Abstract
Androgen deprivation therapy (ADT) with gonadal testosterone depletion is the frontline treatment for advanced prostate cancer. Other hormonal interventions have a role in the treatment of prostate cancer. We sought to examine systematically the evidence for hormonal interventions in prostate cancer, risks of ADT and interventions that mitigate these risks. PubMed and Web of Science were searched for English-language articles using the terms prostate cancer, androgen deprivation therapy and hormone treatment between 1966 and February 2010. Bibliographies from selected articles and meeting abstracts were also reviewed. The highest quality data was emphasized. Results for therapeutic studies were focused primarily on randomized controlled clinical trials and the Jadad scale criteria was used to evaluate the quality of these studies. Four trials of the efficacy of intermittent versus continuous ADT were included. One randomized study analysis and 6 postrandomization analyses were included on the effects of ADT on cardiovascular mortality. Seven randomized controlled trials were included of pharmacologic interventions for the treatment of metabolic effects due to ADT. One randomized trial of gonadotropin-releasing hormone (GnRH)-antagonist versus GnRH-agonist was included. Six phase I/II clinical trials of secondary hormonal therapies with novel mechanisms of action were included. Randomized studies completed to date indicate that intermittent might be equivalent to continuous ADT. Although adverse effects of ADT include risk factors for cardiovascular disease, effects on cardiovascular mortality are uncertain. Bone loss and increased risk of fracture may be effectively treated with pharmacologic interventions. Benefits of ADT must be balanced with a consideration of the risks.
Keywords: Prostate cancer, androgen deprivation therapy, androgens, androgen receptor
Introduction
In 2009 alone, there were an estimated 192,280 new cases of prostate cancer and 27,360 estimated deaths due to prostate cancer in the United States (Jemal, et al. 2009). Depletion of gonadal testosterone through androgen deprivation therapy (ADT) is the frontline treatment for advanced prostate cancer and may be accomplished by medical or surgical castration. Of the approximately 2 million men currently diagnosed with prostate cancer in the United States, over one third have received treatment with ADT (Keating, et al. 2006; Saylor and Smith). Those treated comprise the vast majority of the approximately 27,000 men who die annually from prostate cancer, including men who undergo ADT as primary therapy for localized disease, as an adjunct to radiation therapy for high-risk localized disease, and as treatment for biochemical relapse (prostate-specific antigen [PSA] rise only) after failure of localized therapy, often with uncertain benefits (Sharifi, et al. 2005).
Other hormonal interventions for prostate cancer include further depletion of androgens by inhibition of adrenal androgen synthesis, direct inhibition of the androgen receptor (AR), and inhibition of 5α-reductase, which converts testosterone to the more potent dihydrotestosterone (DHT). New and more potent hormonal agents for the treatment of prostate cancer are in phase III clinical trials. The large number of men treated with hormonal therapy for prostate cancer has increased the urgency to understand and effectively treat adverse effects that accompany these therapies. This review is a critical evaluation of new hormonal therapies for prostate cancer, the adverse effects of ADT, treatments that may ameliorate adverse effects and efficacy of continuous versus intermittent ADT.
Methods
Electronic literature searches of PubMed and Web of Science were conducted for English-language articles published between 1966 and February 2010, using the terms prostate cancer, androgen deprivation therapy and hormone treatment. To specifically identify studies on bone loss, cardiovascular endpoints and intermittent hormonal therapy, the secondary search terms osteopenia, cardiovascular and intermittent were used. Articles retrieved from clinical studies that were not based on randomized design were excluded. References from selected articles were reviewed manually and supplemental searches of meeting abstracts from American Society of Clinical Oncology and American Urological Association annual meetings were performed to further identify relevant studies. Articles were further selected for agents with novel mechanisms of action based on randomized study design for clinical trials on ADT and any phase I/II clinical trial for secondary hormonal therapies. To extract these studies, the search terms GnRH antagonist, abiraterone acetate and MDV3100 were used. The Jadad scale was used to evaluate the quality of randomized controlled clinical trials.
Results
Study Inclusion
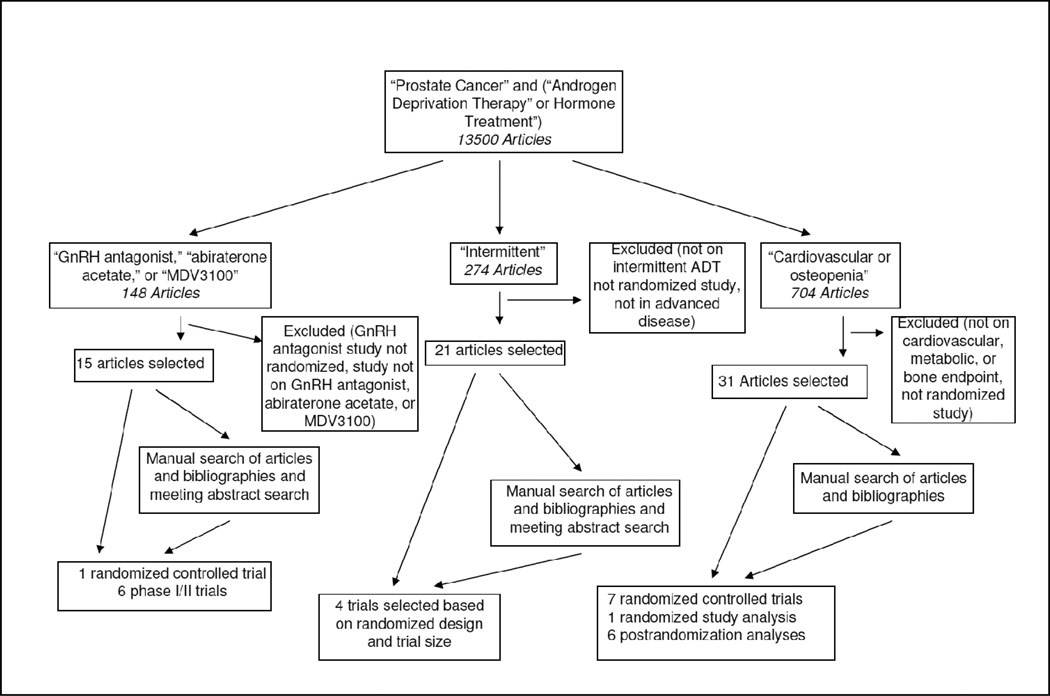
Emphasis was placed on the highest quality of data. Inclusion of data from trials of pharmacologic agents with novel mechanisms of action of ADT was based on randomized controlled trials for comparisons of medical castration (Figure 1). Phase I/II clinical trial data for secondary hormonal therapies with novel mechanisms of action were included only if phase III placebo-controlled trials were ongoing, indicating the potential for eventual Food and Drug Administration approval. Only randomized studies were included to compare the effectiveness of intermittent versus continuous ADT. Further selection of these studies was based on the size of the trial and smaller studies were not included. Although some findings from prospective studies and population-based analyses were used to describe adverse effects of ADT, only data from randomized, placebo-controlled clinical trials were used to assess the effect of therapeutic interventions to prevent or reverse adverse effects. Not included were studies designed to assess changes in skeletal-related adverse events due to bony metastasis. Overall, 15 studies had a Jadad score ≥ 2.
