Abstract
Background
The proportion of elderly (≥65 years) kidney transplant recipients (KTRs) doubled in the United States (US) from 1999 – 2008. Given higher mortality, more medication side effects, and less rejection among elderly KTRs, optimal care of these patients may require tailored decisions about transplant therapeutics. It is unknown whether participants in transplant clinical trials – which generate the best evidence for patient care – are representative of the aging population of KTRs.
Methods
Using PubMed, we identified randomized trials involving KTRs from 1999 – 2008 and determined age-exclusion criteria and the mean age of participants. The mean age of these trial participants was compared to the mean age of the overall population of incident KTRs in the US.
Results
The 87,222 participants in 573 trials were significantly younger than the US KTR population (p<0.05). This age discrepancy worsened over the study period (during the years 2006 – 2008, the mean age was 45 years for trial participants versus 50 years for US KTRs, p<0.05). Thirty percent of trials had an exclusion criterion based on older age, and 16% excluded recipients ≥65 years. In multivariable regression, immunosuppression trials (p<0.01) and trials in higher-impact journals (p=0.03) were more likely to exclude the elderly, but there was no significant difference in exclusion of elderly patients based on a trial’s geographic location.
Conclusions
Trial participants are younger than KTRs in the US and many trials exclude older patients. Transplant investigators should make strong efforts to recruit patients across the total age spectrum.
Keywords: Kidney transplantation, age, generalizability, clinical trial
Introduction
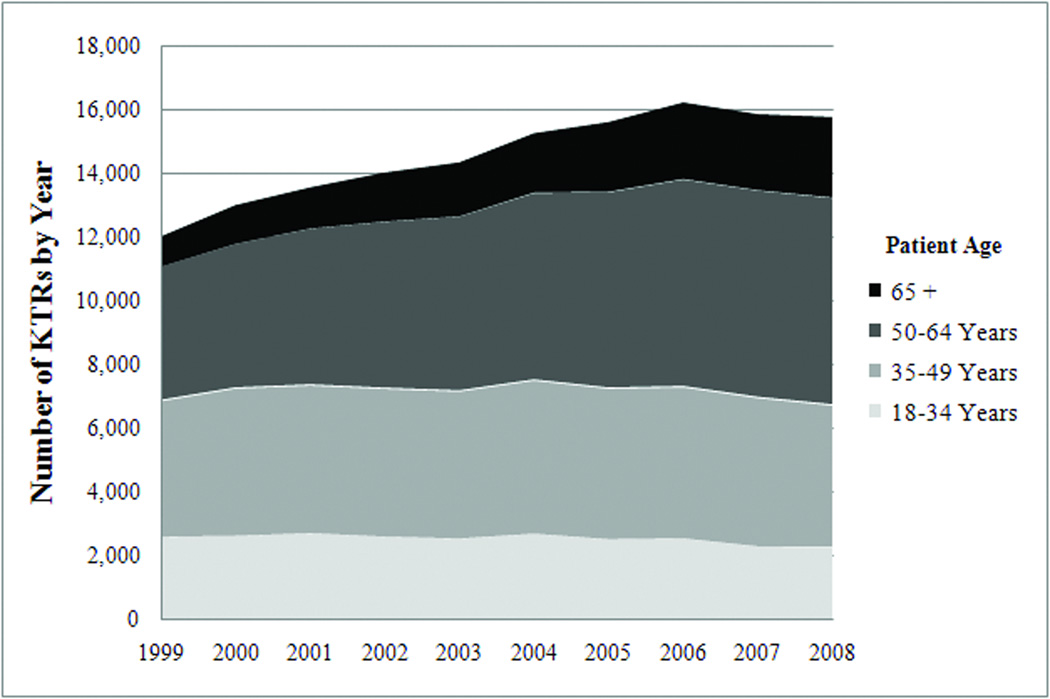
The United States (US) and the global population are aging rapidly, leading to greater focus on the clinical needs of geriatric patients.(1) Older age elevates the risk of kidney disease because of age-related kidney senescence and because older individuals are more likely to have co-morbidities such as diabetes that compromise renal function.(2, 3) As a result, the elderly (defined as ≥65 years) are the fastest growing segment of end-stage renal disease (ESRD) patients and kidney transplant recipients (KTRs) in the US. Approximately half of US incident dialysis patients are elderly, while the percentage of elderly kidney transplant recipients (KTRs) doubled from 7% to 16% during the decade from 1999 – 2008 (Figure 1).(4, 5) Optimal care of elderly KTRs requires tailored decisions about transplant therapeutics because these patients have lower rejection rates, more medication side effects, and higher mortality.(6–8) It is unknown, however, whether participants in transplant clinical trials are representative of the aging population of KTRs in the US, and whether trial results can be generalized to elderly KTRs.
Figure 1.
