Abstract
It is widely believed that calorie restriction (CR) can extend the lifespan of model organisms and protect against aging-related diseases. A potential CR mimetic is resveratrol, which may have beneficial effects against numerous diseases such as type 2 diabetes, cardiovascular diseases, and cancer in tissue culture and animal models. However, resveratrol in its current form is not ideal as therapy, because even at very high doses it has modest efficacy and many downstream effects. Identifying the cellular targets responsible for the effects of resveratrol and developing target-specific therapies will be helpful in increasing the efficacy of this drug without increasing its potential adverse effects. A recent discovery suggests that the metabolic effects of resveratrol may be mediated by inhibiting cAMP phosphodiesterases (PDEs), particularly PDE4. Here, we review the current literature on the metabolic and cardiovascular effects of resveratrol and attempt to shed light on the controversies surrounding its action.
Keywords: resveratrol, PDE, AMPK, Sirt1, calorie restriction, diabetes
Calorie restriction and lifespan
It is said that in the 16th century, conquistador Juan Ponce de Leon searched for the fountain of youth in what is now Florida. Although he may not have been successful in this endeavor, our scientific understanding of aging, and possible strategies to extend lifespan, have increased dramatically over the past decade (Box 1). The most robust means of extending lifespan in mammals is CR, which typically involves reducing caloric intake by 30–40% while maintaining adequate nutrition [1]. CR not only extends the lifespan of a wide range of animals, but also protects against many aging-related diseases such as type 2 diabetes, cardiovascular diseases, cancer and neurodegeneration in rodents. More importantly, studies with rhesus monkeys indicate that long-term CR also decreases aging-related mortality and diseases in non-human primates [2]. However, it is not known whether CR delays the aging process in monkeys, because experimental animals have not yet reached their maximal lifespan. Despite these demonstrated benefits, CR is difficult to maintain for long periods in humans from western countries. Resveratrol [3], a polyphenol that is produced in plants in response to stress and is present in many plant-based foods, has demonstrated properties that mimic CR (Figure 1). This review highlights the biological functions of resveratrol and its implications for treatment of aging-related diseases.
Box 1. Genetic models of whole-body Sirt1 gain of function.
Although Sirt1 overexpression studies in tissue culture cells and transgenic mice overexpressing Sirt1 in a tissue-specific fashion provide valuable insight regarding Sirt1 activity, genetic models of whole-body Sirt1 gain of function are best suited to evaluating the physiological effect of activating Sirt1 with a small molecule. Three such models have been generated thus far. Transgenic mice in which additional copies of the Sirt1 transgene are expressed from its own promoter have been reported by two independent groups [11,84,94]. These transgenic mice, which have physiological patterns of Sirt1 expression, are more insulin sensitive partly due to increased adiponectin levels and decreased hepatic steatosis in obese mice. They also have a lower incidence of certain types of tumors and higher bone density. However, they are also more prone to develop atherosclerosis while on a pro-atherogenic diet [95]. Surprisingly, transgenic mice [84] that are congenic in C57BL6/J background have a reduced metabolic rate and body temperature on a regular diet and have the same amount of fat despite eating less than control mice. In Sirt1 knock-in mice [96], the Sirt1 gene was inserted into the β-actin locus. Unlike the transgenic mice, the knock-in mice had less fat and were more insulin sensitive. One caveat with the knock-in mice is that Sirt1 was overexpressed in fat but not in muscle or liver. It is possible that overexpression of Sirt1 from the β-actin locus impaired adipocyte differentiation during development [97].
Figure 1.
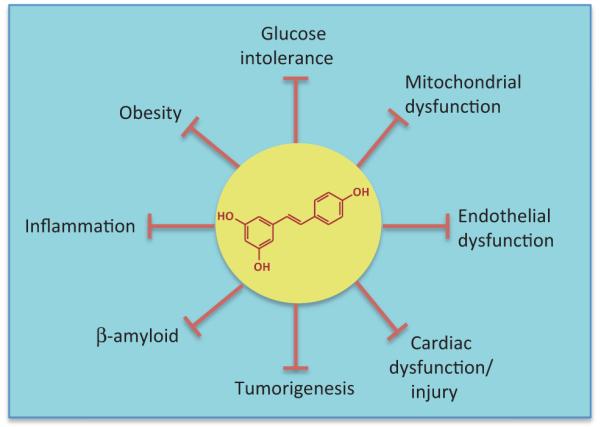
Resveratrol protects against a wide range of aging-related diseases. Resveratrol blocks pathological processes that contribute to aging-related diseases such as type 2 diabetes, cardiovascular diseases, and neurodegenerative diseases including Alzheimer’s diseases.
Controversy regarding the role of Sirt1 in longevity
Increased dosage of Sir2, a protein deacetylase that utilizes NAD+ as a cofactor, extends replicative lifespan in yeast [4]. Early reports suggested that CR-induced longevity in yeast [5], worms [6], and flies [7] is Sir2-dependent. However, subsequent studies have challenged the hypothesis that Sir2 mediates CR-mediated longevity. Sir2-null yeast respond to CR if an additional mutation (fob1) is introduced into it [8]. Moreover, when the genetic background was standardized and appropriate controls were used, CR-mediated extension of lifespan in both Caenorhabditis elegans and Drosophila was Sir2-independent, and over-expression of Sir2 increased lifespan in these organisms only modestly, if at all [9,10]. Increased dosage of Sirt1, the mammalian ortholog closest to Sir2, does not increase lifespan but improves healthy aging in mice [11].
Discovery of resveratrol as a potential CR mimetic
With the premise that activating Sirt1 may improve healthy aging, small molecule activators of Sirt1 were identified. In the initial screen, resveratrol was found to be the most potent Sirt1 activator [12]. The effect of resveratrol on the lifespan of lower eukaryotes is controversial; some studies have reported that it extends lifespan in a Sir2-dependent manner [12,13], whereas others report that resveratrol does not affect lifespan [14,15]. In Notho-branchius furzeri, a short-lived species of fish, resveratrol extends lifespan and delays cognitive decline [16]. In mice, long-term administration of resveratrol induces gene expression patterns that resemble those induced by CR and delays aging-related deterioration, but does not extend lifespan when the mice are fed a regular diet [17,18]. Resveratrol does not mimic all aspects of CR, such as decreases in heart rate and core body temperature [19]. In fact, resveratrol increases metabolic rate and fasting body temperature in mice fed a high-fat diet [20,21].
