SUMMARY
Excess adipose tissue is associated with metabolic disease and reduced lifespan, whereas caloric restriction decreases these risks. Here we show that as mice age, there is down-regulation of Dicer and miRNA processing in adipose tissue resulting in decreases of multiple miRNAs. A similar decline of Dicer with age is observed in C. elegans. This is prevented in both species by caloric restriction. Decreased Dicer expression also occurs in preadipocytes from elderly humans and can be produced in cells by exposure to oxidative stress and UV radiation. Knockdown of Dicer in cells results in premature senescence, and fat-specific Dicer knockout renders mice hypersensitive to oxidative stress. Finally, Dicer loss-of-function mutations in worms reduce lifespan and stress tolerance, while overexpression of Dicer confers stress resistance. Thus, regulation of miRNA processing in adipose-related tissues plays an important role in longevity and the ability of an organism to respond to environmental stress and age-related disease.
INTRODUCTION
Aging is a complex process characterized by a progressive impairment of the organism’s response to environmental stress and general metabolic deterioration. This results in accumulation of cellular damage that can lead to diseases, such as diabetes and cancer, and eventually death (Akerfelt et al., 2010; Vijg and Campisi, 2008). Interventions that prolong healthy lifespan improve the ability of the organism to deal with environmental threats and prevent metabolic complications (Fontana and Klein, 2007; Russell and Kahn, 2007).
Calorie restriction (CR) extends lifespan across species from yeast to primates (Anderson et al., 2009; Bishop and Guarente, 2007; Fontana et al., 2010). Calorie-restricted mice live up to 60% longer than ad libitum fed mice and are protected against metabolic diseases like diabetes and obesity, as well as cancer, cardiovascular disease and other age-related complications (Fontana et al., 2010). The mechanisms through which CR promotes lifespan involve multiple metabolic adaptations, including decreased production of reactive oxygen species, decreased levels of circulating pro-inflammatory cytokines, increased expression of protein chaperones, increases in detoxification pathways, enhanced DNA repair processes, decreased apoptosis and reduced cellular senescence (Cohen et al., 2004; Fontana and Klein, 2007). In mammals, part of the beneficial effects of CR is thought to be mediated by decreases in adipose mass (Huffman and Barzilai, 2009). Indeed, depletion or expansion of adipose tissue using genetic or surgical approaches has been shown to impact the risk of many metabolic diseases and affect mean and maximum lifespan in rodents (Bluher et al., 2003; Huffman and Barzilai, 2009).
Despite evidence for an important role of adipose tissue in mediating lifespan and disease susceptibility, it is not known how aging and CR coordinate processes in this tissue to exert its cell non-autonomous effects. Adipose tissue is known to exert some of its systemic effects through lipid storage and release, secretion of adipokines and by serving as a site of chronic inflammation in obesity. Here we show an additional regulatory role of adipose tissue. We find that aging in mice is associated with down-regulation of multiple microRNAs (miRNAs) in fat, and that these changes are largely prevented by CR. This is due in large part to a decrease in components of the miRNA processing machinery, particularly Dicer. We also show that this phenomenon is present in human preadipocytes and in the nematode C. elegans. Finally, we show that response to environmental stress and lifespan can be positively or negatively affected by up- or down-regulating the levels of Dicer expression in the worm intestine or in mouse adipose tissue, demonstrating an important role for changes in miRNA processing in adipose and related tissues in the cell non-autonomous regulation of aging.
RESULTS
miRNA processing in fat with age and CR
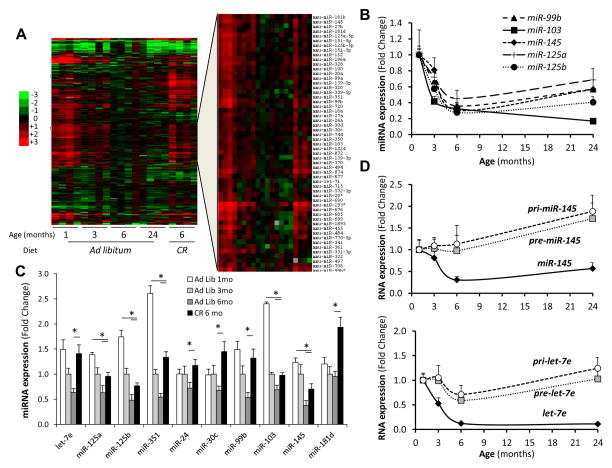
To identify potential mechanisms through which adipose tissue modulates lifespan, we investigated how aging affects expression of miRNAs in subcutaneous adipose tissue by quantifying over 600 miRNAs of C57BL/6J mice at one, three, six and 24 months of age. Of the 265 miRNAs that were detectable, 136 (51%) decreased with age, while only 27 (10%) miRNAs increased (Figure 1A and Table S1). Most of these miRNAs showed an exponential decline with the most robust down-regulation occurring between one and six months of age followed by either a new steady state or a further gradual decline up to 24 months (Figures 1B and S1A). Analysis of adipose tissue from mice subjected to caloric restriction from three to six months of age indicated that CR not only prevented this decline for many miRNAs, but in many cases restored expression to the levels observed at one month of age (Figures 1A, C, S1B and Table S1). Thus, of the 136 miRNAs that decreased with age, 63% were at least partially rescued by CR (Figures S1B).
Figure 1. Regulation of miRNA Expression in Adipose Tissue in Response to Age and Calorie Restriction (CR).
See also Figure S1 and Tables S1 and S6.
(A) Heat map representing the quantitation of 265 miRNAs expressed in the subcutaneous adipose tissue of mice at 1, 3, 6 and 24 months of age and at 6 months of age after 3 months of a 40% CR.
(B) and (C) Quantitation of selected miRNAs by SYBR-Green stem-loop PCR in subcutaneous fat of aged and calorie restricted mice. n=4–5 per group.
(D) RT-qPCR of the pri-, pre- and mature miR-145 and let-7e was performed as described in Experimental Procedures. n=5 per group.
* P < 0.05. Error bars, SEM. Ad Lib, Ad libitum. mo, months.
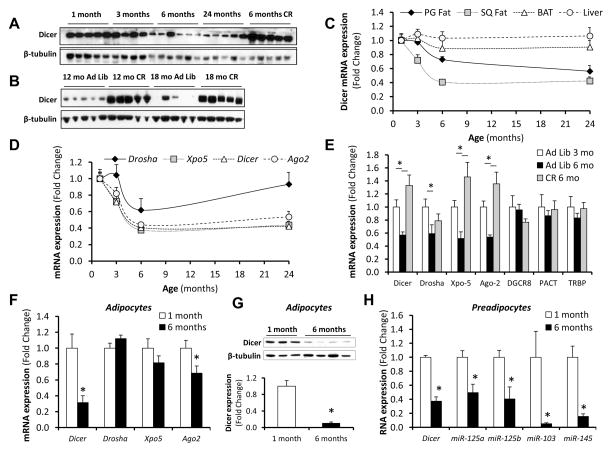
miRNA processing begins with the transcription of primary miRNA molecules (pri-miRNA). These are processed in the nucleus by Drosha to generate precursor miRNAs (pre-miRNAs), which are exported to the cytoplasm where they are cleaved by Dicer to give mature miRNAs (Jinek and Doudna, 2009). In order to identify the specific steps in miRNA processing that are affected by age, we assessed the levels of pre- and pri-miRNAs for several highly represented miRNAs in adipose tissue. While expression of the mature forms of these miRNAs decreased substantially with age, levels of the pre- and pri-miRNAs showed variable patterns, with some showing a mild decrease, some remaining stable, and some increasing throughout the 24 month period, indicating a significant defect in the processing of pre-miRNAs to mature miRNAs with age (Figures 1D and S1C). Consistent with this, Dicer protein in adipose tissue dramatically decreased with age (Figure 2A). This decrease was completely prevented by calorie restriction (Figures 2A and B), paralleling the changes observed with the miRNAs. These changes correlated well with changes in several stress, senescence and longevity biomarkers, including phosphorylated p53, which progressively increased in adipose tissue with age, and SIRT1 and HMGA2, which decreased with age over a similar time course (Figures S2A and S2B). This decrease in Dicer protein was due in large part to a decrease in Dicer mRNA, which declined by 60% between one and six months of age (Figure 2C). Exportin-5, which is responsible for the transport of pri-miRNAs from the nucleus to the cytoplasm, and Argonaute-2, a member of the RNA-Induced Silencing Complex that is required for miRNA-mediated gene silencing, were also down-regulated at the mRNA level by 50–60% with age (Figure 2D). Again, CR reverted the decreases in mRNA levels of these components of the miRNA processing pathway to the levels found in three month-old mice (Figure 2E). Drosha mRNA levels were also reduced with age, but at lesser extent, and were not affected by CR (Figures 2D and E). Thus, age and CR lead to changes in the expression of multiple miRNAs in adipose tissue of mice, and this correlates with changes in components of the miRNA processing pathway, particularly Dicer.