Age-Distribution of Kidney Transplant Recipients (KTRs) in the United States 1999 – 2008(5)
Compared to chronic dialysis, older patients with ESRD derive survival and quality of life benefits from kidney transplantation.(9) Using Organ Procurement and Transplantation Network (OPTN) registry data, Rao et al. examined outcomes among 5667 individuals over 70 years of age in the US.(10) After 18 months, the relative risk of death was 56% lower for transplant patients compared to waitlisted patients (p<0.01). Similar findings of improved survival with transplant have been reported for elderly recipients in Australia and the United Kingdom.(11, 12) The survival benefit of transplantation has also been shown in the subgroup of older recipients who undergo transplantation with expanded criteria donor (ECD) kidneys.(13–15)
Older age leads to alterations in immune function and medication metabolism that provide a conceptual basis for tailoring transplant medical regimens for elderly patients. Studies of older individuals reveal differences in both adaptive and innate immunity, compared to younger controls.(16–19) Multiple lines of evidence also suggest that older KTRs are at lower risk of cellular rejection.(20, 21) Conversely, older KTRs experience a greater incidence of transplant-associated malignancies, including lymphoproliferative disorders.(22) Additionally, older KTRs may have slower metabolism of immunosuppression medications. For example, Falck et al. reported that, compared to young recipients, KTRs >65 years old required lower doses of cyclosporine to achieve similar drug concentrations.(23) Integrating these findings, some leaders in clinical transplantation have proposed using less intense induction and maintenance therapy, and closer surveillance for malignancy when treating older KTRs.(6, 20, 24)
Randomized clinical trials offer the highest quality evidence to guide treatment.(25) The primary goal of this study was to examine the generalizability of recent clinical trials to the aging population of KTRs in the U.S. We hypothesized that trials involving immunosuppression would be more likely to exclude elderly KTRs, that more recent clinical trials would be less likely to exclude elderly KTRs, and that trial participants would be younger than the overall population of US KTRs.
Results
A total of 637 manuscripts were initially identified. Twenty-six manuscripts were eliminated due to duplication of the same study population, 23 because they did not involve adult kidney-alone transplant recipients, 13 because the trials were not randomized, 4 because they did not report sample sizes, and 2 because they were studies of kidney donors. The final analyses included 573 (90% of total) trials with 87,222 patients.
We sought information about missing characteristics of trials – the number enrolled, mean ages or group sizes – from the authors of 29 manuscripts, and received data from 6 authors. Among the final 573 trials analyzed, 484 (84%) reported mean age, and 395 (69%) reported mean age and standard deviation.
Characteristics of clinical trials (See Table 1)
Table 1.
Characteristics of trials
| Overall Trials |
Number of Trials Excluding Recipients ≥65 years (%) |
p- value |
Overall Patients |
Number of Patients in Trials Excluding Recipients ≥65 years (%) |
p-value, weighted by number pts enrolled |
||
|---|---|---|---|---|---|---|---|
| Overall | 573 | 90 (16) | 87,222 | 11,653 (13) | |||
| Era of Publication (%) | |||||||
| 1999 – 2002 | 212 | 34 (16) | 0.98 | 34,745 | 3,873 (11) | <0.01 | |
| 2003 – 2005 | 198 | 31 (16) | 28,780 | 4,470 (16) | |||
| 2006 – 2008 | 163 | 25 (15) | 23,697 | 3,310 (14) | |||
|
Number of Patients Enrolled in Trial (%) |
|||||||
| 0 – 52 | 196 | 35 (18) | 0.55 | 6,100 | 1,092 (18) | <0.01 | |
| 53 – 123 | 189 | 29 (15) | 15,783 | 2,564 (16) | |||
| ≥124 | 188 | 26 (14) | 65,339 | 7,997 (12) | |||
| Location (%) | |||||||
| U.S. Only | 97 | 12 (12) | 0.52 | 13,396 | 1,859 (14) | 0.04 | |
| U.S. & Non-U.S. Countries | 38 | 5 (13) | 13,281 | 1,701 (13) | |||
| Non-U.S. Only | 438 | 73 (17) | 60,545 | 8,093 (13) | |||
| Therapeutic Intervention (%) | |||||||
| Immunosuppression | 386 | 72 (19) | <0.01 | 68,380 | 10,270 (15) | <0.01 | |
| Other | 187 | 18 (10) | 18,842 | 1,383 (7) | |||
| Journal type | 0.07 | <0.01 | |||||
| Highest impact | 212 | 41 (19) | 42,935 | 6,893 (16) | |||
| Other | 361 | 49 (14) | 44,287 | 4,760 (11) | |||
Trials varied widely in number of participants, with a median of 81, a mean of 152, and a range of 2 to 2102. The number of trials declined from 212 published in the period from 1999 – 2002, to 198 in 2003 – 2005, and 163 in 2006 – 2008. Four hundred thirty-eight (76%) trials were conducted entirely outside the US. Three hundred eighty-six (67%) trials had an immunosuppression intervention. The non-immunosuppression interventions were diverse and included anti-viral prophylaxis, vitamin D supplementation, and protocol biopsies.