Resveratrol has antidiabetic effects in mice and humans
In mice fed a high-fat diet, resveratrol protects against obesity, type 2 diabetes, and premature death [20,22,23]. One of the most striking effects of resveratrol is induction of genes important for mitochondrial function, such as PGC-1α, a master regulator of mitochondrial biogenesis, leading to an increase in mitochondrial content and function as well as an increase in physical endurance[20,22,23]. Several clinical trials have been conducted to study the metabolic effects of resveratrol [24–26]. Although these trials have used different subject groups (e.g., obese healthy, type 2 diabetics or older adults with glucose intolerance) and different resveratrol doses (150 mg to 2 g per day), they suggest that resveratrol may improve insulin sensitivity and mimic aspects of CR. However, the therapeutic potential of resveratrol is diminished by the fact that it has only modest efficacy and that it has a very promiscuous target profile [27]. For chronic diseases that require lifelong treatment, such as type 2 diabetes or other aging-related diseases, lead compounds with pleiotropic effects and/or targets are difficult to optimize, with regard to both increasing efficacy and reducing adverse effects. Therefore, in order to translate resveratrol biology into clinical application, it will be helpful to identify the cellular targets of resveratrol that mediate the desired effects and develop therapies specific for those targets.
Resveratrol improves mitochondrial function and glucose tolerance in an AMPK-dependent manner
Subsequent to the report that resveratrol is a direct Sirt1 activator [12], it was discovered that the results of the in vitro screen might have been an artifact derived from the use of a fluorescent substrate [27–30]. The Sirt1 dependency of some resveratrol effects [20,31,32] suggested that resveratrol may indirectly activate Sirt1 as a downstream event of some unknown pathway.
Evidence is mounting that a key mediator of the metabolic effects of resveratrol may be the fuel-sensing AMP-activated kinase (AMPK), which is activated by conditions that increase AMP/ATP and ADP/ATP ratios such as physical exercise, ischemia, and glucose deprivation [33]. AMPK activity is increased by CR [34,35] or the antidiabetic drug metformin [36]. Resveratrol indirectly activates AMPK [23,37,38], and activation of AMPK by other means has been shown to reduce fat accumulation and increase glucose tolerance, insulin sensitivity, mitochondrial biogenesis, and physical endurance [33].
AMPK activity requires one of at least two AMPK kinases: LKB1 or calcium/calmodulin-dependent kinase kinase β (CamKKβ), which phosphorylate T172 in the T-loop of AMPK [33]. Activation of AMPK via energy depletion is thought to be dependent on LKB1 and activation of AMPK via increased intracellular Ca2+ is dependent on CamKKβ [33]. Resveratrol-induced activation of AMPK provides a plausible mechanism for how resveratrol could activate Sirt1 indirectly, because AMPK is known to increase the NAD+ level, which promotes the deacetylation of Sirt1 substrates [22,39–41]. This hypothesis is strengthened by the observation that AMPK is required for resveratrol to produce metabolic benefits such as increased metabolic rate and protection against diet-induced obesity and type 2 diabetes, and to increase NAD+ levels and deacetylation of Sirt1 substrates [22,41].
Resveratrol activates AMPK by inhibiting PDEs and increasing cAMP signaling
How does resveratrol, at a physiologically relevant concentration (low micromolar range), activate AMPK, [42]? One obvious possibility is that it may decrease ATP. Indeed, resveratrol is a known inhibitor of the F1F0-ATPase/ATP synthase [43]. Resveratrol has been shown to decrease intracellular ATP levels and activate AMPK in an AMP/ ATP ratio-dependent manner, but this has been demonstrated only at high concentrations (100–300 μM) [44]. There is no consensus regarding this effect over a lower concentration range. One study found that resveratrol decreases ATP at 50 μM but not at 25 μM [45], whereas others found that resveratrol at 40–100 μM does not decrease ATP [46,47] (Park et al., unpublished). These inconsistencies among the groups may be due to differences in cell type and/or condition. Resveratrol, at both low and high doses, does not change ATP or AMP levels in mouse tissues [45] and can activate AMPK at concentrations of less than 10 μM [37,48,49], at which there is no evidence of changes in nucleotide concentrations. However, the possibility that resveratrol can transiently change nucleotide concentrations thereby contributing to its overall activation of AMPK cannot be excluded.
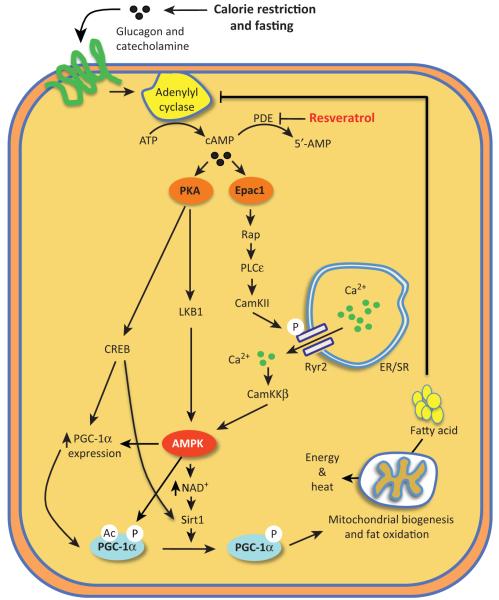
Because resveratrol mimics some aspects of CR, it is possible that CR and resveratrol trigger similar pathways. Fasting and exercise release glucagon and catecholamines, which stimulate adenylylate cyclases (ACs) and cAMP production (Figure 2). Indeed, resveratrol increases cAMP levels in myotubes at concentrations of less than 10 μM [21], which corresponds to the concentration range effective for AMPK activation. In most cells, intracellular cAMP signaling is mediated by two groups of effectors that bind cAMP:protein kinase A (PKA) and the cAMP-regulated guanine nucleotide exchange factors (cAMPGEFs) Epac1 and Epac2 [50]. The role of the two cAMP effectors in resveratrol action depends on the cell type and the conditions used. In HeLa cells, which do not express LKB1, resveratrol activates AMPK in an entirely Epac1-dependent manner [21]. Epac1 increases intracellular Ca2+ levels and activates the CaMKKβ–AMPK pathway via phospholipase C and the ryanodine receptor CaCa2+ -release channel. In myotubes, which express both LKB1 and CaMKKβ, the Epac1–CaMKKβ pathway is required for resveratrol to fully activate AMPK. In these cells, a role of PKA in resveratrol-mediated AMPK activation is also likely, but may be more complicated. PKA is known to stabilize and increase the activity of LKB1 by phosphorylating S431 [51]. However, S431 is not required for the ability of LKB1 to activate AMPK when LKB1 is overexpressed [52] and it has been shown that PKA inhibits AMPK in adipocytes [53].