Figure 1.
Flow of Study Search for Androgen Deprivation Therapy for Prostate Cancer
Androgen Deprivation Therapy
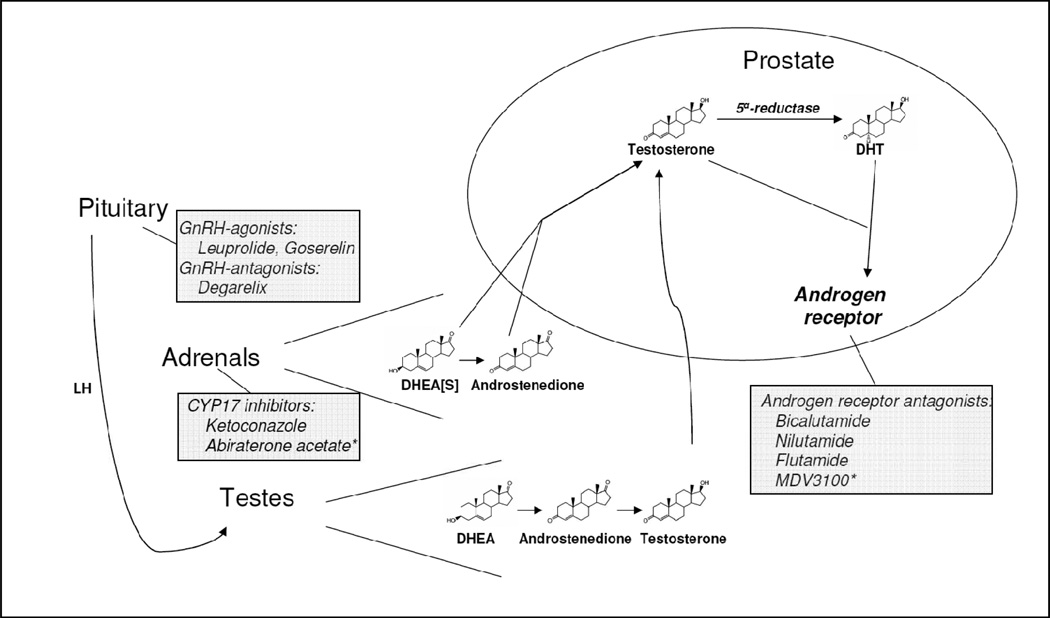
Gonadal testosterone is the main source of circulating androgens (Figure 2). Although there are recognized limitations in measuring serum testosterone concentrations (Rosner, et al. 2007), a total testosterone concentration > 300 ng/dL (10.4 nmol/L) is generally considered normal (Bhasin, et al.). The upper limit of castration concentrations of serum testosterone is considered to be 50 ng/dL (1.7 nmol/L), although lower concentrations (20 ng/dL; 0.7 nmol/L) may be more desirable for optimal therapy (Bubley, et al. 1999). Testosterone has AR agonist activity. However, in the prostate testosterone is rapidly reduced by 5α-reductase to DHT (Bruchovsky and Wilson 1968), a more potent AR agonist required for prostate development (Russell and Wilson 1994). For ADT to be effective against prostate cancer, the decline in serum testosterone must translate to a decrease in intraprostatic androgens. However, despite the approximately 94% decline in serum testosterone with ADT, intraprostatic concentrations of testosterone and DHT decline by only 70%–80% (Page, et al. 2006). The adrenal origin of the residual intraprostatic androgens is suggested by the correlation of serum dehydroepiandrosterone (DHEA) with intraprostatic testosterone and DHT (Page et al. 2006). These findings suggest that, despite the clinical effects of standard ADT, the potential exists to intensify the effects of ADT on prostate tissue.
Figure 2.
Androgen physiology and pharmacologic interventions for prostate cancer. The pituitary regulates testosterone synthesis and release from the testes through luteinizing hormone (LH). Testosterone is synthesized from cholesterol (not shown) with dehydroepiandrosterone (DHEA) and androstenedione as intermediate metabolites, secreted into systemic circulation, and is converted to dihydrotestosterone (DHT) in the prostate by 5α-reductase. Testosterone and DHT both bind and activate the androgen receptor. DHEA and androstenedione, the major source of 19-carbon steroids in the absence of gonadal testosterone, are similarly synthesized in the adrenal cortex, secreted into circulation and are converted to testosterone and DHT in the prostate. Most DHEA in circulation is sulfated (DHEAS). Pharmacologic interventions for the hormonal treatment of prostate cancer are indicated. Asterisks denote investigational agents currently in phase III clinical trials.
ADT is achievable pharmacologically with medical castration or through surgical orchiectomy. Medical castration is generally favored by patients because of the psychological effects and irreversible nature of surgical orchiectomy (McLeod 2003). However, bilateral orchiectomy is significantly less expensive than medical castration (Chon, et al. 2000).