Formal age-exclusion of kidney transplant recipients from clinical trials
One hundred seventy-three trials (30%) involving 29,478 patients had any exclusion criterion related to older age. Ten trials excluded KTRs over 55 years, 31 trials excluded KTRs over 60 years, 49 trials excluded KTRs over 65 years, 43 trials excluded KTRs over 70 years, 32 excluded KTRs over 75 years, and 8 trials excluded KTRs over 80 years. Thus, 90 trials (16%) involving 11,653 patients met the primary outcome of excluding patients >65 years.
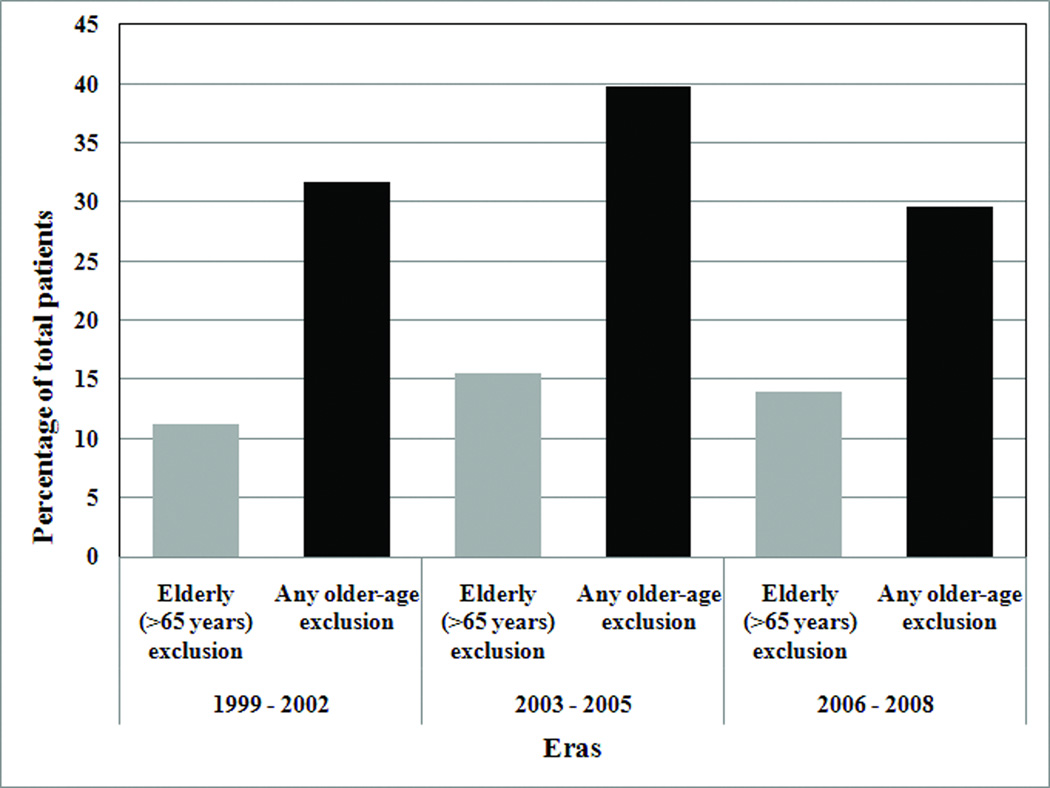
Contrary to our hypothesis, the percentage of trials excluding the elderly (>65 years) did not decrease significantly during the study period (Table 1). Sixteen percent of studies published in the years 1999 – 2002, 16% in 2003 – 2005, and 15% in 2006 – 2008 excluded the elderly (p=0.98 for changes between each era). In secondary analyses, we also examined these temporal trends by 1) weighting each trial according to the number of participants, and 2) measuring the percentage of trials that had any exclusion based on older age (such excluding patients over 70 years). As shown in Figure 2, these analyses did not provide consistent evidence that the exclusion of elderly patients decreased over time.
Figure 2. Percentage of Participants in Clinical Trials Excluding the Elderly and in Trials with Any Older-age Exclusion, by Era a,b.
a Trials with “any older age exclusion” ranged from excluding patients >55 years old to excluding patients > 80 years old.
b Trials were weighted by population size; therefore, percentages reflect the number of participants in trials excluding patients on the basis of older age, not the number of trials.
In univariate analysis, immunosuppression trials were more likely to exclude the elderly. This finding was consistent across non-frequency-weighted (p<0.01) and frequency weighted analyses (p<0.01). As a secondary analysis, we examined whether trials with the goal of immunosuppression minimization were more likely to exclude the elderly than other immunosuppression trials. We found no significant difference between these groups (p=0.60).
In frequency-weighted analyses, larger trials were less likely to exclude the elderly (p<0.01), while trials in higher impact journals were more likely to exclude the elderly (p<0.01). Location of enrollment was also statistically significant, but differences in absolute percentages of excluded patients across trials in different regions were unimportant.
Multivariable logistic regression (Table 2)
Table 2.
