Abstract
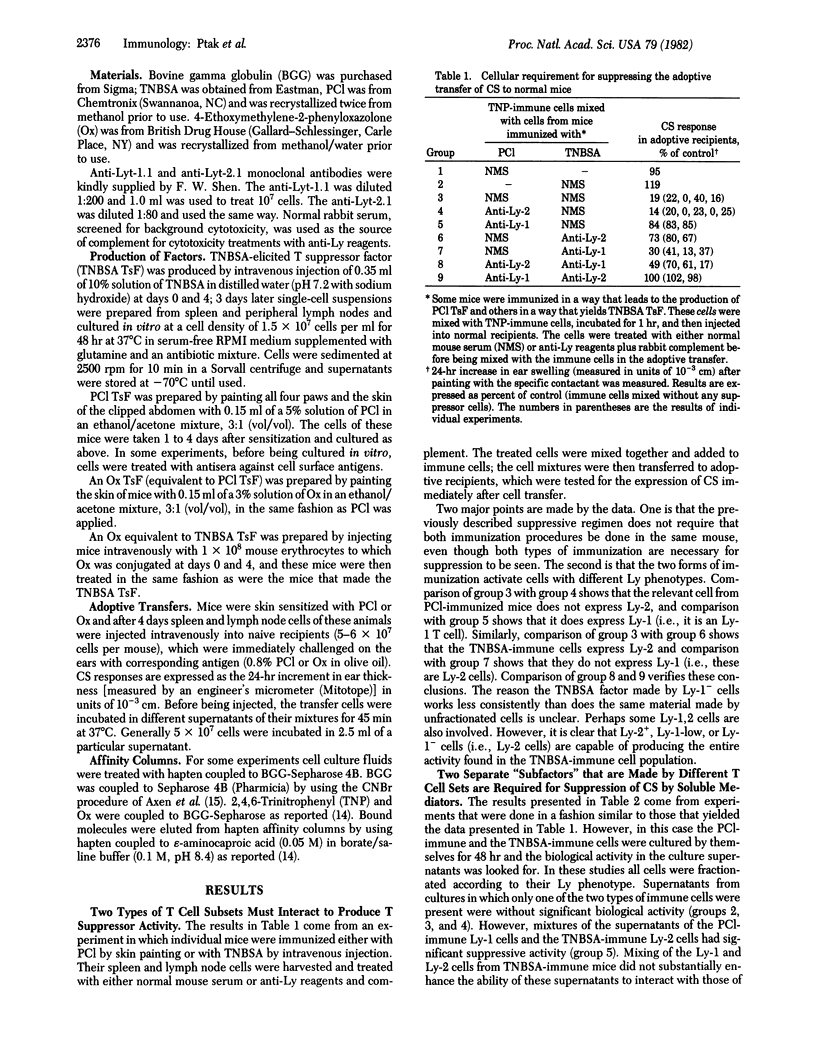
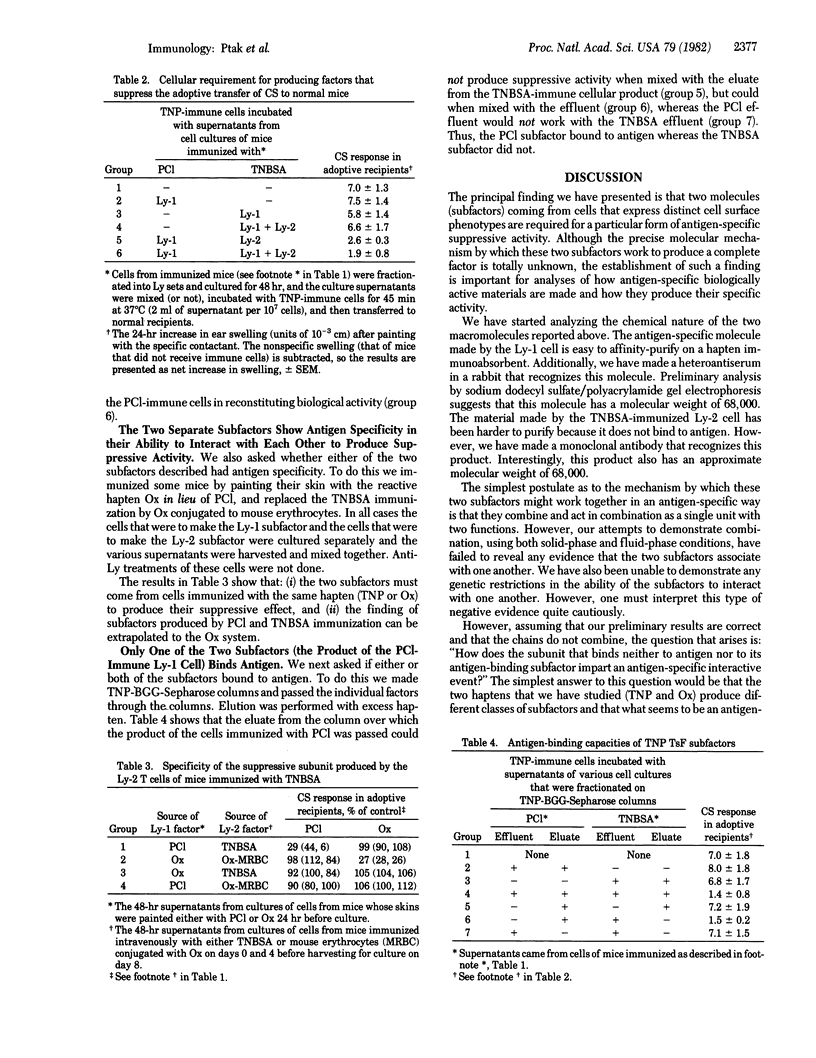
T cells that have been immunized to express optimal levels of contact hypersensitivity upon adoptive transfer to normal animals can be inhibited from doing so by incubating them with an antigen-specific T suppressor factor. This factor is composed of at least two subunits which come from cells expressing different Ly phenotypes; an antigen-specific antigen-binding "subfactor" is made by an Ly-1 cell and a non-antigen-binding one is made by an Ly-2 cell. Neither of these cells nor their products express detectable amounts of major histocompatibility gene products. The mode of immunization plays an important role in determining which of these subfactors will be produced. Painting the skin with a reactive hapten immunizes Ly-1 cells that secrete antigen-binding material, whereas intravenous injection of trinitrobenzenesulfonic acid activates Ly-2 cells to produce a second subunit that does not see antigen. There is reason to believe that the molecule that does not bind to antigen does have some antigen specificity. An analysis of the data at hand suggests that the antigen specificity stems from an interaction of the two subunits described with yet another subunit and that biological activity is dependent upon three macromolecules. Thus, the complex level of cellular interactions that regulate immunity may also be reflected in a similar type of complexity in the interaction between their biologically active cell-free products.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asherson G. L., Zembala M., Thomas W. R., Perera M. A. Suppressor cells and the handling of antigen. Immunol Rev. 1980;50:3–45. doi: 10.1111/j.1600-065x.1980.tb00306.x. [DOI] [PubMed] [Google Scholar]
- Axén R., Porath J., Ernback S. Chemical coupling of peptides and proteins to polysaccharides by means of cyanogen halides. Nature. 1967 Jun 24;214(5095):1302–1304. doi: 10.1038/2141302a0. [DOI] [PubMed] [Google Scholar]
- Cantor H., Gershon R. K. Immunological circuits: cellular composition. Fed Proc. 1979 Jun;38(7):2058–2064. [PubMed] [Google Scholar]
