Abstract
Objective: The ascending reticular activating system (ARAS) modulates circadian wakefulness, which is preserved in a persistent vegetative state (PVS). Its metabolism is preserved. Impairment of metabolism in the polymodal associative cortices (i.e., precuneus) is characteristic of PVS where awareness is abolished. Because the interaction of these 2 structures allows conscious sensory perception, our hypothesis was that an impaired functional connectivity between them participates in the loss of conscious perception.
Methods: 15O-radiolabeled water PET measurement of regional cerebral blood flow (rCBF) was performed at rest and during a proprioceptive stimulation. Ten patients in PVS and 10 controls were compared in a cross-sectional study. The functional connectivity from the primary sensorimotor cortex (S1M1) and the ARAS in both groups was also investigated.
Results: Compared with controls, patients showed significantly less rCBF in posterior medial cortices (precuneus) and higher rCBF in ARAS at rest. During stimulation, bilateral Brodmann area 40 was less activated and not functionally correlated to S1M1 in PVS as it was in controls. Precuneus showed a lesser degree of deactivation in patients. Finally, ARAS whose activity was functionally correlated to that of the precuneus in controls was not in PVS.
Conclusions: Global neuronal workspace theory predicts that damage to long-distance white matter tracts should impair access to conscious perception. During persistent vegetative state, we identified a hypermetabolism in the ascending reticular activating system (ARAS) and impaired functional connectivity between the ARAS and the precuneus. This result emphasizes the functional link between cortices and brainstem in the genesis of perceptual awareness and strengthens the hypothesis that consciousness is based on a widespread neural network.
Persistent vegetative state (PVS) describes a unique disorder in which patients who emerge from coma seem to be awake but show no signs of awareness. The ascending reticular activating system (ARAS) is located at a critical juncture in the inflow of sensory information, and its activity modulates circadian wakefulness. No abnormality has been reported in the ARAS in PVS so far.1 According to some authors, activity in the precuneus is a sign of self-referential processing during which stimulus-independent thought could participate in the emergence of perceptual awareness. Its metabolism is impaired in PVS.2 In healthy subjects, effects of each structure on each other (precuneus and ARAS) exist in the basal state, are opposite, and are thought to predict whether a somatosensory stimulus will be consciously perceived.3 We found it appropriate to evaluate the interactions between the 2 competing systems in the development of conscious perception during a sensory stimulation. Functional connectivity was used to assess this interaction. Basal resting activity was first measured in patients and controls to detect abnormalities in these structures before assessing a putative impaired interaction. On the basis of H2O15 PET measurement of cerebral blood flow during a resting state, we made the hypothesis of a preserved metabolism in the ARAS. Later, we chose a proprioceptive nonpainful stimulus, passive extension of the finger. Because the interaction of the ARAS and the precuneus allows conscious sensory perception,3 our second hypothesis was that an impaired functional connectivity between both structures participates in awareness loss during PVS.
METHODS
Standard protocol approvals, registrations, and patient consents.
The study was approved by the Ethics Committee of the University of Toulouse (France). Written informed consent was obtained from the persons having legal responsibility for the patients and from all controls subjects according to the Declaration of Helsinki.
Participants.
We conducted a prospective study of 10 nonsedated patients in PVS (8 men and 2 women, ranging in age from 19 to 64 years) and 10 healthy volunteers (7 men and 3 women, ranging in age from 21 to 65 years, without significant history or examination abnormalities). Clinical diagnoses were made on the basis of repeated, standardized evaluation and conformed to international established criteria for PVS.4–6 Patients were assessed by experienced practitioners outside the study: 2 weeks and 1 day before scanning, the day of the scan, and 1 month after the scan. None of the patients in PVS showed normal flexion or withdrawal in response to verbal or noxious stimuli. All patients had spontaneous breathing and had retained pupillary, corneal, and vestibular reflexes (table). In 6 cases, the PVS was associated with diffuse axonal injury (road traffic accident), and in 4 cases, the PVS was associated with anoxic brain injury after resuscitation from cardiac arrest. The average time between the initial damage and the PET examination was 109 ± 45 days (more than 30 days in all cases).
Table Clinical data of patients in persistent vegetative state
After admission to the hospital and while in awake periods (as demonstrated by simultaneous polygraphic recordings), patients and healthy subjects underwent scanning.
Data acquisition.