Figure 2.
Resveratrol mimics calorie restriction by inhibiting cAMP phosphodiesterases (PDEs). Food deprivation and exercise increase the levels of glucagon and catecholamines, which bind to their receptors and increase cAMP production by activating adenylate cyclase. Resveratrol increases cAMP levels, not by increasing cAMP production, but by inhibiting cAMP PDEs, which hydrolyze cAMP to AMP. Protein kinase A (PKA) and Epac, two effectors of cAMP signaling, activate parallel pathways that lead to AMP-activated kinase (AMPK) activation. In addition, exercise can increase AMPK activity in skeletal muscle via a decrease in ATP and also via contraction-associated Ca2+ release (not shown). Transcription factor CREB (cAMP response element binding protein), which is activated by PKA phosphorylation, induces transcription of PGC-1α [98] and Sirt1 [99], which are two important mediators of the beneficial effects of resveratrol on mitochondrial function. In addition to improving mitochondrial function, activation of AMPK decreases glucose production in the liver and increases glucose uptake in peripheral tissues such as skeletal muscle. Although PKA signaling increases hepatic glucose production, it also inhibits SREBP-1c and, as a result, inhibits synthesis of triglyceride and development of hepatic steatosis, which may have a dominant effect on insulin sensitivity in the obese state. This may explain why the metabolic benefits of resveratrol are largely limited to animals fed a high-fat diet.
Resveratrol is a cAMP phosphodiesterase inhibitor
Intracellular levels of cAMP are determined by the activities of adenyl cyclases (ACs) and cyclic nucleotide PDEs [54], which hydrolyze cAMP or cGMP to AMP or GMP, respectively. Resveratrol has no effect on AC activity, but inhibits the activity of PDE1, PDE3, and PDE4 but not PDE2 or PDE5 [21]. The effect of resveratrol on the remaining six PDE family members is unknown. Photo-affinity crosslinking and analysis of the kinetics of cAMP hydrolysis indicate that resveratrol inhibits PDEs by binding to the catalytic site and competing with cAMP [21].
In myotubes, PDE4 constitutes most of the cAMP PDE activity [21]. Genetic evidence that resveratrol activates AMPK by inhibiting PDEs comes from the observation that in myotubes expressing an inhibitor-resistant mutant PDE4, resveratrol does not activate AMPK [21]. If the metabolic effects of resveratrol are mediated by PDE inhibition, PDE4 inhibitors should replicate the metabolic effects of resveratrol in skeletal muscle. Indeed, the PDE4-specific inhibitor rolipram activates AMPK and increases mitochondrial biogenesis and function in skeletal muscle and improves physical endurance [21]. In mice fed a high-fat diet, rolipram increases metabolic rate, protects against obesity, and improves glucose tolerance [21].
Metabolic effects of PDE inhibition
Other studies using PDE inhibitors and PDE knockout (KO) mice also illustrate the metabolic benefits of PDE inhibition. PDE3 and PDE4 inhibitors apparently affect distinct cAMP pools that control activation of AMPK in rodent adipocytes [55] and increase insulin secretion from rat pancreatic islets and cultured INS-1 rat insulinoma cells [56]. Pancreatic β-cell PDE3B is an important regulator of Ca2+- and cAMP-stimulated exocytosis of insulin [57], and in pancreatic islets from wild-type (WT) and PDE3B KO mice, glucose, alone or in combination with GLP-1 (Glucagon-like peptide-1), increased insulin secretion to a greater extent from KO islets than from WT islets [58]. Rolipram increases plasma GLP-1 [21] and enhances glucose-induced release of GLP-1 from GLP-1-producing GLUTag cells [59]. Compared with WT littermates, PDE4B KO mice are leaner, and have smaller fat pads and adipocytes and decreased serum leptin levels [60]. On a high-fat diet, KO mice exhibit increased serum adiponectin, with reduced TNF-α expression and reduced macrophage infiltration in white adipose tissue (WAT). Moreover, PDE4 inhibitors are potent anti-inflammatory agents [61]. Although the mechanisms are not entirely clear, the anti-inflammatory effect of PDE4 inhibition may be related to the ability of cAMP-induced signals to inter-fere with the function of the proinflammatory transcription factor nuclear factor-κB.[62] Given the current concept that obesity and type 2 diabetes reflect chronic systemic inflammation [63], these results, taken together, suggest that, by inhibiting PDE4, resveratrol may produce metabolic benefits partly by suppressing inflammation in WAT.
Cardiovascular benefits of resveratrol
The leading cause of mortality in individuals with type 2 diabetes is cardiovascular disease. Independent of its antidiabetic effects, there is extensive literature on the protective effects of resveratrol against cardiovascular diseases [64] (Figure 3). For example, in patients with existing coronary heart disease, acute resveratrol supplementation improves flow-mediated vasodilation, and chronic treatment of patients with resveratrol has been shown to reduce blood pressure [65]. Although the effects of resveratrol may be multifactorial, preclinical work has shown that resveratrol stimulates endothelium-dependent relaxation [66,67] by promoting the bioavailability of nitric oxide (NO) to cause vasodilation as well as by reducing expression of molecules that promote vasoconstriction. However, it is unknown whether the AMPK-mediated vasodilation is due to the well-established fact that AMPK can directly phosphorylate and activate endothelial nitric oxide synthase (eNOS) to increase NO production or whether other mediators such as PKA are involved. However, inhibition of AMPK prevents resveratrol-mediated improvements in endothelium-dependent vasodilation in aortic rings [67], suggesting that AMPK is critical for the ability of resveratrol to increase vasodilation.
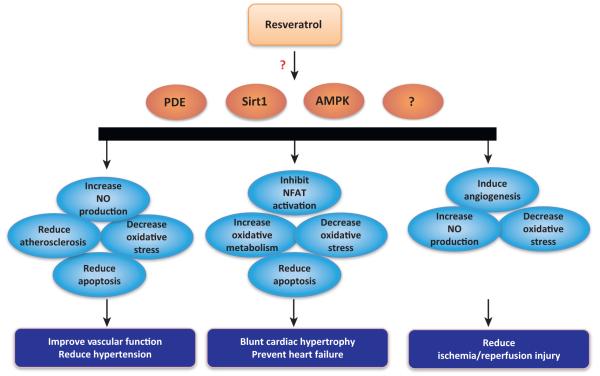
Figure 3.