Figure 2. Regulation of miRNA Processing Pathway in Adipose Tissue in Response to Age and CR.
See also Figure S2.
(A) and (B) Extracts of white adipose tissue (subcutaneous) from mice at different ages and after CR were analyzed by Western blotting with Dicer antibody. β-tubulin was used as the loading control.
(C) Dicer mRNA was quantitated by RT-qPCR in perigonadal (PG) and subcutaneous (SQ) white adipose tissue, interscapular BAT and liver of mice at different ages. (n=5 per group).
(D) and (E) RT-qPCR quantitation of the message of components of the miRNA processing pathway in subcutaneous fat of aged or CR mice. (n=5 per group).
(F) and (G) Dicer expression was assessed in isolated adipocytes from SQ fat of mice at 1 month and 6 months of age by RT-qPCR (n=16 per group) and Western blotting (n=3–4 per group).
(H) Preadipocytes were sorted from the stromovascular fraction of subcutaneous fat of mice at 1 month and 6 months of age using CD34 and Sca1 markers, and Dicer mRNA levels and the expression of selected miRNAs was measured by RT-qPCR. (n=3 per group).
* P < 0.05. Error bars, SEM. Xpo-5, exportin 5. Ago-2, argonaute 2. Ad Lib, Ad libitum. mo, months.
The decline in expression of Dicer with age was not limited to subcutaneous white fat and was also seen in perigonadal white fat at both protein and mRNA levels, as well as to a lesser extent in interscapular brown fat at the protein level (Figures 2C, S2C and D). As with subcutaneous fat, expression of Dicer in these fat depots was increased by CR (Figures S2C and D). A significant, but small, decrease in Dicer with age was also observed in the spleen at the mRNA level and in the brain at both mRNA and protein levels (Figures S2C–E). By contrast, liver, kidney and skeletal muscle showed no changes in Dicer mRNA expression with age (Figures 2C and S2E).
Adipose tissue is composed of adipocytes and cells of the stromovascular fraction, such as preadipocytes, inflammatory cells and vascular cells, and this composition may vary with age and nutritional state. To examine whether the changes observed occurred in adipocytes, subcutaneous fat pads were digested with collagenase and separated into adipocytes and stromovascular fraction by centrifugation. This revealed that Dicer expression at the mRNA and protein level was decreased in older mice in both the adipocyte and stromovascular fractions (Figures 2F, 2G and S2F). By contrast, isolated adipocytes showed no down-regulation of Drosha or Exportin-5, and Argonaute-2 expression was only modestly decreased (Figure 2F). Isolation of preadipocytes from the stromovascular fraction by cell sorting revealed that the decrease in expression of Dicer and miRNAs with age in this fraction was due to changes in the preadipocytes (Figure 2H), while other cells in the stromovascular fraction showed no change in Dicer expression with age (Figure S2G).
Decreases in Dicer expression in adipose as a function of age and CR were not due to changes in fat accumulation or obesity-related inflammation, since Dicer expression was no different between high fat diet fed obese mice and controls (Figures S2H and I). Likewise, mice with fat-specific knockout of the insulin receptor (FIRKO mice), which are leaner and live longer than their littermate controls (Bluher et al., 2003; Bluher et al., 2002), did not show alterations in Dicer expression in relation to controls in either subcutaneous or perigonadal fat when young (<3 months) or old (>24 months) mice were compared (Figures S2J and K). Finally, 24 h fasting did not impact Dicer mRNA or protein levels (Figures S2L and M), indicating that the regulation of miRNA processing in fat tissue in response to calorie restriction is not an acute phenomenon.
Dicer, stress, and nutrient deprivation
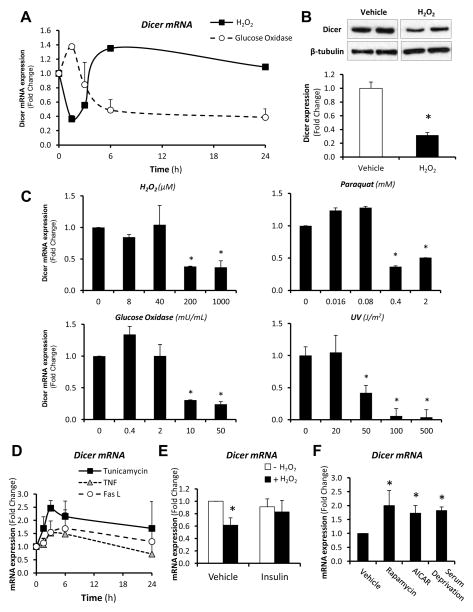
To gain insight as to the factors that might influence Dicer expression with age, we challenged 3T3-F442A preadipocytes with stressors associated with aging and metabolic diseases. Acute treatment with sub-lethal doses of a number of agents that induce oxidative stress, including H2O2, glucose oxidase and paraquat, resulted in reduced expression of Dicer (Figures 3A–C). Most of these effects occurred rapidly, in a dose-dependent manner and were accompanied by parallel decreases in Dicer protein. Sub-lethal doses of UV radiation also resulted in a robust reduction of Dicer mRNA levels (Figure 3C). In contrast, treatment with tunicamycin, which induces the unfolded protein response, increased Dicer expression, while treatment with TNF-α and Fas ligand had no significant effect (Figure 3D). Interestingly, pre-treatment of cells with insulin, an intervention that protects preadipocytes from stress-induced apoptosis (Tseng et al., 2002), prevented the effects of H2O2 on Dicer expression (Figure 3E). Likewise, serum deprivation or treatment with rapamycin or AICAR, increased Dicer mRNA expression (Figure 3F), correlating with the fact that both mTor and AMP kinase, the targets of rapamycin and AICAR, have been implicated in control of lifespan and stress response in multiple organisms (Greer et al., 2007; Harrison et al., 2009; Robida-Stubbs et al., 2012). Thus, stressors associated with the aging process have a negative impact on Dicer expression, while models of nutrient deprivation in vitro have a positive impact on Dicer expression. Furthermore, the effect of stressors can be prevented by acute insulin treatment.
Figure 3. Regulation of Dicer Expression in 3T3-F442A Preadipocytes in Response to Stress.
(A) 3T3-F442A preadipocytes were treated with sub-lethal doses of different stress agents (1 mM H2O2, 50 mU/mL glucose oxidase). Dicer mRNA expression was assessed by RT-qPCR at the indicated time-points. After 90 min, medium containing H2O2 was replaced by H2O2-free medium. Glucose oxidase was maintained in the medium throughout the entire experiment. Experiments were repeated twice in duplicate.
(B) Dicer expression was analyzed by Western blotting in 3T3-F442A preadipocytes in response to 1 mM H2O2 for 3h.
(C) Dose-response of Dicer mRNA expression to different stress agents in 3T3-F442A preadipocytes as measured by RT-qPCR. Time-points were 1.5h for H2O2, 3h for glucose oxidase and UV, and 24h for paraquat. (n=4 per group).
(D) 3T3-F442A preadipocytes were treated with sub-lethal doses of different stress agents (2 μg/mL tunicamycin, 25 ng/mL TNFα, 100 ng/mL Fas ligand) and Dicer mRNA expression was assessed by RT-qPCR at the indicated time-points. Experiments were repeated twice in duplicate.
(E) 3T3-F442A preadipocytes were pre-treated with 150 nM Insulin for 1h, followed by treatment with 1 mM H2O2 for 90 min. Dicer mRNA expression was determined by RT-qPCR. (n=4 per group).
(F) 3T3-F442A preadipocytes were serum starved (6h) or treated with agents to mimic nutrient deprivation (1 mM AICAR for 6h and 1 μM rapamycin for 3h), and Dicer mRNA expression was assessed by RT-qPCR. Experiments were repeated twice in duplicate.
* P < 0.05. Error bars, SEM.
