Abstract
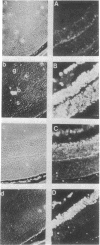
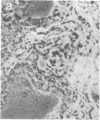
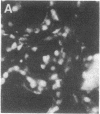
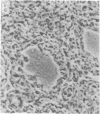
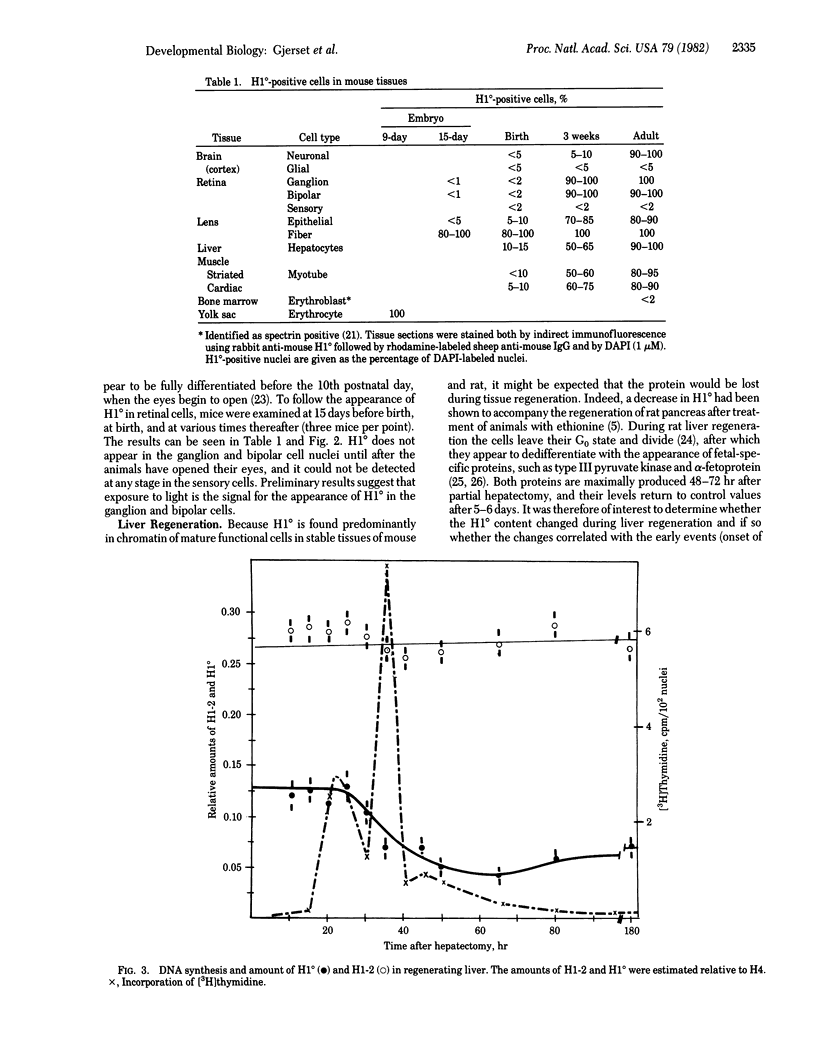
The tissue and cellular distribution and regulation of the chromatin protein H1 degrees has been examined in developing and adult mouse and in rat. The protein appears in specific cell types of solid tissues only when the cells have terminated their maturation. This was found for brain, retina, striated and cardiac muscle, and liver. In tissues that depend on hormones for their function and maintenance, the expression of H1 degrees is dependent on the continued presence of the specific maintenance hormone. In regenerating rat liver the amount of H1 degrees decreases to one-third after the onset of DNA synthesis. The possible role of H1 degrees is discussed in light of these results.