Cardiovascular effects of resveratrol. Resveratrol and/or Sirt1/AMP-activated kinase (AMPK) activation and/or phosphodiesterase (PDE) inhibition have been shown to improve vascular function and reduce hypertension via increased nitric oxide (NO) production, reduced atherosclerosis, decreased oxidative stress, and reduced apoptosis. Decreased oxidative stress and reduced apoptosis also play a role in blunting cardiac hypertrophy and preventing heart failure. Resveratrol also lessens the severity of these cardiac syndromes by reducing activation of nuclear factor of activated T cells (NFAT)-mediated and increasing oxidative metabolism. Resveratrol and/or Sirt1 activation has also been shown to reduce ischemia/reperfusion injury by inducing angiogenesis, increasing NO production, and decreasing oxidative stress.
In addition to preventing cardiac hypertrophy by reducing hemodynamic load [64], there is evidence that resveratrol can act directly on cardiomyocytes to lessen hypertrophic growth [68], even in the presence of hypertension [69]. Although resveratrol has been shown to inhibit cardiac hypertrophy via AMPK [68], others have shown that resveratrol prevented left ventricular hypertrophy (LVH) in mice fed a diet high in fat and sucrose; this effect was AMPK independent and may have been direct or secondary to the resolution of metabolic abnormalities in these mice [70].
Resveratrol also improves post-ischemic functional recovery [66] and reduces myocardial infarct size in model systems [71]. Similarly, resveratrol reduced infarct size and improved cardiac function along with decreasing the incidence of ventricular arrhythmias in rats subjected to permanent left anterior descending artery occlusion in vivo [72]. Moreover, in rat models of both type 1 and type 2 diabetes, resveratrol significantly improved cardiac function during reperfusion following ischemia in ex vivo perfused hearts [73,74].
Although considerable research effort has gone into defining the precise mechanism of action of resveratrol on the cardiovascular system in various disease states, the effects of resveratrol are likely to be pleiotropic and multiple cellular changes in various organs may be involved. Nevertheless, these findings indicate that resveratrol may be beneficial for a diverse array of cardiovascular diseases.
Does resveratrol activate Sirt1 in vivo?
Resveratrol does not activate Sirt1 against native substrates in vitro, but it is generally believed that it increases Sirt1 activity above its basal level in vivo. However, this notion is based solely on the observation that, in cells treated with resveratrol, Sirt1 substrates are less acetylated[12,20]. The acetylation state of any protein is determined not only by the rate of deacetylation (e.g., by Sirt1), but also by the rate of acetylation by an acetyl transferase such as p300 [75]. However, because p300 is also inhibited by AMPK [76], it is possible that resveratrol decreases acetylation of Sirt1 substrates, at least in part or even entirely in some contexts, by decreasing acetylation rather than by increasing Sirt1-mediated deacetylation (Figure 4). The observation that the effects of resveratrol are diminished in Sirt1 KO or knock-down models [3] may indicate that Sirt1 basal activity plays an important role in the effects of resveratrol, not necessarily that it is activated by resveratrol (Figure 2). Developing a direct marker of Sirt1 activity will be required to answer this question.
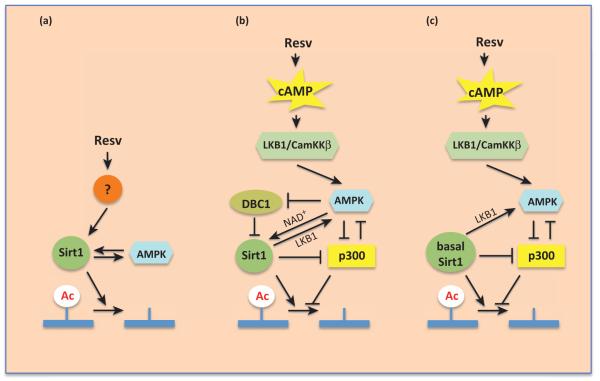
Figure 4.
Understanding how resveratrol regulates AMP-activated kinase (AMPK) and Sirt1. The acetylation state of a Sirt1 substrate is determined not only by Sirt1 but also by acetyl transferases such as p300. Sirt1, p300, and AMPK regulate each other, making knock-down or knockout approaches difficult to interpret. (a) A model in which resveratrol activates Sirt1 before AMPK. (b) A model in which resveratrol activates Sirt1 via AMPK. (c) A model in which resveratrol activates AMPK but does not activate Sirt1. In this model, basal Sirt1 activity is still required for resveratrol to have an effect.
AMPK-Sirt1 hierarchy
Numerous groups have shown that the metabolic effects of resveratrol, including deacetylation of Sirt1 substrates, do not occur in AMPK-deficient cells or mice, indicating that AMPK is upstream of Sirt1 [22,41,45]. It is generally assumed that AMPK increases Sirt1 activity [39,40], but the mechanism is unclear. + Although NAD levels rise in response to resveratrol in an AMPK-dependent manner[22,41], this is relevant + to Sirt1 activity only if the NAD level in the basal state is rate limiting for Sirt1 activity, and this has not been proven. Another manner by which AMPK may activate Sirt1 is by AMPK-dependent dissociation of Sirt1 from its inhibitor DBC1 [77].
The role of Sirt1 in the action of resveratrol has been difficult to study due to the severe developmental abnormalities and early death of germline Sirt1 KO mice[78,79]. To circumvent these problems, adult-inducible Sirt1 KO mice that exhibit efficient deletion of Sirt1 in various tissues were generated [45]. Studies with these mice showed that, with low-dose resveratrol (25–30 mg/kg/day), the increase in mitochondrial content and function in obese mice is Sirt1 dependent, but with high dose resveratrol (215–230 mg/kg/day) it is Sirt1 independent. Activation of AMPK with low-dose resveratrol is Sirt1 dependent, but with high-dose resveratrol it is Sirt1-independent [45]. Based on these findings, it has been proposed that, at low dose, Sirt1 is upstream of AMPK and activates AMPK in a feed-forward fashion, possibly by deacetylating LKB1 [45,80,81]. Indeed, it has been reported that resveratrol does not activate AMPK in LKB1-deficient mouse embryo fibroblasts [68] or in HeLa cells, which are LKB1 deficient [81]. However, others have shown that LKB1 is not always required for resveratrol to activate AMPK, because resveratrol increases Ca2+ and activates AMPK via CaMKKβ in HeLa cells, even at a low dose (10 μM) [21,47]. In addition, resveratrol activates AMPK in Sirt1 KO mouse embryo fibroblasts [22]. These discrepancies may be due to differences in cell type or condition before resveratrol treatment. Immortalized cell lines, particularly if they have been in a confluent state, often have high basal AMPK activity and/or fail to respond to moderate doses of resveratrol. Synchronizing cells with 0.2% fetal calf serum overnight before addition of resveratrol often improves sensitivity to resveratrol [21].