The common mechanism of the various means of medical castration is suppression of the release of luteinizing hormone (LH) from the anterior pituitary. Gonadotropin-releasing hormone (GnRH) is a peptide hormone that is synthesized in the hypothalamus and regulates pituitary LH release (Conn and Crowley 1994). However, the LH response depends on the nature of stimulation by GnRH, and LH is released only in response to pulsatile GnRH secretion (Conn and Crowley 1994). Although administration of estrogens, such as diethylstilbestrol (DES), suppresses pituitary release of LH and the resultant testosterone secretion from the Leydig cells of the testes (Cox and Crawford 1995), treatment with DES is also associated with cardiovascular deaths (Byar 1973) and is therefore no longer used. On the other hand, GnRH agonists are commonly used for medical castration. GnRH agonists are administered subcutaneously or intramuscularly for sustained release. Continuous pituitary stimulation by GnRH agonists overcomes endogenous pulsatile GnRH and suppresses LH release, resulting in low serum testosterone (Tolis, et al. 1982). Synthetic GnRH agonists include leuprolide, buserelin, goserelin, and histrelin. A potential disadvantage of GnRH agonists is the initial rise in serum testosterone concentrations when beginning treatment and the potential to induce a consequent stimulation of prostate cancer growth. However, the effects of the initial testosterone surge can be blocked by AR antagonists (Kuhn, et al. 1989). Alternatively, administration of GnRH antagonists, such as Degarelix (Doehn, et al. 2009), does not induce a testosterone surge. A three-armed, randomized phase III study compared a starting dose of 240 mg degarelix, followed by 80- or 160-mg monthly subcutaneous doses, with monthly 7.5-mg intramuscular doses of leuprolide in 610 previously untreated patients (Klotz, et al. 2008). Concomitant treatment with an AR antagonist in the leuprolide arm to prevent an initial testosterone surge and tumor flare was at the discretion of the investigator. By day 3, the median testosterone concentration rose from 384 ng/dL (13.1 nmol/L) to 630 ng/dL (21.4 nmol/L) in the leuprolide arm. In contrast, median testosterone concentrations were 24 ng/dL (0.82 nmol/L) and 26 ng/dL (0.88 nmol/L), and 96.1% and 95.5% of patients in the degarelix 240/80 mg and 240/160 mg arms, respectively, were below the testosterone castrate threshold of 50 ng/dL (1.7 nmol/L). The PSA decline at day 14 was significantly greater in the degarelix arms, reflecting the faster onset of testosterone decline, although this difference was no longer statistically significant at day 35. However, the PSA decline was similar between the degarelix arms and men who received leuprolide in addition to AR antagonist. Testosterone suppression to < 50 ng/dL (1.7 nmol/L) for all monthly assessments up to 1 year was achieved in 97.2%, 98.3%, and 96.4% of patients in the degarelix 240/80 mg, degarelix 240/160 mg, and leuprolide arms, respectively. Although there were no allergic reactions, in contrast to other trials of GnRH antagonists, treatment in the degarelix arms was associated with a 40% chance of injection-site reactions.
The benefits of ADT in advanced disease include fewer tumor-associated events, such as spinal cord compression, extraskeletal metastases, pathological fracture, and ureteral obstruction (1997). ADT adjuvant to radiation therapy increases survival in men with intermediate, high-risk, and locally advanced disease (Bolla, et al. 2009; D'Amico, et al. 2004; Souhami, et al. 2009). Furthermore, there is a survival benefit for men treated with ADT after radical prostatectomy who also have lymph node involvement (Messing, et al. 1999).
Other Hormonal Interventions
Although the majority of prostate tumors initially respond to ADT, metastatic disease almost invariably progresses eventually to castration-resistant prostate cancer (CRPC). Paradoxically, CRPC often remains responsive to other hormonal therapies (McPhaul 2008; Scher and Sawyers 2005; Sharifi 2010), in large part due to the intratumoral regeneration of androgens (Montgomery, et al. 2008; Titus, et al. 2005). Therefore, after the development of CRPC, patients are often treated with secondary hormonal therapies that further deplete androgen concentrations, or directly bind and inhibit AR (Ryan and Small 2005).
Bicalutamide, nilutamide, and flutamide are nonsteroidal AR antagonists frequently used as secondary hormonal therapy in the United States (Ryan and Small 2005). Of these, bicalutamide binds AR with the highest affinity, has the longest half-life, and is generally the most favored (Gao, et al. 2005). Importantly, these nonsteroidal AR antagonists may have AR agonist activity, particularly under certain circumstances associated with CRPC (Chen, et al. 2004; Figg, et al. 1995; Kelly and Scher 1993; Taplin, et al. 2003). Ketoconazole is an antifungal imidazole that inhibits cytochrome P450 enzymes, including 17-hydroxylase/17,20-lyase (CYP17A1), in the adrenal, which is required for androgen synthesis (De Coster, et al. 1996). Secondary hormonal therapy with ketoconazole inhibits the synthesis of adrenal androgens and leads to frequent PSA declines in CRPC (Figg, et al. 2005). Treatment with ketoconazole can cause adrenal insufficiency due to declines in other adrenal steroids; patients are therefore supplemented with hydrocortisone (Figg et al. 2005; Khosla, et al. 1989). Unfortunately, no survival advantage for CRPC has ever been definitely demonstrated with any of these standard secondary hormonal therapies.
Two investigational hormonal therapies with novel mechanisms of action and promising activity in phase I/II clinical trials are currently in phase III placebo-controlled trials. MDV3100 is a member of the new class of diarylthiohydantoin AR antagonists (Tran, et al. 2009). This drug binds AR with a 5- to 8-fold higher affinity than bicalutamide, inhibits AR nuclear translocation, and has reduced agonist activity, distinguishing it from the 3 nonsteroidal AR antagonists used in current clinical practice. A phase I/II clinical trial of MDV3100 demonstrated PSA declines of > 50% in 57% of CRPC patients not previously treated with chemotherapy and 45% of patients who progressed on docetaxel chemotherapy (Scher, et al.; Tran et al. 2009). A phase III randomized placebo-controlled trial of MDV3100 is currently underway in patients with CRPC previously treated with chemotherapy.
Compared with ketoconazole, abiraterone acetate is a more specific and potent inhibitor of CYP17A1, with unique clinical activity and adverse effect profiles (Barrie, et al. 1994; Haidar, et al. 2003). Several phase I/II clinical trials of abiraterone acetate have been completed, both in patients who have previously received and those who have never been treated with ketoconazole and/or chemotherapy (Attard, et al. 2009; Attard, et al. 2008; Danila, et al.; Reid, et al.; Ryan, et al.). In 2 trials, serum testosterone was further suppressed from baseline after treatment with abiraterone acetate (median 7 ng/dL at baseline, < 1 ng/dL by day 8 (Attard et al. 2008); mean 4 ng/dL at baseline, < 1 ng/dL at day 28 (Ryan et al.)). Serum DHEA and DHEA-sulfate (DHEA-S) concentrations also declined in these trials (median DHEA 282.4 ng/dL at baseline, 83.6 ng/dL at day 28 (Attard et al. 2008); median DHEA-S 39 µg/dL at baseline, < 15 µg/dL at day 28 (Attard et al. 2008); mean DHEA-S 49 µg/dL at baseline, < 15 µg/dL at day 28 (Ryan et al.)). Inhibition of CYP17A1 activity with abiraterone acetate, which blocks the pathway to androgens and other 19-carbon steroids, shunts the steroidogenic pathway to mineralocorticoids. This results in 10-fold and 40-fold increases in deoxycorticosterone and corticosterone, respectively (Attard et al. 2008). As might be predicted, the adverse effect profile includes hypertension, hypokalemia, and edema, which are manageable with the mineralocorticoid receptor antagonist eplerenone (Attard et al. 2008). However, low-dose glucocorticoids, which suppress adrenocorticotropic hormone (ACTH) and adrenal steroidogenesis, may ameliorate these adverse effects (Attard et al. 2009). Two phase III randomized, placebo-controlled clinical trials are ongoing and will ultimately determine the role of abiraterone acetate for the treatment of CRPC.