Dicer knockout in adipocytes increases sensitivity to stressors
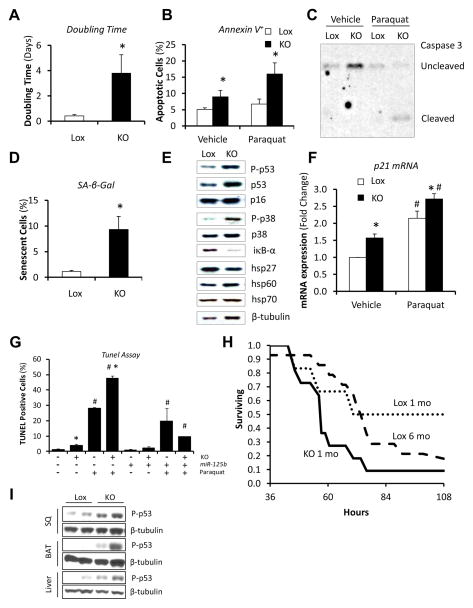
To investigate the role of reduced Dicer expression in adipocyte biology, preadipocytes were isolated from the subcutaneous fat of mice with floxed Dicer allele (Dicerlox/lox), and the Dicer gene was inactivated in vitro using adenoviruses harboring Cre recombinase (Figures S3A–E). These Dicer knockout cells were viable and able to differentiate into adipocytes, but showed significantly increased doubling time (Figure 4A) and a trend towards decreased proliferation (Figure S3F). Dicer knockout cells also exhibited hypercondensed chromatin and fragmented nuclei (Figure S3G, arrows), consistent with early changes of apoptosis. This was confirmed by increased levels of Annexin V staining (Figure 4B) and caspase 3 cleavage (Figure 4C). In addition, the percentage of senescent cells as estimated by β-galactosidase staining was increased 8-fold after Dicer ablation (Figures 4D and S3H). Gene expression profiling in Dicer knockout cells using microarrays revealed a pattern of gene expression that resembled that of senescent cells (Sahin and Depinho, 2010), with down-regulation of genes involved in DNA repair and up-regulation of genes involved in mitochondrial stress pathways and inflammation (Table S2 and Figures S3I and J). Likewise, both phosphorylated (active) and total p53, a critical component of cellular senescence, were significantly increased in Dicer knockout preadipocytes (Figures 4E and S3K), as was the expression of the p53 target p21WAF1 (Figure 4F). There was also increased phosphorylation of p38 MAPK and reductions of the inhibitor of NF-κB signaling, iκB-α, and heat shock protein 27 (hsp27) (Figures 4E and S3K). These changes could all contribute to the increased sensitivity of Dicer knockout cells to apoptosis in response to different stressors (Figures 4B, C, F and S3K).
Figure 4. Premature Senescence and Increased Susceptibility to Stress upon Dicer Knockout in Fat Cells.
See also Figures S3, S4 and Table S2.
Dicerlox/lox preadipocytes were transduced with adenoviruses harboring GFP (Lox) or CRE (KO). After 4 days, cells were analyzed.
(A) Doubling time was estimated by counting cell number over 4–6 days with cells under normal growth conditions. (n=4 per group).
(B) Percentage of early apoptotic cells as determined by Annexin V+/PI− staining using flow cytometry. Cells were treated with 2 mM paraquat or vehicle for 16h prior to the analysis. Experiments were repeated three times in duplicate.
(C) Degree of apoptosis as determined by caspase 3 cleavage. Cells were treated with 2 mM paraquat or vehicle for 16h prior to the analysis. Representative blot of at least two independent experiments.
(D) Percentage of cells with senescence-associated β-galactosidase (SA-β-Gal) activity. Quantitation of two fields of three independent samples per group. Experiments were repeated twice.
(E) Senescence markers, stress markers, and heat shock proteins were analyzed by Western blotting. Representative blots of at least two independent experiments.
(F) Quantitation of p21 mRNA by RT-qPCR. Cells were treated with 2 mM paraquat or vehicle for 16h prior to the analysis. (n=4 per group).
(G) TUNEL assay of Dicer knockout cells or controls where miR-125b expression was rescued by transfection. For transfection control, a non-silencing miRNA (NS) was used. Cells were treated with 2 mM paraquat or vehicle for 24h prior to the analysis. Quantitation of two fields of three independent samples per group.
(H) Survival of mice in response to intraperitoneal injection with of paraquat. mo, months of age. Lox, controls. KO, fat-specific Dicer knockout mice. (n = 15–17 per age group).
(I) Phosphorylation of p53 (Ser15) in subcutaneous white adipose tissue (SQ), interscapular brown adipose tissue (BAT) and liver of 4-month old fat-specific Dicer knockout mice (KO) and Lox controls.
* P < 0.05 vs. Lox; # P < 0.05 vs. 6 months. Error bars, SEM.
High levels of miR-125b have been shown to inhibit apoptosis by targeting genes in the p53 network (Le et al., 2009). Interestingly, miR-125b and other members of the miR-125/lin-4 family were decreased in expression with age in the fat of mice (Figures 1A and B), and this correlated with increased levels of phosphorylated p53 in adipose tissue with age (Figure S2A). miR-125b was also decreased in Dicer knockout cells (Figure S3E), negatively correlating with p53 activation. To determine if down-regulation of miR-125/lin-4 may mediate some of the effects of lack of Dicer on cellular senescence and stress response, Dicer knockout cells were transfected with a miR-125b mimic (Figures S4A and B). This resulted in a reduction in the levels of phospho-p53, total p53, and cleaved caspase 3 in the knockout cells to levels approaching that of the control (Figure S4C). More importantly, the miR-125b mimic reversed the susceptibility of Dicer knockout cells to paraquat-induced apoptosis (Figures 4G and S4D). Thus, preadipocytes with reduced Dicer expression were senescent and more susceptible to stress, and this could be attributed, at least in part, to the down-regulation of the miR-125/lin-4 family and a consequent up-regulation of the p53 pathway in these cells.
To assess if alterations in Dicer expression in adipose tissue could affect stress resistance at the level of the organism, we generated fat-specific Dicer knockout mice (AdicerKO) by breeding Dicerlox/lox mice and mice with an adiponectin promoter-Cre transgene (Figure S4E–G). We then injected these and control mice with the superoxide generator paraquat, which results in tissue failure that has been shown to be prevented, in part, by calorie restriction (Sun et al., 2001). As expected, we found that six-month old mice are more sensitive to paraquat-induced mortality than one-month old animals (Figure 4H). Interestingly, when Dicer levels in fat were prematurely down-regulated by gene inactivation, one-month old mice became as sensitive to paraquat as six-month old control mice (Figure 4H). This occurred without any obvious morphological abnormality that could indirectly affect susceptibility to oxidative stress (Table S3). Consistent with this being a model of premature aging, four-month old AdicerKO mice displayed higher levels of phosphorylated p53 in both adipose and non-adipose tissues (Figure 4I), indicating a cell non-autonomous role for Dicer in adipose tissue to regulate aging.
Evolutionary conservation in humans and nematodes
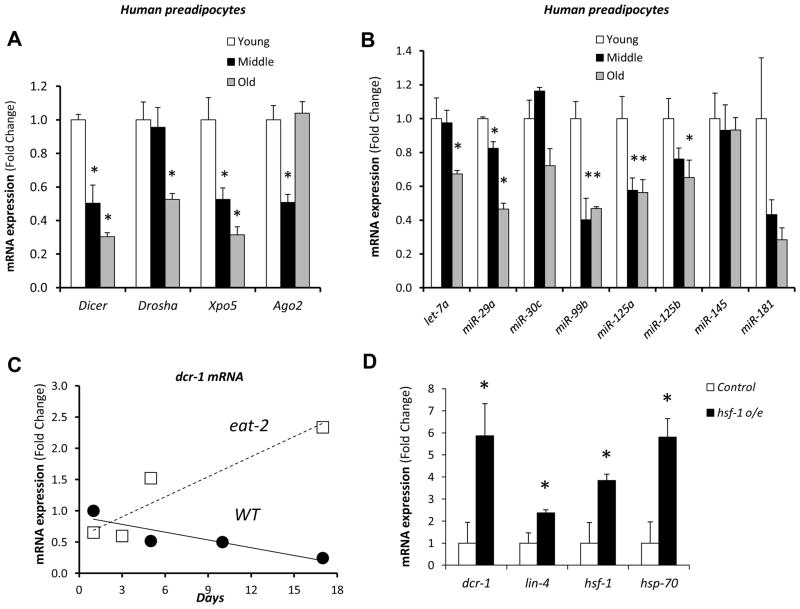
To assess if regulation in the miRNA processing pathway also occurred with age in humans, we studied preadipocytes in culture obtained from subcutaneous fat from young (26±4.1 years), middle-aged (46±2.2 years) and old (74±3.7 years) human donors (Figure S5A). We have previously shown that preadipocytes from older donors have decreased differentiation capacity and increased expression of proinflammatory cytokines, indicating that they maintain an altered phenotype reflecting the age of the donor even after multiple passages in culture (Tchkonia et al., 2010). As in rodent fat, Dicer, Drosha and Exportin-5 mRNAs were all decreased in expression in preadipocytes from old human donors in comparison to young human donors, with the greatest difference being for Dicer (Figure 5A). Consistent with the decrease in these processing enzymes, many miRNAs, including members of the miR-125/lin-4 family, showed decreased expression in preadipocytes from the older individuals (Figure 5B). Interestingly, with the exception of miR-30c and miR-145, which were down-regulated by aging in mice but not in humans, all the other miRNAs followed a very similar kinetics of down-regulation with age across these species. This pattern of age-related decreases in Dicer expression in human preadipocytes was not observed in cells that were aged in culture by serial passage (Figures S5B–D).