Basal Sirt1 activity may also play a role in AMPK activation without being upstream of AMPK in the resveratrol response. Because Sirt1 is an inhibitor of p300 [82] and p300 is an AMPK inhibitor [83], AMPK may be in asuppressed state in Sirt1 KO cells. Sirt1 may also play a role in AMPK activity in a more indirect manner. For example, Sirt1 regulates secretion of AMPK-activating adipokines such as adiponectin [84], leading to the possibility that Sirt1 KO cells may have altered tissue crosstalk that regulates AMPK activity. Additional work will be required to determine the significance of the dose-dependent effect of resveratrol on AMPK activity.
The antidiabetic effect of resveratrol is AMPK dependent but Sirt1 independent
One of the primary indications for resveratrol is type 2 diabetes. Although overexpression of Sirt1 is known to improve glucose tolerance, the antidiabetic effect of resveratrol is AMPK dependent [22] but Sirt1-independent [45] (Figure 5). Another primary indication for resveratrol is fatty liver [23], a condition that is associated with obesity and can lead to cirrhosis. Here again, overexpression of Sirt1 is known to lower hepatic triglyceride, but resveratrol-induced reduction in hepatic triglyceride is Sirt1 independent [45]. These observations indicate that Sirt1 and resveratrol may act in a parallel but independent manner for these indications (Figure 5). The observation that the glucose-lowering effect of resveratrol is Sirt1 independent even though the mitochondrial effects are Sirt1 dependent reflects the conflicted relationship between type 2 diabetes and skeletal muscle mitochondrial function [85]. Increasing mitochondrial function by overexpressing PGC-1α in skeletal muscle paradoxically worsens glucose tolerance in young obese mice [86], but improves glucose tolerance in old mice [87].
Figure 5.
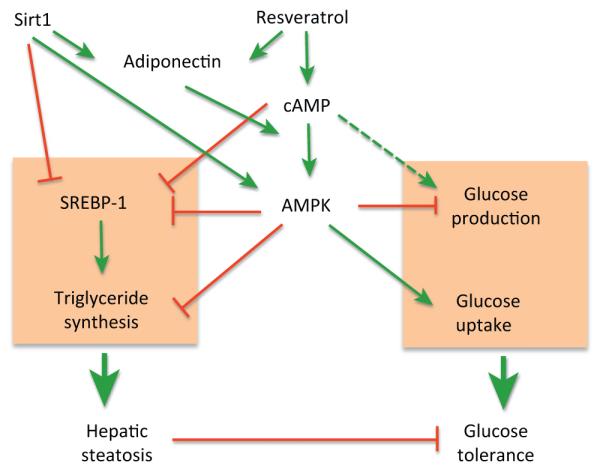
Proposed model of AMP-activated kinase (AMPK) dependence and Sirt1 independence of the antidiabetic effects of resveratrol. Resveratrol increases adiponectin [100], cAMP signaling [21], and AMPK activity [21], which contribute to the inhibition of SREBP-1 [101], the transcription factor [102] that promotes hepatic triglyceride synthesis and development of hepatic steatosis, a known instigator of insulin resistance [103]. AMPK also suppresses hepatic glucose production and stimulates glucose uptake in skeletal muscle [33]. It should be noted that protein kinase A (PKA) also increases hepatic glucose production (dotted line). In a parallel manner, Sirt1 also inhibits SREBP-1 [104] and stimulates AMPK activity and adiponectin production [84].
Intracellular concentration of resveratrol
One conundrum in the biology of resveratrol is that the in vitro concentration of resveratrol required for its diverse array of effects is 5–50 μM, yet the serum concentration of unmodified resveratrol in animals is no more than 3 μM [42]. The serum level of unmodified resveratrol is low because most resveratrol in serum is conjugated to glucuronide or sulfate [88]. One possible explanation for the efficacy of resveratrol at such a low serum concentration is that the intracellular levels of lipophilic compounds such as resveratrol could be significantly higher than serum levels. Moreover, tissues such as skeletal muscle can express glucuronidases [89], which can potentially remove the conjugates and increase the tissue levels of resveratrol. The concentration of resveratrol to which the liver is exposed before its hepatic metabolism may be significantly higher than that in systemic circulation. Because the metabolic functions of almost all tissues are affected by hepatic status (e.g., hepatic steatosis), it is likely that some of the effects of resveratrol in peripheral tissues may be indirect effects of the resveratrol-induced changes in the liver.
Concluding remarks
In addition to the metabolic and cardiovascular effects, resveratrol treatment has an array of other benefits that may contribute to its overall CR-mimetic effects; most notably, anticancer and anti-inflammation effects [3]. The targets that mediate these effects have not been identified. However, PDE4 inhibition also induces expression of the cell cycle inhibitors p21 and p27 and has anticancer properties [90]. The well known anti-inflammatory effects of PDE4 inhibition [61] may play a role in the overall anti-inflammatory effects of resveratrol. However, it is possible that numerous other potential resveratrol targets such as cyclooxygenases 1 [91] and 2 [92], which catalyze the first step in the synthesis of prostaglandins, may also be involved.
Since the beneficial effects of resveratrol were first recognized, much progress has been made in our understanding of the underlying biology. However, fundamental questions such as what targets mediate the effects of resveratrol and whether resveratrol affects Sirt1 activity remain controversial. The biochemical pathways that have been proposed to mediate the beneficial effects of resveratrol are highly interconnected (Figures 2 and 4). Therefore, traditional genetic strategies such as knock down or KO, which can lead to an array of indirect effects, not only within a cell but on tissue crosstalk, have limited utility in pinpointing the role of resveratrol in each pathway. This means that the precise mechanism of resveratrol action may be difficult to elucidate with absolute clarity. However, the timing of Sirt1 and AMPK activation may provide a clue to the AMPK-Sirt1 epistasis or hierarchy. Although the timing differences between resveratrol-mediated activation of AMPK and Sirt1 have not been carefully analyzed, resveratrol-mediated increase in cAMP precedes both [21].