Continuous versus Intermittent ADT
The antitumor effect of intermittent versus continuous ADT has been debated since preclinical studies first suggested that intermittent ADT might allow for multiple cycles and delayed resistance to ADT (Akakura, et al. 1993). Furthermore, given the adverse effects of ADT, there may be beneficial effects and potential cost savings in time off therapy with intermittent treatment, particularly if suppressive effects on prostate cancer are equivalent to continuous ADT (Seruga and Tannock 2008).
A randomized trial of intermittent versus continuous ADT in 335 patients with advanced (lymph node-positive or metastatic) prostate cancer demonstrated equivalent survival (51.4 versus 53.8 months, P = 0.658) (Miller, et al. 2007). Patients in the intermittent arm were off treatment > 40% of the time. However, it is important to note that testosterone recovery after discontinuation of GnRH agonist is often delayed and may depend on treatment duration, age, baseline testosterone, and ethnicity (Gulley, et al. 2008). In a trial of intermittent versus continuous ADT for advanced prostate cancer, 193 patients were randomized and, after a mean follow-up of 34 months, no difference in survival was observed (P value not stated) (Langenhuijsen, et al. 2008). A larger trial randomized 312 men to continuous and 314 men to intermittent ADT (Calais da Silva, et al. 2009). With a median follow-up of 51 months from randomization, there were fewer cancer deaths (84 versus 106), more cardiovascular deaths (52 versus 41), and an equivalent number of total deaths (169 versus 170) in the continuous versus intermittent arms, respectively. Median time off ADT was 52 weeks for patients in the intermittent arm (Calais da Silva et al. 2009). It should be noted that the randomization criteria for all of these trials is a PSA decline of 80%–90%, or to < 4 ng/mL, on initial ADT. Furthermore, ADT for all of these trials included treatment with an AR antagonist (Calais da Silva et al. 2009; Langenhuijsen et al. 2008; Miller et al. 2007).
Although current evidence suggests that intermittent ADT may be reasonable for some patients with hormone-sensitive prostate cancer (Seruga and Tannock 2008), there are still questions about patient selection, timing, and methodology of intermittent ADT (Keizman and Carducci 2009). SWOG 9346 is an ongoing randomized trial with an accrual goal of 1512 patients, with a primary objective to determine whether treatment of men with newly diagnosed, hormone-sensitive, metastatic prostate cancer with intermittent and continuous ADT leads to equivalent survival (Hussain, et al. 2009). This would be the largest randomized trial of continuous versus intermittent ADT to date and is expected to yield more definitive results.
Adverse Effects of ADT
Prostate cancer is generally a disease of older men, many of whom already have other significant comorbidities. In this population, the potential benefits of therapy must be tempered with a consideration of its adverse effects (Table 1). Prospective clinical trials of ADT for men with prostate cancer demonstrate the development of multiple risk factors for cardiovascular disease, including increases in serum cholesterol and triglycerides, insulin resistance, body mass index, and fat body mass, along with decreases in lean body mass (Levine, et al.; Sharifi et al. 2005). Population-based analyses further suggest that treatment with ADT is associated with an elevated risk for diabetes (Alibhai, et al. 2009; Keating et al. 2006).
Table 1.
Possible Adverse Effects of Androgen Deprivation Therapy
| Metabolic effects | Hyperlipidemia. insulin resistance and diabetes, osteoporosis, increased risk of fracture and anemia |
| Physical changes | Increased fat mass, decreased muscle mass, loss of body hair, gynecomastia and hot flashes |
| Mental changes | Decreased cognition and emotional changes |
| Sexual effects | Decreased libido and erectile dysfunction |
Cardiovascular Risk
Randomized study analyses and postrandomization analyses from several clinical trials have examined whether these longitudinal changes in cardiovascular risk factors translate to an increased risk of cardiovascular death. In a pooled analysis of 1372 men in 3 randomized trials of men receiving radiation for localized prostate cancer and 0 versus 3 versus 6, 3 versus 8, or 0 versus 6 months of ADT, men 65 years of age or older receiving 6 months of ADT had shorter times to fatal myocardial infarction compared with men not receiving ADT (P = 0.017) (D'Amico, et al. 2007). No significant difference was observed in men younger than 65 years of age, or in men 65 years of age or older receiving 6–8 months versus 3 months of ADT (D'Amico et al. 2007). In a randomized trial of 206 men receiving 6 months of ADT plus radiation versus radiation alone for localized prostate cancer, 13 deaths from myocardial infarction occurred in each group (D'Amico, et al. 2008). However, in men receiving ADT, 11 deaths occurred in men with moderate to severe comorbidities, leading to a loss in overall survival benefit in these men.
On the other hand, other studies from randomized clinical trials do not suggest that ADT confers an increased risk of cardiovascular events. In a randomized trial in 945 men with locally advanced prostate cancer receiving adjuvant ADT with radiation versus radiation and salvage ADT on disease recurrence (RTOG 85-31), the treatment arm was not significantly associated with risk of cardiovascular mortality after censoring for salvage ADT (Efstathiou, et al. 2009). In a trial of 1554 men with locally advanced prostate cancer receiving radiation therapy and randomized to 4 versus 30 months of ADT (RTOG 92-02), duration of ADT was not significantly associated with 5-year risk of cardiovascular mortality (4.8% versus 5.9%; P = 0.16) (Efstathiou, et al. 2008). The 10-year rate of fatal cardiac events in a randomized trial of short-term neoadjuvant ADT for locally advanced prostate cancer (RTOG 8610) was not significantly different in the arm receiving 2 months of ADT versus no ADT (12.5% versus 9.1%; P = 0.32) (Roach, et al. 2008). A randomized trial of radiation plus 6 versus 30 months of ADT in 1113 men with locally advanced prostate cancer (EORTC 22961) showed no difference in the cumulative incidence of fatal cardiac events at 5 years (4.0% versus 3.0%) (Bolla et al. 2009). A randomized trial of immediate versus deferred ADT in 985 men with localized prostate cancer not suitable for local treatment demonstrated no increase in cardiovascular mortality in the immediate ADT arm (17.9% versus 19.7%) (Studer, et al. 2006).
