Abstract
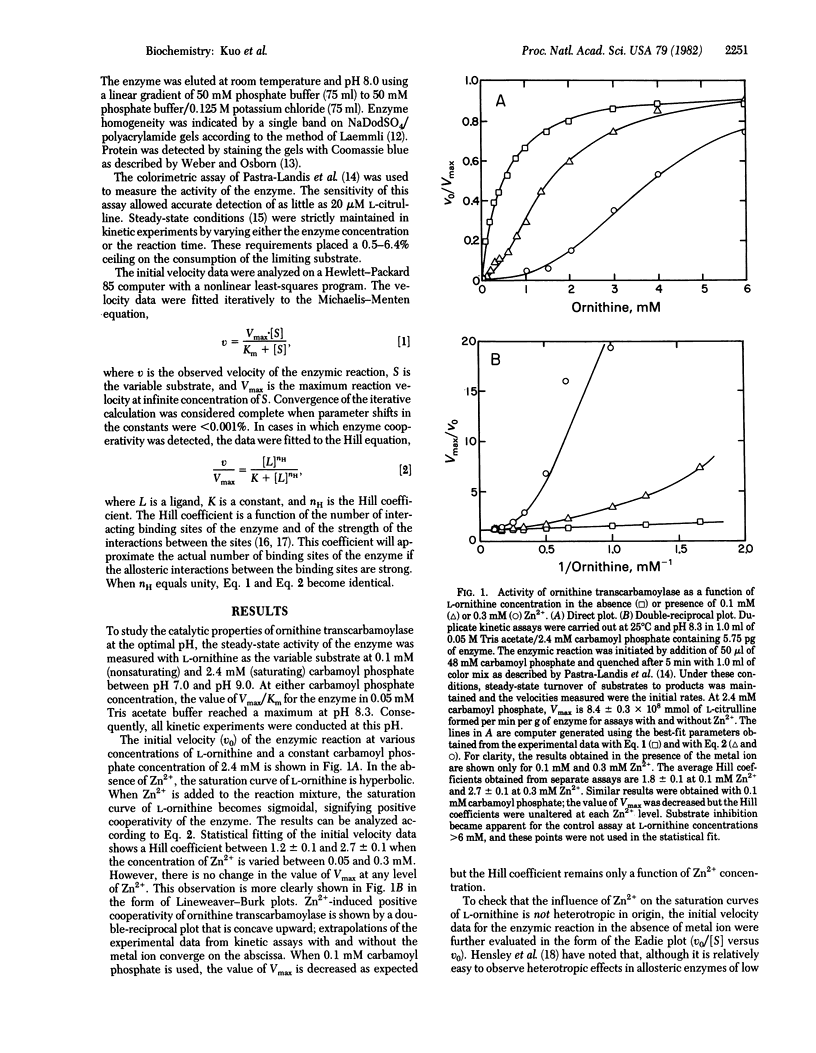
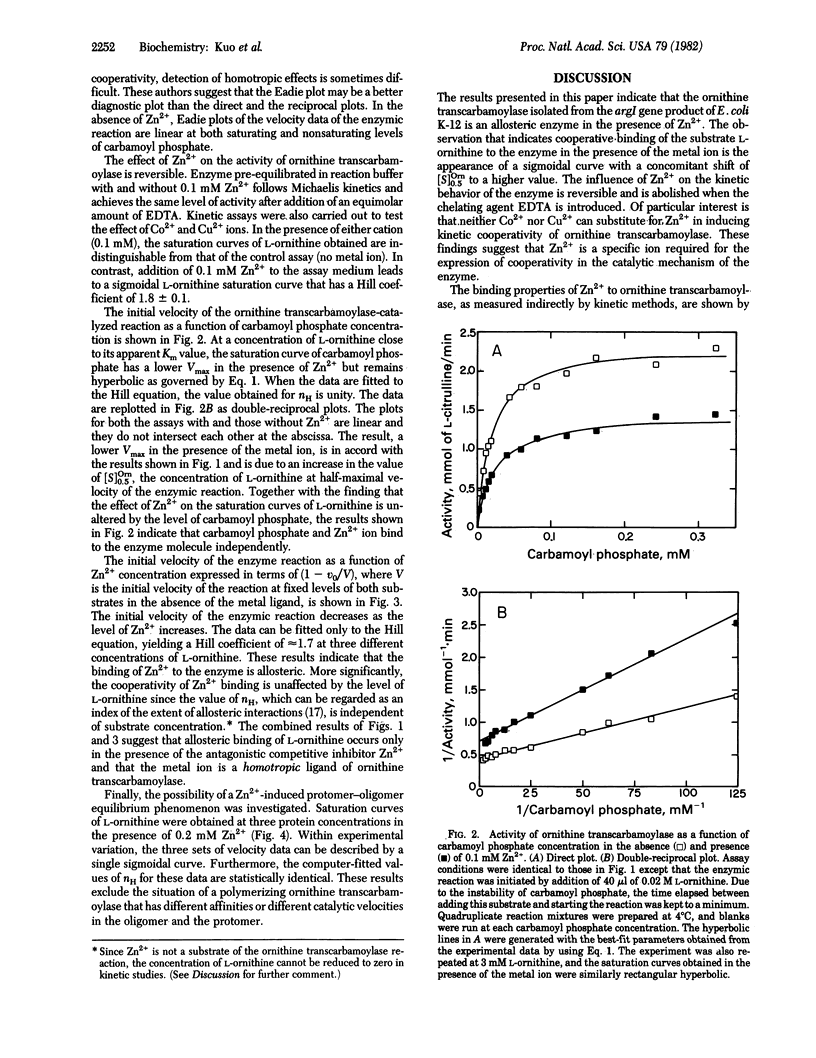
The steady-state reaction of ornithine transcarbamoylase (ornithine carbamoyltransferase, carbamoyl phosphate:L-ornithine carbamoyltransferase, EC 2.1.3.3) purified from the argI gene product of Escherichia coli strain K-12 exhibits Michaelis-Menten kinetics over an extended range of concentration for both L-ornithine and carbamoyl phosphate. In the presence of Zn2+, however, the saturation curve of L-ornithine becomes sigmoidal, revealing positive cooperativity for this anabolic enzyme. The kinetic data give a limiting Hill coefficient of 2.7 for this substrate at 0.3 mM Zn2+. The allosteric effect of Zn2+ on the enzyme is not altered by the concentration of carbamoyl phosphate, and the saturation curve of carbamoyl phosphate remains hyperbolic in the presence of the metal ion. At fixed substrate concentrations, initial velocity data obtained at 0.-0.3 mM Zn2+ indicate cooperative binding of the metal ion to ornithine transcarbamoylase; a Hill coefficient of 1.7 +/- 0.1 is found that is independent of the level of L-ornithine. These results suggest competitive and exclusive binding to the enzyme between L-ornithine and Zn2+ with conformational changes induced in the subunits of the enzyme only by the metal ligand. Neither Co2+ nor Cu2+ exerts an effect on the kinetic behavior of the enzyme. This finding reveals not only specific allosteric control of ornithine transcarbamoylase by Zn2+ but also the possibility of an interlocking metabolic regulation between the urea cycle and the pathway for pyrimidine biosynthesis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison R. D., Purich D. L. Practical considerations in the design of initial velocity enzyme rate assays. Methods Enzymol. 1979;63:3–22. doi: 10.1016/0076-6879(79)63003-3. [DOI] [PubMed] [Google Scholar]