The next chapter in resveratrol biology that may be more fruitful, at least for clinical application, is to reconstruct the beneficial effects of resveratrol in humans with small molecule inhibitors of proteins such as PDE4 that are directly targeted by resveratrol. This new chapter may have already begun. Shortly after the finding that inhibiting PDE4 inhibition has an antidiabetic effect [21], it was reported that the PDE4 inhibitor roflumilast, which was recently approved by the US FDA for treatment of chronic obstructive pulmonary disease, unexpectedly lowered fasting glucose by more than 1 mM in individuals with type 2 diabetes [93]. This provides a proof of principle that identifying, and selectively targeting, the proteins that mediate resveratrolaction may be an effective therapeutic strategy, not only for type 2 diabetes but also for other aging-related diseases.
Acknowledgments
This work was supported by the Intramural Research Program, the National Heart Lung and Blood Institute, the National Institutes of Health, and the Canadian Institutes of Health Research. We thank Dr Alexandra Brown for helpful comments and manuscript preparation.
References
- 1.McCay CM, et al. The effect of retarded growth upon the lebgth of life span and ultimate body size. J. Nutr. 1935;10:63–79. [PubMed] [Google Scholar]
- 2.Colman RJ, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baur JA. Resveratrol, sirtuins, and the promise of a DR mimetic. Mech. Ageing Dev. 2010;131:261–269. doi: 10.1016/j.mad.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guarente L. Franklin H. Epstein Lecture: sirtuins, aging, and medicine. N. Engl. J. Med. 2011;364:2235–2244. doi: 10.1056/NEJMra1100831. [DOI] [PubMed] [Google Scholar]
- 5.Lin SJ, et al. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Tissenbaum HA. Overlapping and distinct functions for a Caenorhabditis elegans SIR2 and DAF-16/FOXO. Mech. Ageing Dev. 2006;127:48–56. doi: 10.1016/j.mad.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc. Natl. Acad. Sci. U.S.A. 2004;101:15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaeberlein M, et al. Sir2-independent life span extension by calorie restriction in yeast. PLoS Biol. 2004;2:e296. doi: 10.1371/journal.pbio.0020296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burnett C, et al. Absence of effects of Sir2 overexpression on lifespan in C. elegans and Drosophila. Nature. 2011;477:482–485. doi: 10.1038/nature10296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Viswanathan M, Guarente L. Regulation of Caenorhabditis elegans lifespan by sir-2.1 transgenes. Nature. 2011;477:E1–E2. doi: 10.1038/nature10440. [DOI] [PubMed] [Google Scholar]
- 11.Herranz D, et al. Sirt1 improves healthy ageing and protects from metabolic syndrome-associated cancer. Nat. Commun. 2010;1:1–8. doi: 10.1038/ncomms1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howitz KT, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 13.Wood JG, et al. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 14.Bass TM, et al. Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditis elegans. Mech. Ageing Dev. 2007;128:546–552. doi: 10.1016/j.mad.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Kaeberlein M, et al. Substrate-specific activation of sirtuins by resveratrol. J. Biol. Chem. 2005;280:17038–17045. doi: 10.1074/jbc.M500655200. [DOI] [PubMed] [Google Scholar]
- 16.Valenzano DR, et al. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr. Biol. 2006;16:296–300. doi: 10.1016/j.cub.2005.12.038. [DOI] [PubMed] [Google Scholar]
- 17.Pearson KJ, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barger JL, et al. A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS ONE. 2008;3:e2264. doi: 10.1371/journal.pone.0002264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayers JR, et al. Resveratrol treatment in mice does not elicit the bradycardia and hypothermia associated with calorie restriction. FASEB J. 2009;23:1032–1040. doi: 10.1096/fj.08-115923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lagouge M, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Park SJ, et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell. 2012;148:421–433. doi: 10.1016/j.cell.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Um JH, et al. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes. 2010;59:554–563. doi: 10.2337/db09-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baur JA, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brasnyo P, et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br. J. Nutr. 2011;106:383–389. doi: 10.1017/S0007114511000316. [DOI] [PubMed] [Google Scholar]
- 25.Crandall JP, et al. Pilot study of resveratrol in older adults with impaired glucose tolerance. J. Gerontol. A: Biol. Sci. Med. Sci. 2012 doi: 10.1093/gerona/glr235. http://dx.doi.org/10.1093/gerona/glr235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Timmers S, et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14:612–622. doi: 10.1016/j.cmet.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pacholec M, et al. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J. Biol. Chem. 2010;285:8340–8351. doi: 10.1074/jbc.M109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borra MT, et al. Mechanism of human SIRT1 activation by resveratrol. J. Biol. Chem. 2005;280:17187–17195. doi: 10.1074/jbc.M501250200. [DOI] [PubMed] [Google Scholar]
- 29.Kaeberlein M, et al. Substrate-specific activation of sirtuins by resveratrol. J. Biol. Chem. 2005;280:17038–17045. doi: 10.1074/jbc.M500655200. [DOI] [PubMed] [Google Scholar]
- 30.Beher D, et al. Resveratrol is not a direct activator of SIRT1 enzyme activity. Chem. Biol. Drug Des. 2009;74:619–624. doi: 10.1111/j.1747-0285.2009.00901.x. [DOI] [PubMed] [Google Scholar]
- 31.Breen DM, et al. Stimulation of muscle cell glucose uptake by resveratrol through sirtuins and AMPK. Biochem. Biophys. Res. Commun. 2008;374:117–122. doi: 10.1016/j.bbrc.2008.06.104. [DOI] [PubMed] [Google Scholar]
- 32.Csiszar A, et al. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2009;297:H13–H20. doi: 10.1152/ajpheart.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hardie DG, et al. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edwards AG, et al. Life-long caloric restriction elicits pronounced protection of the aged myocardium: a role for AMPK. Mech. Ageing Dev. 2010;131:739–742. doi: 10.1016/j.mad.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palacios OM, et al. Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1alpha in skeletal muscle. Aging (Albany N. Y.) 2009;1:771–783. doi: 10.18632/aging.100075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou G, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc. Natl. Acad. Sci. U.S.A. 2007;104:7217–7222. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zang M, et al. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes. 2006;55:2180–2191. doi: 10.2337/db05-1188. [DOI] [PubMed] [Google Scholar]