The protocol consisted of H2O15 PET cerebral image acquisition (ECAT EXAT HR, Siemens, Munich, Germany) during rest or during somatosensory stimulation. Six H2O15 scans were acquired at 8-minute intervals in 3-dimensional mode. Each scan consisted of 2 frames. The slow IV water infusion began just before the second frame to observe the head curve rising within the first 10 s of the second frame. Eight millicuries (296 MBq) was injected for each scan. The infusion was totally automated. Anatomic images were acquired using NMR (Magneton Vision Siemens, 1.5 T) to assist the subsequent analysis of the functional images.
The same paradigm was used for both study groups. The somatosensory stimulation was obtained by passive execution of an extension (amplitude 30°, frequency 0.5 Hz) of the metacarpophalangeal joint of the right index finger.7–10 An automatic device was used to ensure the reproducibility and synchronization of the task (Spacelabs, Issaquah, WA).7 The distal part of the finger was immobilized by an individual cap, which could effectively abolish the pressure or tactile sense caused by the passive movement.9 Other fingers of the right hand were fixed to the device. The movement produced by the equipment was completely noiseless. The passive movement was induced 30 seconds before the image acquisition was started.
During the resting state, subjects (patients and healthy volunteers) were asked to keep their eyes open and to let their thoughts wander freely.11–13 These 2 states (passive finger movement and resting state) were repeated twice with an 8-minute interval between repetitions, and were randomized. The subjects' vital signs were monitored during the procedure (heart rate, mean arterial blood pressure, pulse oximetry, capnometry).
Data analysis.
The PET data were realigned, mapped to the standard Montreal Neurological Institute MRI template, and smoothed (8 mm3). The images were processed on the basis of a pixel-by-pixel comparison of the images acquired during the passive movement phases against those obtained during the rest phases. The statistical analysis was based on the general linear regression model implemented in the SPM2 program (Welcome Department of Cognitive Neurology, Institute of Neurology, London, UK). A fixed effects comparison was performed between the 2 states. The contrasts between the maps for the different groups were calculated using repeated measures (analysis of covariance). A comparison between patients with traumatic brain injury and patients with anoxia was made on resting regional cerebral blood flow (rCBF) (2-sample t test). A comparison between patients and controls was also made (2-sample t test). Results are given for a threshold p < 0.05 corrected for multiple comparisons (family-wise error [FWE]). Maps of the activations and deactivations, and differences in activation and deactivation are presented with a p threshold of 0.001 and corrected with a cluster size >40 voxels to reject false positives.14,15
Finally, a “psychophysiological interaction”1,16–20 study was performed between the different groups. This enabled us to study functional connectivity between chosen regions of interest and the rest of the brain using a fixed effects approach. The results were considered significant for a whole-brain FWE-corrected p value of 0.05.
RESULTS
The vital signs recorded (heart rate, mean arterial blood pressure, pulse oximetry, capnometry) in the healthy subjects and patients did not change during the study procedure. All patients remained in PVS 1 month after PET scanning.
Cerebral blood flow in resting state.
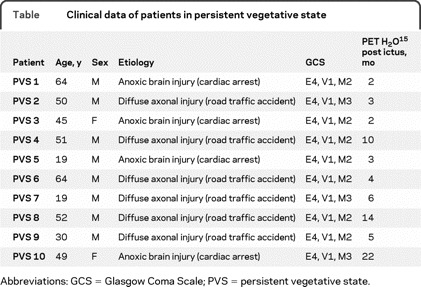
Patients essentially showed less rCBF in the posterior medial associative cortices (precuneus) (small volume correction [SVC] of a 12-mm-radius sphere around predetermined coordinates from healthy subjects, pFWE corrected <0.05; figure 1A), in accord with the literature.19–21 Moreover, during the same condition, we identified a higher rCBF in the midbrain tegmentum (ARAS)22 in patients in PVS compared with healthy subjects (pFWE corrected [SVC] <0.05; figure 1B). No significant differences were observed at rest between patients in PVS from traumatic origin compared with patients with anoxic brain injury (pFWE corrected [SVC] <0.05).
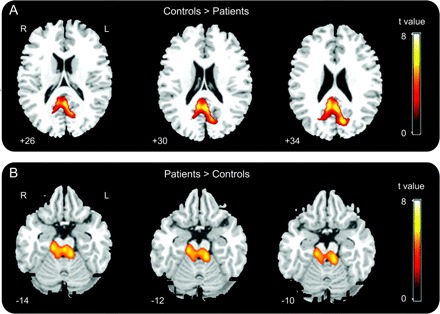
Figure 1 Comparison of regional cerebral blood flow at rest between controls and patients in persistent vegetative state
Two-sample t test, family-wise error corrected p < 0.05; images are displayed at a noncorrected threshold p < 0.001.
Deactivation maps.