Full text
PDF

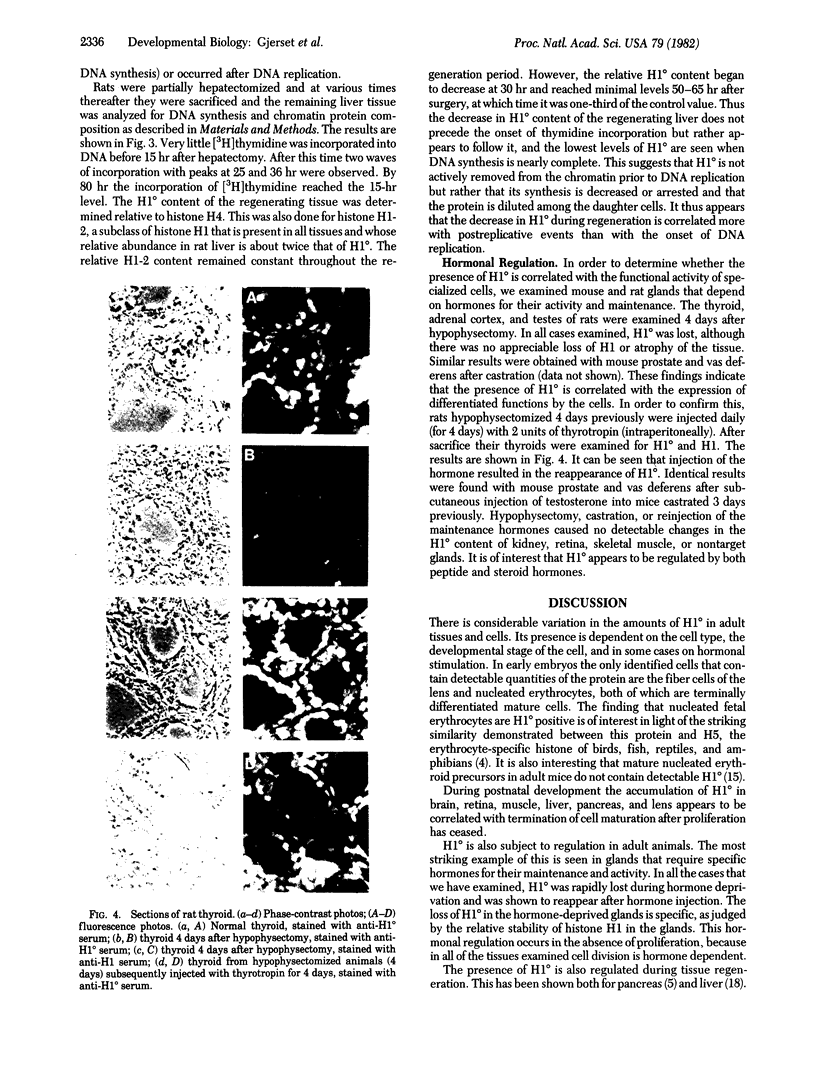

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUCHER N. L. REGENERATION OF MAMMALIAN LIVER. Int Rev Cytol. 1963;15:245–300. doi: 10.1016/s0074-7696(08)61119-5. [DOI] [PubMed] [Google Scholar]
- Bonney R. J., Hopkins H. A., Walker P. R., Potter V. R. Glycolytic isoenzymes and glycogen metabolism in regenerating liver from rats on controlled feeding schedules. Biochem J. 1973 Sep;136(1):115–124. doi: 10.1042/bj1360115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candido E. P., Reeves R., Davie J. R. Sodium butyrate inhibits histone deacetylation in cultured cells. Cell. 1978 May;14(1):105–113. doi: 10.1016/0092-8674(78)90305-7. [DOI] [PubMed] [Google Scholar]
- D'Anna J. A., Gurley L. R., Becker R. R., Barham S. S., Tobey R. A., Walters R. A. Amino acid analysis and cell cycle dependent phosphorylation of an H1-like, butyrate-enhanced protein (BEP; H1(0); IP25) from Chinese hamster cells. Biochemistry. 1980 Sep 2;19(18):4331–4341. doi: 10.1021/bi00559a029. [DOI] [PubMed] [Google Scholar]
- Eisen H., Bach R., Emery R. Induction of spectrin in erythroleukemic cells transformed by Friend virus. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3898–3902. doi: 10.1073/pnas.74.9.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjerset R., Ibarrando F., Saragosti S., Eisen H. Distribution of IP25 in chromatin and its possible involvement in chromatin condensation. Biochem Biophys Res Commun. 1981 Mar 31;99(2):349–357. doi: 10.1016/0006-291x(81)91752-6. [DOI] [PubMed] [Google Scholar]
- Gorka C., Lawrence J. J. The distribution of histone H1 subfractions in chromatin subunits. Nucleic Acids Res. 1979 Sep 25;7(2):347–359. doi: 10.1093/nar/7.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewish D. R., Burgoyne L. A. Chromatin sub-structure. The digestion of chromatin DNA at regularly spaced sites by a nuclear deoxyribonuclease. Biochem Biophys Res Commun. 1973 May 15;52(2):504–510. doi: 10.1016/0006-291x(73)90740-7. [DOI] [PubMed] [Google Scholar]
- Keppel F., Allet B., Eisen H. Appearance of a chromatin protein during the erythroid differentiation of Friend virus-transformed cells. Proc Natl Acad Sci U S A. 1977 Feb;74(2):653–656. doi: 10.1073/pnas.74.2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks D. B., Kanefsky T., Keller B. J., Marks A. D. The presence of histone H1degree in human tissues. Cancer Res. 1975 Apr;35(4):886–889. [PubMed] [Google Scholar]