- 39.Canto C, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fulco M, et al. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev. Cell. 2008;14:661–673. doi: 10.1016/j.devcel.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Canto C, et al. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab. 2010;11:213–219. doi: 10.1016/j.cmet.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crowell JA, et al. Resveratrol-associated renal toxicity. Toxicol. Sci. 2004;82:614–619. doi: 10.1093/toxsci/kfh263. [DOI] [PubMed] [Google Scholar]
- 43.Zheng J, Ramirez VD. Inhibition of mitochondrial proton F0F1-ATPase/ATP synthase by polyphenolic phytochemicals. Br. J. Pharmacol. 2000;130:1115–1123. doi: 10.1038/sj.bjp.0703397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hawley SA, et al. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab. 2010;11:554–565. doi: 10.1016/j.cmet.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Price NL, et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15:675–690. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suchankova G, et al. Concurrent regulation of AMP-activated protein kinase and SIRT1 in mammalian cells. Biochem. Biophys. Res. Commun. 2009;378:836–841. doi: 10.1016/j.bbrc.2008.11.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vingtdeux V, et al. AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-beta peptide metabolism. J. Biol. Chem. 2010;285:9100–9113. doi: 10.1074/jbc.M109.060061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park CE, et al. Resveratrol stimulates glucose transport in C2C12 myotubes by activating AMP-activated protein kinase. Exp. Mol. Med. 2007;39:222–229. doi: 10.1038/emm.2007.25. [DOI] [PubMed] [Google Scholar]
- 49.Feige JN, et al. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab. 2008;8:347–358. doi: 10.1016/j.cmet.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 50.Gloerich M, Bos JL. Epac: defining a new mechanism for cAMP action. Annu. Rev. Pharmacol. Toxicol. 2010;50:355–375. doi: 10.1146/annurev.pharmtox.010909.105714. [DOI] [PubMed] [Google Scholar]
- 51.Shelly M, et al. LKB1/STRAD promotes axon initiation during neuronal polarization. Cell. 2007;129:565–577. doi: 10.1016/j.cell.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 52.Fogarty S, Hardie DG. C-terminal phosphorylation of LKB1 is not required for regulation of AMP-activated protein kinase, BRSK1, BRSK2, or cell cycle arrest. J. Biol. Chem. 2009;284:77–84. doi: 10.1074/jbc.M806152200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Djouder N, et al. PKA phosphorylates and inactivates AMPKalpha to promote efficient lipolysis. EMBO J. 2010;29:469–481. doi: 10.1038/emboj.2009.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Conti M, Beavo J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu. Rev. Biochem. 2007;76:481–511. doi: 10.1146/annurev.biochem.76.060305.150444. [DOI] [PubMed] [Google Scholar]
- 55.Omar B, et al. Regulation of AMP-activated protein kinase by cAMP in adipocytes: roles for phosphodiesterases, protein kinase B, protein kinase A, Epac and lipolysis. Cell. Signal. 2009;21:760–766. doi: 10.1016/j.cellsig.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Waddleton D, et al. Phosphodiesterase 3 and 4 comprise the major cAMP metabolizing enzymes responsible for insulin secretion in INS-1 (832/13) cells and rat islets. Biochem. Pharmacol. 2008;76:884–893. doi: 10.1016/j.bcp.2008.07.025. [DOI] [PubMed] [Google Scholar]
- 57.Walz HA, et al. Beta-cell PDE3B regulates Ca2+-stimulated exocytosis of insulin. Cell. Signal. 2007;19:1505–1513. doi: 10.1016/j.cellsig.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 58.Choi YH, et al. Alterations in regulation of energy homeostasis in cyclic nucleotide phosphodiesterase 3B-null mice. J. Clin. Invest. 2006;116:3240–3251. doi: 10.1172/JCI24867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ong WK, et al. The role of the PDE4D cAMP phosphodiesterase in the regulation of glucagon-like peptide-1 release. Br. J. Pharmacol. 2009;157:633–644. doi: 10.1111/j.1476-5381.2009.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang R, et al. Reduced adiposity and high-fat diet-induced adipose inflammation in mice deficient for phosphodiesterase 4B. Endocrinology. 2009;150:3076–3082. doi: 10.1210/en.2009-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Torphy TJ. Phosphodiesterase isozymes: molecular targets for novel antiasthma agents. Am. J. Respir. Crit. Care Med. 1998;157:351–370. doi: 10.1164/ajrccm.157.2.9708012. [DOI] [PubMed] [Google Scholar]
- 62.Gerlo S, et al. Cyclic AMP: a selective modulator of NF-kappaB action. Cell. Mol. Life Sci. 2011;68:3823–3841. doi: 10.1007/s00018-011-0757-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 64.Dolinsky VW, Dyck JR. Calorie restriction and resveratrol in cardiovascular health and disease. Biochim. Biophys. Acta. 2011;1812:1477–1489. doi: 10.1016/j.bbadis.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 65.Timmers S, et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14:612–622. doi: 10.1016/j.cmet.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goh SS, et al. The red wine antioxidant resveratrol prevents cardiomyocyte injury following ischemia-reperfusion via multiple sites and mechanisms. Antioxid. Redox Signal. 2007;9:101–113. doi: 10.1089/ars.2007.9.101. [DOI] [PubMed] [Google Scholar]
- 67.Karuppagounder SS, et al. Dietary supplementation with resveratrol reduces plaque pathology in a transgenic model of Alzheimer’s disease. Neurochem. Int. 2009;54:111–118. doi: 10.1016/j.neuint.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chan AY, et al. Resveratrol inhibits cardiac hypertrophy via AMP-activated protein kinase and Akt. J. Biol. Chem. 2008;283:24194–24201. doi: 10.1074/jbc.M802869200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dolinsky VW, et al. Resveratrol prevents the prohypertrophic effects of oxidative stress on LKB1. Circulation. 2009;119:1643–1652. doi: 10.1161/CIRCULATIONAHA.108.787440. [DOI] [PubMed] [Google Scholar]
- 70.Qin F, et al. The polyphenols resveratrol and s17834 prevent the structural and functional sequelae of diet-induced metabolic heart disease in mice. Circulation. 2012;125:1757–1764. doi: 10.1161/CIRCULATIONAHA.111.067801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dai JM, et al. SIRT1 interacts with p73 and suppresses p73-dependent transcriptional activity. J. Cell. Physiol. 2007;210:161–166. doi: 10.1002/jcp.20831. [DOI] [PubMed] [Google Scholar]
- 72.Chen D, et al. Tissue-specific regulation of SIRT1 by calorie restriction. Genes Dev. 2008;22:1753–1757. doi: 10.1101/gad.1650608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lekli I, et al. Protective mechanisms of resveratrol against ischemia-reperfusion-induced damage in hearts obtained from Zucker obese rats: the role of GLUT-4 and endothelin. Am. J. Physiol. Heart Circ. Physiol. 2008;294:H859–H866. doi: 10.1152/ajpheart.01048.2007. [DOI] [PubMed] [Google Scholar]