Figure 5. Evolutionary Conservation of miRNA Processing Pathway Regulation with Age in Human Preadipocytes and Worms.
See also Figure S5 and S6.
(A) and (B) Preadipocytes from humans [young (26±4.1 years), middle-aged (46±2.2 years) and old (74±3.7 years)] (n=3) were isolated. The mRNA levels of (A) the components of the miRNA processing pathway and (B) selected miRNAs were assessed by RT-qPCR. * P < 0.05 vs. young group. Values represent mean of 3 independent samples per age group, where each sample represents cells of a single individual. Variance among individuals for Dicer mRNA expression was 0.0032 (young), 0.0349 (middle-aged) and 0.0018 (old).
(C) RT-qPCR of Dicer mRNA in whole N2 (WT) and eat-2 (a genetic model of calorie restriction) worms at different ages.
(D) RT-qPCR of Dicer mRNA in whole N2 (WT) and hsf-1 overexpressing worms (hsf-1 o/e).
In worms, each point represent mean of at least 2 independent pools containing 30 worms each.
Error bars, SEM. * P < 0.05.
Previous studies have revealed that many miRNAs are also down-regulated with age in C. elegans (Ibanez-Ventoso et al., 2006; Kato et al., 2011). In agreement with the mammalian data, this decline in miRNAs was associated with a progressive decline in Dicer and Drosha mRNA with age in C. elegans, so that in old animals (31 days), they were reduced by 90% (Figures 5C and S6A and B). The expression of Argonaute (alg-1 and alg-2) and Exportin-1 (xpo-1) mRNAs showed transient increases from day 5 to day 10 and then decreases between days 10 to 31 (Figure S6B). Consistent with the changes in Dicer, expression of lin-4 and let-7 miRNAs also decreased with age (Figure S6B). As in mice, the reduction of Dicer expression with age was delayed in calorically-restricted, long-lived worms, such as worms with the eat-2 mutation (Figure 5C). Dietary restriction by growth in a liquid medium promoted a similar trend towards an increase in Dicer mRNA (Figure S6C).
Both heat shock factors and the insulin/IGF-1 signaling pathway have been proposed to mediate the effects of dietary restriction in C. elegans (Greer et al., 2007; Steinkraus et al., 2008). To test if these factors could be placed upstream of Dicer, we measured Dicer mRNA in worms that overexpress heat shock factor-1 (hsf-1) and worms with impairment of the insulin/IGF receptor orthologue daf-2. Dicer mRNA levels were increased 5.9-fold in animals that overexpress hsf-1 (Figure 5D), but were markedly reduced in young animals in all three daf-2 loss-of-function models studied (e1370, e1368 and RNAi) (Figure S6A). Thus, while hsf-1 positively regulates Dicer, insulin/IGF-1 signaling seems to act through a parallel pathway to modulate the effects of dietary restriction, consistent with our data in the FIRKO mouse.
Dicer, lifespan, and stress resistance
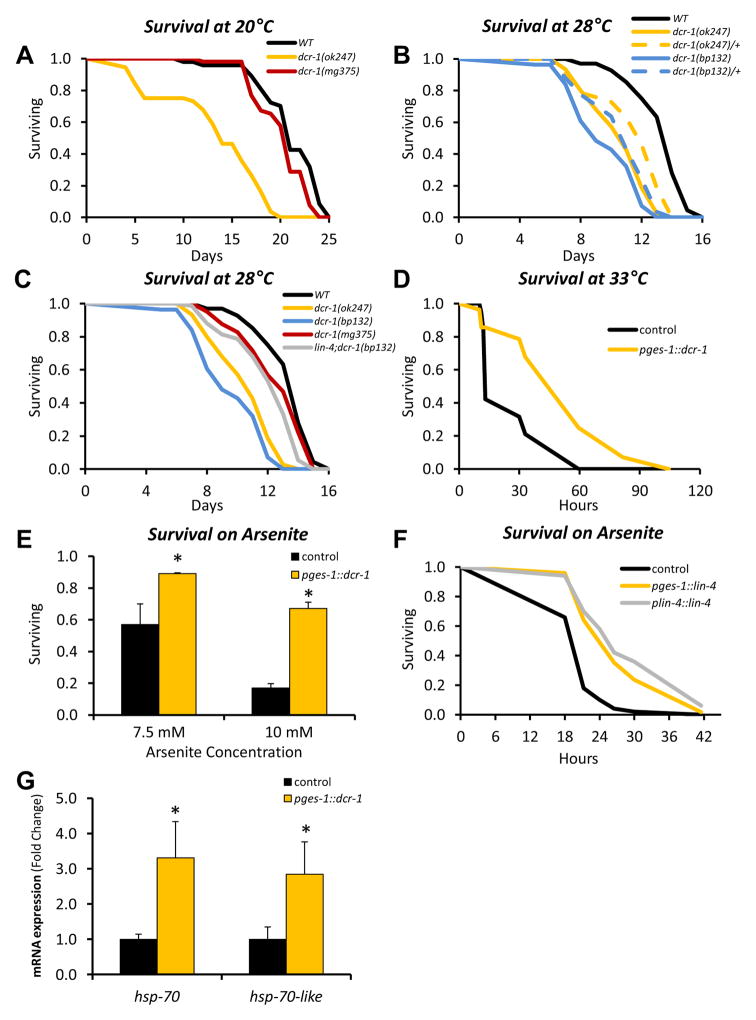
We took advantage of this evolutionary conservation to use C. elegans to determine whether modulation of Dicer function might influence aging and stress responses at the organismal level. The Dicer null mutant dcr-1(ok247) develops to adulthood, presumably because of maternally supplied Dicer, but cannot produce mature miRNAs or endo-siRNAs and is sterile. Under normal growth conditions (20°C) or modest heat stress (≥ 28°C), dcr-1(ok247) exhibited marked decreases in mean and maximum lifespan (Figure 6A and B and Table S4). Resistance to heat was similarly reduced in dcr-1(bp132) mutants, which are partially defective in miRNA processing but are fertile (Ren and Zhang, 2010) (Figure 6B and Table S4). Importantly, survival was reduced in dcr-1(ok247) and dcr-1(bp132) heterozygotes at each temperature examined (Figure 6B and Table S4). This indicates that Dicer haploinsufficiency renders worms short-lived, and argues that the role of Dicer in stress resistance and lifespan is independent of its developmental functions. In contrast, lifespan and heat tolerance were decreased only slightly by a Dicer mutation (mg375) (Pavelec et al., 2009) that impairs its helicase domain and endo-siRNA production, but leaves miRNA synthesis intact (Figure 6A and B and Table S4), suggesting that the effect of Dicer on lifespan and stress defense may be largely associated with altered miRNA processing.
Figure 6. miRNA Processing in the Worm Intestine Affects Organism Survival and Stress Resistance in a Cell Non-Autonomous Manner.
See also Figure S7 and Table S4.
(A) Survival of Dicer loss-of-function mutants dcr-1(ok247) and dcr-1(mg375), and wild type (WT) N2 worms at 20°C (n=41–52 per group).
(B,C) Survival of Dicer loss-of-function mutants [dcr-1(ok247), dcr-1(bp132), and dcr-1(mg375)], lin-4 gain-of-function mutants [lin-4(bp238)] and wild type (WT) N2 worms at 28°C (n=57–81 per group). In these cases, lifespan was counted from hatching, not from adulthood as in all the other cases.
(D) Survival at 33°C of worms with selective overexpression of Dicer in the intestine (pges-1::dcr-1) (n=19–28 per group).
(E) Survival of worms after 8h in different concentrations of arsenite (n=24–50 per group). Experiments were performed twice using multiple lines. The graph shows a representative result obtained with pges-1::dcr-1 # 6. * P < 0.05. Error bars, SEM.
