Summary
Neuronal function depends on the retrograde relay of growth and survival signals from the synaptic terminal, where the neuron interacts with its targets, to the nucleus, where gene transcription is regulated. Activation of the Bone Morphogenetic Protein (BMP) pathway at the Drosophila larval neuromuscular junction results in nuclear accumulation of the phosphorylated form of the transcription factor Mad in the motoneuron nucleus. This in turn regulates transcription of genes that control synaptic growth. How BMP signaling at the synaptic terminal is relayed to the cell body and nucleus of the motoneuron to regulate transcription is unknown. We show that the BMP receptors are endocytosed at the synaptic terminal and transported retrogradely along the axon. Furthermore, this transport is dependent on BMP pathway activity, as it decreases in the absence of ligand or receptors. We further demonstrate that receptor traffic is severely impaired when Dynein motors are inhibited, a condition that has previously been shown to block BMP pathway activation. In contrast to these results, we find no evidence for transport of phosphorylated Mad along the axons, and axonal traffic of Mad is not affected in mutants defective in BMP signaling or retrograde transport. These data support a model in which complexes of activated BMP receptors are actively transported along the axon towards the cell body to relay the synaptogenic signal, and that phosphorylated Mad at the synaptic terminal and cell body represent two distinct molecular populations.
Key words: BMP, Smad, Axonal transport, Endosome traffic, Molecular motors
Introduction
Neurons have long processes, resulting in a very long distance between the cell body, which is responsible for biosynthesis, and the synaptic terminal where cell signaling occurs. Communication between cell body and synaptic terminal is accomplished through specialized forms of active transport referred to as axonal transport (Hirokawa and Takemura, 2004). Axonal retrograde transport goes from the synaptic terminal to the cell body and is driven by Dynein and Dynactin. Anterograde transport moves cargo from the cell body to the synaptic terminal using molecular motors of the Kinesin superfamily (Holzbaur, 2004). Blockages in axonal transport result in death of the neuron and, if widespread, nervous system disorders (Goldstein, 2003). Errors in retrograde transport have been shown to cause neuron degeneration in diseases such as motor neuron disease, amyotrophic lateral sclerosis, and spinal muscle atrophy (Hafezparast et al., 2003; Levy et al., 2006).
Retrograde signaling is cued by a target-derived factor synthesized in the postsynaptic cell that is presented to the innervating neuron and is critical for coordinated growth of neurons and innervated cells during development (Fitzsimonds and Poo, 1998). Bidirectional communication between neuron and target is required for synaptic homeostasis during development and synaptic plasticity (Davis, 2006). During larval development of the Drosophila neuromuscular junction (NMJ) BMP retrograde signaling is required for synaptic terminal growth and electrophysiological refinement. The muscle-derived BMP ligand, Glass bottom boat (Gbb), signals through the neuronal BMP receptors Wishful thinking (Wit, type II), Thick veins (Tkv, type I) and Saxophone (type I) (Aberle et al., 2002; Marqués et al., 2002; McCabe et al., 2004; McCabe et al., 2003; Rawson et al., 2003). This results in phosphorylation and nuclear accumulation of the down-stream Smad transcription factor, Mothers against decapentaplegic (Mad). Both endogenous (McCabe et al., 2004) and transgenic receptors expressed with a motoneuron driver localize to the synaptic terminal (McCabe et al., 2004; O'Connor-Giles et al., 2008; Wang et al., 2007). Muscle expression of Gbb is required to establish proper synaptic terminal size as well as nuclear accumulation of phosphorylated Mad (pMad) (McCabe et al., 2003). These data suggest that muscle-secreted Gbb activates the BMP receptor complex at the NMJ, inducing accumulation of pMad in the motoneuron nuclei and growth of the synaptic terminal (McCabe et al., 2003). Transcriptional targets of the BMP pathway in motoneurons have recently been described (Ball et al., 2010; Kim and Marqués, 2010) confirming its role in regulation of gene expression.
There is evidence that, similar to neurotrophin signaling (Heerssen et al., 2004), neuronal BMP pathway activation requires intact retrograde axonal transport. A truncated form of the Dynactin component p150/Glued (DN-Glued) has been shown to block assembly of Dynein retrograde motors (Martin et al., 1999; Waterman-Storer and Holzbaur, 1996). Overexpression of DN-Glued in neurons leads to inhibition of the BMP signaling pathway, as shown by small synaptic terminals and elimination of pMad from motoneuron nuclei; similar to the phenotype of BMP pathway mutants (Aberle et al., 2002; Eaton et al., 2002; Marqués et al., 2002; McCabe et al., 2004; McCabe et al., 2003; Rawson et al., 2003). Blockage of pathway signaling by inhibition of Dynein suggests that an integral component of the BMP pathway, upon activation, is retrogradely trafficked along the axon from the synaptic terminal to the cell body and nuclei to relay the signaling event at the NMJ. While the identity of this component is unknown, candidates for long-range transport of BMP signaling are pMad and the activated receptors, but neither has been shown to be transported along the axon. Since pMad has been found at the synaptic terminal (O'Connor-Giles et al., 2008; Wang et al., 2007) and the motoneuron nucleus (Marqués et al., 2002; Marqués et al., 2003), it is a plausible candidate for long range axonal transport from the synaptic terminal to the nucleus. However, other retrograde factors, such as Nerve Growth Factor (NGF) and Brain Derived Neurotrophic Factor, depend on axonal transport of a receptor signaling endosome (Delcroix et al., 2003; Ibáñez, 2007; Ye et al., 2003). It is possible that the Gbb signal is relayed by transport of the BMP receptors from the synaptic terminal to the motoneuron soma, where they would phosphorylate Mad for nuclear translocation. Previous studies support the interaction of BMP and TGF-β receptors with transport machinery (Machado et al., 2003).
In this study we find that the BMP receptors form endosomes at the NMJ and are transported along the axon. Additionally we demonstrate colocalization of Wit and Tkv at the cell body, as well as in dynamic vesicles at the synaptic terminal and along the axon. Immunostaining confirmed pMad localization at the nucleus and synaptic terminal, but not along the axon. We found that receptor vesicle formation is influenced by pathway activity, and that transport of vesicles containing both Tkv and Wit is attenuated in gbb mutant larvae. Moreover, expression of DN-Glued, which blocks the BMP pathway, inhibited axonal transport of the receptors, but not the Mad protein. Thus, our findings support a receptor-based model, in which a BMP signaling endosome relays the Gbb signal from the synaptic terminal to the neuron soma.
Results
Wit and Tkv localize to early endosomes in the synaptic terminal
To investigate the localization and dynamics of the BMP receptors we tagged the type I receptor, Tkv, and the type II receptor, Wit, with different color variants of fluorescent proteins. These fusion proteins are functional as verified by their ability to rescue the cognate mutant phenotypes listed in the methods section (see Materials and Methods) (Marqués et al., 2003). Mutant Drosophila melanogaster that expressed the transgene were analyzed further for survival and NMJ phenotypes (supplementary material Fig. S1A,B). Additionally we verified that the expression levels of the transgenes were close to endogenous levels, Wit-GFP being expressed at 1.6 fold excess over the endogenous Wit protein (supplementary material Fig. S1C). We also verified that the fluorescent signal was not a result of in vivo cleavage of the tagged proteins resulting in ‘free’ fluorescent tags (supplementary material Fig. S1D), indicating that we are strictly analyzing the fluorescent fusion proteins in our subsequent results.
Upon expression of these fusion proteins with the motoneuron Gal4 driver OK6, both receptors were detected at the plasma membrane of synaptic boutons and in punctate structures within the synaptic terminal, as well as along the axons and at the cell body of the motoneuron (Fig. 1). The localization of the receptors to the synaptic terminal is consistent with epitope tagged Tkv (McCabe et al., 2004; O'Connor-Giles et al., 2008; Wang et al., 2007) and our previous results with endogenous Tkv (McCabe et al., 2004). Vesicular receptors within the synaptic terminal are subject to complex movements that include fusion and fission of vesicles (supplementary material Movie 1).
Fig. 1.
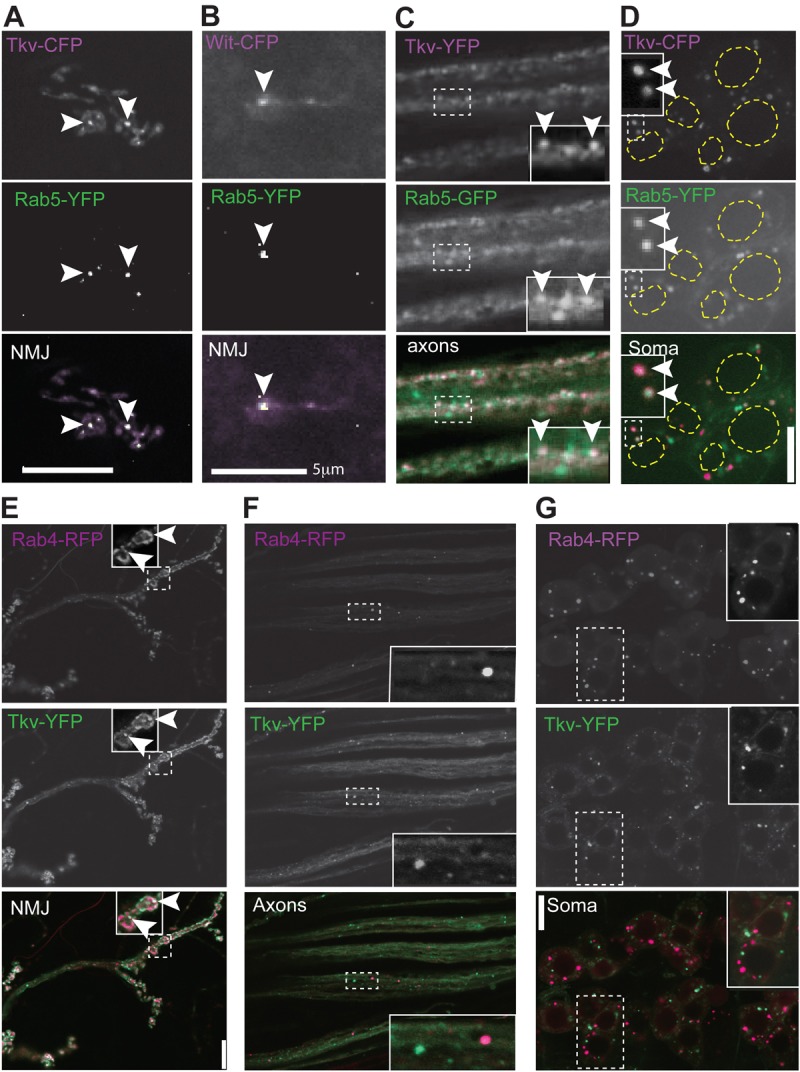
BMP receptors colocalize with Rab5 and Rab4 in early endosomes. (A) Fluorescent fusion proteins of Tkv and the early endosome protein Rab5 partially colocalize (arrowheads) in motoneuron neuromuscular junctions (NMJs). (B) Colocalization is also seen for Wit and Rab5 at the NMJ. (C,D) Additional colocalization is seen between Tkv and Rab5 along the axons (C) and cell bodies (D). The dashed yellow lines highlight the nuclei. (E–G) Tkv–YFP partially colocalizes with early/recycling endosome protein Rab4–RFP at the synaptic terminal (E), but not in axons (F) or at the cell body (G). Note that Tkv (E) also localizes to the plasma membrane at the synaptic terminal. Enlarged images of the regions within the dashed boxes are shown in the insets. Scale bars: 5 µm (E); 10 µm (all panels except E).