- Marsh W. H., Fitzgerald P. J. Pancreas acinar cell regeneration. 13. Histone synthesis and modification. Fed Proc. 1973 Nov;32(11):2119–2125. [PubMed] [Google Scholar]
- Medvedev Z. A., Medvedeva M. N., Huschtscha L. I. Age changes of the pattern of F1 histone subfractions in rat liver and spleen chromatin. Gerontology. 1977;23(5):334–341. doi: 10.1159/000212205. [DOI] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. A new histone found only in mammalian tissues with little cell division. Biochem Biophys Res Commun. 1969 Dec 4;37(6):1042–1049. doi: 10.1016/0006-291x(69)90237-x. [DOI] [PubMed] [Google Scholar]
- Pehrson J., Cole R. D. Histone H10 accumulates in growth-inhibited cultured cells. Nature. 1980 May 1;285(5759):43–44. doi: 10.1038/285043a0. [DOI] [PubMed] [Google Scholar]
- Piha R. S., Valkonen K. H. Changes in liver nuclear histones during bovine ontogeny. FEBS Lett. 1979 Dec 15;108(2):326–330. doi: 10.1016/0014-5793(79)80556-6. [DOI] [PubMed] [Google Scholar]
- Sell S., Nichols M., Becker F. F., Leffert H. L. Hepatocyte proliferation and alpha 1-fetoprotein in pregnant, neonatal, and partially hepatectomized rats. Cancer Res. 1974 Apr;34(4):865–871. [PubMed] [Google Scholar]
- Seyedin S. M., Kistler W. S. Levels of chromosomal protein high mobility group 2 parallel the proliferative activity of testis, skeletal muscle, and other organs. J Biol Chem. 1979 Nov 25;254(22):11264–11271. [PubMed] [Google Scholar]
- Smith B. J., Johns E. W. Isolation and characterisation of subfractions of nuclear protein H1 degree. FEBS Lett. 1980 Jan 28;110(1):25–29. doi: 10.1016/0014-5793(80)80014-7. [DOI] [PubMed] [Google Scholar]
- Smith B. J., Walker J. M., Johns E. W. Structural homology between a mammalian H1(0) subfraction and avian erythrocyte-specific histone H5. FEBS Lett. 1980 Mar 24;112(1):42–44. doi: 10.1016/0014-5793(80)80122-0. [DOI] [PubMed] [Google Scholar]
- Varricchio F. Postnatal increase in histone H1a in the rat pancreas. Arch Biochem Biophys. 1977 Mar;179(2):715–717. doi: 10.1016/0003-9861(77)90161-8. [DOI] [PubMed] [Google Scholar]