Association of trial characteristics with the outcome of trial exclusion of participants over 65 years of age in multivariable logistic regression a
| Trial characteristic | Odds Ratio | 95% C.I. | p-value |
|---|---|---|---|
|
Era 1999 – 2002 2003 – 2005 2006 – 2008 |
Reference 0.84 0.80 |
Reference 0.49, 1.46 0.45, 1.43 |
Reference 0.54 0.45 |
|
Number of patients enrolled 0 – 52 53 – 123 ≥124 |
Reference 0.82 0.59 |
Reference 0.47, 1.42 0.32, 1.07 |
Reference 0.48 0.08 |
|
Location of Patient Enrollment Enrollment only in the U.S. Enrollment in the U.S. and Non-U.S. countries Enrollment only in non-U.S. |
Reference 1.08 1.62 |
Reference 0.34, 3.39 0.82, 3.18 |
Reference 0.90 0.17 |
|
Immunosuppression trial (versus non-immunosuppression) |
2.40 | 1.36, 4.22 | 0.01 |
| Higher impact journal (versus lower) | 1.74 | 1.07, 2.84 | 0.03 |
The multivariable logistic model included all trial characteristics listed in the table
Trials involving immunosuppression (OR 2.40; CI 1.36, 4.22; p<0.01) and trials published in high impact journals (OR 1.74, CI 1.07, 2.84; p=0.03) were more likely to exclude elderly KTRs than other trials. There was no significant difference in the odds of excluding elderly patients between trials conducted only in the US versus in other locations.
Mean ages of kidney transplant recipients
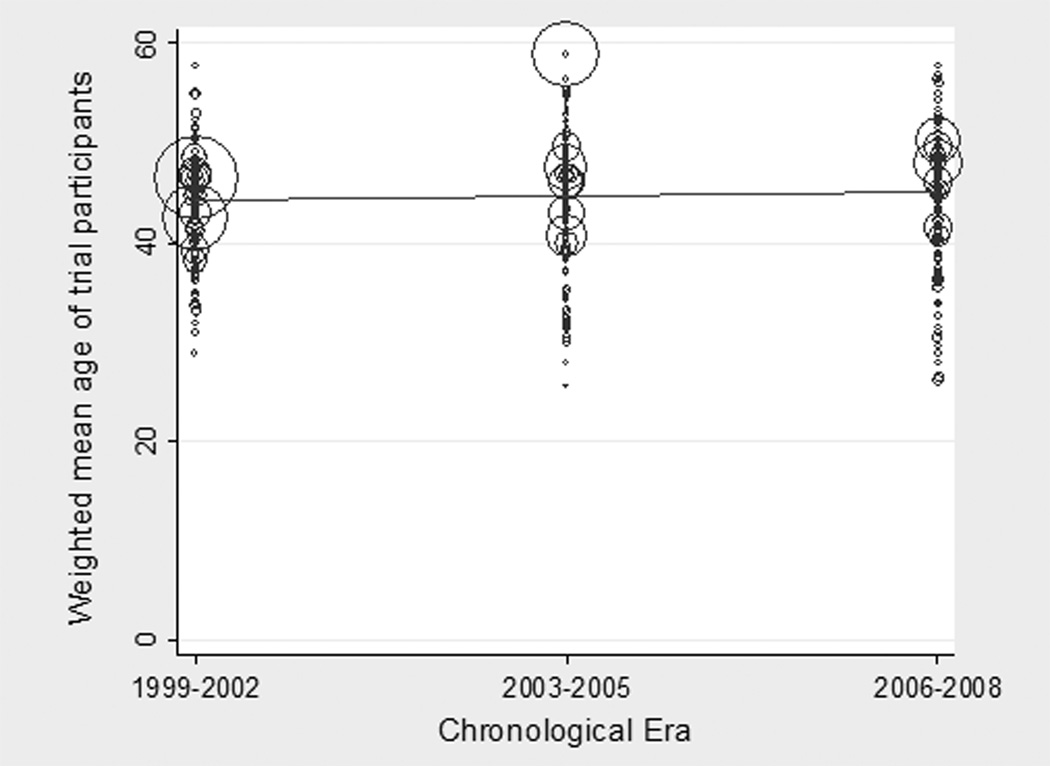
KTRs in clinical trials were younger than the KTR population in the US (45 versus 48 years, p<0.05). Although elderly KTRs represented an increasing proportion of the US KTR population from 1999 – 2008, the mean age of trial participants did not rise significantly during this period. Figure 3 displays the ages of KTRs in clinical trials, with each bubble’s size proportionate to the number of participants. The red line reflects the relative stability of the weighted mean age of participants across each era (p=0.42 for change between reference era 1 and era 2; p=0.50 for change between era 1 and era 3).
Figure 3. Mean Age of Participants in Randomized Trials in Kidney Transplantation, by Era a.
a The size of each bubble is proportionate to the size of the trial
Contrary to our expectation, the difference between the mean age of trial participants and that of the overall US KTR population widened during the study period. From 1999 –2002, the mean age of trial participants was 44 years versus 47 years for US KTRs overall; from 2003 – 2005, it was 45 years versus 49 years respectively; and from 2006 – 2008, it was 45 years versus 50 years respectively (p<0.05).