To further investigate the nature of these vesicles, we analyzed the colocalization of the receptors with endosomal proteins. Rab5 is specifically involved in early endosome functions and regulates early endosome fusion and motility (Clague and Urbé, 2001). Rab4 is an early endosome protein that specifically controls the recycling of early endosome cargo to the plasma membrane (Somsel Rodman and Wandinger-Ness, 2000). Coexpression of Tkv–YFP and Rab5–GFP, Tkv–CFP and Rab5–YFP, or Rab5–YFP and Wit–CFP in motoneurons revealed partial colocalization of Rab5 and the receptors at the synaptic terminal, along the axon, and at the cell body (Fig. 1A–D). However, expression of Rab4–RFP along with Tkv–YFP or Wit–GFP resulted in colocalization only at the synaptic terminal (Fig. 1E; supplementary material Fig. S2A), but not in the axons or cell bodies (Fig. 1F,G; supplementary material Fig. S2B,C). This is in agreement with the more restricted localization of Rab4 to early and rapidly recycling endosomes (McCaffrey et al., 2001; Sönnichsen et al., 2000), suggesting that receptor internalization and recycling to the plasma membrane occur mainly, if not exclusively, at the synaptic terminal. These data support that ligand–receptor binding, receptor activation, and endocytosis occurs at the synaptic terminal, as expected for a retrograde signal originating at the muscle (McCabe et al., 2003). These results are consistent with the presence of pMad at the NMJ described by others (O'Connor-Giles et al., 2008; Wang et al., 2007), and suggest that Mad is phosphorylated by the receptor complex at the synaptic terminal.
Wit and Tkv traffic bidirectionally along the axon
Upon motoneuron expression of fluorescently tagged Wit or Tkv, vesicular movement of both receptors was observed in a bidirectional manner along the motoneuron axons, with less than 20% of receptor vesicles not moving within the duration of our experiments (60 s runs; Fig. 2A–C,F; supplementary material Movies 2, 3). The proportion of vesicles moving anterogradely and retrogradely, and the velocity distribution of those vesicles were quantified. Approximately 1/3 of moving particles moved in an anterograde direction and 2/3 moved in a retrograde direction for both Tkv–YFP and Wit–GFP (Fig. 2C,F).
Fig. 2.
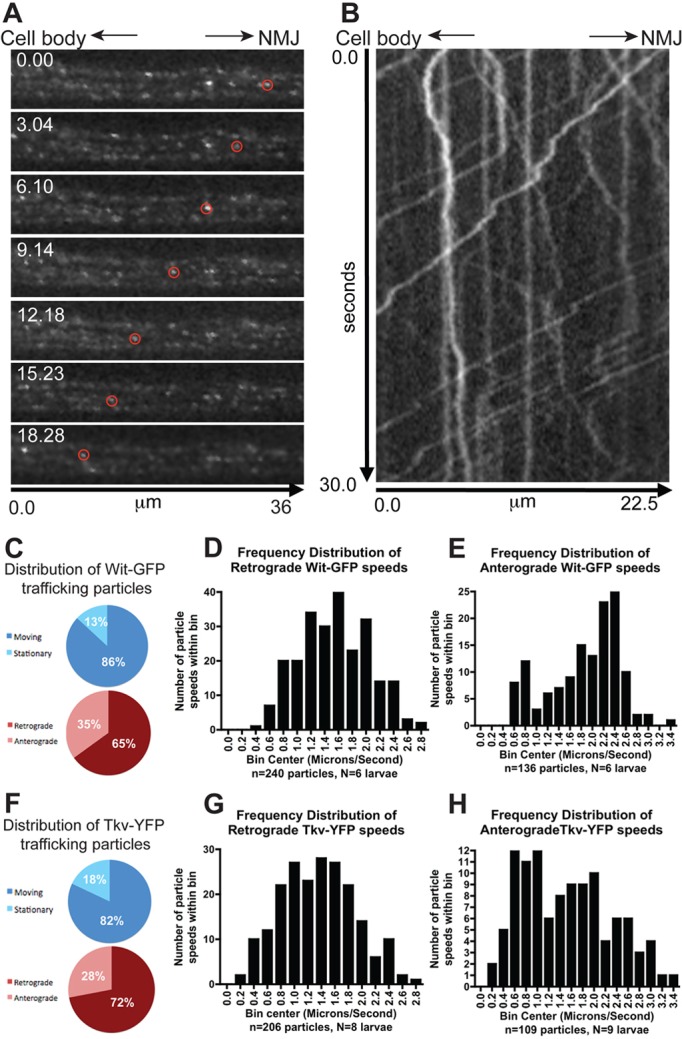
BMP receptors are transported bidirectionally along the axon. (A) Frames from a time-lapse movie showing the retrograde movement (towards the cell body; left) of a Wit–GFP vesicle (circled). The time stamp is in seconds. (B) Kymograph plot displaying the trajectory of many Wit–GFP vesicles. The kymograph demonstrates retrograde Wit vesicle movement (ascending slope projections), anterograde Wit vesicle transport (descending slope projections) and stationary Wit vesicles (vertical lines). (C) Quantification of moving and stationary Wit–GFP vesicles and directionality of traffic. (D) Speed distribution of Wit–GFP retrograde traffic; range 0.47–2.8 µm/s. (E) Speed distribution of Wit–GFP anterograde traffic; range 0.53–3.33 µm/s. (F) Quantification of moving and stationary Tkv–YFP vesicles and directionality of traffic. (G) Speed distribution of Tkv–YFP retrograde traffic; range 0.27–2.7 µm/s. (H) Speed distribution of Tkv–YFP anterograde traffic, range 0.19–3.36 µm/s.
The retrograde velocities displayed a uni-modal distribution (Fig. 2D,G), suggesting a single transport mechanism or motor mediates BMP receptor trafficking. The receptors moved at similar retrograde speeds (Wit 1.55±0.03 µm/s, n = 240; Tkv 1.36±0.04 µm/s, n = 206; P = 0.0001) compatible with Dynein-mediated fast axonal transport, the main motor responsible for retrograde traffic (Holzbaur, 2004). Anterograde speeds ranged between 0.52–3.4 µm/s for Wit–GFP and 0.2–3.4 µm/s for Tkv–YFP. Although the two receptors anterograde mean speeds were significantly different (Wit 1.87±0.06, n = 136; Tkv 1.50±0.07, n = 109; P<0.0001), the speed distributions largely overlap and are in the range of fast axonal transport (Fig. 2E,H).
Phosphorylated Mad is not detected along the axon
We next examined the distribution of the R-SMAD effector Mad (Sekelsky et al., 1995). Previous studies have shown that pMad accumulates in motoneuron nuclei, and this accumulation depends on Wit (Marqués et al., 2002; Marqués et al., 2003). Additional studies have described pMad at the synaptic terminal post (Dudu et al., 2006) and presynaptically (O'Connor-Giles et al., 2008; Wang et al., 2007). All these studies used the polyclonal rabbit antiserum to human pSmad1 developed by Persson et al. (Persson et al., 1998). Considering that Mad is the substrate for the receptors, that the receptors are activated at the synaptic terminal, and that pMad is found both at the synaptic terminal and the nucleus, it is possible that newly phosphorylated Mad at the NMJ is transported retrogradely to the cell body and nucleus to regulate transcription. In order to test this possibility, the subcellular localization of pMad and its dependence on BMP signaling was determined. As expected, pMad expression was found at the synaptic terminal (Fig. 3A–C) and nucleus (Fig. 3D). Furthermore, pMad disappears from the NMJ in wit mutants, indicating that the presence of pMad at the NMJ depends on BMP signaling (Fig. 3A,C). Given that wit is expressed and required only presynaptically (Aberle et al., 2002; Marqués et al., 2002), this result is highly suggestive that all synaptic pMad is presynaptic. More detailed analysis of the synaptic pMad signal, using a single confocal section, revealed that post-synaptic Dlg staining largely surrounds the pMad staining in the synaptic terminal; further indicating that pMad staining is presynaptic (Fig. 3B). We sometimes detect nuclear postsynaptic pMad in the muscle, but it is inconsistent and does not depend on wit activity (see below). The discrepancy with the results of Dudu et al. (Dudu et al., 2006) could be due to postsynaptic TGF-β signaling (Ellis et al., 2010) combined with the variable specificity of the antibodies to phosphorylated Mad (see below). Since pMad expression was found to localize both to the motoneuron nucleus and at the synaptic terminal in a Wit dependent manner, we next examined whether newly generated pMad at the NMJ could serve as the relay molecule for BMP pathway activation. However, specific axonal pMad staining was not detected along the axon (Fig. 3E), arguing against a role for pMad in transporting the BMP signal from the synaptic terminal to the nucleus.
Fig. 3.
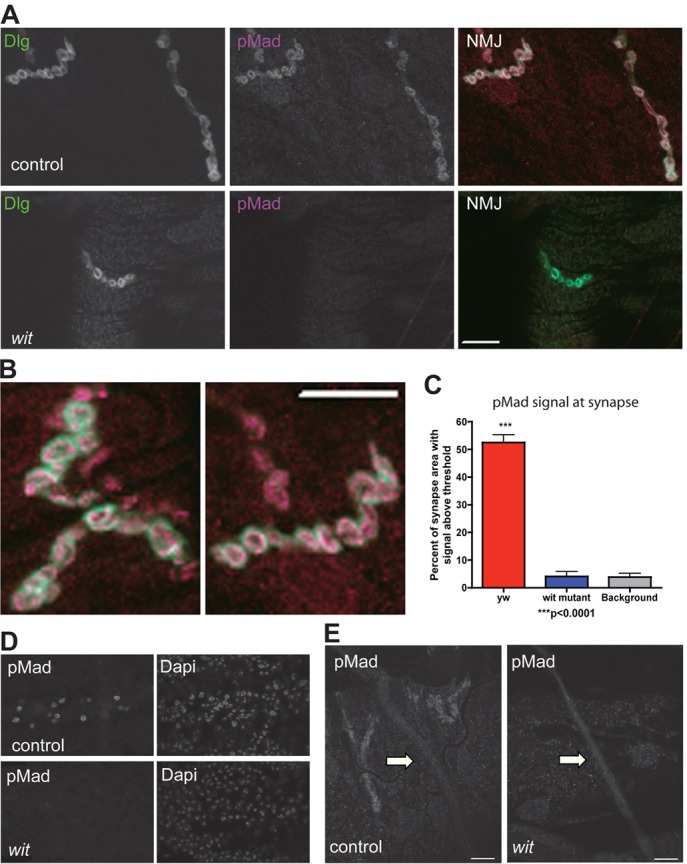
Distribution of phosphorylated Mad in motoneurons. (A) Confocal single section images showing synaptic terminal of muscle 4 in control motoneurons (upper panel, yw) and in motoneurons in the absence of BMP signaling (lower panel, witA12/witB11) stained with antibodies to the post-synaptic protein Discs large (Dlg) and pMad. Note pMad is absent in wit mutants. (B) Confocal single section images at high magnification of control muscle 4 synaptic terminals showing post-synaptic Dlg (green) surrounding the pMad signal (magenta), indicating that pMad is pre-synaptic. (C) Quantification of pMad staining intensity at the NMJ in controls (yw), wit mutants (witA12/witB11) and controls without pMad primary antibody (background staining). (D) wit mutants lose pMad staining in the motoneuron nuclei. Nuclei counterstained with DAPI. (E) No pMad staining above background levels (wit mutant) was seen along the axons (arrows) of control larvae, despite the positive neighboring synaptic staining. Immunofluorescence study of pMad carried out with the PS1-P antiserum (Persson et al., 1998). Scale bars: 10 µm.
Mad–YFP localizes to the synaptic terminal, axon and cell body and moves along the axon
In order to further characterize localization and axonal movement of Mad, we constructed a Mad protein tagged with YFP at the N-terminus. This protein is fully functional and rescues Mad mutants to viability (details in Methods). When expressed with the OK6 motoneuron driver, Mad–YFP localized to the synaptic terminal (Fig. 4A,B), axons (Fig. 4C) and cell body cytoplasm and nucleus (Fig. 4D). Nuclear localization in addition to diffuse cytoplasmic staining of Mad has also been reported in muscle, where Mad has been described previously to shuttle in and out of the nucleus in an inactive basal state. However, upon phosphorylation, the nuclear export rate of Mad is decreased, resulting in an accumulation of Mad within the nucleus and concomitant depletion from the cytoplasm (Dudu et al., 2006). Similar results have been described for mammalian R-Smads (Hill, 2009). Since Mad–YFP was not found in cytoplasmic punctae, Mad does not appear to be associated with vesicular structures in motoneurons. To study Mad axonal movement, fluorescence recovery after photobleaching (FRAP) of Mad–YFP was analyzed in the axons (Fig. 4E). After bleaching, recovery of Mad–YFP fluorescence occurred slowly from both sides of the bleached area, indicating that Mad–YFP can move along the axon. Quantification of the recovery kinetics revealed Mad–YFP had similar motion parameters as free GFP, while Tau–GFP (a microtubule-associated protein) was much slower as demonstrated by a lower diffusion coefficient and higher time to half recovery (Fig. 4F,G).