- Fortin A. F., Hauber J. M., Kantrowitz E. R. Comparison of the essential arginine residue in Escherichia coli ornithine and aspartate transcarbamylases. Biochim Biophys Acta. 1981 Nov 13;662(1):8–14. doi: 10.1016/0005-2744(81)90216-3. [DOI] [PubMed] [Google Scholar]
- GERHART J. C., PARDEE A. B. The enzymology of control by feedback inhibition. J Biol Chem. 1962 Mar;237:891–896. [PubMed] [Google Scholar]
- Glansdorff N., Sand G., Verhoef C. The dual genetic control of ornithine transcarbamylase synthesis in Escherichia coli K12. Mutat Res. 1967 Nov-Dec;4(6):743–751. doi: 10.1016/0027-5107(67)90083-8. [DOI] [PubMed] [Google Scholar]
- Hensley P., Yang Y. R., Schachman H. K. On the detection of homotropic effects in enzymes of low co-operativity. Application to modified aspartate transcarbamoylase. J Mol Biol. 1981 Oct 15;152(1):131–152. doi: 10.1016/0022-2836(81)90098-x. [DOI] [PubMed] [Google Scholar]
- Kirtley M. E., Koshland D. E., Jr Models for cooperative effects in proteins containing subunits. Effects of two interacting ligands. J Biol Chem. 1967 Sep 25;242(18):4192–4205. [PubMed] [Google Scholar]
- Knight D. M., Jones E. E. Regulation of Escherichia coli ornithine transcarbamylase by orotate. J Biol Chem. 1977 Sep 10;252(17):5928–5930. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Legrain C., Halleux P., Stalon V., Glansdorff N. The dual genetic control of ornithine carbamolytransferase in Escherichia coli. A case of bacterial hybrid enzymes. Eur J Biochem. 1972 May;27(1):93–102. doi: 10.1111/j.1432-1033.1972.tb01814.x. [DOI] [PubMed] [Google Scholar]
- Legrain C., Stalon V. Ornithine carbamoyltransferase from Escherichia coli W. Purification, structure and steady-state kinetic analysis. Eur J Biochem. 1976 Mar 16;63(1):289–301. doi: 10.1111/j.1432-1033.1976.tb10230.x. [DOI] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Madsen N. B., Shechosky S. Allosteric properties of phosphorylase b. II. Comparison with a kinetic model. J Biol Chem. 1967 Jul 25;242(14):3301–3307. [PubMed] [Google Scholar]