In subgroup analyses, KTRs in immunosuppression trials, and in trials published in higher impact journals were significantly younger than the overall population of US KTRs (p<0.05 for each comparison). We also closely examined trials performed only in the US. We found that KTRs in trials conducted only within the US were younger than US KTRs overall (p<0.05). However, when examining only KTRs in trials, we found that the mean age of KTRs in US trials was older (46 years) than participants in trials conducted outside the US (44 years, p<0.05).
Results were unchanged in sensitivity analyses and not shown.
Trials focused on older patients
No trials had the explicit goal of enrolling older KTRs. Three trials specifically enrolled recipients of ECD kidneys, but were not restricted to elderly KTRs. The mean age of KTRs in these trials was 57 years.
Discussion
The population of elderly KTRs is growing in the US and many other countries. These patients experience lower rates of rejection, but higher rates of infection, cancer, adverse events and death.(26) Our study reveals that a substantial proportion of clinical trials in kidney transplantation exclude patients on the basis of older age, that trial participants are younger than US KTRs overall, and that the mean age of trial participants is not increasing. These findings raise concerns about the quality of evidence available to clinicians in treating elderly KTRs, and suggest that investigators leading future trials should make strong efforts to recruit patients across a wide age spectrum.
Investigators might have diverse motivations for excluding older patients from trials. These include legitimate fear of harming these patients and a desire to demonstrate a favorable side effect profile for a new medication. Concern about side effects in older individuals is consistent with our finding that trials involving immunosuppression were more likely to exclude elderly patients, who are more vulnerable to infections.(8) On the other hand, investigators and trial sponsors may have failed to anticipate the growth of the elderly KTR population. No randomized trials that we reviewed targeted elderly patients specifically and only three trials focused on ECD kidney recipients, who are often older. From this standpoint, failing to enroll elderly KTRs could have negative consequences for marketing and dissemination of new treatments.(15, 27)
Trial participants were significantly younger than the overall population of KTRs in the US. This finding could have resulted from formal age-exclusion criteria, or indirectly from criteria based on co-morbidities, such as cardiovascular disease, malignancy or other conditions that increase in prevalence with age.(28) This finding might also be expected given that clinical trials are conducted in countries throughout the world, including nations with a smaller geriatric patient population than in the US or where resource-intensive treatments such as transplantation are less often offered to the elderly. However, we found that trial participants were younger in multiple subgroups, including trials performed only in the US. Unfortunately, as shown in Figure 3, the mean age of KTRs in trials has not risen appreciably, and thus, the age-disparity between KTRs in trials and US KTRs is widening and growing more clinically important. The stable mean age of KTRs in trials suggests that enrollment strategies have not been responsive to the changing age-demographics in places with substantial kidney transplant populations including the US, Europe and Canada. This study should provide a cautionary lesson for clinicians caring for elderly KTRs. For the elderly, the risk/benefit profile of many medications may not have been sufficiently investigated (29). These age-related limitations to our knowledge base may not be highlighted in commonly-used reference materials including Micromedex or UpToDate.(30, 31)
Achieving greater age-inclusiveness in clinical trials might be accomplished through several means.(32) Agencies such as the Food and Drug Administration (FDA) and the National Institutes of Health should encourage investigators to provide strong evidence that age-exclusion criteria are warranted. The FDA might also require post-marketing surveillance studies of transplant medications aimed at characterizing side effects among under-represented subgroups such as elderly KTRs. Notably, the exclusion of older patients from clinical trials has also been reported in general nephrology, cardiovascular medicine and oncology, suggesting that regulatory agencies might emphasize the need for enrollment of older patients in trials across disciplines.(33–35) On the other hand, the majority of transplant clinical trials are performed outside the US and participants in these trials are slightly younger than participants in trials conducted only with US patients. US regulatory and funding agencies may have limited abilities to influence the design of these trials conducted outside their purview.
In the future, journals considering clinical trials should require clear reporting of age-exclusion policies, detailed descriptive measures of age, and, where relevant, subgroup analyses of elderly patients.(36, 37) The association found in our analysis between studies that excluded elderly KTRs and higher impact journals was not anticipated. It is possible that these trials were more likely to show “favorable” findings because elderly patients were excluded, and therefore they were more likely to be accepted by high impact journals. In any case, higher impact journals are in a strong position to require subgroup analyses among older participants or acknowledgement of limited generalizability where the elderly are not well-represented.
This study has limitations. First, we did not analyze trials published in non-English language journals. Second, some trials may have had unreported age-exclusion policies, or recruitment procedures that excluded the elderly in practice, such as coordinators that didn’t enroll elderly patients out of concern for their wellbeing. However, these possibilities, if substantiated, would indicate that we underestimated the proportion of trials excluding older patients. Additionally, it is likely that we underestimated the difference in mean age between trial participants and KTRs in the US generally, since the OPTN provides the age at transplant and the trials enrolled a mixture of incident and prevalent patients.