Fig. 4.
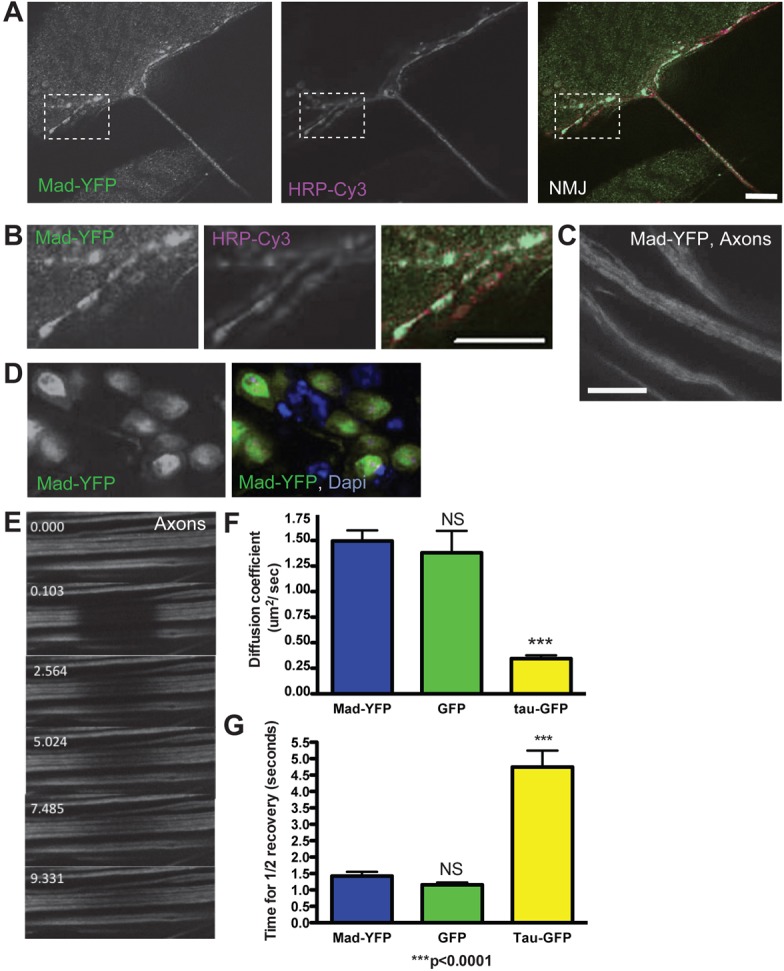
Mad–YFP localizes to the synaptic terminal, axon and cell body. (A) Mad–YFP signal (green) is seen at the synaptic terminal labeled with the presynaptic marker HRP (magenta). (B) An enlarged area of the synaptic terminal (boxed region in A). (C) Mad–YFP is seen along the axon as a diffuse signal. (D) In the motoneuron cell body Mad–YFP localizes predominantly to the nucleus (labeled with DAPI, blue), but it is also present in the cytoplasm. (E) Frames from a time-lapse recording of a FRAP experiment showing axonal Mad–YFP. Recovery of Mad–YFP signal indicates Mad mobility along the axon. The time stamp is in seconds. (F,G) Quantification of FRAP parameters of axonal Mad–YFP shows similar motion parameters to unconjugated GFP and faster movement than the microtubule binding protein Tau–GFP.
Traffic of Tkv–YFP is decreased in wit mutants
Since BMP signaling requires the relay of a BMP pathway component from the synaptic terminal to the cell body, traffic of this component may be affected by pathway activity. If, as expected from the biochemistry of BMP receptor activation (Marom et al., 2011; Moustakas and Heldin, 2009), the endocytosis and transport of the receptors occurs in a complex containing both type I and type II receptors, a difference in the amount of Tkv receptor vesicles and their retrograde transport when Wit is not present and the pathway is inactive would be expected. Indeed, compared to Tkv–YFP expression in the control motoneurons (Materials and Methods; supplementary material Fig. S3), expression in the motoneurons of wit mutants revealed a significant decrease in Tkv–YFP vesicular distribution in both the synaptic terminal and cell body (Fig. 5A,B). Tkv–YFP levels at the synaptic terminal were decreased 2.7 fold in wit mutants (supplementary material Fig. S4A; control: 18.8±1.9% of total synaptic area, n = 5; wit mutant: 6.9±0.4%, n = 4, P = 0.0009). Importantly, expression of the OK6 Gal4 line used to drive expression of Tkv–YFP was found to be unaffected in wit mutants (supplementary material Fig. S5).
Fig. 5.
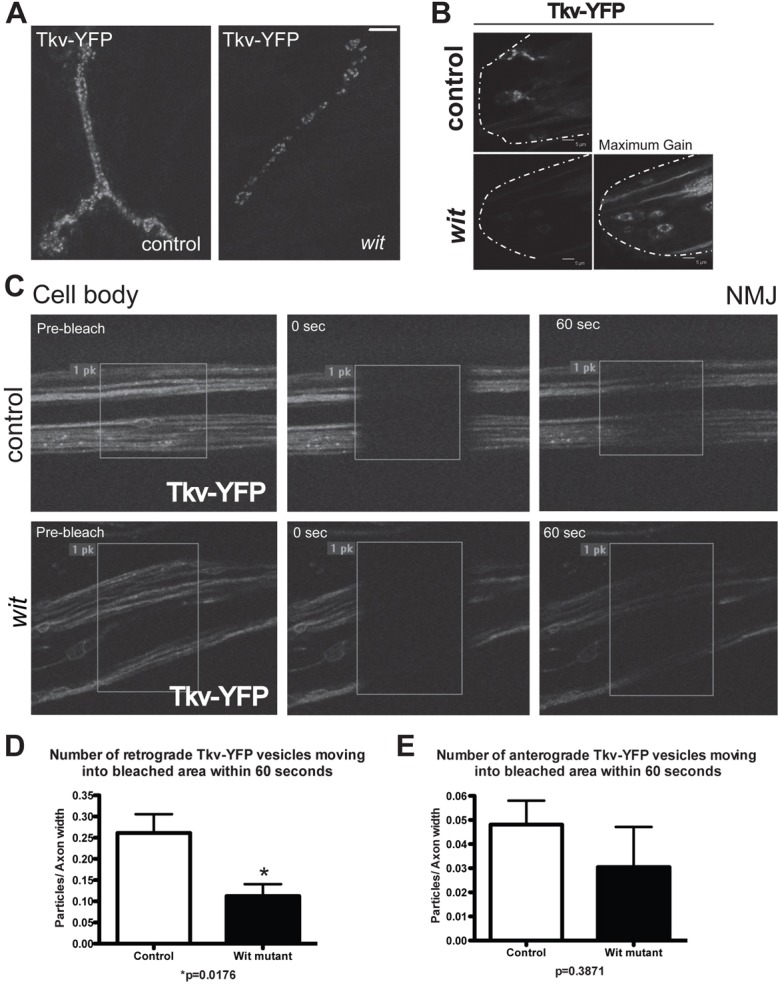
Vesicular transport of Tkv is dependent on Wit. (A) Vesicular Tkv–YFP at the synaptic terminal of muscle 4 in control (left panel) and wit mutant (right panel) animals. Compared to control (n = 5), wit mutants (n = 4) had a 63% decrease in Tkv–YFP intensity at the synaptic terminal, normalized to synaptic terminal size (data shown in supplementary material Fig. S4A; see Materials and Methods and supplementary material Fig. S3 for experimental details). (B) Tkv–YFP vesicles in motoneuron cell bodies of control (upper panel) and wit mutants (lower panels). Increasing the gain to maximum (right lower panel) some remaining vesicular Tkv–YFP can be seen in wit mutants. The dashed lines indicate the posterior end of the ventral ganglion. (C) Axons of Tkv–YFP-expressing motoneurons in control (upper panels) and wit mutant animals (lower panels) were bleached and the number of vesicles moving into the bleached area within 60 seconds quantified. (D) Using the axonal FRAP method illustrated in C, there was a 57% decrease in retrograde Tkv vesicle movement into the bleached area in wit mutants. (E) There was no significant change between the number of anterograde-moving Tkv–YFP vesicles in controls and wit mutants. Scale bars: 5 µm.
It has been previously reported that general fast axonal transport, in both anterograde and retrograde directions, is impaired in tkv mutants and when Spict, a modulator of the BMP pathway, is overexpressed (Wang et al., 2007). In that study, fast axonal transport of Synaptotagmin (Syt)–GFP was disturbed and led to a dramatic accumulation of large non-motile Syt–GFP aggregates. To determine whether altered Tkv localization is the result of a general trafficking impairment due to loss of BMP pathway activity, we next analyzed the axonal traffic of Tkv–YFP via live imaging in wit mutants followed by kymograph analysis (supplementary material Fig. S4B). Tkv–YFP transport occurred in both directions in wit mutants, (supplementary material Movies 4, 5) and, although not reaching significance, there was a trend for less anterograde moving vesicles in the wit mutant (supplementary material Fig. S4C). A slight, but significant, decrease in average retrograde velocity of Tkv–YFP was observed in wit mutants compared with Tkv–YFP retrograde velocity in control (supplementary material Fig. S4D; control: 1.2±0.04 µm/s, n = 140; wit mutant: 1.0±0.04 µm/s, n = 101, P = 0.0149), but the Tkv–YFP vesicles retained movement. There was no change in the anterograde velocity of Tkv–YFP in wit mutants (supplementary material Fig. S4E; control: 1.3±0.08 µm/s, n = 59; wit mutant: 1.1±0.07 µm/s, n = 151, P = 0.0741) nor formation of aggregates. In summary, loss of BMP pathway activity in wit mutants results in decreased formation of Tkv-containing vesicles without a notable change in the motility of these vesicles.
To determine whether attenuated vesicular Tkv–YFP in the cell body and synaptic terminal of wit mutants was reflected in the number of axonal transport vesicles containing Tkv–YFP, we bleached a central portion of the axon field and then quantified the particles that were transported into the bleached area in 60 seconds (Fig. 5C), thus reflecting the number and velocity of the vesicles. In the wit mutant, the number of Tkv–YFP vesicles transported in the retrograde direction was significantly decreased by 57% when compared to Tkv–YFP vesicle number in control (Fig. 5D; control: 0.26±0.04 vesicles/axon width, n = 6; wit mutant: 0.11±0.03 vesicles/axon width, n = 6, P = 0.018), but no change was observed in the number of vesicles moving in an anterograde direction (Fig. 5E; control: 0.048±0.001 vesicles/axon width, n = 6; wit mutant: 0.031 ±0.017 vesicles/axon width, n = 6, P = 0.39) The decrease in the number of retrograde Tkv–YFP vesicles in the wit mutant compared to control indicates that BMP pathway activity regulates type I receptor retrograde transport, further supporting that BMP receptors relay the BMP signal from the NMJ to the cell body.
Wit and Tkv colocalized vesicle movement is sensitive to BMP pathway activity
Ligand binding results in stabilization of a heterocomplex of type I and type II BMP receptors (Marom et al., 2011). If such a complex is formed and relays the signal from NMJ to cell body, it is likely that the receptors colocalize in transport vesicles along the axon. To test this hypothesis, we used two-color live imaging of motoneurons expressing Wit–CFP and Tkv–YFP. The receptors partially colocalized in punctate structures that moved within the synaptic terminal (Fig. 6A; supplementary material Movie 6; Fig. S6A,B). While the convoluted traffic pattern of the vesicles impedes quantification of their movement in the synaptic terminal, this result suggests active receptor heterocomplexes are formed at the NMJ. Partial receptor colocalization was also detected in vesicles moving in retrograde and anterograde directions along the axon (Fig. 6B; supplementary material Fig. S6C,D; Movie 7, quantification of traffic velocity) and in the cell body (Fig. 6C). The colocalized presence of these receptors at the synaptic terminal, axon, and soma is consistent with a model in which the receptors relay the active pathway signal from the synaptic terminal to the cell body.
Fig. 6.