- 74.Thirunavukkarasu M, et al. Resveratrol alleviates cardiac dysfunction in streptozotocin-induced diabetes: role of nitric oxide, thioredoxin, and heme oxygenase. Free Radic. Biol. Med. 2007;43:720–729. doi: 10.1016/j.freeradbiomed.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goodman RH, Smolik S. CBP/p300 in cell growth, transformation, and development. Genes Dev. 2000;14:1553–1577. [PubMed] [Google Scholar]
- 76.Yang W, et al. Regulation of transcription by AMP-activated protein kinase: phosphorylation of p300 blocks its interaction with nuclear receptors. J. Biol. Chem. 2001;276:38341–38344. doi: 10.1074/jbc.C100316200. [DOI] [PubMed] [Google Scholar]
- 77.Nin V, et al. Role of deleted in breast cancer 1 (DBC1) in SIRT1 activation induced by protein kinase A and AMP activated protein kinase. J. Biol. Chem. 2012;287:23489–23501. doi: 10.1074/jbc.M112.365874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cheng HL, et al. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 2003;100:10794–10799. doi: 10.1073/pnas.1934713100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McBurney MW, et al. The mammalian SIR2alpha protein has a role in embryogenesis and gametogenesis. Mol. Cell. Biol. 2003;23:38–54. doi: 10.1128/MCB.23.1.38-54.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lan F, et al. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J. Biol. Chem. 2008;283:27628–27635. doi: 10.1074/jbc.M805711200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hou X, et al. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J. Biol. Chem. 2008;283:20015–20026. doi: 10.1074/jbc.M802187200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bouras T, et al. SIRT1 deacetylation and repression of p300 involves lysine residues 1020/1024 within the cell cycle regulatory domain 1. J. Biol. Chem. 2005;280:10264–10276. doi: 10.1074/jbc.M408748200. [DOI] [PubMed] [Google Scholar]
- 83.Lin YY, et al. Functional dissection of lysine deacetylases reveals that HDAC1 and p300 regulate AMPK. Nature. 2012;482:251–255. doi: 10.1038/nature10804. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 84.Banks AS, et al. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab. 2008;8:333–341. doi: 10.1016/j.cmet.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schiff M, et al. Mitochondria and diabetes mellitus: untangling a conflictive relationship? J. Inherit. Metab. Dis. 2009;32:684–698. doi: 10.1007/s10545-009-1263-0. [DOI] [PubMed] [Google Scholar]
- 86.Choi CS, et al. Paradoxical effects of increased expression of PGC-1alpha on muscle mitochondrial function and insulin-stimulated muscle glucose metabolism. Proc. Natl. Acad. Sci. U.S.A. 2008;105:19926–19931. doi: 10.1073/pnas.0810339105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wenz T, et al. Increased muscle PGC-1alpha expression protects from sarcopenia and metabolic disease during aging. Proc. Natl. Acad. Sci. U.S.A. 2009;106:20405–20410. doi: 10.1073/pnas.0911570106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 88.Meng X, et al. Urinary and plasma levels of resveratrol and quercetin in humans, mice, and rats after ingestion of pure compounds and grape juice. J. Agric. Food Chem. 2004;52:935–942. doi: 10.1021/jf030582e. [DOI] [PubMed] [Google Scholar]
- 89.Vihko V, et al. beta-Glucuronidase activity in trained red and white skeletal muscle of mice. Eur. J. Appl. Physiol. Occup. Physiol. 1978;39:255–261. doi: 10.1007/BF00421449. [DOI] [PubMed] [Google Scholar]
- 90.Chen TC, et al. The type IV phosphodiesterase inhibitor rolipram induces expression of the cell cycle inhibitors p21(Cip1) and p27(Kip1), resulting in growth inhibition, increased differentiation, and subsequent apoptosis of malignant A-172 glioma cells. Cancer Biol. Ther. 2002;1:268–276. doi: 10.4161/cbt.80. [DOI] [PubMed] [Google Scholar]
- 91.Szewczuk LM, et al. Resveratrol is a peroxidase-mediated inactivator of COX-1 but not COX-2: a mechanistic approach to the design of COX-1 selective agents. J. Biol. Chem. 2004;279:22727–22737. doi: 10.1074/jbc.M314302200. [DOI] [PubMed] [Google Scholar]
- 92.Zykova TA, et al. Resveratrol directly targets COX-2 to inhibit carcinogenesis. Mol. Carcinog. 2008;47:797–805. doi: 10.1002/mc.20437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wouters EF, et al. Effect of the phosphodiesterase 4 inhibitor roflumilast on glucose metabolism in patients with treatment-naive, newly diagnosed type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2012 doi: 10.1210/jc.2011-2886. http://dx.doi.org/10.1210/jc.2011-2886. [DOI] [PubMed] [Google Scholar]
- 94.Pfluger PT, et al. Sirt1 protects against high-fat diet-induced metabolic damage. Proc. Natl. Acad. Sci. U.S.A. 2008;105:9793–9798. doi: 10.1073/pnas.0802917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Qiang L, et al. Proatherogenic abnormalities of lipid metabolism in SirT1 transgenic mice are mediated through Creb deacetylation. Cell Metab. 2011;14:758–767. doi: 10.1016/j.cmet.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bordone L, et al. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell. 2007;6:759–767. doi: 10.1111/j.1474-9726.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- 97.Picard F, et al. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Herzig S, et al. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature. 2001;413:179–183. doi: 10.1038/35093131. [DOI] [PubMed] [Google Scholar]
- 99.Noriega LG, et al. CREB and ChREBP oppositely regulate SIRT1 expression in response to energy availability. EMBO Rep. 2011;12:1069–1076. doi: 10.1038/embor.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang A, et al. Up-regulation of adiponectin by resveratrol: the essential roles of the Akt/FOXO1 and AMP-activated protein kinase signaling pathways and DsbA-L. J. Biol. Chem. 2011;286:60–66. doi: 10.1074/jbc.M110.188144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li Y, et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011;13:376–388. doi: 10.1016/j.cmet.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Horton JD, et al. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Invest. 2002;109:1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Roden M. Mechanisms of disease: hepatic steatosis in type 2 diabetes–pathogenesis and clinical relevance. Nat. Clin. Pract. Endocrinol. Metab. 2006;2:335–348. doi: 10.1038/ncpendmet0190. [DOI] [PubMed] [Google Scholar]
- 104.Walker AK, et al. Conserved role of SIRT1 orthologs in fasting-dependent inhibition of the lipid/cholesterol regulator SREBP. Genes Dev. 2010;24:1403–1417. doi: 10.1101/gad.1901210. [DOI] [PMC free article] [PubMed] [Google Scholar]