Conclusion
Despite the growing population of elderly KTRs, many clinical trials in kidney transplantation exclude these patients and the mean age of trial participants has not increased appreciably. Given that elderly recipients often have worse outcomes and a greater likelihood of adverse effects in kidney transplantation, this study raises concerns that the findings of many clinical trials may not be generalizable to the higher-risk cohort of elderly KTRs. Transplant clinicians should carefully examine the age-distribution of trial participants before applying trial results to their elderly patients. Investigators designing future trials should provide a strong rationale for excluding older patients and make appropriate efforts to recruit patients across a representative age range.
Methods
Identification of clinical trials
In June 2009, we performed a systematic search of the PubMed database to identify manuscripts reporting results from randomized clinical trials involving adult (≥18 years) KTRs, published in English from 1999 through 2008. We selected this time interval because transplantation of elderly KTRs in the US doubled from 1999 – 2008, and because the interval was recent enough that these trials could be considered relevant to current practice.
Manuscripts were excluded if transplantation was an intervention or desired outcome of the trial, if the number of participants could not be determined, or if the sample population was used for more than one manuscript. When duplicate populations were identified in >1 trial, we used the largest trial and excluded the others.
The study met criteria for exemption from our institution’s review board because no data with patient identifiers were used.
Outcomes
The primary outcome was a study’s explicit exclusion of KTRs ≥65 years of age according to eligibility criteria. Studies that excluded KTRs using a lower age threshold (e.g. a study excluding patients ≥60 years) were also categorized as meeting the primary outcome of excluding participants ≥65 years old. For simplicity, we did not distinguish between studies that excluded patients >65 years versus ≥65 years.
The secondary outcome was mean age of a study’s clinical trial participants.
Characterization of trials
Trials were categorized as immunosuppression versus other intervention and by site of patient enrollment (US only, US and non-US sites, non-US sites only). Trials were empirically divided into tertiles according to number of patients (<52, ≥53 and <124, and ≥124 participants). To examine temporal trends related to age, trials were categorized according to chronological era of publication year (1999 – 2002, 2003 – 2005, and 2006 – 2008). Lastly, trials were categorized as to whether they were published in a “higher impact” journal as measured by impact factor reported by ISI Web of Knowledge in June 2010.(38) We restricted this category to the five highest impact general medicine journals (New England Journal of Medicine, Journal of the American Medical Association, the Lancet, Annals of Internal Medicine, and the British Medical Journal), the two highest impact transplantation journals (the American Journal of Transplantation, and Transplantation) and the two highest impact nephrology journals (Journal of the American Society of Nephrology and Kidney International).
Characterization of kidney transplant recipients in the United States
We used registry data from OPTN to determine the mean age of adult (≥18 years) KTRs in the United States overall from 1999 – 2008. We excluded recipients of multi-organ transplants.
Statistical analysis
Comparisons of age-exclusion policies across types of trials were made using chi-square. Chi-square analyses were also performed weighted by the number of participants in each trial (frequency weight command in Stata) in order to account for differences in sizes of trial populations.
Some trials did not report an overall mean age and/or standard deviation for randomized patients. We derived these missing data by calculating the weighted mean of the ages of patients in each trial arm and/or the overall variance in each trial, if standard deviations and numbers of patients were provided only for each trial arm (and not overall). We used meta-regression models to estimate the mean age of the total population of patients in trials, and to analyze temporal trends in the ages of trial participants across eras; for these calculations, we assumed random effects for each trial.(39) For meta-regression, when standard deviation was missing from a trial, we imputed the standard deviation using the mean standard deviation of KTRs in trials that did report these data. Additionally, to ensure that imputation did not affect our conclusions, we performed sensitivity analyses for the meta-regression models where the 25th and 75th percentile values of standard deviations were imputed for missing values.
Multivariable logistic regression was used to examine associations between trial characteristics and the primary outcome of having a stated criterion to exclude elderly KTRs. All trial characteristics considered in univariate analysis (immunosuppression intervention, site of patient enrollment, size, era, and type of journal) were included in the model. The Hosmer-Lemeshow goodness-of-fit hypothesis was not rejected (p>0.05). Analyses were performed using StataMP 11 (Stata Corporation, College Station, TX, USA).
Acknowledgements
Funding sources: Dr. Reese was supported by NIH grant K23 DK078688, and by a grant jointly sponsored by the American Society of Nephrology, the Atlantic Philanthropies, the John A. Hartford Foundation and the Association of Specialty Professors.
The authors wish to thank James Guevara, MD for his helpful comments and advice about the approach to analysis.
Abbreviations
- ECD
Expanded criteria donor
- KTR
Kidney transplant recipients
- OPTN
Organ Procurement and Transplantation Network
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Funding sources and participation:
1 Christopher D Blosser: Participated in research design, data collection, data analysis, interpreting the data and writing the manuscript.