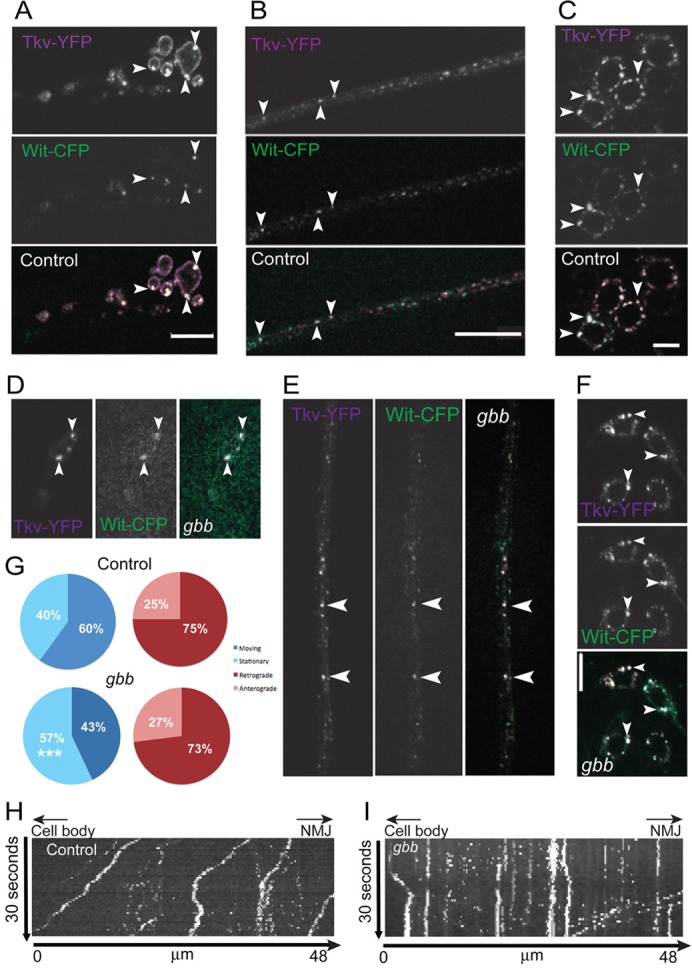
Decreased axonal movement of vesicular receptors in the absence of ligand. (A–C) Coexpressed Wit–CFP (green) and Tkv–YFP (magenta) partially colocalize (arrowheads, white) in motoneuron synaptic terminals (A), motoneuron axons (B) and cell bodies (C) of control animals. (D–F) Colocalization in the NMJ (D), axons (E) and cell bodies (F) is maintained in gbb mutants. (G–I) Quantification (G) of moving and stationary colocalized Tkv–YFP and Wit–CFP vesicles and directionality of axonal traffic in controls and gbb mutants using data from kymographs of controls (H) and gbb mutants (I). Cell body to the left, synaptic terminal to the right. Descending traces represent anterograde traffic, ascending traces retrograde traffic, vertical traces non-motile vesicles. Scale bars: 10 µm.
If the colocalized vesicles carry the BMP signal from the NMJ to the cell soma in the form of an activated receptor complex of Tkv and Wit, it is likely that the retrograde traffic is a signaling-dependent process, as is the case with the neurotrophin receptors TrkA and TrkB (Heerssen et al., 2004). To test this hypothesis, we quantified the traffic of colocalized receptors in controls and gbb null mutants, which lack ligand (Wharton et al., 1999) and have no BMP pathway activity in motoneurons (McCabe et al., 2003). We found that in gbb mutants Wit–CFP and Tkv–YFP still partially colocalize at the synaptic terminal, axons and cell body (Fig. 6D–F; supplementary material Movie 8). This suggests that the vesicles containing both receptors are a mix of two populations, one in which the receptors form a complex and a second population that contains independent receptors, perhaps forming transient complexes (Marom et al., 2011).
To determine whether trafficking of the colocalized receptor is specifically affected when the BMP pathway is inactive, we quantified the vesicular transport of receptors (Fig. 6G–I). In gbb mutants there is a significantly smaller fraction of moving vesicles containing both receptors than moving vesicles containing both receptors in control (control: 204 (60%) moving vesicles and 134 (40%) stationary vesicles; gbb mutant: 123 (43%) moving vesicles and 163 (57%) stationary vesicles, P<0.0001). However, the distribution of the direction of the moving vesicles was unchanged in gbb mutants [Fig. 6G; control: 52 (25%) anterograde vesicles and 152 (75%) retrograde vesicles; gbb mutant: 33 (27%) anterograde vesicles and 90 (73%) retrograde vesicles, P = 0.7891]. A small, but significant decrease in both the anterograde (control: 0.58±0.030 µm/s n = 53; gbb mutant: 0.43±0.031 µm/s n = 40, P = 0.0011) and retrograde (control: 0.58±0.02 µm/s n = 153; gbb mutant: 0.50±0.02 µm/s n = 94, P = 0.0051) velocities of colocalized vesicles was observed between the control and gbb mutant. Since more stationary vesicles were found in the gbb mutant, this indicates that colocalized vesicle traffic is regulated in response to pathway activity, consistent with a receptor-based model of signal relay.
Disruption of Dynein function blocks BMP receptor traffic
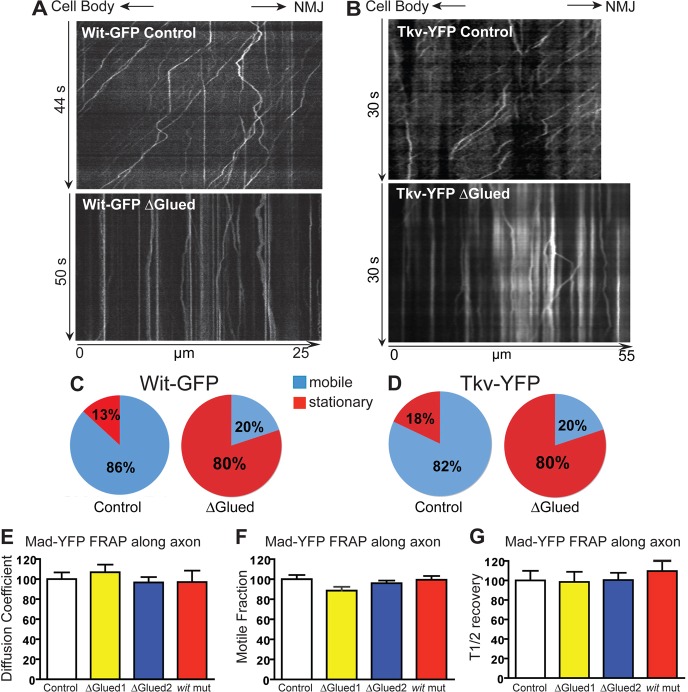
We next determined whether axonal trafficking of the receptors is impaired in an alternative method of BMP pathway inhibition. DN-Glued has been shown to inhibit the BMP pathway in motoneurons (Marqués et al., 2003; McCabe et al., 2003), causing loss of pMad nuclear accumulation and defective synaptic growth (Eaton et al., 2002; McCabe et al., 2003). Glued is a subunit of the Dynein/Dynactin complex, and the truncated form DN-Glued acts as a dominant negative to inhibit Dynein activity (Waterman-Storer and Holzbaur, 1996). The block of BMP signaling by DN-Glued is attributed to the failure of retrograde relay of the BMP signal from the NMJ to the cell body, but it is not known which pathway component traffic is inhibited. When DN-Glued is coexpressed with Tkv–YFP or Wit–GFP a large increase in stationary receptor vesicles (vertical tracks in kymographs) was observed (Fig. 7A,B). 78% and 77% decreases in the fraction of moving vesicles containing Tkv–YFP or Wit–GFP, respectively, were found in response to DN-Glued expression (Fig. 7C,D). These results confirm that Dynein is required for retrograde transport of the receptors, and suggests that this transport is critical for BMP signal relay and nuclear pMad accumulation.
Fig. 7.
Disruption of retrograde motors reduces the transport of BMP receptors. Wit–GFP (A) or Tkv–YFP (B) were expressed in motoneurons without (top panels) or with DN-Glued (bottom panels). Kymograph analyses show severely impaired vesicle movement along the axon as demonstrated by an increase in stationary vesicles (vertical lines). (C) Quantification of the proportion of moving Wit–GFP vesicles. (D) Quantification of the proportion of moving Tkv–YFP vesicles. (E–G) Using the axonal FRAP technique shown in Fig. 4, Mad–YFP movement along the axon was quantified. This quantification of the recovery of Mad–YFP fluorescence shows that there is no difference in the motion parameters between Mad–YFP in controls (white bars, normalized to 100%), wit mutants (witA12/witB11, red bars) or when retrograde motors are disrupted by coexpression of DN-Glued (yellow and blue bars – two different transgenes – see Materials and Methods).
Mad axonal movement is not affected by loss of BMP signaling or blockade of retrograde axonal transport
Since loss of BMP signaling in wit mutants resulted in decreased volume and average speed of Tkv retrograde transport, we determined if transport of Mad was also affected in wit mutants. FRAP experiments of Mad–YFP along the axon in wit mutant larvae and controls revealed that all mobility parameters of Mad–YFP (motile fraction, diffusion coefficient and half-time of recovery; Fig. 7E–G) were unchanged in the absence of Wit, indicating that, as opposed to Tkv trafficking, BMP signaling does not influence Mad axonal motion.
Finally, the effect of blocking retrograde transport with DN-Glued on Mad–YFP mobility in the axon was assessed. No significant difference in Mad motility between controls and DN-Glued over expression (Fig. 7E–G) were observed under the same conditions that result in substantial disruption of BMP receptor traffic (Fig. 7A–D). Since DN-Glued presumably inhibits BMP pathway activity by blocking the retrograde axonal transport of the pathway component that relays the signal from NMJ to cell body, our combined data support a model in which activated BMP receptors act directly to relay the signal from synaptic terminal to the neuronal soma.
What to make then of the presence of phospho-Mad at the NMJ and the cell nucleus if pMad is not the carrier of the BMP signal? One hypothesis is that these two forms of pMad represent the same molecular species that is transported from NMJ to cell body and nucleus. However this is not consistent with the absence of pMad in axons and the insensitivity of Mad axonal motion to disruption of BMP signaling or retrograde axonal transport. An alternative hypothesis consistent with these findings is that pMad detected at the synaptic terminal and pMad at the motoneuron nucleus represent different populations with potentially different roles. To test this possibility we extended our characterization of the polyclonal antibody PS1 of ten Dijke (Fig. 3; PS1-P) (Persson et al., 1998) to polyclonal PS1 from Laufer (PS1-L) (Crickmore and Mann, 2007) and two commercially available monoclonals to p-Smad proteins. The Epitomics pSmad3 monoclonal antibody (PS3) recognizes pMad in the nucleus of motoneurons (Fig. 8A; Materials and Methods) and this signal goes away in wit mutants (Fig. 8B, left panels). Similarly, PS1-L (Fig. 8B, center panels) and monoclonal PS1-CS (Fig. 8B, right panels) recognize pMad in motoneuron nuclei and the signal depends on BMP signaling. These three antibodies then stain motoneuron nuclei as the previously described PS1-P (Fig. 3) (Marqués et al., 2002; Marqués et al., 2003). The polyclonal antisera PS1-L also recognizes pMad at the NMJ (Fig. 8C, left panels), similar to polyclonal PS1-P (Fig. 3) (O'Connor-Giles et al., 2008; Wang et al., 2007). However, the monoclonal antibodies PS3 and PS1-CS that recognize pMad in the motoneuron nucleus (Fig. 8A,B) cannot detect pMad at the synaptic terminal (Fig. 8C, center and right panels). We think both nuclear and synaptic signal are phosphorylated Mad (as opposed to an accidental cross reaction with an unrelated antigen) because both nuclear (Fig. 3D, Fig. 8B) and NMJ signal (Fig. 3A,C, for PS1-P; Fig. 8D, for PS1-L) disappear in wit mutants. PS3 was raised against the phosphorylated form of human Smad3, a homolog of the other R-Smad in Drosophila, dSmad2 (Brummel et al., 1999). To verify that the nuclear signal we detect with PS3 (Fig. 8A) corresponds to pMad and not to p-dSmad2 we stained controls (Fig. 9A), animals lacking Mad (9B, Mad mutants), animals without neuronal BMP signaling (9C, wit mutants) and animals without dSmad2 (9D, dSmad2 mutants). Loss of BMP signaling or Mad eliminates the nuclear signal detected by the PS3 antibody, signal that is not affected by the loss of dSmad2. This indicates that PS3 recognizes the phosphorylated, active form of Mad, and not p-dSmad2. This is not entirely unexpected, as the C-terminal phosphorylation motif of Smad3 is more similar to Mad than to dSmad2 (Fig. 9E). Our interpretation of these data is that there are two different populations of phosphorylated Mad, that the monoclonal antibodies recognize only the nuclear population involved in transcriptional regulation, and that this form is different form the pMad species recognized by the polyclonal antisera at the NMJ. This hypothesis is consistent with the broader specificity of polyclonal antibodies.