It is presently unclear whether there is a causal relationship between ADT and cardiovascular mortality. The differences in outcome among studies that have examined this issue may be due to study design, characteristics of the study populations, or competing risks. ADT may affect cardiovascular mortality in a subset of these study populations. Alternatively, there may be no causal relationship. It may be prudent to carefully consider the potential risks and benefits before initiating ADT, particularly in patients with coronary artery disease. Patients with cardiac disease who initiate ADT should receive particular attention to secondary preventive interventions (Levine et al.).
Intervention for Hyperlipidemia
Favorable modification of cardiac risk factors may be beneficial for patients receiving ADT. In an interim analysis of 188 patients receiving ADT in a phase III, randomized, double-blind, placebo-controlled trial of toremifene, patients in the toremifene arm had favorable changes in serum lipid profile at 1 year of treatment (Smith, et al. 2008b). In the toremifene arm, mean total cholesterol, low-density cholesterol (LDL), and triglycerides declined by 8.1%, 8.2%, and 13.2%, respectively, and high-density cholesterol (HDL) increased by 0.5%. In the placebo arm, total cholesterol decreased by 1.0%, LDL increased by 0.8%, HDL decreased by 4.9%, and triglycerides increased by 6.9%. All comparisons between the placebo and toremifene arms were statistically significant (Smith et al. 2008b). However, the effects of toremifene on cardiovascular events and mortality are unknown.
Treatment of ADT Adverse Effects
Bone Density and Fracture Risk
The conversion of testosterone to estradiol by aromatase in bone is important in maintaining bone density (Guise, et al. 2007). Through this mechanism, ADT may lead to a relative estrogen deficiency in bone that may be comparable to the postmenopausal state. Prospective clinical trials have shown that ADT leads to significant decreases in bone mineral density (BMD) (Saylor and Smith). Furthermore, a retrospective study of 50,000 men suggests that men treated with ADT have an increased fracture risk (Shahinian, et al. 2005). Interventions are therefore required to prevent bone loss and decrease fracture risk in patients receiving ADT.
Bisphosphonates decrease bone loss by inhibiting osteoclast function and bone resorption (Drake, et al. 2008). Randomized, placebo-controlled clinical trials of several agents in this class, including pamidronate (Diamond, et al. 2001; Smith, et al. 2001), alendronate (Greenspan, et al. 2007), and zoledronic acid (Michaelson, et al. 2007; Smith, et al. 2003), have demonstrated that bisphosphonates increase BMD in patients treated with ADT. However, none of these bisphosphonate studies was large enough to determine the impact on fractures due to ADT (Saylor and Smith).
Given that bone loss from ADT is due to a deficiency of estrogen (Guise et al. 2007), replacement of estrogenic function with selective estrogen receptor modulators (SERMs) may favorably affect bone density. In a phase III randomized study of placebo versus toremifene (80 mg daily), patients in the toremifene arm had significantly increased BMD in the hip and spine (Smith, et al. 2008a). Furthermore, the 2-year incidence of new vertebral fractures was significantly lower in the toremifene arm (2.5%) than in the placebo arm (4.9%; P = 0.05) (Smith, et al. In press). However, the toremifene arm had more than twice the number of venous thromboembolic events.
The genesis, function, and survival of osteoclasts are critically dependent upon the receptor activator of nuclear factor-κB ligand (RANKL) (Lacey, et al. 1998). Denosumab is a human monoclonal antibody against RANKL that inhibits osteoclast activity. A randomized, double-blind, placebo-controlled clinical trial compared denosumab (60 mg subcutaneously) with placebo, given every 6 months, in 1468 men on ADT for nonmetastatic, hormone-responsive prostate cancer (Smith, et al. 2009). At 2 years, patients in the denosumab arm had significantly higher BMD than those in the placebo arm, with 4.8, 3.9, 5.5, and 4.0 percent increases (P < 0.001) in total hip, femoral neck, distal third of radius, and whole body BMD values. The relative risk of vertebral fractures for men in the denosumab arm compared to placebo at 1, 2, and 3 years was 0.15, 0.31, and 0.38 (P ≤ 0.006). Over 36 months, fractures at any site developed in 5.2% and 7.2% of patients in the denosumab and placebo groups respectively, although the difference was not statistically significant (P = 0.10) (Smith et al. 2009).
Conclusions
ADT with gonadal depletion of testosterone is widely used as the frontline therapy for advanced prostate cancer, and to treat localized disease in combination with other therapies. Other hormonal therapies further reduce androgen synthesis, or directly and competitively inhibit the AR. Intermittent and continuous ADT may be equivalent, but more definitive results await completion of a larger clinical trial.
Adverse effects of ADT include metabolic changes such as hyperlipidemia, increased fat mass, insulin resistance, and diabetes. Although many of the metabolic effects induced by ADT are risk factors for cardiovascular disease, the effects on cardiovascular risk are uncertain. Pharmacologic intervention may decrease bone loss and reverse increased risk of fracture due to ADT.
Acknowledgments
Funding: This work was supported in part by a Howard Hughes Medical Institute Physician-Scientist Early Career Award, an award from the Prostate Cancer Foundation and from grant number PC80193 from the U.S. Army Medical Research and Materiel Command to NS and the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Footnotes
Declarations of interest: The authors have no real or potential conflicts of interest to declare.