2 Ari Huverserian: Participated in data collection.
3 Roy D Bloom: Participated in research design and data interpretation.
4 Peter D Abt: Participated in research design and data interpretation.
5 Simin Goral: Participated in data interpretation.
6 Arwin Thomasson: Participated in research design, data analysis and interpretation.
7 Justine Shults: Participated in research design, data analysis and interpretation.
8 Peter P Reese: Participated in research design, data analysis, interpreting the data and writing the manuscript.
Conflicts of interest: The authors have no financial conflicts of interest to disclose.
Disclosures: Preliminary results of this study were presented at the 2010 American Transplant Congress.
References
- 1.UN. World Population Ageing: 1950–2050. vol. New York: Department of Economic and Social Affairs, Population Division; 2010. [Google Scholar]
- 2.Fliser D, Franek E, Ritz E. Renal function in the elderly--is the dogma of an inexorable decline of renal function correct? Nephrol Dial Transplant. 1997;12(8):1553. doi: 10.1093/ndt/12.8.1553. [DOI] [PubMed] [Google Scholar]
- 3.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 4.United States Renal Data System 2007 Annual Data Report: Atlas of Chronic Kidney Disease & End-Stage Renal Disease in the United States. American Journal of Kidney Diseases. 2008;51(1):S20. doi: 10.1053/j.ajkd.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Organ Procurement and Transplantation Network. Transplants in the U.S. by Recipient Age. [Accessed 10/04/2010]; http://optn.transplant.hrsa.gov/latestData/rptData.asp.
- 6.Danovitch GM, Gill J, Bunnapradist S. Immunosuppression of the elderly kidney transplant recipient. Transplantation. 2007;84(3):285. doi: 10.1097/01.tp.0000275423.69689.dc. [DOI] [PubMed] [Google Scholar]
- 7.Kauffman HM, McBride MA, Cors CS, Roza AM, Wynn JJ. Early mortality rates in older kidney recipients with comorbid risk factors. Transplantation. 2007;83(4):404. doi: 10.1097/01.tp.0000251780.01031.81. [DOI] [PubMed] [Google Scholar]
- 8.Meier-Kriesche HU, Ojo AO, Hanson JA, Kaplan B. Exponentially increased risk of infectious death in older renal transplant recipients. Kidney Int. 2001;59(4):1539. doi: 10.1046/j.1523-1755.2001.0590041539.x. [DOI] [PubMed] [Google Scholar]
- 9.Knoll GA. Is kidney transplantation for everyone? The example of the older dialysis patient. Clin J Am Soc Nephrol. 2009;4(12):2040. doi: 10.2215/CJN.04210609. [DOI] [PubMed] [Google Scholar]
- 10.Rao PS, Merion RM, Ashby VB, Port FK, Wolfe RA, Kayler LK. Renal transplantation in elderly patients older than 70 years of age: results from the Scientific Registry of Transplant Recipients. Transplantation. 2007;83(8):1069. doi: 10.1097/01.tp.0000259621.56861.31. [DOI] [PubMed] [Google Scholar]
- 11.Johnson DW, Herzig K, Purdie D, et al. A comparison of the effects of dialysis and renal transplantation on the survival of older uremic patients. Transplantation. 2000;69(5):794. doi: 10.1097/00007890-200003150-00020. [DOI] [PubMed] [Google Scholar]
- 12.Oniscu GC, Brown H, Forsythe JL. How great is the survival advantage of transplantation over dialysis in elderly patients? Nephrol Dial Transplant. 2004;19(4):945. doi: 10.1093/ndt/gfh022. [DOI] [PubMed] [Google Scholar]
- 13.Frei U, Noeldeke J, Machold-Fabrizii V, et al. Prospective age-matching in elderly kidney transplant recipients--a 5-year analysis of the Eurotransplant Senior Program. Am J Transplant. 2008;8(1):50. doi: 10.1111/j.1600-6143.2007.02014.x. [DOI] [PubMed] [Google Scholar]
- 14.Schold JD, Meier-Kriesche HU. Which renal transplant candidates should accept marginal kidneys in exchange for a shorter waiting time on dialysis? Clin J Am Soc Nephrol. 2006;1(3):532. doi: 10.2215/CJN.01130905. [DOI] [PubMed] [Google Scholar]
- 15.Merion RM, Ashby VB, Wolfe RA, et al. Deceased-donor characteristics and the survival benefit of kidney transplantation. JAMA. 2005;294(21):2726. doi: 10.1001/jama.294.21.2726. [DOI] [PubMed] [Google Scholar]
- 16.Lansdorp PM, Dragowska W, Thomas TE, Little MT, Mayani H. Age-related decline in proliferative potential of purified stem cell candidates. Blood Cells. 1994;20(2–3):376. [PubMed] [Google Scholar]
- 17.Douek DC, Vescio RA, Betts MR, et al. Assessment of thymic output in adults after haematopoietic stem-cell transplantation and prediction of T-cell reconstitution. Lancet. 2000;355(9218):1875. doi: 10.1016/S0140-6736(00)02293-5. [DOI] [PubMed] [Google Scholar]