Fig. 8.
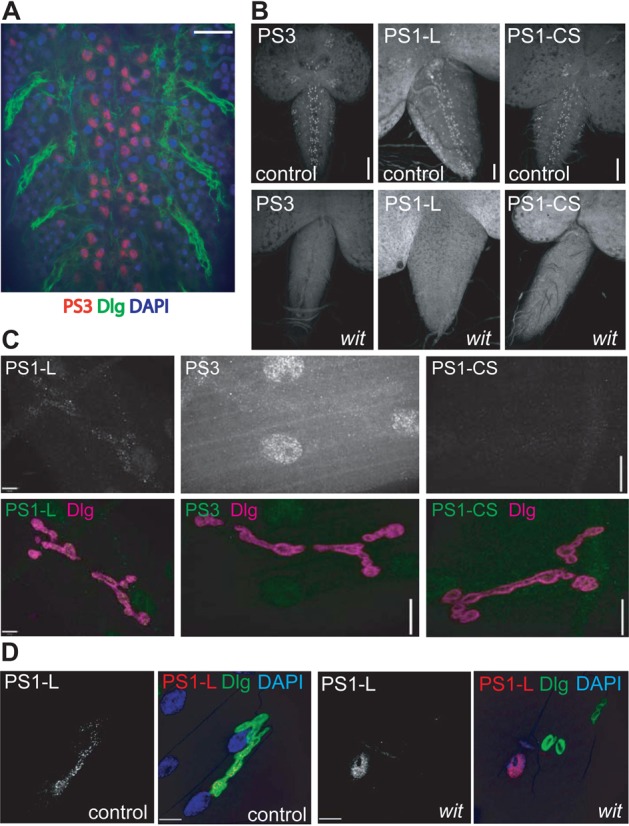
Different species of phosphorylated Mad exist in specific neuronal domains. (A) Staining of pMad (red) in control CNS with Epitomics anti-human pSmad3 monoclonal antibody, PS3. Note the characteristic pattern of dorsomedial motoneurons highlighted by Dlg (green) staining. (B) In the absence of BMP signaling (left lower panel) the pMad reactivity is absent. The pMad antiserum PS1-L from Ed Laufer (Crickmore and Mann, 2007) shows a similar pattern of motoneuron staining (B, upper center panel) that disappears in the absence of BMP signal (B, lower center panel). pSmad1 from Cell Signaling (PS1-CS) also recognizes motoneurons in a Wit-dependent manner (B, right panels). (C) PS1-L shows synaptic terminal pMad staining (gray or green, left panels). Neither PS3 (C, central panels) nor PS1-CS (C, right panels) recognize pMad at the NMJ. Dlg (magenta) was used to highlight the NMJ. (D) The synaptic terminal pMad signal obtained with the PS1-L antisera (left panels, grey/red) is dependent on BMP signaling, because it disappears in wit mutants (right panels). Dlg (green) was used to highlight the NMJ. All images of ventral ganglia are single optical slices at the level of the dorsomedial motoneurons. The NMJ panels show a maximum intensity projection (Z-stack) of a muscle-4 synaptic terminal. Scale bars: A, 10 µm; B, 50 µm right and left, 40 µm center panels (identical for matched samples); C, 5 µm left panels and 10 µm for center and right panels; D, 10 µm.
Fig. 9.
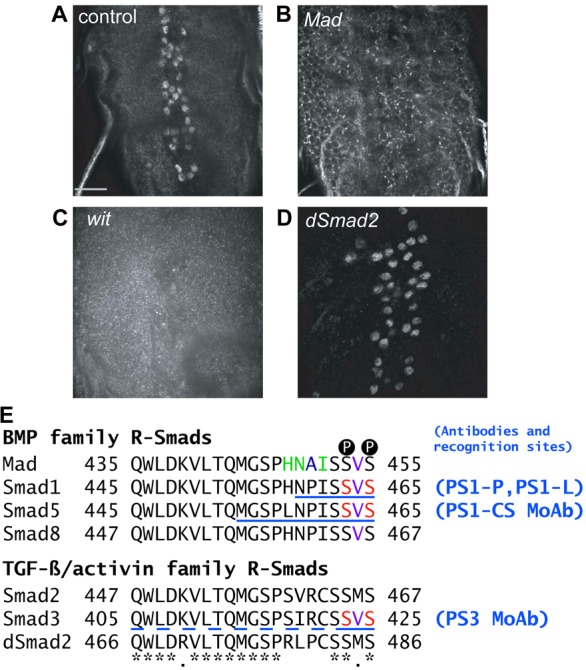
The PS3 monoclonal antibody is specific for pMad. (A–D) Staining of pMad in the CNS of control (w1118; A), Mad (Mad12/Df(2L)BSC28; B), wit (witA12/witB11; C) and dSmad2 (dSmad2F4; D) mutants with Epitomics anti-human pSmad3 monoclonal antibody, PS3. A shows the characteristic pattern of dorsomedial motoneurons. In the absence of Mad (B) or BMP signaling (C) the nuclear pMad reactivity is absent. dSmad2 mutants show normal signaling, indicating that PS3 does not recognize p-dSmad2. All images are of a single optical section. Staining, image acquisition and display parameters were identical for all panels. (E) Sequence alignment of the C-terminal sequences of R-Smads. *: identical residues; red: phospho-amino-acids in the immunogen; blue: different in Mad versus PS1 and PS3 immunogens; green: different in Mad versus PS3 immunogen; purple: Met in all TGF-β R-Smads except Smad3, which has Val like all BMP R-Smads; underlined: immunogen peptide (dashed, uncertain). Black circled Ps indicate the two Ser residues that are phosphorylated during R-Smad activation.
Discussion
Axonal transport is essential for neuronal function and survival (Goldstein, 2003; Hafezparast et al., 2003; Holzbaur, 2004). In Drosophila motoneurons the BMP pathway is activated at the synaptic terminal but ultimately results in regulation of transcription in the nucleus (Ball et al., 2010; Kim and Marqués, 2010), thus this signaling pathway is dependent on long-range signal propagation. Our results indicate that signal relay is mediated by a signaling endosome containing an activated receptor complex formed by Wit and Tkv.
Evidence in favor of a BMP signaling endosome
A target-derived factor that is critical to ensure the survival and growth of selected neurons, NGF, utilizes a receptor signaling endosome to propagate pathway activity (Delcroix et al., 2003; Ye et al., 2003). NGF binds its receptors at the synaptic terminal and the complex is endocytosed and retrogradely transported along the axon in a Dynein-dependent manner to activate downstream effector molecules (Cosker et al., 2008). We find evidence that the BMP receptors colocalize with endosomal markers and are likely endocytosed at the synaptic terminal. This is supported by the exclusive colocalization of the receptors with Rab4 at the synaptic terminal, indicating that the receptors are subject to rapid membrane recycling in this subcellular compartment (Bottger et al., 1996; Hoogenraad et al., 2010; McCaffrey et al., 2001; Sönnichsen et al., 2000). This result agrees with the source of the BMP ligand Gbb being the muscle and signaling locally to the synaptic terminal (McCabe et al., 2003). The BMP receptors colocalize with each other and are actively transported along the axon. In addition, we find that both motoneuron BMP signaling (in agreement with previous reports) (Marqués et al., 2003; McCabe et al., 2003) and BMP receptor traffic are dependent on retrograde motor activity, similar to NGF signaling. Several published reports support our findings. First, TGF-β2 was found to be transported along mammalian motoneuron axons (Jiang et al., 2000). The dependence on Gbb for endosome motility strongly suggests that the ligand is part of the signaling endosome complex. Second, several studies have shown that the endocytic pathway regulates BMP signaling in Drosophila motoneurons. Spichthyin (Spict) and Nervous wreck (Nwk) downregulate the BMP signal at the synaptic terminal and, when mutated, cause BMP-dependent synaptic terminal overgrowth (O'Connor-Giles et al., 2008; Wang et al., 2007). The late endosomal/lysosomal protein Spinster, when mutated, causes enhanced/misregulated BMP pathway signaling resulting in synaptic terminal expansion (Sweeney and Davis, 2002). Loss of Vps35, an endocytic sorting protein, leads to BMP-dependent upregulation of synaptic size (Korolchuk et al., 2007), and a similar phenotype is observed in mutants of the novel endosomal protein Ema, with increased levels of Tkv and pMad at the NMJ (Kim et al., 2010). Finally, a recent report has proposed that sorting nexin SNX16 interacts with Nwk to regulate BMP signaling-dependent synaptic growth through endocytic routing of activated Tkv (Rodal et al., 2011). Taken together, these reports show that endocytic proteins regulate BMP signaling and that a signaling endosome is a plausible mechanism for signal sorting and attenuation in this pathway. This again parallels the situation of neurotrophin receptors and the endocytic pathway they share with neurotoxins (Deinhardt and Schiavo, 2005).
An important question is the mechanism to preferentially select and transport active signaling endosomes as opposed to other vesicles that contain BMP receptors. The receptors, Wit and Tkv, could be transported individually, they could be in the same endosome without being in a complex, or the receptors could be in an active complex. All of these endosomes can either be degraded or destined for long-range trafficking, and in the case of those containing active receptor complexes ultimately lead to the phosphorylation of Mad in the soma. The skewed directionality of receptor traffic (2:1 ratio for retrograde to anterograde) is difficult to interpret with our current knowledge, and a mechanism by which the transport machinery selects the endosome that is positive for an active receptor complex and carries it to its final destination in the cell body has yet to be determined. Somehow this mechanism must be linked to BMP signaling itself, considering the diminished receptor traffic in the absence of either Wit or Gbb. Adaptor proteins that function to attach cargo to motor proteins may provide a specialized mechanism to preferentially select and transport active signaling endosomes, as has been shown in other cases of axonal transport (Horiuchi et al., 2007). In this regard it is worth noting that the regulatory light chain of the Dynein complex Tctex interacts physically with the mammalian orthologue of Wit, BMPR-II (Machado et al., 2003). Dynein light and intermediate chains also interact with the NGF receptors TrkA and TrkB (Yano et al., 2001), further supporting the parallelism between NGF and BMP signaling endosomes.
The existence of a BMP signaling endosome in Drosophila larval motoneurons also raises questions as to how general this mechanism is for sorting and regulating the TGF–β signal. Smad Anchor for Receptor Activation (SARA) is an endocytic protein that regulates the subcellular distribution of Smad proteins and presents these R-Smads for phosphorylation to the activated TGF-β receptor complex (Tsukazaki et al., 1998). In mammalian cells SARA regulates TGF–β signaling by sorting the receptor complex to degradation or signaling pathways (Di Guglielmo et al., 2003). In Drosophila wing imaginal discs, BMP signaling depends on SARA to properly distribute a gradient of the morphogen Decapentaplegic (Dpp). SARA, the ligand Dpp, and the type I receptor Tkv were found in a population of endosomes that associated with the spindle machinery. This association allows equal distribution of the Dpp morphogen into the two daughter cells during mitosis and proper activation of Mad (Bökel et al., 2006). It seems then that BMP signaling spatial regulation through endocytic traffic is a general phenomenon, and not a specialization of extremely long cells such as motoneurons.