(F) Survival of worms on 5 mM arsenite (n=40–50 per group). Lines assessed were pges-1::lin-4 # 2 and plin-4::lin-4 # 4. This experiment was repeated twice with identical results. P < 0.05 comparing lin-4 transgenics and control.
(G) mRNA levels were assessed in day 3 worms by RT-qPCR. Pools of at least 30 worms were used for the RT-qPCR analyses. * P < 0.05. Error bars, SEM.
Experiments with transgenic lines were performed in parallel with a co-injection control, in which worms were germline transformed with the plasmids pUN24 and pJK590 only. For statistics refer to Table S4.
In C. elegans, Dicer is expressed at high levels throughout the intestine (Figure S7A), the closest analogue of fat in mammals. To investigate whether up-regulation of Dicer in C. elegans intestine could affect survival and stress resistance, we generated transgenic lines that overexpressed Dicer 2- to 6-fold in the intestine (pges-1::dcr-1) (Figure S7B and C). At middle age, these transgenic worms maintained Dicer levels to levels comparable to those of young (day 3) wild type animals (Figure S7D). Intestinal Dicer overexpression significantly enhanced resistance to various stresses. In the higher-expressing pges-1::dcr-1 lines, mean and maximum lifespan at 33°C were increased 2- to 3- and 2.1- to 2.8-fold, respectively (Figure 6D and Table S4). These worms were also resistant to oxidative stress from paraquat or arsenite and had a slightly increased lifespan under normal conditions (20°C)(Figures 6E, S7E, S7F and Table S4). To determine if Dicer expression in the intestine is sufficient to promote survival, we introduced the pges-1::dcr-1 transgene into the Dicer loss of function mutant dcr-1(ok247) (Figures S7G and H). At high temperature, intestinal Dicer expression increased survival of this mutant from 88 to 97 hours, i.e. similar to the effect of whole body expression using the dcr-1 promoter (mean lifespan 88 vs. 106 hours) (Figure S7I and Table S4). Thus, Dicer function in selective tissues, such as the intestine, can increase resistance of the organism to stress.
While it is likely that the effect of Dicer on longevity and stress resistance involves multiple miRNAs, Dicer overexpression in intestine resulted in 1.5- to 5-fold increases in the levels of miRNAs let- 7, miR-231, and lin-4 (Figures S7C), the last of which has been shown to promote longevity (Boehm and Slack, 2005). Furthermore, the impaired heat resistance of dcr-1(bp132) was substantially rescued by a mutation that allows lin-4 to be processed more readily [lin-4(bp238)] (Ren and Zhang, 2010) (Figure 6C and Table S4). This suggests that while many miRNAs influence lifespan (Pincus et al., 2011), lin-4 may be especially important for the stress defense and longevity functions of Dicer. Accordingly, intestinal overexpression of lin-4 increased resistance to arsenite (Figure 6F, S7J, and S7K). Overexpression of Dicer in the intestine upregulated the chaperone genes hsp-70 and hsp-70-like (F44E5.4) to a similar extent seen in animals overexpressing hsf-1 (Figures 5D and 6G), and modestly increased expression of other stress response genes (Figure S7L). Together, these data suggest that the Dicer influences stress resistance and longevity through multiple mechanisms that involve lin-4 and presumably other miRNAs, along with chaperone defenses.
DISCUSSION
Studies in species across the evolutionary spectrum from yeast to mammals have shown that calorie restriction can promote longevity and stress resistance (Fontana et al., 2010). In mammals, this appears to be due in large part to the effect of calorie restriction on adipose tissue, since reducing fat mass by surgical (Huffman and Barzilai, 2009) or genetic approaches (Bluher et al., 2003) without reducing food intake can mimic this effect. The exact molecular mediators linking adipose tissue and calorie restriction to longevity, however, remain poorly understood. In this study, we show that in adipose tissue of mice there is a coordinated decline in many miRNAs with age and that this correlates with a down-regulation of miRNA processing enzymes, especially Dicer. This time-course coincides with a period of significant growth and plasticity of the adipose tissue in rodents. This also suggests that metabolic studies and studies of age-related diseases like type 2 diabetes using animals of this age may be limited because of the changing environment caused by changing levels of numerous miRNAs. A similar decline in Dicer and miRNAs is found in nematodes as they age and in human preadipocytes taken from older individuals. Importantly, in both mice and worms this pattern is almost completely reversed by calorie restriction. Further, we show that changing the levels of Dicer and miRNA expression in fat or its counterpart in worms, the intestine, by either knockout or overexpression can alter organismal survival and stress resistance. Thus, the regulation of miRNA processing in fat tissue or its analogue with aging is an evolutionarily conserved phenomenon which appears to integrate metabolic signals and coordinate the detrimental effects of stress and the beneficial effects of caloric restriction on the aging process.
Dicer is a RNase III endoribonuclease whose primary function is to cleave double-stranded RNA into 20–25 nucleotide double-stranded RNA fragments that mediate RNA interference (Jinek and Doudna, 2009). Dicer is required for miRNA processing, cleaving the hairpin loop structures of pre miRNAs to release mature miRNAs that can bind to complementary sequences at the 3′UTR of target mRNAs to induce mRNA degradation and/or to inhibit protein translation (Guo et al., 2010; Jinek and Doudna, 2009). Dicer is also required for synthesis of small interfering RNAs (e.g. endo-siRNAs) that have been implicated in gene silencing and epigenetic regulation in both somatic and germline cells (Duchaine et al., 2006), and has been show to be involved in the degradation of Alu RNA sequences (Kaneko et al., 2011). These Alu RNAs have been associated with macular degeneration in aging in humans, and accumulation of Alu RNAs correlates with a down-regulation of Dicer in the retina of aged individuals (Kaneko et al., 2011). Despite the multiple roles for Dicer in producing or degrading small RNAs, our data with different mutant alleles of the Dicer gene in worms suggest that the effects of Dicer down-regulation on stress defense is primarily mediated by impaired miRNA biogenesis.
Although Dicer is an important enzyme in the miRNA processing pathway and its down-regulation can result in the down-regulation of many miRNAs, not all miRNAs are affected to the same degree, suggesting that Dicer expression level is not the only factor determining miRNA levels. Indeed, factors like the rate of transcription of pri-miRNAs, splicing of miRtrons, RNA editing, levels of the dsRNA precursors or the pre-miRNAs, level of Dicer partners, post-transcriptional modification of Dicer, and miRNA turnover all contribute to miRNA expression (Winter et al., 2009). Consistent with this, potentiation of Dicer-mediated miRNA processing by increasing TRBP phosphorylation results in increased expression of many miRNAs. However, in this case, some miRNAs are reduced even though Dicer activity is increased (Paroo et al., 2009), suggesting a complex regulation of miRNA processing. Nonetheless, alterations in Dicer expression that affect miRNA processing appear to play a critical role in stress response, as C. elegans carrying Dicer mutations that block (homozygous) or diminish (heterozygous) miRNA synthesis are both hypersensitive to stress. This suggests that as Dicer declines with age there may be a graded effect or perhaps some threshold at which the decrease affects stress defense and lifespan, even without a complete loss of the enzyme.
miRNAs themselves are important regulators and play critical roles in fine-tuning the transcriptome, helping to preserve cell identity and survival (Ebert and Sharp, 2012). Dysregulation of miRNAs has been associated with alterations in development and multiple pathologies, including various age-related diseases such as diabetes, cardiovascular disease, neurodegenerative disease and cancer (Bernstein et al., 2003; Boehm and Slack, 2005; Kanellopoulou et al., 2005; Lanceta et al., 2010). miRNAs have also been shown to influence pathways involved in senescence, like the p53/p21 pathway, the p16/RB pathway, the pathways involved in control of a variety of secretory proteins associated with senescence and chronic disease (such as IL-6 and IL-8), as well as transcriptional and post-transcriptional factors involved in control of cellular senescence, such as HMGA2 (Bhaumik et al., 2009; Gorospe and Abdelmohsen, 2011).
