Abstract
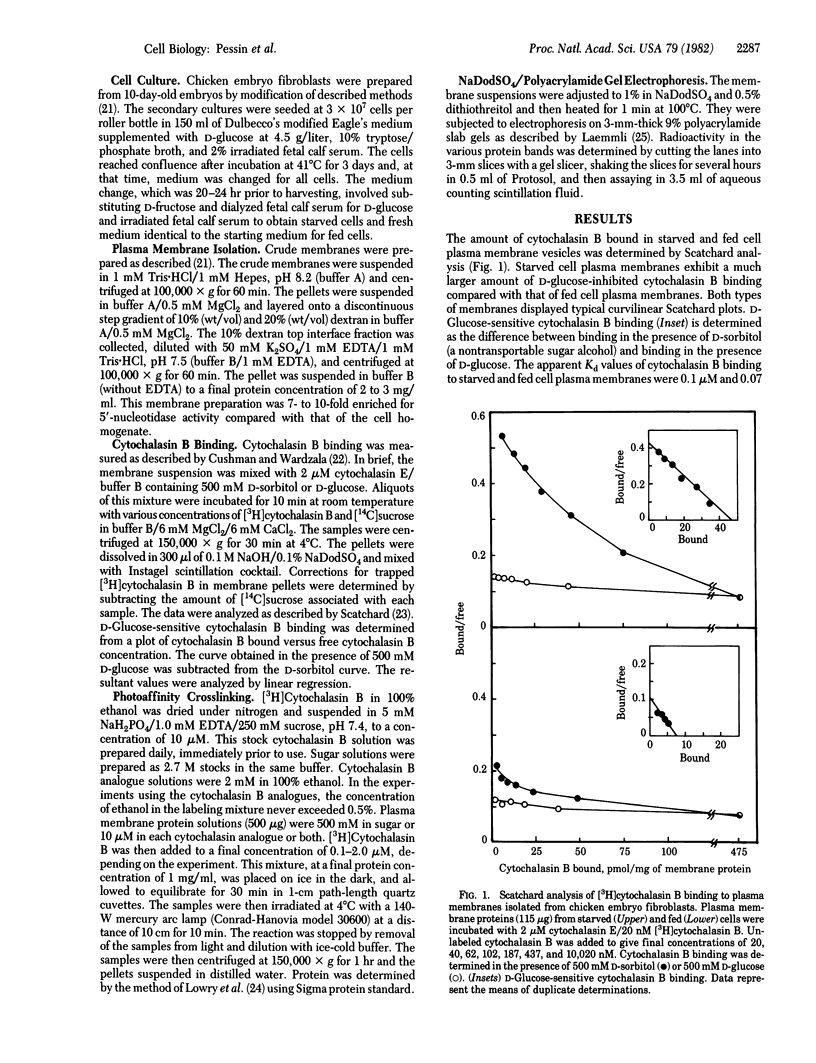
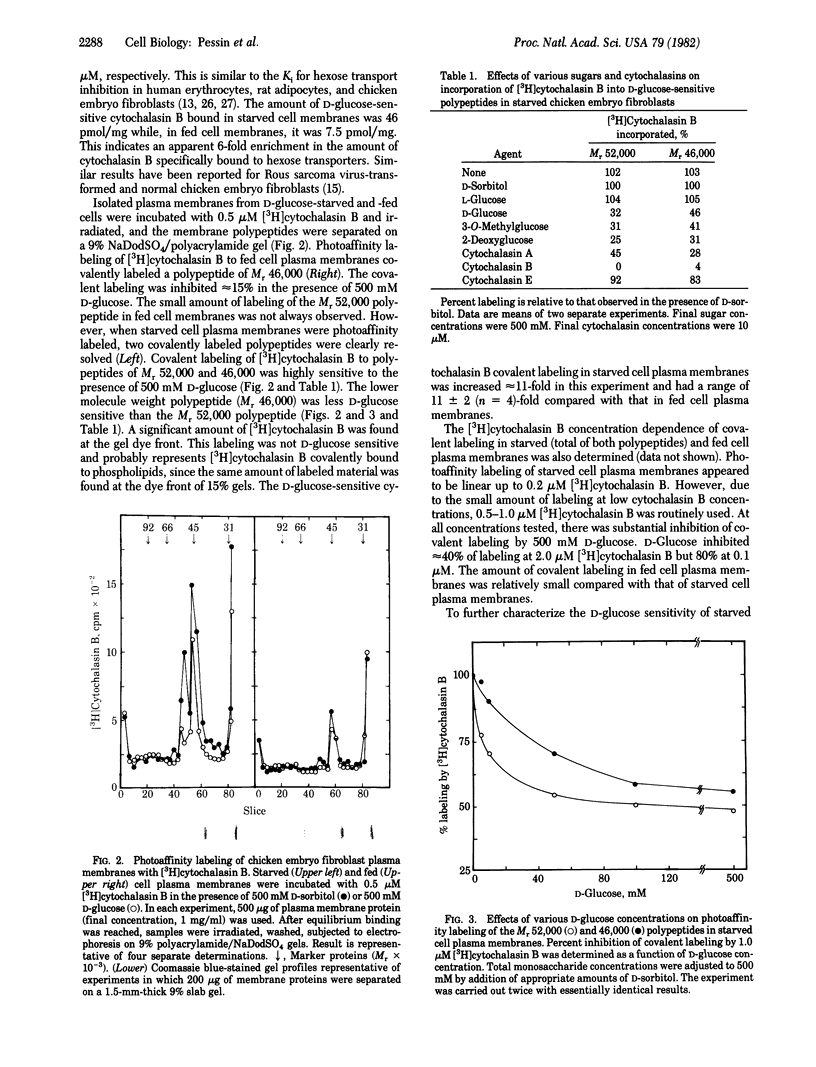
When deprived of D-glucose for 24 hr, chicken embryo fibroblasts exhibit a marked increase in hexose transport activity compared with that of control cells. Scatchard analysis of [3H]cytochalasin B binding to starved cell plasma membranes (46 pmol/mg) indicated a six-fold increase compared with fed cell plasma membranes (7.5 pmol/mg). Irradiation of starved cell plasma membranes with high-intensity UV light in the presence of 0.5 microM [3H]cytochalasin B resulted in covalent labeling of polypeptides of Mr 52,000 and 46,000. In fed cell plasma membranes irradiated under the same conditions, both polypeptides were labeled but at greatly decreased levels. In fact, labeling of the Mr 52,000 polypeptide was barely detectable. The amount of D-glucose-sensitive [3H]cytochalasin B covalent insertion into these membrane components was increased 11 +/- 2 (n = 4)-fold in starved versus fed cell plasma membranes. Photoaffinity labeling of both polypeptides in starved cell plasma membranes was inhibited by D-glucose, 3-O-methylglucose, 2-deoxyglucose, cytochalasin B, and cytochalasin A but not by D-sorbitol, L-glucose, or cytochalasin E. Half-maximal inhibition of labeling of the Mr 52,000 polypeptide occurred at 8 mM D-glucose whereas, for the Mr 46,000 polypeptide, half-maximal inhibition occurred at 40 mM D-glucose. It is concluded that (i) two hexose transport proteins, one of Mr 46,000 and one of Mr 52,000, have been identified in chicken embryo fibroblasts and (ii) the increased affinity labeling of these transporter components after cell starvation may reflect increased numbers of transporters in the plasma membrane.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin S. A., Baldwin J. M., Gorga F. R., Lienhard G. E. Purification of the cytochalasin B binding component of the human erythrocyte monosaccharide transport system. Biochim Biophys Acta. 1979 Mar 23;552(1):183–188. doi: 10.1016/0005-2736(79)90257-8. [DOI] [PubMed] [Google Scholar]
- Christopher C. W., Colby W. W., Ullrey D. Derepression and carrier turnover: evidence for two distinct mechanisms of hexose transport regulation in animal cells. J Cell Physiol. 1976 Dec;89(4):683–692. doi: 10.1002/jcp.1040890427. [DOI] [PubMed] [Google Scholar]
- Christopher C. W. Hexose transport regulation in cultured hamster cells. J Supramol Struct. 1977;6(4):485–494. doi: 10.1002/jss.400060403. [DOI] [PubMed] [Google Scholar]
- Christopher C. W., Kohlbacher M. S., Amos H. Transport of sugars in chick-embryo fibroblasts. Evidence for a low-affinity system and a high-affinity system for glucose transport. Biochem J. 1976 Aug 15;158(2):439–450. doi: 10.1042/bj1580439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher C. W., Morgan R. A. Are lysosomes involved in hexose transport regulation? Turnover of hexose carriers and the activity of thiol cathepsins are arrested by cyanate and ammonia. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4416–4420. doi: 10.1073/pnas.78.7.4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher C. W., Ullrey D., Colby W., Kalckar M. Paradoxical effects of cycloheximide and cytochalasin B on hamster cell hexose uptake. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2429–2433. doi: 10.1073/pnas.73.7.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman S. W., Wardzala L. J. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell. Apparent translocation of intracellular transport systems to the plasma membrane. J Biol Chem. 1980 May 25;255(10):4758–4762. [PubMed] [Google Scholar]
- Czech M. P. Insulin action and the regulation of hexose transport. Diabetes. 1980 May;29(5):399–409. doi: 10.2337/diab.29.5.399. [DOI] [PubMed] [Google Scholar]
- Dolberg D. S., Bassham J. A., Bissell M. J. Selective inhibition of the facilitated mode of sugar uptake by cytochalasin B in cultured chick fibroblasts. Exp Cell Res. 1975 Nov;96(1):129–137. doi: 10.1016/s0014-4827(75)80045-0. [DOI] [PubMed] [Google Scholar]
- Gorga F. R., Baldwin S. A., Lienhard G. E. The monosaccharide transporter from human erythrocytes is heterogeneously glycosylated. Biochem Biophys Res Commun. 1979 Dec 14;91(3):955–961. doi: 10.1016/0006-291x(79)91972-7. [DOI] [PubMed] [Google Scholar]
- Inui K. I., Tillotson L. G., Isselbacher K. J. Hexose and amino acid transport by chicken embryo fibroblasts infected with temperature-sensitive mutant of Rous sarcoma virus. Comparison of transport properties of whole cells and membrane vesicles. Biochim Biophys Acta. 1980 Jun 6;598(3):616–627. doi: 10.1016/0005-2736(80)90041-3. [DOI] [PubMed] [Google Scholar]
- Jung C. Y., Rampal A. L. Cytochalasin B binding sites and glucose transport carrier in human erythrocyte ghosts. J Biol Chem. 1977 Aug 10;252(15):5456–5463. [PubMed] [Google Scholar]
- Kahlenberg A., Zala C. A. Reconstitution of D-glucose transport in vesicles composed of lipids and intrinsic protein (zone 4.5) of the human erythrocyte membrane. J Supramol Struct. 1977;7(3-4):287–300. doi: 10.1002/jss.400070303. [DOI] [PubMed] [Google Scholar]
- Kalckar H. M., Christopher C. W., Ullrey D. Uncouplers of oxidative phosphorylation promote derepression of the hexose transport system in cultures of hamster cells. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6453–6455. doi: 10.1073/pnas.76.12.6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalckar H. M., Ullrey D. Two distinct types of enhancement of galactose uptake into hamster cells: tumor-virus transformation and hexose starvation. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2502–2504. doi: 10.1073/pnas.70.9.2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara M., Hinkle P. C. Reconstitution and purification of the D-glucose transporter from human erythrocytes. J Biol Chem. 1977 Oct 25;252(20):7384–7390. [PubMed] [Google Scholar]