Two different populations of phosphorylated Mad in motoneurons
The dual localization of pMad at the synaptic terminal (O'Connor-Giles et al., 2008; Wang et al., 2007) and nucleus (Marqués et al., 2002; Marqués et al., 2003) has been well characterized. This suggests that pMad is the molecular carrier of the signaling event, purportedly transported from synaptic terminal to cell nucleus. While this model has been proposed (Bayat et al., 2011; Fuentes-Medel and Budnik, 2010), no experimental evidence supports it, and our results show that axonal transport of pMad is an unlikely mechanism for pathway relay. Similar to the situation with the BMP receptors, we find Mad in the cell body, nucleus, axon and synaptic terminal. However, we find no evidence of the active form pMad along the axon when ample signal is observed at the synaptic terminal and nucleus. Furthermore, while disrupting Dynein retrograde motors abrogates BMP signaling and substantially interrupts the axonal traffic of BMP receptors, axonal movement of Mad is not affected. It is possible that a small amount – undetectable by standard imaging techniques – of pMad is present along the axon, and it is also possible that a small fraction of the total YFP–Mad in our FRAP experiments is subject to active retrograde traffic towards the nucleus. In neurotrophin signaling the transcription factor CREB is transported along the axon with the receptor complex to act as a second messenger (Cox et al., 2008). However, efforts to detect axonal colocalization of YFP–Mad and Tkv–CFP containing endosomes have been unsuccessful (R.B.S. and G.M., unpublished). The presence of Mad along the axons without detectable phosphorylation by the activated receptor complex brings up the issue of substrate accessibility. It is possible that factors that help Mad phosphorylation, such as SARA (Bökel et al., 2006; Tsukazaki et al., 1998) are not present or active in the axons. It is also possible that Mad cannot access the active receptor complex due to hindrance by the linkers between receptor and molecular motors (Machado et al., 2003).
It is worth noting that the two pMad populations show different immunofluorescence patterns, punctate at the NMJ and diffuse in the nucleus. The molecular nature of these different pMad isoforms is unclear, but at least part of the difference may stem from single versus double phosphorylation of the SVS Mad sequence (Fig. 9E). This is conceptually similar to ERK isoforms that are differentially phosphorylated by neurotrophin receptors at the synaptic terminal and the cell soma (Cosker et al., 2008; Watson et al., 2001). An alternative explanation is that identically phosphorylated forms of Mad bind different partners at NMJ and nucleus, and that the complex formed at the NMJ cannot be recognized by the monoclonal antibodies due to steric hindrance. We propose that synaptic pMad is a different population with a distinct local role, perhaps signaling through cross talk with other pathways (Guo and Wang, 2009; Moustakas and Heldin, 2005), while nuclear pMad is the result of phosphorylation of Mad in the soma by activated receptors retrogradely transported from the synaptic terminal. Consistent with our model of different pMad isoforms with different roles in larval motoneurons, a recent study found that synaptic pMad is regulated differently than nuclear pMad. Synaptic pMad was found to be absent in importinb11 mutants, but nuclear pMad was unaffected (Higashi-Kovtun et al., 2010).
Identifying the mechanism by which the BMP signal is relayed along the axon is a first step towards understanding the regulation of synaptic plasticity by this critical pathway. Intriguingly, a recent study has linked spinal muscular atrophy (SMA) and the BMP pathway. Spinal muscle atrophy is a recessive hereditary neurodegenerative disease in humans that results in early onset lethality, motor neuron loss, and skeletal muscle atrophy. Using a Drosophila ortholog to model this disease, the Wit receptor and Mad transcription factor were identified to modify the disease phenotype (Chang et al., 2008). The authors propose that increasing BMP signaling could be a possible therapeutic approach for SMA patients. Our results describing the mechanism of BMP signaling in Drosophila motoneurons helps understand the pathological consequences of pathway disruption and will open new avenues to understand human neurodegenerative disorders that involve TGF-β signaling (Bayat et al., 2011; Katsuno et al., 2010). Additionally, our results in conjunction with other reports (Bökel et al., 2006; Di Guglielmo et al., 2003) suggest that signaling endosome traffic is a general mechanism in TGF-β signaling.
Materials and Methods
Fly stocks and genetics
Gal4 drivers OK6 (Aberle et al., 2002), OK371 (Mahr and Aberle, 2006) and BG380 (Budnik et al., 1996) are expressed in Drosophila larval motoneurons. elav, armadillo and daughterless Gal4 drivers were from Bloomington Drosophila Stock Center. UAS-DN-Glued was obtained from Tom Hays (Reddy et al., 1997; Li et al., 2004). Fluorescent balancers were from Greg Beitel (Le et al., 2006) and the null allele dSmad2F4, was a gift from Mike O'Connor (Peterson et al., 2012). Rab protein markers used included UAS>Rab5–GFP (Entchev et al., 2000), UAS>Rab5–YFP and UAS>Rab4–RFP (Bloomington). See FlyBase (http://flybase.org/) for other mutant alleles used: witA12, witB11, witG15, gbb1, gbb2, tkv5, tkv8, Mad12, Mad1, Df(2L)BSC28 (Mad).
Constructs
Fluorescent fusion proteins were constructed by standard methods (Marqués et al., 2003), sequenced to verify PCR accuracy and subcloned into pUAST (Brand and Perrimon, 1993). Details available upon request. All constructs were injected for germ line transformation by Model Systems Genomics, Duke University.
Transgene functionality
When expressed with a pan-neuronal (elav) Gal4 driver, Wit tagged with EGFP or CFP fully rescued the lethality of witA12/witB11 mutants (Marqués et al., 2002; Marqués et al., 2003). Briefly, animals of the genotype w; P{elav>Gal4}/CyO, P{Dfd-EYFP}2; witA12, st/TM6B, Tb were crossed to y, w; P{UAS>wit-XFP}/CyO, P{Dfd-EYFP}2; witB11, st/TM6B, Tb. wit mutants were identified by the lack of the Tb marker, and animals carrying both the UAS transgene and the Gal4 driver (either elav or OK6) by the presence of Wit–XFP fluorescence in the CNS and absence of the Dfd-EYFP marker. The expression of this marker is distinct and can be separated from the fluorescent transgenes (Le et al., 2006). These animas were analyzed for rescue of viability and synaptic size. Both UAS>wit-GFP#7 and UAS>wit-CFP#5DHL rescued viability and the synaptic terminal size of witA12/witB11 mutant larvae (supplementary material Fig. S1A; data not shown). For the rescue experiments of tkv by a tkv-YFP transgene, animals of the genotype w; tkv5/CyO, Dfd-EYFP; arm>Gal4/TM6B, Tb were crossed to y, w; tkv8/CyO, Dfd-EYFP; UAS>tkv-YFP#9/TM6B, Tb. tkv mutants were identified by the lack of the Dfd-EYFP marker, and animals carrying both the UAS transgene and the arm>Gal4 driver by the absence of the Tb marker and the presence of generalized Tkv-YFP fluorescence. When driven by the ubiquitous driver arm>Gal4, Tkv–YFP rescued the embryonic lethality phenotype of tkv8/tkv5 to pupal stage, and the tkv larva rescued from this cross had normal synaptic terminal size (supplementary material Fig. S1B). To examine the function of Mad–YFP, flies of the genotype w; Mad1/CyO; UAS>YFP-Mad#26D were crossed to w; Mad12/CyO; da>Gal4/TM6B, Tb. Mad mutants were identified as adults by the lack of the Cy wing phenotype. Rescue occurred only in the absence of the Tb marker, indicating that ubiquitous expression of Mad–YFP by simultaneous presence of da>Gal4 and UAS>YFP-Mad is enough to rescue the Mad12/Mad1 mutant phenotype.
Western blot
Western blot performed essentially as in Marqués et al. (Marqués et al., 1997). Briefly, larval CNS were dissected in PBS and homogenized in SDS-PAGE sample buffer. One to four CNSs were loaded per lane of 7% or 4–20% gels. Transferred membranes were probed sequentially with the antibody of interest followed by anti-tubulin to correct for unequal loads.
Immunofluorescence
Antibodies utilized were rabbit polyclonal anti-pSmad1 [PS1-P, gift from Carl-Henrik Heldin (Persson et al., 1998)]; rabbit polyclonal anti-pSmad1 (PS1-L, gift from Ed Laufer, Columbia (Crickmore and Mann, 2007)]; rabbit monoclonal anti-pSmad1 (PS1-CS, Cell Signaling Ref# 9516); and rabbit monoclonal anti-pSmad3 (PS3, Epitomics Ref# 1880-1). This antibody recognizes Drosophila phosphorylated Mad (Figs 8, 9) (Dejima et al., 2011), but does not recognize phosphorylated dSmad2 (Mike O'Connor, personal communication) (Fig. 9). Anti-HRP-Cy3 was from Jackson Immunoresearch. The mouse anti-Dlg and anti-Wit monoclonals developed by Corey Goodman were obtained from the Developmental Studies Hybridoma Bank. DM1A anti-tubulin was from Sigma and mouse anti-β-galactosidase from Promega. Anti-GFP Living Colors from was from Clontech. Secondary antibodies were goat anti-rabbit Alexa568 and Alexa488, goat anti-mouse Alexa488 and Alexa568 (Invitrogen), and donkey anti-goat Cy3 (Jackson Immunoresearch). HRP-conjugated secondaries for western blot were from Promega.
Standard immunofluorescence protocol
Third instar larvae were dissected live in HL3 medium (Stewart et al., 1994) and fixed for 10–20 minutes with 4% PFA containing 0.1% Triton X-100 in PBS. Larvae were blocked for 1 hour with PBS containing 0.1% Triton X-100 (PBT) and 2.5% BSA. Primary antibody was incubated overnight at 4°C in block, washed with PBT and secondary antibody and DAPI were incubated in block at room temperature for 3–4 hours. After secondary wash, samples were cleared in 80% glycerol–PBS and mounted in Permafluor (Thermo Scientific). All samples that were compared to one another were stained simultaneously in the same dissecting dish. For quantifying Tkv–YFP in fixed tissue, the same protocol was used but fixation time with 4% PFA was decreased to 5 minutes to avoid quenching YFP.
Microscope confocal imaging and time-lapse movies
Confocal imaging of fixed tissues for synaptic and ventral ganglion comparisons was completed on a Leica SP2. All compared images were taken at the same settings and processed identically. Time-lapse movies were obtained in a Nikon Eclipse TE2000-U equipped with a Perkin Elmer Ultraview confocal head using Ultraview or Volocity software. The animals were dissected live in HL3 medium (Stewart et al., 1994), pinned to Sylgard in a specially designed imaging chamber, eviscerated keeping CNS, nerves and muscle intact and covered with a coverslip. Axonal transport was imaged with 1.49 or 1.30 NA oil 100× objectives. When imaging one receptor, exposure times of 100–200 ms were used (GFP 488 nm laser, emission filter 527 nm pass band center, 55 nm half-power bandwidth; YFP 514 nm laser, emission filter 575 nm nm bandpass center, 100 nm half-power bandwidth) for acquisition speeds of 9–5 frames per second (fps). For dual color imaging, spectral separation setting was used, with exposure times of 30–300 ms for Tkv–YFP (settings as above) and 200–300 ms for Wit–CFP (440 nm laser, emission filter 477 nm bandpass center, 45 nm half-power bandwidth), for a combined acquisition speed of 0.5–1 fps. Receptor colocalization analysis was done using the following genotypes: OK6>Gal4, UAS>Wit-CFP#5DHL, gbb2/UAS>Tkv-YFP#66, gbb1 (mutant) and OK6>Gal4, UAS>Wit-CFP#5DHL, gbb2/UAS>Tkv-YFP#66 (control).
For FRAP analysis, larvae with the following genotypes were imaged in the Perkin Elmer spinning disk confocal using Volocity software: OK6>Gal4, UAS-Mad-YFP/+ (control, line #60 used throughout); OK6>Gal4/UAS-Tau-GFP; OK6>Gal4/UAS-GFP; OK6>Gal4, UAS-Mad-YFP/UAS-DN-Glued (retrograde transport disrupted); OK6>Gal4, UAS-Mad-YFP/+; witA12/witB11 (no BMP signaling).
Spectral separation between GFP and YFP
The Leica settings to spectrally separate YFP and GFP were as follows. YFP channel: excitation laser, 514 nm 100% power; emission band pass 565–612, pinhole 370, dichroic mirror DD 458/514. GFP channel: excitation laser, 488 nm 100%, emission band pass 496–515 nm, pinhole 370, dichroic mirror RSP500. Controls containing only YFP or GFP were used to ensure lack of cross talk between the channels.
Quantification of Tkv–YFP and pMad at the synaptic terminal
The synaptic area identified by Dlg or HRP staining was outlined in IPLab and designated as an ROI. The ROI was transferred to the synaptic pMad or Tkv–YFP channel. A threshold was established and areas with signal above threshold were summed and quantified as percent of the ROI area (supplementary material Fig. S3). Because this measurement is a percent of area, it takes into consideration different size synaptic terminals. IPlab was used to quantify the fluorescent intensity.