Of the miRNAs that have been shown to be involved in control of aging and stress resistance, those in the lin-4/miR-125 family are of particular interest. Previous studies have shown that reducing lin-4 expression will reduce lifespan in worms, while increasing its level will prolong lifespan (Boehm and Slack, 2005). Likewise, miR-125b, the mammalian orthologue of lin-4, has been shown to be a negative regulator of the important senescence network coordinated by p53 in mammalian cells (Le et al., 2009). Here we show that Dicer overexpression in intestine of C. elegans can prevent the decline of lin-4 with aging, and this is associated with increased levels of the molecular chaperone hsp-70, a target gene of hsf-1, which has been demonstrated to mediate some of the effects of lin-4 on longevity (Boehm and Slack, 2005). In addition, we find that lin-4 overexpression in the intestine is sufficient to confer resistance to stress and a compensatory lin-4 mutation largely rescues the dcr-1(bp132) defect in heat stress survival. Moreover, as Dicer levels fall with age, levels of members of the miR-125/lin-4 family are reduced in cells of the adipose tissue of both mice and humans. In mouse preadipocytes, we show that restoration of this down-regulation can reverse some components of senescence and the activation of stress pathways. Thus, although the decline in Dicer affects many miRNAs that are likely involved in aging and age-related disease, our data demonstrate that alterations in the expression of members of the miR-125/lin-4 family in adipose-related tissues play an important role in stress response and contribute to the ability of fat to promote organismal survival in a cell non-autonomous manner.
The insulin/IGF-1 signaling and the heat shock pathways have been proposed to be involved in some of the beneficial effects of calorie restriction on health and lifespan (Greer et al., 2007; Russell and Kahn, 2007; Steinkraus et al., 2008). In worms, Dicer expression is increased by hsf-1 overexpression, but is independent of daf-2 function, suggesting that changes in miRNA processing may be more closely linked to the heat shock pathway than the insulin/IGF-1 pathway. Worms with daf-2 loss-of-function mutations, which are known to have increased longevity and stress resistance (Kenyon et al., 1993; Wang and Ruvkun, 2004), exhibit relatively low levels of Dicer expression when young, and these further decline with aging. Likewise, Dicer levels in the adipose tissue of FIRKO mice, which have an inactivated insulin receptor gene in fat, are similar to those in controls, despite their longevity phenotype (Bluher et al., 2003). The fact that the effects of insulin/IGF-1 signaling on longevity and stress resistance are independent of those related to changes in Dicer is further supported by the finding that acute treatment of preadipocytes in culture with insulin does not affect Dicer expression, but prevents the down-regulation of Dicer in response to oxidative stress. Thus, Dicer seems to act in a different, and possibly complementary, pathway from insulin/IGF-1 signaling in its ability to mediate effects on survival and stress resistance (model summarized in Figure 7).
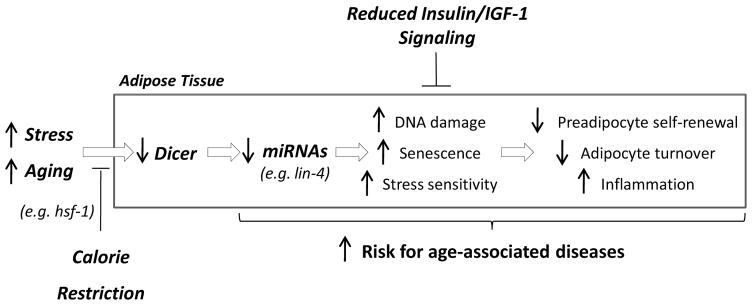
Figure 7.
Aging and calorie restriction modulate miRNA processing in adipose tissue to confer resistance to stress and promote healthy lifespan in organisms across the evolutionary spectrum.
Exactly how adipose and related tissues mediate their cell non-autonomous effects on aging remains unknown. In mammals, adipose tissue is the site of production of many hormones and adipokines and releases metabolic intermediates such as free fatty acids (Waki and Tontonoz, 2007). Adipose tissue can also serve as a site for production or storage of toxic intermediates, including products of reactive oxygen species production, small lipophilic molecules, and other metabolic by products (Picard and Guarente, 2005; Wisse et al., 2007). It is also possible that the systemic effects of adipose tissue on aging could be mediated by some sterol metabolites, such as the dafachronic acids previously identified in worms (Gerisch et al., 2007) or products of miRNA processing itself. Interestingly, small RNAs can be detected in the circulation in mammals (Cortez et al., 2011), suggesting that the intercellular transport of miRNAs or other small RNAs might contribute to stress resistance and longevity. Consistent with this idea, the adipocyte has been shown to secrete vesicles carrying small RNAs, which could function in intercellular communication (Muller et al., 2011; Ogawa et al., 2010), and some studies have suggested that circulating miRNAs might serve as biomarkers of cardiovascular disease, cancer and other age-related diseases (Cortez et al., 2011). In addition, changes in miRNAs in preadipocytes may have autonomous effects on the ability of these preadipocytes to self-renew. This could lead to an accumulation of large, insulin resistant fat cells in adipose tissue, which would in turn promote an inflammatory response that could contribute to systemic insulin resistance and many of the diseases associated with aging (Bhaumik et al., 2009; Tchkonia et al., 2010). Thus, Dicer and miRNA expression in fat could serve as a coordinating site for the cell autonomous and cell non-autonomous events that regulate the response of an organism to environmental stress in response to metabolic fluctuations.
In summary, our work demonstrates that down-regulation of miRNAs and miRNA processing is an evolutionarily conserved physiological phenomenon associated with age. In mammals, this regulation takes place most prominently in adipose tissue, and in both mammals and worms, this process appears to participate in the modulation of the ability of the organism to respond to stress. Interventions that preserve miRNA processing in adipose tissue, therefore, may provide a new approach for prevention of some of the complications associated with aging and age-related diseases such as diabetes.
EXPERIMENTAL PROCEDURES
Mice
Mice were maintained on a 12h light-dark cycle with ad libitum access to tap water (reverse osmosis purified) and chow diet (Mouse Diet 9F, PharmaServ) until the date of sacrifice, unless otherwise indicated. Male mice were used throughout this study. For the feeding paradigm, 6 week-old C57BL/6J mice were given a low-fat (Rodent NIH-31M Auto, Taconic) or high-fat (TD.93075, Harlan Teklad) diet for 18 weeks prior to the sacrifice. For the fasting paradigm, 8 week-old C57BL/6J mice were submitted to 24 h food deprivation. FIRKO mice and littermate controls (IRlox/lox) were as previously described (Bluher et al., 2003). Aged and calorie restricted C57BL/6J mice were obtained from the National Institute on Aging. Calorie restriction was initiated at 14 weeks of age with a 10% decrease in calories, increased to 25% restriction at 15 weeks, and to 40% restriction at 16 weeks, which was maintained until the indicated age, when the mice were sacrificed. Mice were killed by cervical dislocation. Subcutaneous flank white adipose, perigonadal white adipose, interscapular brown adipose, liver, gastrocnemius skeletal muscle, brain, spleen, and kidney were collected, snap frozen in N2, and stored at −80 °C. Protocols for animal use were reviewed and approved by the Animal Care Committee of the Joslin Diabetes Center and Brandeis University and were in accordance with the National Institutes of Health guidelines.
Fat-specific Dicer knockout mice (AdicerKO) were generated by breeding Dicerlox/lox mice with mice carrying the Cre recombinase driven by the adiponectin promoter (kindly provided by Evan Rosen, Beth Israel Deaconess Hospital, Boston, MA). Paraquat was injected intraperitoneally at 0.65 mg/Kg body weight and survival was monitored periodically.