- Marshall M., Cohen P. P. Ornithine transcarbamylase from Streptococcus faecalis and bovine liver. I. Isolation and subunit structure. J Biol Chem. 1972 Mar 25;247(6):1641–1653. [PubMed] [Google Scholar]
- Marshall M., Cohen P. P. Ornithine transcarbamylase from Streptococcus faecalis and bovine liver. II. Multiple binding sites for carbamyl-P and L-norvaline, correlation with steady state kinetics. J Biol Chem. 1972 Mar 25;247(6):1654–1668. [PubMed] [Google Scholar]
- Neet K. E. Cooperativity in enzyme function: equilibrium and kinetic aspects. Methods Enzymol. 1980;64:139–192. doi: 10.1016/s0076-6879(80)64009-9. [DOI] [PubMed] [Google Scholar]
- Pastra-Landis S. C., Foote J., Kantrowitz E. R. An improved colorimetric assay for aspartate and ornithine transcarbamylases. Anal Biochem. 1981 Dec;118(2):358–363. doi: 10.1016/0003-2697(81)90594-7. [DOI] [PubMed] [Google Scholar]
- Rubin M. M., Changeux J. P. On the nature of allosteric transitions: implications of non-exclusive ligand binding. J Mol Biol. 1966 Nov 14;21(2):265–274. doi: 10.1016/0022-2836(66)90097-0. [DOI] [PubMed] [Google Scholar]
- Wargnies B., Legrain C., Stalon V. Anabolic ornithine carbamoyltransferase of Escherichia coli and catabolic ornithine carbamoyltransferase of Pseudomonas putida. Steady-state kinetic analysis. Eur J Biochem. 1978 Aug 15;89(1):203–212. doi: 10.1111/j.1432-1033.1978.tb20914.x. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wells B. D., Stewart T. A., Fisher J. R. Interpretation of nonlinear steady state enzyme kinetics--cyclic and mathematical properties of cooperative, second-site and random pathway models. J Theor Biol. 1976 Jul 21;60(01):209–221. doi: 10.1016/0022-5193(76)90165-x. [DOI] [PubMed] [Google Scholar]