Quantification of Tkv–YFP vesicle numbers
To examine the effect of Wit activity on Tkv vesicle formation, third instar larvae of the following genotypes were dissected live in HL3 medium: OK6>Gal4, UAS>Tkv-YFP#66/+ (control), OK6>Gal4, UAS>Tkv-YFP#66/+; witA12/witB11 (wit mutant). Using Ultraview software, we imaged the axon and bleached a center portion for a clear view of moving vesicles. The time-lapse movies were uploaded into ImageJ and, to eliminate viewer bias, we assigned a fluorescence intensity threshold to the movies counting only those vesicles that reached or surpassed the threshold. All particles that met or surpassed the threshold and crossed the initial bleaching line of the ROI within 60 seconds were counted. In different samples the number of axon bundles imaged would vary, so we normalized the particle data to the total width of the axons analyzed.
Statistical analysis and graphs
Statistical analysis was performed with Prism4 statistical software (GraphPad Software Inc.). Comparing the experimental data set to the control yielded all P-values. A Student's unpaired t-test with one-tailed P-value was completed for all data sets. χ2-tests were applied to population distributions to get the P-values for the comparison pie charts. The pie charts were made in Excel (Microsoft). All data are shown as mean ± s.e.m.
Kymograph generation
We used ImageJ (Version 1.38x, NIH) and the plug-in ‘Kymograph’ (http://www.embl-heidelberg.de/eamnet/html/body_kymograph.html) to create kymograph plots. The cell body was always to the left of axon movies. Particles with ascending slopes are moving retrograde, towards the ventral ganglion, while particles with descending slopes are moving anterograde toward the synaptic terminal.
Velocity measurements
Using the straight-line tool in ImageJ, a line was drawn over the longest pathway section of movement of each trajectory. Then the Macros plug-in ‘tsp050706’ was used. If the particle changed speeds, the longest continuous section of one speed was measured. For most kymographs, all sloping (moving) pathway lengths were measured and it was recorded which direction each particle was traveling. Kymographs were excluded from the population distribution analysis if all of the sloping trajectories within the kymograph were not measured.
Kymograph analysis of colocalized Tkv–YFP and Wit–CFP
Using ImageJ, the two channels of each movie were opened as different stacks. Using the ‘Colocalization Analysis’ plug-in, an eight-bit stack was created where the colocalized particles in each frame were highlighted with white. After this stack was created we generated a kymograph and analyzed the velocity of colocalized particles using the methods described above. The number of vesicles that were going retrograde, anterograde, or stationary were quantified and graphed.
Supplementary Material
Acknowledgments
We thank Ed Laufer, Carl-Henrik Heldin and the Developmental Studies Hybridoma Bank, developed under the auspices of the NICHD and maintained by the University of Iowa for antibodies. Hugo Bellen, Hermann Aberle, Greg Beitel and the Bloomington Drosophila Stock Center supplied essential fly stocks. FlyBase (Flybase.org) provided critical information used in this work. We thank Albert Tousson (UAB High Resolution Imaging Facility) for help and advice, and Brad Yoder for use of his Perkin Elmer confocal microscope. Sarah Mische helped generate some early receptor traffic data for this project. We thank Mike O'Connor for sharing unpublished information and reagents and Nisha Badders for helpful comments on the manuscript.
Footnotes
Funding
R.B.S. and J.B.M. received Carmichael Scholarships from the University of Alabama Medical Alumni Association; J.B.M. was supported by an National Institutes of Health Predoctoral Training Grant in Cell and Molecular Biology [grant number T32 GM008111]. This work was supported by National Institutes of Health – National Institute of Neurological Disorders and Stroke [grant number R21NS051319-01]; and a Pew Scholars Program in Biomedical Sciences Award [subcontract number 3779sc] to G. M. Deposited in PMC for release after 12 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.094292/-/DC1
References
- Aberle H., Haghighi A. P., Fetter R. D., McCabe B. D., Magalhães T. R., Goodman C. S. (2002). wishful thinking encodes a BMP type II receptor that regulates synaptic growth in Drosophila. Neuron 33, 545–558 10.1016/S0896-6273(02)00589-5 [DOI] [PubMed] [Google Scholar]
- Ball R. W., Warren–Paquin M., Tsurudome K., Liao E. H., Elazzouzi F., Cavanagh C., An B-S., Wang T-T., White J. H., Haghighi A. P. (2010). Retrograde BMP signaling controls synaptic growth at the NMJ by regulating trio expression in motor neurons. Neuron 66, 536–549 10.1016/j.neuron.2010.04.011 [DOI] [PubMed] [Google Scholar]
- Bayat V., Jaiswal M., Bellen H. J. (2011). The BMP signaling pathway at the Drosophila neuromuscular junction and its links to neurodegenerative diseases. Curr. Opin. Neurobiol. 21, 182–188 10.1016/j.conb.2010.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bökel C., Schwabedissen A., Entchev E., Renaud O., González–Gaitán M. (2006). Sara endosomes and the maintenance of Dpp signaling levels across mitosis. Science 314, 1135–1139 10.1126/science.1132524 [DOI] [PubMed] [Google Scholar]
- Bottger G., Nagelkerken B., van der Sluijs P. (1996). Rab4 and Rab7 define distinct nonoverlapping endosomal compartments. J. Biol. Chem. 271, 29191–29197 10.1074/jbc.271.46.29191 [DOI] [PubMed] [Google Scholar]
- Brand A. H., Perrimon N. (1993). Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401–415 [DOI] [PubMed] [Google Scholar]
- Brummel T., Abdollah S., Haerry T. E., Shimell M. J., Merriam J., Raftery L., Wrana J. L., O'Connor M. B. (1999). The Drosophila activin receptor baboon signals through dSmad2 and controls cell proliferation but not patterning during larval development. Genes Dev. 13, 98–111 10.1101/gad.13.1.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budnik V., Koh Y-H., Guan B., Hartmann B., Hough C., Woods D., Gorczyca M. (1996). Regulation of synapse structure and function by the Drosophila tumor suppressor gene dlg. Neuron 17, 627–640 10.1016/S0896-6273(00)80196-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H. C-H., Dimlich D. N., Yokokura T., Mukherjee A., Kankel M. W., Sen A., Sridhar V., Fulga T. A., Hart A. C., Van Vactor D.et al. (2008). Modeling spinal muscular atrophy in Drosophila. PLoS ONE 3, e3209 10.1371/journal.pone.0003209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clague M. J., Urbé S. (2001). The interface of receptor trafficking and signalling. J. Cell Sci. 114, 3075–3081 [DOI] [PubMed] [Google Scholar]
- Cosker K. E., Courchesne S. L., Segal R. A. (2008). Action in the axon: generation and transport of signaling endosomes. Curr. Opin. Neurobiol. 18, 270–275 10.1016/j.conb.2008.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox L. J., Hengst U., Gurskaya N. G., Lukyanov K. A., Jaffrey S. R. (2008). Intra-axonal translation and retrograde trafficking of CREB promotes neuronal survival. Nat. Cell Biol. 10, 149–159 10.1038/ncb1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crickmore M. A., Mann R. S. (2007). Hox control of morphogen mobility and organ development through regulation of glypican expression. Development 134, 327–334 10.1242/dev.02737 [DOI] [PubMed] [Google Scholar]
- Davis G. W. (2006). Homeostatic control of neural activity: from phenomenology to molecular design. Annu. Rev. Neurosci. 29, 307–323 10.1146/annurev.neuro.28.061604.135751 [DOI] [PubMed] [Google Scholar]
- Deinhardt K., Schiavo G. (2005). Endocytosis and retrograde axonal traffic in motor neurons. Biochem. Soc. Symp. 72, 139–150 [DOI] [PubMed] [Google Scholar]
- Dejima K., Kanai M. I., Akiyama T., Levings D. C., Nakato H. (2011). Novel contact-dependent bone morphogenetic protein (BMP) signaling mediated by heparan sulfate proteoglycans. J. Biol. Chem. 286, 17103–17111 10.1074/jbc.M110.208082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcroix J-D., Valletta J. S., Wu C., Hunt S. J., Kowal A. S., Mobley W. C. (2003). NGF signaling in sensory neurons: evidence that early endosomes carry NGF retrograde signals. Neuron 39, 69–84 10.1016/S0896-6273(03)00397-0 [DOI] [PubMed] [Google Scholar]
- Di Guglielmo G. M., Le Roy C., Goodfellow A. F., Wrana J. L. (2003). Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat. Cell Biol. 5, 410–421 10.1038/ncb975 [DOI] [PubMed] [Google Scholar]
- Dudu V., Bittig T., Entchev E., Kicheva A., Jülicher F., González–Gaitán M. (2006). Postsynaptic mad signaling at the Drosophila neuromuscular junction. Curr. Biol. 16, 625–635 10.1016/j.cub.2006.02.061 [DOI] [PubMed] [Google Scholar]
- Eaton B. A., Fetter R. D., Davis G. W. (2002). Dynactin is necessary for synapse stabilization. Neuron 34, 729–741 10.1016/S0896-6273(02)00721-3 [DOI] [PubMed] [Google Scholar]
- Ellis J. E., Parker L., Cho J., Arora K. (2010). Activin signaling functions upstream of Gbb to regulate synaptic growth at the Drosophila neuromuscular junction. Dev. Biol. 342, 121–133 10.1016/j.ydbio.2010.03.012 [DOI] [PubMed] [Google Scholar]
- Entchev E. V., Schwabedissen A., González–Gaitán M. (2000). Gradient formation of the TGF-β homolog Dpp. Cell 103, 981–992 10.1016/S0092-8674(00)00200-2 [DOI] [PubMed] [Google Scholar]
- Fitzsimonds R. M., Poo M-M. (1998). Retrograde signaling in the development and modification of synapses. Physiol. Rev. 78, 143–170 [DOI] [PubMed] [Google Scholar]
- Fuentes–Medel Y., Budnik V. (2010). Ménage à Trio during BMP-Mediated Retrograde Signaling at the NMJ. Neuron 66, 473–475 10.1016/j.neuron.2010.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein L. S. B. (2003). Do disorders of movement cause movement disorders and dementia? Neuron 40, 415–425 10.1016/S0896-6273(03)00630-5 [DOI] [PubMed] [Google Scholar]
- Guo X., Wang X-F. (2009). Signaling cross-talk between TGF-β/BMP and other pathways. Cell Res. 19, 71–88 10.1038/cr.2008.302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafezparast M., Klocke R., Ruhrberg C., Marquardt A., Ahmad–Annuar A., Bowen S., Lalli G., Witherden A. S., Hummerich H., Nicholson S.et al. (2003). Mutations in dynein link motor neuron degeneration to defects in retrograde transport. Science 300, 808–812 10.1126/science.1083129 [DOI] [PubMed] [Google Scholar]
- Heerssen H. M., Pazyra M. F., Segal R. A. (2004). Dynein motors transport activated Trks to promote survival of target-dependent neurons. Nat. Neurosci. 7, 596–604 10.1038/nn1242 [DOI] [PubMed] [Google Scholar]
- Higashi–Kovtun M. E., Mosca T. J., Dickman D. K., Meinertzhagen I. A., Schwarz T. L. (2010). Importin-beta11 regulates synaptic phosphorylated mothers against decapentaplegic, and thereby influences synaptic development and function at the Drosophila neuromuscular junction. J. Neurosci. 30, 5253–5268 10.1523/JNEUROSCI.3739-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C. S. (2009). Nucleocytoplasmic shuttling of Smad proteins. Cell Res. 19, 36–46 10.1038/cr.2008.325 [DOI] [PubMed] [Google Scholar]
- Hirokawa N., Takemura R. (2004). Molecular motors in neuronal development, intracellular transport and diseases. Curr. Opin. Neurobiol. 14, 564–573 10.1016/j.conb.2004.08.011 [DOI] [PubMed] [Google Scholar]