References
- Immediate versus deferred treatment for advanced prostatic cancer: initial results of the Medical Research Council Trial. The Medical Research Council Prostate Cancer Working Party Investigators Group. Br J Urol. 1997;79:235–246. doi: 10.1046/j.1464-410x.1997.d01-6840.x. [DOI] [PubMed] [Google Scholar]
- Akakura K, Bruchovsky N, Goldenberg SL, Rennie PS, Buckley AR, Sullivan LD. Effects of intermittent androgen suppression on androgen-dependent tumors. Apoptosis and serum prostate-specific antigen. Cancer. 1993;71:2782–2790. doi: 10.1002/1097-0142(19930501)71:9<2782::aid-cncr2820710916>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Alibhai SM, Duong-Hua M, Sutradhar R, Fleshner NE, Warde P, Cheung AM, Paszat LF. Impact of androgen deprivation therapy on cardiovascular disease and diabetes. J Clin Oncol. 2009;27:3452–3458. doi: 10.1200/JCO.2008.20.0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attard G, Reid AH, A'Hern R, Parker C, Oommen NB, Folkerd E, Messiou C, Molife LR, Maier G, Thompson E, et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2009;27:3742–3748. doi: 10.1200/JCO.2008.20.0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attard G, Reid AH, Yap TA, Raynaud F, Dowsett M, Settatree S, Barrett M, Parker C, Martins V, Folkerd E, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26:4563–4571. doi: 10.1200/JCO.2007.15.9749. [DOI] [PubMed] [Google Scholar]
- Barrie SE, Potter GA, Goddard PM, Haynes BP, Dowsett M, Jarman M. Pharmacology of novel steroidal inhibitors of cytochrome P450(17) alpha (17 alpha-hydroxylase/C17–20 lyase) J Steroid Biochem Mol Biol. 1994;50:267–273. doi: 10.1016/0960-0760(94)90131-7. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 95:2536–2559. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- Bolla M, de Reijke TM, Van Tienhoven G, Van den Bergh AC, Oddens J, Poortmans PM, Gez E, Kil P, Akdas A, Soete G, et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med. 2009;360:2516–2527. doi: 10.1056/NEJMoa0810095. [DOI] [PubMed] [Google Scholar]
- Bruchovsky N, Wilson JD. The conversion of testosterone to 5-alpha-androstan-17-betaol-3-one by rat prostate in vivo and in vitro. J Biol Chem. 1968;243:2012–2021. [PubMed] [Google Scholar]
- Bubley GJ, Carducci M, Dahut W, Dawson N, Daliani D, Eisenberger M, Figg WD, Freidlin B, Halabi S, Hudes G, et al. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 1999;17:3461–3467. doi: 10.1200/JCO.1999.17.11.3461. [DOI] [PubMed] [Google Scholar]
- Byar DP. Proceedings: The Veterans Administration Cooperative Urological Research Group's studies of cancer of the prostate. Cancer. 1973;32:1126–1130. doi: 10.1002/1097-0142(197311)32:5<1126::aid-cncr2820320518>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Calais da Silva FE, Bono AV, Whelan P, Brausi M, Marques Queimadelos A, Martin JA, Kirkali Z, Calais da Silva FM, Robertson C. Intermittent androgen deprivation for locally advanced and metastatic prostate cancer: results from a randomised phase 3 study of the South European Uroncological Group. Eur Urol. 2009;55:1269–1277. doi: 10.1016/j.eururo.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, Rosenfeld MG, Sawyers CL. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- Chon JK, Jacobs SC, Naslund MJ. The cost value of medical versus surgical hormonal therapy for metastatic prostate cancer. J Urol. 2000;164:735–737. doi: 10.1097/00005392-200009010-00027. [DOI] [PubMed] [Google Scholar]
- Conn PM, Crowley WF., Jr Gonadotropin-releasing hormone and its analogs. Annu Rev Med. 1994;45:391–405. doi: 10.1146/annurev.med.45.1.391. [DOI] [PubMed] [Google Scholar]
- Cox RL, Crawford ED. Estrogens in the treatment of prostate cancer. J Urol. 1995;154:1991–1998. [PubMed] [Google Scholar]
- D'Amico AV, Chen MH, Renshaw AA, Loffredo M, Kantoff PW. Androgen suppression and radiation vs radiation alone for prostate cancer: a randomized trial. JAMA. 2008;299:289–295. doi: 10.1001/jama.299.3.289. [DOI] [PubMed] [Google Scholar]
- D'Amico AV, Denham JW, Crook J, Chen MH, Goldhaber SZ, Lamb DS, Joseph D, Tai KH, Malone S, Ludgate C, et al. Influence of androgen suppression therapy for prostate cancer on the frequency and timing of fatal myocardial infarctions. J Clin Oncol. 2007;25:2420–2425. doi: 10.1200/JCO.2006.09.3369. [DOI] [PubMed] [Google Scholar]
- D'Amico AV, Manola J, Loffredo M, Renshaw AA, DellaCroce A, Kantoff PW. 6-month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: a randomized controlled trial. JAMA. 2004;292:821–827. doi: 10.1001/jama.292.7.821. [DOI] [PubMed] [Google Scholar]
- Danila DC, Morris MJ, de Bono JS, Ryan CJ, Denmeade SR, Smith MR, Taplin ME, Bubley GJ, Kheoh T, Haqq C, et al. Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer. J Clin Oncol. 2010;28:1496–1501. doi: 10.1200/JCO.2009.25.9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Coster R, Wouters W, Bruynseels J. P450-dependent enzymes as targets for prostate cancer therapy. J Steroid Biochem Mol Biol. 1996;56:133–143. doi: 10.1016/0960-0760(95)00230-8. [DOI] [PubMed] [Google Scholar]
- Diamond TH, Winters J, Smith A, De Souza P, Kersley JH, Lynch WJ, Bryant C. The antiosteoporotic efficacy of intravenous pamidronate in men with prostate carcinoma receiving combined androgen blockade: a double blind, randomized, placebo-controlled crossover study. Cancer. 2001;92:1444–1450. doi: 10.1002/1097-0142(20010915)92:6<1444::aid-cncr1468>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Doehn C, Sommerauer M, Jocham D. Degarelix for prostate cancer. Expert Opin Investig Drugs. 2009;18:851–860. doi: 10.1517/13543780902954713. [DOI] [PubMed] [Google Scholar]
- Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 2008;83:1032–1045. doi: 10.4065/83.9.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstathiou JA, Bae K, Shipley WU, Hanks GE, Pilepich MV, Sandler HM, Smith MR. Cardiovascular mortality and duration of androgen deprivation for locally advanced prostate cancer: analysis of RTOG 92-02. Eur Urol. 2008;54:816–823. doi: 10.1016/j.eururo.2008.01.021. [DOI] [PubMed] [Google Scholar]
- Efstathiou JA, Bae K, Shipley WU, Hanks GE, Pilepich MV, Sandler HM, Smith MR. Cardiovascular mortality after androgen deprivation therapy for locally advanced prostate cancer: RTOG 85-31. J Clin Oncol. 2009;27:92–99. doi: 10.1200/JCO.2007.12.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figg WD, Liu Y, Arlen P, Gulley J, Steinberg SM, Liewehr DJ, Cox MC, Zhai S, Cremers S, Parr A, et al. A randomized, phase II trial of ketoconazole plus alendronate versus ketoconazole alone in patients with androgen independent prostate cancer and bone metastases. J Urol. 2005;173:790–796. doi: 10.1097/01.ju.0000147013.09157.8e. [DOI] [PubMed] [Google Scholar]