C. elegans
Worms were derived from the wild-type N2 strain and cultured at 20°C on Nematode Growth Medium plates that were seeded with a lawn of E. coli strain OP50-1 unless otherwise indicated. The following alleles were used in this study: daf-2(e1370), daf-2(e1368), dcr-1(ok247)(PD8753), dcr-1(bp132), dcr-1(mg375), lin-4(bp238); LG IV uuIs1(sur-5::GFP;pRF4phsp16::GFP[IR]); eat-2(ad1116) II; ccIs4251[myo-3::GFP + dpy-20(+)] I; qtIs3[myo-2::GFP dsRNA hairpin] III; mIs11[myo-2::GFP + pes-10::GFP + gut::GFP] IV; Ex[lin-4p::lin-4; myo-2p::GFP]; and Is[hsf-1p::hsf-1;rol-6]. The dcr-1 gene, including all intronic regions, was amplified from the fosmid clone WRM066bH04 (Geneservice) using Phusion High-Fidelity DNA Polymerase (Finnzymes) and the oligos 5′-AGCATGCATGGTCAGGGTAAGAGCTGAT-3′ and 5′-ACCCGGGGAACAGTTGTTAATGATGGGC-3′, and subcloned into pGem-T Easy vector (Promega). The gene was then transferred to the pPD95.75.pges-1 vector using the Spe I and Xma I sites. The presumptive dcr-1 promoter was obtained by amplifying 0.6 kb of the upstream genomic sequence using the oligos 5′-TAAGCTTAAAACTCACCATCAGGCATTCT-3′ and 5′-ACCCGGGGAACAGTTGTTAATGATGGGC-3′ and cloned upstream of dcr-1::GFP insert. Lin-4 precursor was amplified from N2 genomic DNA using the oligos 5′-ATCTAGAATGCTTCCGGCCTGTTCC-3′ and 5′-AGGATCCATCTGCTCAAACCGTCCT-3′ and similarly cloned into pPD95.75.pges-1 vector. Full length lin-4 gene, including its own promoter, was also amplified from N2 genomic DNA using the oligos 5′-ACAATAAAAGTCGACGAGACGC-3′ and 5′-ACTTCTGAAAATAATCGTTTGACCC-3′ and cloned into pPD95.75. All germline transformations were performed using pUN24 (pY66H1B.3::Y66H1B.3::GFP) (Kovacevic and Cram, 2010) and pJK590 (plag-2::GFP) as co-injection markers. All plasmids were injected at 10 ng/ul as described (Mello et al., 1991). Multiple lines were generated for each genotype and screened for dcr-1 and miRNA overexpression. At least three lines of each genotype were analyzed in subsequent experiments. Experiments with transgenic lines were always performed in parallel with a co-injection marker control line, in which worms were germline transformed with the plasmids pUN24 and pJK590 only. Lifespan analysis was conducted essentially as described (Robida-Stubbs et al., 2012). Experiments were performed in the absence of fluorodeoxyduridine, and worms were separated from their progeny every two days during the reproductive phase. Oxidative stress resistance was assessed by survival at 20°C on NGM/OP50-1 plates containing 50 mM paraquat (Sigma) and percentage of survival at 20°C on liquid medium containing arsenite (Sigma). Optimal arsenite concentrations were predefined based on the overall sensitivity of the control strain measured by prior dose-response experiments. Heat shock assays were performed by transferring young adult worms from 20°C to 28–33°C and scoring survival periodically. Liquid dietary restriction was performed by a modification of a published protocol (Mair et al., 2009), which consistently increased N2 lifespan and stress resistance (Blackwell lab, unpublished). All assays were initiated on Day 1 of adulthood unless otherwise indicated. Survival plots and p values (Log-Rank) were determined using JMP 8.0.2 software. Microscopy images were obtained using Zeiss Axioskop 2 and analyzed using Axiovision software release 4.6.3.
Cell culture
Adipocyte precursor cells were isolated from the subcutaneous adipose tissue of new born Dicerlox/lox mice upon collagenase digestion (1.5 mg/ml; Worthington Biochemical). After two days in culture, cells were immortalized using retrovirus harboring the pBabe SV40 Large T antigen puromycin vector. The pool of puromycin-resistant clones was amplified and transduced with 750 MOI adenoviruses harboring GFP (Ad5CMVeGFP) or Cre recombinase (Ad5CMVCre-eGFP) (Gene Transfer Vector Core, University of Iowa). Four days after adenovirus infection, Dicer knockout cells (KO) and controls (Lox) were analyzed. Abdominal subcutaneous fat tissue from 3 young (age 26 ± 4.1 yrs), 3 middle-aged (age 46 ± 2.2 yrs), and 3 old (age 74 ± 3.7 yrs) lean female subjects was obtained during laparoscopic intra-abdominal procedures, and human preadipocytes were isolated as previously described (Tchkonia et al., 2007).
Additional methods can be found in the Online Supplement.
Supplementary Material
HIGHLIGHTS.
miRNAs and Dicer decline with age in mouse fat, human preadipocytes and C. elegans
In mice and C. elegans, this is prevented by calorie restriction
In cultured cells, Dicer is down-regulated by oxidative and UV stress
Dicer levels in mouse fat or worm intestine influence stress defense and longevity
Acknowledgments
We thank J. LaVecchio, G. Buruzula, J. Schroeder, M. Rourk, G. Smyth, S. Rath and N. Moroz for help with experiments. We also thank M. Merkenschlager and H. M. Kronenberg for providing us with the Dicerlox/lox mice, E. Rosen for providing us with the Adiponectin-Cre mice, E. Cram and I. Kovacevic for providing us with plasmids, B. L. Bass, F. Slack, H. Zhang, S. Kennedy and the Caenorhabditis Genetics Center for providing us with C. elegans strains, and N. Giorgadze for help in culturing human preadipocytes.
M. A. Mori, Y. Macotela, J. Boucher, S.J. Russell, and C. R. Kahn were supported by grants from the NIH - DK082659, DK033201 and AG032869 - as well as grants from the Ellison Foundation, the Joslin Diabetes and Endocrinology Research Center cores (DK036836) and the Mary K. Iacocca Professorship. P. Raghavan, S. Robida-Stubbs and T.K. Blackwell were supported by the NIH GM62891, and the Beatson and Myra Reinhard Family Foundations. T. Thomou and J.L. Kirkland were supported by grants from the NIH – AG13925 and AG31736 - as well as the Ted Nash Long Life Foundation and the Noaber Foundation Professorship in Aging Research.
Footnotes
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
M.A.M., P.R., J.L.K., T.K.B. and C.R.K. conceived and designed the experiments. M.A.M., P.R., T.T., J.B., S.R., Y.M., S.J.R. performed the experiments. All authors analyzed the data and edited/reviewed the manuscript. M.A.M., P.R., T.T., T.K.B. and C.R.K. wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akerfelt M, Morimoto RI, Sistonen L. Heat shock factors: integrators of cell stress, development and lifespan. Nat Rev Mol Cell Biol. 2010;11:545–555. doi: 10.1038/nrm2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RM, Shanmuganayagam D, Weindruch R. Caloric restriction and aging: studies in mice and monkeys. Toxicol Pathol. 2009;37:47–51. doi: 10.1177/0192623308329476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein E, Kim SY, Carmell MA, Murchison EP, Alcorn H, Li MZ, Mills AA, Elledge SJ, Anderson KV, Hannon GJ. Dicer is essential for mouse development. Nat Genet. 2003;35:215–217. doi: 10.1038/ng1253. [DOI] [PubMed] [Google Scholar]
- Bhaumik D, Scott GK, Schokrpur S, Patil CK, Orjalo AV, Rodier F, Lithgow GJ, Campisi J. MicroRNAs miR-146a/b negatively modulate the senescence-associated inflammatory mediators IL-6 and IL-8. Aging (Albany NY) 2009;1:402–411. doi: 10.18632/aging.100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop NA, Guarente L. Genetic links between diet and lifespan: shared mechanisms from yeast to humans. Nat Rev Genet. 2007;8:835–844. doi: 10.1038/nrg2188. [DOI] [PubMed] [Google Scholar]
- Bluher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299:572–574. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- Bluher M, Michael MD, Peroni OD, Ueki K, Carter N, Kahn BB, Kahn CR. Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Dev Cell. 2002;3:25–38. doi: 10.1016/s1534-5807(02)00199-5. [DOI] [PubMed] [Google Scholar]
- Boehm M, Slack F. A developmental timing microRNA and its target regulate life span in C. elegans. Science. 2005;310:1954–1957. doi: 10.1126/science.1115596. [DOI] [PubMed] [Google Scholar]
- Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- Cortez MA, Bueso-Ramos C, Ferdin J, Lopez-Berestein G, Sood AK, Calin GA. MicroRNAs in body fluids--the mix of hormones and biomarkers. Nat Rev Clin Oncol. 2011;8:467–477. doi: 10.1038/nrclinonc.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchaine TF, Wohlschlegel JA, Kennedy S, Bei Y, Conte D, Jr, Pang K, Brownell DR, Harding S, Mitani S, Ruvkun G, et al. Functional proteomics reveals the biochemical niche of C. elegans DCR-1 in multiple small-RNA-mediated pathways. Cell. 2006;124:343–354. doi: 10.1016/j.cell.2005.11.036. [DOI] [PubMed] [Google Scholar]