- Holzbaur E. L. F. (2004). Motor neurons rely on motor proteins. Trends Cell Biol. 14, 233–240 10.1016/j.tcb.2004.03.009 [DOI] [PubMed] [Google Scholar]
- Hoogenraad C. C., Popa I., Futai K., Martinez–Sanchez E., Wulf P. S., van Vlijmen T., Dortland B. R., Oorschot V., Govers R., Monti M.et al. (2010). Neuron specific Rab4 effector GRASP-1 coordinates membrane specialization and maturation of recycling endosomes. PLoS Biol. 8, e1000283 10.1371/journal.pbio.1000283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi D., Collins C. A., Bhat P., Barkus R. V., Diantonio A., Saxton W. M. (2007). Control of a kinesin-cargo linkage mechanism by JNK pathway kinases. Curr. Biol. 17, 1313–1317 10.1016/j.cub.2007.06.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibáñez C. F. (2007). Message in a bottle: long-range retrograde signaling in the nervous system. Trends Cell Biol. 17, 519–528 10.1016/j.tcb.2007.09.003 [DOI] [PubMed] [Google Scholar]
- Jiang Y., McLennan I. S., Koishi K., Hendry I. A. (2000). Transforming growth factor-beta 2 is anterogradely and retrogradely transported in motoneurons and up-regulated after nerve injury. Neuroscience 97, 735–742 10.1016/S0306-4522(00)00084-1 [DOI] [PubMed] [Google Scholar]
- Katsuno M., Adachi H., Minamiyama M., Waza M., Doi H., Kondo N., Mizoguchi H., Nitta A., Yamada K., Banno H.et al. (2010). Disrupted transforming growth factor-beta signaling in spinal and bulbar muscular atrophy. J. Neurosci. 30, 5702–5712 10.1523/JNEUROSCI.0388-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N. C., Marqués G. (2010). Identification of downstream targets of the bone morphogenetic protein pathway in the Drosophila nervous system. Dev. Dyn. 239, 2413–2425 10.1002/dvdy.22368 [DOI] [PubMed] [Google Scholar]
- Kim S., Wairkar Y. P., Daniels R. W., DiAntonio A. (2010). The novel endosomal membrane protein Ema interacts with the class C Vps-HOPS complex to promote endosomal maturation. J. Cell Biol. 188, 717–734 10.1083/jcb.200911126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korolchuk V. I., Schütz M. M., Gómez–Llorente C., Rocha J., Lansu N. R., Collins S. M., Wairkar Y. P., Robinson I. M., O'Kane C. J. (2007). Drosophila Vps35 function is necessary for normal endocytic trafficking and actin cytoskeleton organisation. J. Cell Sci. 120, 4367–4376 10.1242/jcs.012336 [DOI] [PubMed] [Google Scholar]
- Le T., Liang Z., Patel H., Yu M. H., Sivasubramaniam G., Slovitt M., Tanentzapf G., Mohanty N., Paul S. M., Wu V. M.et al. (2006). A new family of Drosophila balancer chromosomes with a w− dfd-GMR yellow fluorescent protein marker. Genetics 174, 2255–2257 10.1534/genetics.106.063461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. R., Sumner C. J., Caviston J. P., Tokito M. K., Ranganathan S., Ligon L. A., Wallace K. E., LaMonte B. H., Harmison G. G., Puls I.et al. (2006). A motor neuron disease-associated mutation in p150Glued perturbs dynactin function and induces protein aggregation. J. Cell Biol. 172, 733–745 10.1083/jcb.200511068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M. G., Serr M., Newman E. A., Hays T. S. (2004). The Drosophila tctex-1 light chain is dispensable for essential cytoplasmic dynein functions but is required during spermatid differentiation. Mol. Biol. Cell 15, 3005–3014 10.1091/mbc.E04-01-0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado R. D., Rudarakanchana N., Atkinson C., Flanagan J. A., Harrison R., Morrell N. W., Trembath R. C. (2003). Functional interaction between BMPR-II and Tctex-1, a light chain of Dynein, is isoform-specific and disrupted by mutations underlying primary pulmonary hypertension. Hum. Mol. Genet. 12, 3277–3286 10.1093/hmg/ddg365 [DOI] [PubMed] [Google Scholar]
- Mahr A., Aberle H. (2006). The expression pattern of the Drosophila vesicular glutamate transporter: a marker protein for motoneurons and glutamatergic centers in the brain. Gene Expr. Patterns 6, 299–309 10.1016/j.modgep.2005.07.006 [DOI] [PubMed] [Google Scholar]
- Marom B., Heining E., Knaus P., Henis Y. I. (2011). Formation of stable homomeric and transient heteromeric bone morphogenetic protein (BMP) receptor complexes regulates Smad protein signaling. J. Biol. Chem. 286, 19287–19296 10.1074/jbc.M110.210377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marqués G., Musacchio M., Shimell M. J., Wünnenberg–Stapleton K., Cho K. W., O'Connor M. B. (1997). Production of a DPP activity gradient in the early Drosophila embryo through the opposing actions of the SOG and TLD proteins. Cell 91, 417–426 10.1016/S0092-8674(00)80425-0 [DOI] [PubMed] [Google Scholar]
- Marqués G., Bao H., Haerry T. E., Shimell M. J., Duchek P., Zhang B., O'Connor M. B. (2002). The Drosophila BMP type II receptor Wishful Thinking regulates neuromuscular synapse morphology and function. Neuron 33, 529–543 10.1016/S0896-6273(02)00595-0 [DOI] [PubMed] [Google Scholar]
- Marqués G., Haerry T. E., Crotty M. L., Xue M., Zhang B., O'Connor M. B. (2003). Retrograde Gbb signaling through the Bmp type 2 receptor wishful thinking regulates systemic FMRFa expression in Drosophila. Development 130, 5457–5470 10.1242/dev.00772 [DOI] [PubMed] [Google Scholar]
- Martin M., Iyadurai S. J., Gassman A., Gindhart J. G., Jr, Hays T. S., Saxton W. M. (1999). Cytoplasmic dynein, the dynactin complex, and kinesin are interdependent and essential for fast axonal transport. Mol. Biol. Cell 10, 3717–3728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe B. D., Marqués G., Haghighi A. P., Fetter R. D., Crotty M. L., Haerry T. E., Goodman C. S., O'Connor M. B. (2003). The BMP homolog Gbb provides a retrograde signal that regulates synaptic growth at the Drosophila neuromuscular junction. Neuron 39, 241–254 10.1016/S0896-6273(03)00426-4 [DOI] [PubMed] [Google Scholar]
- McCabe B. D., Hom S., Aberle H., Fetter R. D., Marqués G., Haerry T. E., Wan H., O'Connor M. B., Goodman C. S., Haghighi A. P. (2004). Highwire regulates presynaptic BMP signaling essential for synaptic growth. Neuron 41, 891–905 10.1016/S0896-6273(04)00073-X [DOI] [PubMed] [Google Scholar]
- McCaffrey M. W., Bielli A., Cantalupo G., Mora S., Roberti V., Santillo M., Drummond F., Bucci C. (2001). Rab4 affects both recycling and degradative endosomal trafficking. FEBS Lett. 495, 21–30 10.1016/S0014-5793(01)02359-6 [DOI] [PubMed] [Google Scholar]
- Moustakas A., Heldin C. H. (2005). Non-Smad TGF-beta signals. J. Cell Sci. 118, 3573–3584 10.1242/jcs.02554 [DOI] [PubMed] [Google Scholar]
- Moustakas A., Heldin C. H. (2009). The regulation of TGFbeta signal transduction. Development 136, 3699–3714 10.1242/dev.030338 [DOI] [PubMed] [Google Scholar]
- O'Connor–Giles K. M., Ho L. L., Ganetzky B. (2008). Nervous wreck interacts with thickveins and the endocytic machinery to attenuate retrograde BMP signaling during synaptic growth. Neuron 58, 507–518 10.1016/j.neuron.2008.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson U., Izumi H., Souchelnytskyi S., Itoh S., Grimsby S., Engström U., Heldin C-H., Funa K., ten Dijke P. (1998). The L45 loop in type I receptors for TGF-β family members is a critical determinant in specifying Smad isoform activation. FEBS Lett. 434, 83–87 10.1016/S0014-5793(98)00954-5 [DOI] [PubMed] [Google Scholar]
- Peterson A. J., Jensen P. A., Shimell M. J., Stefancsik R., Wijayatonge R., Herder R., Raftery L. A., O’Connor M. B. (2012). R-Smad Competition Controls Activin Receptor Output in Drosophila. PLoS ONE 7, e36548 10.1371/journal.pone.0036548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson J. M., Lee M., Kennedy E. L., Selleck S. B. (2003). Drosophila neuromuscular synapse assembly and function require the TGF-beta type I receptor saxophone and the transcription factor Mad. J. Neurobiol. 55, 134–150 10.1002/neu.10189 [DOI] [PubMed] [Google Scholar]
- Reddy S., Jin P., Trimarchi J., Caruccio P., Phillis R., Murphey R. K. (1997). Mutant molecular motors disrupt neural circuits in Drosophila. J. Neurobiol. 33, 711–723 [DOI] [PubMed] [Google Scholar]
- Rodal A. A., Blunk A. D., Akbergenova Y., Jorquera R. A., Buhl L. K., Littleton J. T. (2011). A presynaptic endosomal trafficking pathway controls synaptic growth signaling. J. Cell Biol. 193, 201–217 10.1083/jcb.201009052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekelsky J. J., Newfeld S. J., Raftery L. A., Chartoff E. H., Gelbart W. M. (1995). Genetic characterization and cloning of mothers against dpp, a gene required for decapentaplegic function in Drosophila melanogaster. Genetics 139, 1347–1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somsel Rodman J., Wandinger–Ness A. (2000). Rab GTPases coordinate endocytosis. J. Cell Sci. 113, 183–192 [DOI] [PubMed] [Google Scholar]
- Sönnichsen B., De Renzis S., Nielsen E., Rietdorf J., Zerial M. (2000). Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J. Cell Biol. 149, 901–914 10.1083/jcb.149.4.901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart B. A., Atwood H. L., Renger J. J., Wang J., Wu C-F. (1994). Improved stability of Drosophila larval neuromuscular preparations in haemolymph-like physiological solutions. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 175, 179–191 10.1007/BF00215114 [DOI] [PubMed] [Google Scholar]
- Sweeney S. T., Davis G. W. (2002). Unrestricted synaptic growth in spinster-a late endosomal protein implicated in TGF-beta-mediated synaptic growth regulation. Neuron 36, 403–416 10.1016/S0896-6273(02)01014-0 [DOI] [PubMed] [Google Scholar]
- Tsukazaki T., Chiang T. A., Davison A. F., Attisano L., Wrana J. L. (1998). SARA, a FYVE domain protein that recruits Smad2 to the TGFbeta receptor. Cell 95, 779–791 10.1016/S0092-8674(00)81701-8 [DOI] [PubMed] [Google Scholar]
- Wang X., Shaw W. R., Tsang H. T. H., Reid E., O'Kane C. J. (2007). Drosophila spichthyin inhibits BMP signaling and regulates synaptic growth and axonal microtubules. Nat. Neurosci. 10, 177–185 10.1038/nn1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman–Storer C. M., Holzbaur E. L. F. (1996). The product of the Drosophila gene, Glued, is the functional homologue of the p150Glued component of the vertebrate dynactin complex. J. Biol. Chem. 271, 1153–1159 10.1074/jbc.271.2.1153 [DOI] [PubMed] [Google Scholar]
- Watson F. L., Heerssen H. M., Bhattacharyya A., Klesse L., Lin M. Z., Segal R. A. (2001). Neurotrophins use the Erk5 pathway to mediate a retrograde survival response. Nat. Neurosci. 4, 981–988 10.1038/nn720 [DOI] [PubMed] [Google Scholar]
- Wharton K. A., Cook J. M., Torres–Schumann S., de Castro K., Borod E., Phillips D. A. (1999). Genetic analysis of the bone morphogenetic protein-related gene, gbb, identifies multiple requirements during Drosophila development. Genetics 152, 629–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano H., Lee F. S., Kong H., Chuang J., Arevalo J., Perez P., Sung C., Chao M. V. (2001). Association of Trk neurotrophin receptors with components of the cytoplasmic dynein motor. J. Neurosci. 21, RC125 (1–7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye H., Kuruvilla R., Zweifel L. S., Ginty D. D. (2003). Evidence in support of signaling endosome-based retrograde survival of sympathetic neurons. Neuron 39, 57–68 10.1016/S0896-6273(03)00266-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.