- Figg WD, Sartor O, Cooper MR, Thibault A, Bergan RC, Dawson N, Reed E, Myers CE. Prostate specific antigen decline following the discontinuation of flutamide in patients with stage D2 prostate cancer. Am J Med. 1995;98:412–414. doi: 10.1016/S0002-9343(99)80323-4. [DOI] [PubMed] [Google Scholar]
- Gao W, Bohl CE, Dalton JT. Chemistry and structural biology of androgen receptor. Chem Rev. 2005;105:3352–3370. doi: 10.1021/cr020456u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan SL, Nelson JB, Trump DL, Resnick NM. Effect of once-weekly oral alendronate on bone loss in men receiving androgen deprivation therapy for prostate cancer: a randomized trial. Ann Intern Med. 2007;146:416–424. doi: 10.7326/0003-4819-146-6-200703200-00006. [DOI] [PubMed] [Google Scholar]
- Guise TA, Oefelein MG, Eastham JA, Cookson MS, Higano CS, Smith MR. Estrogenic side effects of androgen deprivation therapy. Rev Urol. 2007;9:163–180. [PMC free article] [PubMed] [Google Scholar]
- Gulley JL, Aragon-Ching JB, Steinberg SM, Hussain MH, Sartor O, Higano CS, Petrylak DP, Chatta GS, Arlen PM, Figg WD, et al. Kinetics of serum androgen normalization and factors associated with testosterone reserve after limited androgen deprivation therapy for nonmetastatic prostate cancer. J Urol. 2008;180:1432–1437. doi: 10.1016/j.juro.2008.06.017. discussion 1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haidar S, Ehmer PB, Barassin S, Batzl-Hartmann C, Hartmann RW. Effects of novel 17alpha-hydroxylase/C17, 20-lyase (P450 17, CYP 17) inhibitors on androgen biosynthesis in vitro and in vivo. J Steroid Biochem Mol Biol. 2003;84:555–562. doi: 10.1016/s0960-0760(03)00070-0. [DOI] [PubMed] [Google Scholar]
- Hussain M, Goldman B, Tangen C, Higano CS, Petrylak DP, Wilding G, Akdas AM, Small EJ, Donnelly BJ, Sundram SK, et al. Prostate-specific antigen progression predicts overall survival in patients with metastatic prostate cancer: data from Southwest Oncology Group Trials 9346 (Intergroup Study 0162) and 9916. J Clin Oncol. 2009;27:2450–2456. doi: 10.1200/JCO.2008.19.9810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- Keating NL, O'Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–4456. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- Keizman D, Carducci MA. Intermittent androgen deprivation: questions remain. Nat Rev Urol. 2009;6:412–414. doi: 10.1038/nrurol.2009.145. [DOI] [PubMed] [Google Scholar]
- Kelly WK, Scher HI. Prostate specific antigen decline after antiandrogen withdrawal: the flutamide withdrawal syndrome. J Urol. 1993;149:607–609. doi: 10.1016/s0022-5347(17)36163-3. [DOI] [PubMed] [Google Scholar]
- Khosla S, Wolfson JS, Demerjian Z, Godine JE. Adrenal crisis in the setting of high-dose ketoconazole therapy. Arch Intern Med. 1989;149:802–804. [PubMed] [Google Scholar]
- Klotz L, Boccon-Gibod L, Shore ND, Andreou C, Persson BE, Cantor P, Jensen JK, Olesen TK, Schroder FH. The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. BJU Int. 2008;102:1531–1538. doi: 10.1111/j.1464-410X.2008.08183.x. [DOI] [PubMed] [Google Scholar]
- Kuhn JM, Billebaud T, Navratil H, Moulonguet A, Fiet J, Grise P, Louis JF, Costa P, Husson JM, Dahan R, et al. Prevention of the transient adverse effects of a gonadotropin-releasing hormone analogue (buserelin) in metastatic prostatic carcinoma by administration of an antiandrogen (nilutamide) N Engl J Med. 1989;321:413–418. doi: 10.1056/NEJM198908173210701. [DOI] [PubMed] [Google Scholar]
- Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- Langenhuijsen J, Schasfoort E, Heathcote P, Lock M, Zerbib M, Dijkema H, Vergunst H, Srougi M, Van de Beek C, Jimenez Rios M, et al. Intermittent androgen suppression in patients with advanced prostate cancer: an update of the TULP survival data. Eur Urol Suppl. 2008;7:205. [Google Scholar]
- Levine GN, D'Amico AV, Berger P, Clark PE, Eckel RH, Keating NL, Milani RV, Sagalowsky AI, Smith MR, Zakai N. Androgen-deprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society, and American Urological Association: endorsed by the American Society for Radiation Oncology. Circulation. 2010;121:833–840. doi: 10.1161/CIRCULATIONAHA.109.192695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod DG. Hormonal therapy: historical perspective to future directions. Urology. 2003;61:3–7. doi: 10.1016/s0090-4295(02)02393-2. [DOI] [PubMed] [Google Scholar]
- McPhaul MJ. Mechanisms of prostate cancer progression to androgen independence. Best Pract Res Clin Endocrinol Metab. 2008;22:373–388. doi: 10.1016/j.beem.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Messing EM, Manola J, Sarosdy M, Wilding G, Crawford ED, Trump D. Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with node-positive prostate cancer. N Engl J Med. 1999;341:1781–1788. doi: 10.1056/NEJM199912093412401. [DOI] [PubMed] [Google Scholar]
- Michaelson MD, Kaufman DS, Lee H, McGovern FJ, Kantoff PW, Fallon MA, Finkelstein JS, Smith MR. Randomized controlled trial of annual zoledronic acid to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer. J Clin Oncol. 2007;25:1038–1042. doi: 10.1200/JCO.2006.07.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K, Steiner U, Lingnau A, Keilholz U, Witzsch U, Haider A, Wachter U, Russel C, Altwein J. Randomised prospective study of intermittent versus continuous androgen suppression in advanced prostate cancer. J Clin Oncol. 2007;25(18S):5015. [Google Scholar]
- Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS, True LD, Nelson PS. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–4454. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page ST, Lin DW, Mostaghel EA, Hess DL, True LD, Amory JK, Nelson PS, Matsumoto AM, Bremner WJ. Persistent intraprostatic androgen concentrations after medical castration in healthy men. J Clin Endocrinol Metab. 2006;91:3850–3856. doi: 10.1210/jc.2006-0968. [DOI] [PubMed] [Google Scholar]
