [PubMed] [Google Scholar]
- Schaaper R. M., Loeb L. A. Depurination causes mutations in SOS-induced cells. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1773–1777. doi: 10.1073/pnas.78.3.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoog L. An enzymatic method for the determination of dCTP and dGTP in picomole amounts. Eur J Biochem. 1970 Dec;17(2):202–208. doi: 10.1111/j.1432-1033.1970.tb01154.x. [DOI] [PubMed] [Google Scholar]
- Tong C., Fazio M., Williams G. M. Cell cycle-specific mutagenesis at the hypoxanthine phosphoribosyltransferase locus in adult rat liver epithelial cells. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7377–7379. doi: 10.1073/pnas.77.12.7377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker I. G., Ewart D. F. The nature of single-strand breaks in DNA following treatment of L-cells with methylating agents. Mutat Res. 1973 Sep;19(3):331–341. doi: 10.1016/0027-5107(73)90234-0. [DOI] [PubMed] [Google Scholar]
- Weith H. L., Wiebers J. L., Gilham P. T. Synthesis of cellulose derivatives containing the dihydroxyboryl group and a study of their capacity to form specific complexes with sugars and nucleic acid components. Biochemistry. 1970 Oct 27;9(22):4396–4401. doi: 10.1021/bi00824a021. [DOI] [PubMed] [Google Scholar]