- Cantor H., Hugenberger J., McVay-Boudreau L., Eardley D. D., Kemp J., Shen F. W., Gershon R. K. Immunoregulatory circuits among T-cell sets. Identification of a subpopulation of T-helper cells that induces feedback inhibition. J Exp Med. 1978 Oct 1;148(4):871–877. doi: 10.1084/jem.148.4.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claman H. N., Miller S. D., Sy M. S., Moorhead J. W. Suppressive mechanisms involving sensitization and tolerance in contact allergy. Immunol Rev. 1980;50:105–132. doi: 10.1111/j.1600-065x.1980.tb00309.x. [DOI] [PubMed] [Google Scholar]
- Germain R. N., Benacerraf B. Helper and suppressor T cell factors. Springer Semin Immunopathol. 1980 May;3(1):93–127. doi: 10.1007/BF00199927. [DOI] [PubMed] [Google Scholar]
- Greene M. I., Benacerraf B. Studies on hapten specific T cell immunity and suppression. Immunol Rev. 1980;50:163–186. doi: 10.1111/j.1600-065x.1980.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Ptak W., Zembala M., Gershon R. K. Intermediary role of macrophages in the passage of suppressor signals between T-cell subsets. J Exp Med. 1978 Aug 1;148(2):424–434. doi: 10.1084/jem.148.2.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstein R. W., Murray J. H., Cone R. E., Ptak W., Iverson G. M., Gershon R. K. Isolation and partial characterization of an antigen-specific T-cell factor associated with the suppression of delayed type hypersensitivity. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5821–5825. doi: 10.1073/pnas.78.9.5821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi M., Saito T., Takei I., Tokuhisa T. Presence of interchain disulfide bonds between two gene products that compose the secreted form of an antigen-specific suppressor factor. J Exp Med. 1981 Jun 1;153(6):1672–1677. doi: 10.1084/jem.153.6.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taussig M. J., Holliman A. Structure of an antigen-specific suppressor factor produced by a hybrid T-cell line. Nature. 1979 Jan 25;277(5694):308–310. doi: 10.1038/277308a0. [DOI] [PubMed] [Google Scholar]
- Yamauchi K., Murphy D., Cantor H., Gershon R. K. Analysis of an antigen-specific H-2-restricted cell-free products(s) made by "I-J-" Ly-2 cells (Ly-2 TsF) that suppresses Ly-2 cell-depleted spleen cell activity. Eur J Immunol. 1981 Nov;11(11):913–918. doi: 10.1002/eji.1830111111. [DOI] [PubMed] [Google Scholar]
- Yamauchi K., Murphy D., Cantor H., Gershon R. K. Analysis of antigen-specific, Ig-restricted cell-free material made by I-J+ Ly-1 cells (Ly-1 TsiF) that induces Ly-2+ cells to express suppressive activity. Eur J Immunol. 1981 Nov;11(11):905–912. doi: 10.1002/eji.1830111110. [DOI] [PubMed] [Google Scholar]