- 18.Whisler RL, Beiqing L, Chen M. Age-related decreases in IL-2 production by human T cells are associated with impaired activation of nuclear transcriptional factors AP-1 and NF-AT. Cell Immunol. 1996;169(2):185. doi: 10.1006/cimm.1996.0109. [DOI] [PubMed] [Google Scholar]
- 19.Gregg R, Smith CM, Clark FJ, et al. The number of human peripheral blood CD4+ CD25high regulatory T cells increases with age. Clin Exp Immunol. 2005;140(3):540. doi: 10.1111/j.1365-2249.2005.02798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meier-Kriesche HU, Ojo A, Hanson J, et al. Increased immunosuppressive vulnerability in elderly renal transplant recipients. Transplantation. 2000;69(5):885. doi: 10.1097/00007890-200003150-00037. [DOI] [PubMed] [Google Scholar]
- 21.Martins PN, Pratschke J, Pascher A, et al. Age and immune response in organ transplantation. Transplantation. 2005;79(2):127. doi: 10.1097/01.tp.0000146258.79425.04. [DOI] [PubMed] [Google Scholar]
- 22.Kasiske BL, Snyder JJ, Gilbertson DT, Wang C. Cancer after kidney transplantation in the United States. Am J Transplant. 2004;4(6):905. doi: 10.1111/j.1600-6143.2004.00450.x. [DOI] [PubMed] [Google Scholar]
- 23.Falck P, Asberg A, Byberg KT, et al. Reduced elimination of cyclosporine A in elderly (>65 years) kidney transplant recipients. Transplantation. 2008;86(10):1379. doi: 10.1097/TP.0b013e31818aa4b6. [DOI] [PubMed] [Google Scholar]
- 24.Ismail N, Hakim RM, Helderman JH. Renal replacement therapies in the elderly: Part II. Renal transplantation. Am J Kidney Dis. 1994;23(1):1. doi: 10.1016/s0272-6386(12)80805-5. [DOI] [PubMed] [Google Scholar]
- 25.Gordis L. Epidemiology. Philadelphia: Elsevier Saunders; 2004. Chapter 8 Randomized Trials: Some Further Issues; p. 130. [Google Scholar]
- 26.Huang E, Segev DL, Rabb H. Kidney transplantation in the elderly. Semin Nephrol. 2009;29(6):621. doi: 10.1016/j.semnephrol.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sung RS, Guidinger MK, Lake CD, et al. Impact of the expanded criteria donor allocation system on the use of expanded criteria donor kidneys. Transplantation. 2005;79(9):1257. doi: 10.1097/01.tp.0000161225.89368.81. [DOI] [PubMed] [Google Scholar]
- 28.Hartmann EL, Wu C. The evolving challenge of evaluating older renal transplant candidates. Adv Chronic Kidney Dis. 2010;17(4):358. doi: 10.1053/j.ackd.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 29.Kuypers DR. Immunotherapy in elderly transplant recipients: a guide to clinically significant drug interactions. Drugs Aging. 2009;26(9):715. doi: 10.2165/11316480-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 30.Basow D. UpToDate. Waltham, MA: 2010. [Google Scholar]
- 31.Micromedex® Healthcare Series [Internet database] Greenwood Village, Colo: Thomson Reuters (Healthcare) Inc.; Updated periodically. [Google Scholar]
- 32.Cherubini A, Signore SD, Ouslander J, Semla T, Michel JP. Fighting against age discrimination in clinical trials. J Am Geriatr Soc. 2010;58(9):1791. doi: 10.1111/j.1532-5415.2010.03032.x. [DOI] [PubMed] [Google Scholar]
- 33.Coca SG, Krumholz HM, Garg AX, Parikh CR. Underrepresentation of renal disease in randomized controlled trials of cardiovascular disease. JAMA. 2006;296(11):1377. doi: 10.1001/jama.296.11.1377. [DOI] [PubMed] [Google Scholar]
- 34.Gurwitz JH, Col NF, Avorn J. The exclusion of the elderly and women from clinical trials in acute myocardial infarction. JAMA. 1992;268(11):1417. [PubMed] [Google Scholar]
- 35.Townsley CA, Selby R, Siu LL. Systematic review of barriers to the recruitment of older patients with cancer onto clinical trials. J Clin Oncol. 2005;23(13):3112. doi: 10.1200/JCO.2005.00.141. [DOI] [PubMed] [Google Scholar]
- 36.Van Spall HG, Toren A, Kiss A, Fowler RA. Eligibility criteria of randomized controlled trials published in high-impact general medical journals: a systematic sampling review. JAMA. 2007;297(11):1233. doi: 10.1001/jama.297.11.1233. [DOI] [PubMed] [Google Scholar]
- 37.Avorn J. Including elderly people in clinical trials. BMJ. 1997;315(7115):1033. doi: 10.1136/bmj.315.7115.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152(11):726. doi: 10.7326/0003-4819-152-11-201006010-00232. [DOI] [PubMed] [Google Scholar]
- 39.Sterne JAC. Meta-Analysis in Stata. College Station: Stata Press; 2009. [Google Scholar]