- Kletzien R. F., Perdue J. F. Induction of sugar transport in chick embryo fibroblasts by hexose starvation. Evidence for transcriptional regulation of transport. J Biol Chem. 1975 Jan 25;250(2):593–600. [PubMed] [Google Scholar]
- Kletzien R. F., Perdue J. F. Sugar transport in chick embryo fibroblasts. II. Alterations in transport following transformation by a temperature-sensitive mutant of the Rous sarcoma virus. J Biol Chem. 1974 Jun 10;249(11):3375–3382. [PubMed] [Google Scholar]
- Kletzien R. F., Perdue J. F. The inhibition of sugar transport in chick embryo fibroblasts by cytochalasin B. Evidence for a membrane-specific effect. J Biol Chem. 1973 Jan 25;248(2):711–719. [PubMed] [Google Scholar]
- LEFEVRE P. G. Sugar transport in the red blood cell: structure-activity relationships in substrates and antagonists. Pharmacol Rev. 1961 Mar;13:39–70. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Martineau R., Kohlbacher M., Shaw S. N., Amos H. Enhancement of hexose entry into chick fibroblasts by starvation: differential effect on galactose and glucose. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3407–3411. doi: 10.1073/pnas.69.11.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plagemann P. G., Richey D. P. Transport of nucleosides, nucleic acid bases, choline and glucose by animal cells in culture. Biochim Biophys Acta. 1974 Dec 16;344(3-4):263–305. doi: 10.1016/0304-4157(74)90010-0. [DOI] [PubMed] [Google Scholar]
- Rampal A. L., Pinkofsky H. B., Jung C. Y. Structure of cytochalasins and cytochalasin B binding sites in human erythrocyte membranes. Biochemistry. 1980 Feb 19;19(4):679–683. doi: 10.1021/bi00545a011. [DOI] [PubMed] [Google Scholar]
- Salter D. W., Weber M. J. Glucose-specific cytochalasin B binding is increased in chicken embryo fibroblasts transformed by Rous sarcoma virus. J Biol Chem. 1979 May 10;254(9):3554–3561. [PubMed] [Google Scholar]
- Shanahan M. F., Czech M. P. Partial purification of the D-glucose transport system in rat adipocyte plasma membranes. J Biol Chem. 1977 Sep 25;252(18):6554–6561. [PubMed] [Google Scholar]
- Ullrey D., Gammon M. T., Kalckar H. M. Uptake patterns and transport enhancements in cultures of hamster cells deprived of carbohydrates. Arch Biochem Biophys. 1975 Apr;167(2):410–416. doi: 10.1016/0003-9861(75)90481-6. [DOI] [PubMed] [Google Scholar]
- Wardzala L. J., Cushman S. W., Salans L. B. Mechanism of insulin action on glucose transport in the isolated rat adipose cell. Enhancement of the number of functional transport systems. J Biol Chem. 1978 Nov 25;253(22):8002–8005. [PubMed] [Google Scholar]
- Weber M. J. Hexose transport in normal and in Rous sarcoma virus-transformed cells. J Biol Chem. 1973 May 10;248(9):2978–2983. [PubMed] [Google Scholar]
- Zala C. A., Perdue J. F. Stereospecific D-glucose transport in mixed membrane and plasma membrane vesicles derived from cultured chick embryo fibroblasts. Biochim Biophys Acta. 1980 Jul 16;600(1):157–172. doi: 10.1016/0005-2736(80)90421-6. [DOI] [PubMed] [Google Scholar]