- Reid AH, Attard G, Danila DC, Oommen NB, Olmos D, Fong PC, Molife LR, Hunt J, Messiou C, Parker C, et al. Significant and sustained antitumor activity in post-docetaxel, castration-resistant prostate cancer with the CYP17 inhibitor abiraterone acetate. J Clin Oncol. 2010;28:1489–1495. doi: 10.1200/JCO.2009.24.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach M, 3rd, Bae K, Speight J, Wolkov HB, Rubin P, Lee RJ, Lawton C, Valicenti R, Grignon D, Pilepich MV. Short-term neoadjuvant androgen deprivation therapy and external-beam radiotherapy for locally advanced prostate cancer: long-term results of RTOG 8610. J Clin Oncol. 2008;26:585–591. doi: 10.1200/JCO.2007.13.9881. [DOI] [PubMed] [Google Scholar]
- Rosner W, Auchus RJ, Azziz R, Sluss PM, Raff H. Position statement: Utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society position statement. J Clin Endocrinol Metab. 2007;92:405–413. doi: 10.1210/jc.2006-1864. [DOI] [PubMed] [Google Scholar]
- Russell DW, Wilson JD. Steroid 5 alpha-reductase: two genes/two enzymes. Annu Rev Biochem. 1994;63:25–61. doi: 10.1146/annurev.bi.63.070194.000325. [DOI] [PubMed] [Google Scholar]
- Ryan CJ, Small EJ. Secondary hormonal manipulations in prostate cancer. Curr Oncol Rep. 2005;7:228–233. doi: 10.1007/s11912-005-0078-x. [DOI] [PubMed] [Google Scholar]
- Ryan CJ, Smith MR, Fong L, Rosenberg JE, Kantoff P, Raynaud F, Martins V, Lee G, Kheoh T, Kim J, et al. Phase I clinical trial of the CYP17 inhibitor abiraterone acetate demonstrating clinical activity in patients with castration-resistant prostate cancer who received prior ketoconazole therapy. J Clin Oncol. 2010;28:1481–1488. doi: 10.1200/JCO.2009.24.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saylor PJ, Smith MR. Adverse effects of androgen deprivation therapy: defining the problem and promoting health among men with prostate cancer. J Natl Compr Canc Netw. 2010;8:211–223. doi: 10.6004/jnccn.2010.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher HI, Beer TM, Higano CS, Anand A, Taplin ME, Efstathiou E, Rathkopf D, Shelkey J, Yu EY, Alumkal J, et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1–2 study. Lancet. 375:1437–1446. doi: 10.1016/S0140-6736(10)60172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005;23:8253–8261. doi: 10.1200/JCO.2005.03.4777. [DOI] [PubMed] [Google Scholar]
- Seruga B, Tannock IF. Intermittent androgen blockade should be regarded as standard therapy in prostate cancer. Nat Clin Pract Oncol. 2008;5:574–576. doi: 10.1038/ncponc1180. [DOI] [PubMed] [Google Scholar]
- Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352:154–164. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- Sharifi N. New agents and strategies for the hormonal treatment of castration-resistant prostate cancer. Expert Opin Investig Drugs. 2010;19:837–846. doi: 10.1517/13543784.2010.494178. [DOI] [PubMed] [Google Scholar]
- Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294:238–244. doi: 10.1001/jama.294.2.238. [DOI] [PubMed] [Google Scholar]
- Smith M, Morton R, Barnette G, Sieber P, Malkowicz S, Rodrigues D, Hancock M, Steiner M. Toremifene to reduce fracture risk in men receiving androgen deprivation therapy for prostate cancer. J Urol. doi: 10.1016/j.juro.2012.11.016. In press. [DOI] [PubMed] [Google Scholar]
- Smith MR, Eastham J, Gleason DM, Shasha D, Tchekmedyian S, Zinner N. Randomized controlled trial of zoledronic acid to prevent bone loss in men receiving androgen deprivation therapy for nonmetastatic prostate cancer. J Urol. 2003;169:2008–2012. doi: 10.1097/01.ju.0000063820.94994.95. [DOI] [PubMed] [Google Scholar]
- Smith MR, Egerdie B, Hernandez Toriz N, Feldman R, Tammela TL, Saad F, Heracek J, Szwedowski M, Ke C, Kupic A, et al. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med. 2009;361:745–755. doi: 10.1056/NEJMoa0809003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MR, Malkowicz SB, Chu F, Forrest J, Price D, Sieber P, Barnette KG, Rodriguez D, Steiner MS. Toremifene increases bone mineral density in men receiving androgen deprivation therapy for prostate cancer: interim analysis of a multicenter phase 3 clinical study. J Urol. 2008a;179:152–155. doi: 10.1016/j.juro.2007.08.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MR, Malkowicz SB, Chu F, Forrest J, Sieber P, Barnette KG, Rodriquez D, Steiner MS. Toremifene improves lipid profiles in men receiving androgen-deprivation therapy for prostate cancer: interim analysis of a multicenter phase III study. J Clin Oncol. 2008b;26:1824–1829. doi: 10.1200/JCO.2007.13.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MR, McGovern FJ, Zietman AL, Fallon MA, Hayden DL, Schoenfeld DA, Kantoff PW, Finkelstein JS. Pamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancer. N Engl J Med. 2001;345:948–955. doi: 10.1056/NEJMoa010845. [DOI] [PubMed] [Google Scholar]
- Souhami L, Bae K, Pilepich M, Sandler H. Impact of the duration of adjuvant hormonal therapy in patients with locally advanced prostate cancer treated with radiotherapy: a secondary analysis of RTOG 85-31. J Clin Oncol. 2009;27:2137–2143. doi: 10.1200/JCO.2008.17.4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studer UE, Whelan P, Albrecht W, Casselman J, de Reijke T, Hauri D, Loidl W, Isorna S, Sundaram SK, Debois M, et al. Immediate or deferred androgen deprivation for patients with prostate cancer not suitable for local treatment with curative intent: European Organisation for Research and Treatment of Cancer (EORTC) Trial 30891. J Clin Oncol. 2006;24:1868–1876. doi: 10.1200/JCO.2005.04.7423. [DOI] [PubMed] [Google Scholar]
- Taplin ME, Rajeshkumar B, Halabi S, Werner CP, Woda BA, Picus J, Stadler W, Hayes DF, Kantoff PW, Vogelzang NJ, et al. Androgen receptor mutations in androgen-independent prostate cancer: Cancer and Leukemia Group B Study 9663. J Clin Oncol. 2003;21:2673–2678. doi: 10.1200/JCO.2003.11.102. [DOI] [PubMed] [Google Scholar]
- Titus MA, Schell MJ, Lih FB, Tomer KB, Mohler JL. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin Cancer Res. 2005;11:4653–4657. doi: 10.1158/1078-0432.CCR-05-0525. [DOI] [PubMed] [Google Scholar]
- Tolis G, Ackman D, Stellos A, Mehta A, Labrie F, Fazekas AT, Comaru-Schally AM, Schally AV. Tumor growth inhibition in patients with prostatic carcinoma treated with luteinizing hormone-releasing hormone agonists. Proc Natl Acad Sci U S A. 1982;79:1658–1662. doi: 10.1073/pnas.79.5.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, Wongvipat J, Smith-Jones PM, Yoo D, Kwon A, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–790. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]