- Ebert MS, Sharp PA. Roles for microRNAs in conferring robustness to biological processes. Cell. 2012;149:515–524. doi: 10.1016/j.cell.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Klein S. Aging, adiposity, and calorie restriction. Jama. 2007;297:986–994. doi: 10.1001/jama.297.9.986. [DOI] [PubMed] [Google Scholar]
- Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerisch B, Rottiers V, Li D, Motola DL, Cummins CL, Lehrach H, Mangelsdorf DJ, Antebi A. A bile acid-like steroid modulates Caenorhabditis elegans lifespan through nuclear receptor signaling. Proc Natl Acad Sci U S A. 2007;104:5014–5019. doi: 10.1073/pnas.0700847104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorospe M, Abdelmohsen K. MicroRegulators come of age in senescence. Trends Genet. 2011;27:233–241. doi: 10.1016/j.tig.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, Dowlatshahi D, Banko MR, Villen J, Hoang K, Blanchard D, Gygi SP, Brunet A. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr Biol. 2007;17:1646–1656. doi: 10.1016/j.cub.2007.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010;466:835–840. doi: 10.1038/nature09267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman DM, Barzilai N. Role of visceral adipose tissue in aging. Biochim Biophys Acta. 2009;1790:1117–1123. doi: 10.1016/j.bbagen.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez-Ventoso C, Yang M, Guo S, Robins H, Padgett RW, Driscoll M. Modulated microRNA expression during adult lifespan in Caenorhabditis elegans. Aging Cell. 2006;5:235–246. doi: 10.1111/j.1474-9726.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Jinek M, Doudna JA. A three-dimensional view of the molecular machinery of RNA interference. Nature. 2009;457:405–412. doi: 10.1038/nature07755. [DOI] [PubMed] [Google Scholar]
- Kaneko H, Dridi S, Tarallo V, Gelfand BD, Fowler BJ, Cho WG, Kleinman ME, Ponicsan SL, Hauswirth WW, Chiodo VA, et al. DICER1 deficit induces Alu RNA toxicity in age-related macular degeneration. Nature. 2011;471:325–330. doi: 10.1038/nature09830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanellopoulou C, Muljo SA, Kung AL, Ganesan S, Drapkin R, Jenuwein T, Livingston DM, Rajewsky K. Dicer-deficient mouse embryonic stem cells are defective in differentiation and centromeric silencing. Genes Dev. 2005;19:489–501. doi: 10.1101/gad.1248505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Chen X, Inukai S, Zhao H, Slack FJ. Age-associated changes in expression of small, noncoding RNAs, including microRNAs, in C. elegans. RNA. 2011;17:1804–1820. doi: 10.1261/rna.2714411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Kovacevic I, Cram EJ. FLN-1/filamin is required for maintenance of actin and exit of fertilized oocytes from the spermatheca in C. elegans. Dev Biol. 2010;347:247–257. doi: 10.1016/j.ydbio.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanceta J, Prough RA, Liang R, Wang E. MicroRNA group disorganization in aging. Exp Gerontol. 2010;45:269–278. doi: 10.1016/j.exger.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Le MT, Teh C, Shyh-Chang N, Xie H, Zhou B, Korzh V, Lodish HF, Lim B. MicroRNA-125b is a novel negative regulator of p53. Genes Dev. 2009;23:862–876. doi: 10.1101/gad.1767609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair W, Panowski SH, Shaw RJ, Dillin A. Optimizing dietary restriction for genetic epistasis analysis and gene discovery in C. elegans. PloS one. 2009;4:e4535. doi: 10.1371/journal.pone.0004535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello CC, Kramer JM, Stinchcomb D, Ambros V. Efficient gene transfer in C.elegans: extrachromosomal maintenance and integration of transforming sequences. Embo J. 1991;10:3959–3970. doi: 10.1002/j.1460-2075.1991.tb04966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller G, Schneider M, Biemer-Daub G, Wied S. Microvesicles released from rat adipocytes and harboring glycosylphosphatidylinositol-anchored proteins transfer RNA stimulating lipid synthesis. Cell Signal. 2011;23:1207–1223. doi: 10.1016/j.cellsig.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Ogawa R, Tanaka C, Sato M, Nagasaki H, Sugimura K, Okumura K, Nakagawa Y, Aoki N. Adipocyte-derived microvesicles contain RNA that is transported into macrophages and might be secreted into blood circulation. Biochem Biophys Res Commun. 2010;398:723–729. doi: 10.1016/j.bbrc.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Paroo Z, Ye X, Chen S, Liu Q. Phosphorylation of the human microRNA-generating complex mediates MAPK/Erk signaling. Cell. 2009;139:112–122. doi: 10.1016/j.cell.2009.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavelec DM, Lachowiec J, Duchaine TF, Smith HE, Kennedy S. Requirement for the ERI/DICER complex in endogenous RNA interference and sperm development in Caenorhabditis elegans. Genetics. 2009;183:1283–1295. doi: 10.1534/genetics.109.108134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard F, Guarente L. Molecular links between aging and adipose tissue. Int J Obes (Lond) 2005;29(Suppl 1):S36–39. doi: 10.1038/sj.ijo.0802912. [DOI] [PubMed] [Google Scholar]
- Pincus Z, Smith-Vikos T, Slack FJ. MicroRNA predictors of longevity in Caenorhabditis elegans. PLoS Genet. 2011;7:e1002306. doi: 10.1371/journal.pgen.1002306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H, Zhang H. Wnt signaling controls temporal identities of seam cells in Caenorhabditis elegans. Dev Biol. 2010;345:144–155. doi: 10.1016/j.ydbio.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Robida-Stubbs S, Glover-Cutter K, Lamming DW, Mizunuma M, Narasimhan SD, Neumann-Haefelin E, Sabatini DM, Blackwell TK. TOR Signaling and Rapamycin Influence Longevity by Regulating SKN-1/Nrf and DAF-16/FoxO. Cell Metab. 2012;15:713–724. doi: 10.1016/j.cmet.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell SJ, Kahn CR. Endocrine regulation of ageing. Nat Rev Mol Cell Biol. 2007;8:681–691. doi: 10.1038/nrm2234. [DOI] [PubMed] [Google Scholar]
- Sahin E, Depinho RA. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature. 2010;464:520–528. doi: 10.1038/nature08982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinkraus KA, Smith ED, Davis C, Carr D, Pendergrass WR, Sutphin GL, Kennedy BK, Kaeberlein M. Dietary restriction suppresses proteotoxicity and enhances longevity by an hsf-1-dependent mechanism in Caenorhabditis elegans. Aging Cell. 2008;7:394–404. doi: 10.1111/j.1474-9726.2008.00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Muthukumar AR, Lawrence RA, Fernandes G. Effects of calorie restriction on polymicrobial peritonitis induced by cecum ligation and puncture in young C57BL/6 mice. Clin Diagn Lab Immunol. 2001;8:1003–1011. doi: 10.1128/CDLI.8.5.1003-1011.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchkonia T, Lenburg M, Thomou T, Giorgadze N, Frampton G, Pirtskhalava T, Cartwright A, Cartwright M, Flanagan J, Karagiannides I, et al. Identification of depot-specific human fat cell progenitors through distinct expression profiles and developmental gene patterns. Am J Physiol Endocrinol Metab. 2007;292:E298–307. doi: 10.1152/ajpendo.00202.2006. [DOI] [PubMed] [Google Scholar]
- Tchkonia T, Morbeck DE, Von Zglinicki T, Van Deursen J, Lustgarten J, Scrable H, Khosla S, Jensen MD, Kirkland JL. Fat tissue, aging, and cellular senescence. Aging Cell. 2010;9:667–684. doi: 10.1111/j.1474-9726.2010.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng YH, Ueki K, Kriauciunas KM, Kahn CR. Differential roles of insulin receptor substrates in the anti-apoptotic function of insulin-like growth factor-1 and insulin. J Biol Chem. 2002;277:31601–31611. doi: 10.1074/jbc.M202932200. [DOI] [PubMed] [Google Scholar]
- Vijg J, Campisi J. Puzzles, promises and a cure for ageing. Nature. 2008;454:1065–1071. doi: 10.1038/nature07216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waki H, Tontonoz P. Endocrine functions of adipose tissue. Annu Rev Pathol. 2007;2:31–56. doi: 10.1146/annurev.pathol.2.010506.091859. [DOI] [PubMed] [Google Scholar]
- Wang D, Ruvkun G. Regulation of Caenorhabditis elegans RNA interference by the daf-2 insulin stress and longevity signaling pathway. Cold Spring Harb Symp Quant Biol. 2004;69:429–431. doi: 10.1101/sqb.2004.69.429. [DOI] [PubMed] [Google Scholar]
- Winter J, Jung S, Keller S, Gregory RI, Diederichs S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol. 2009;11:228–234. doi: 10.1038/ncb0309-228. [DOI] [PubMed] [Google Scholar]
- Wisse BE, Kim F, Schwartz MW. Physiology. An integrative view of obesity. Science. 2007;318:928–929. doi: 10.1126/science.1148032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.