In both groups, we identified a significant decrease in rCBF between the resting state and the execution of the passive movement, in the following structures: precuneus, anterior cingulate gyrus, left posterolateral parietal cortex (Brodmann area [BA] 39), and dorsal medial prefrontal cortex (BA 9, 10) (table e-1 on the Neurology® Web site at www.neurology.org). These results are consistent with the data in the literature on the deactivation phenomenon within the “default-mode network” (DMN).11–13 Then, we performed a functional connectivity analysis.1,16–20 The ARAS, whose activity was functionally correlated to that of the precuneus in healthy subjects, showed no correlation with this structure in the patient group (corrected p value <0.05; figure 2). Comparison of deactivations between the 2 groups showed a significantly smaller degree of deactivation in the precuneus in the patient group than in the control group (SVC of a 12-mm-radius sphere applied on precuneus, pFWE corrected <0.05; figure 3). Deactivations patterns were not significantly different between patients in PVS from traumatic origin compared with patients with anoxic brain injury (pFWE corrected <0.05).
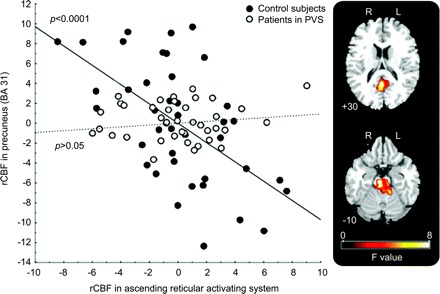
Figure 2 Plot of the regression of neural activity in ascending reticular activating system and in precuneus
F values, corrected p < 0.05 in controls (full circles: slope, r = 0.58, p < 0.0001) and in patients in persistent vegetative state (PVS) (open circles: slope, r = 0.08, p = not significant). During PVS, an impaired functional connectivity was found between the ascending reticular activating system and precuneus (difference between slopes F = 16.3, p < 0.0001). rCBF = regional cerebral blood flow.
Figure 3 Brain regions that showed less decrease in activity during stimulation in persistent vegetative state than in controls
Interaction (rest vs finger movement) × (patients vs controls) (images are displayed at a noncorrected threshold p < 0.005; small volume correction applied on precuneus, family-wise error corrected p < 0.05).
Activation maps.
In the control subjects, the proprioceptive stimulus caused a significant increase in rCBF relative to the resting state, in the sensorimotor cortex (S1M1) contralateral to the right finger movement and the inferior parietal lobule (IPL; BA 40) bilaterally (SVC applied on contralateral S1M1 and BA 40 areas, pFWE corrected <0.05; figure e-1), in accord with the literature.8–10 For patients in PVS, execution of the same paradigm was associated with an increase in rCBF only in the left S1M1 contralateral to the movement (SVC of a 12-mm-radius sphere applied on contralateral S1M1, pFWE corrected <0.05; figure e-1). Furthermore, we identified the brain regions that showed a smaller degree of increased activity between the resting state and the passive movement in patients in PVS: the IPL (BA 40) bilaterally (figure e-2). This pattern was common at all patients in PVS, independently of their etiology (pFWE corrected <0.05). Finally, a psychophysiological interaction1,16–20 analysis of the activity signal of the left S1M1 cortex (proprioceptive stimulus − rest) enabled us to demonstrate a loss of functional connectivity between this primary area (left S1M1) and the high-level associative structures recruited by the task (right and left BA 40) in patients, whereas functional connectivity was found in the controls (corrected p value <0.05; figure 4). Nevertheless, the functional link between these primary and secondary areas was different between the ipsilateral and contralateral sides in patients in PVS (figure 4). Indeed, functional interactions were diminished but persisted in the side contralateral to the passive movement, whereas they were abolished in the ipsilateral side in patients in PVS.
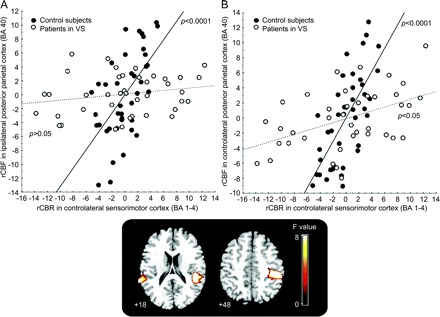
Figure 4 Plot of the regression of neural activity in contralateral S1M1 and bilateral BA 40
Both control (full circles) and persistent vegetative state (PVS; open circles) groups are shown (F values, corrected p < 0.05). (A) During PVS, primary sensorimotor cortex (S1M1; Brodmann area [BA] 1–4) showed no functional correlation with ipsilateral parietal cortex (BA 40) (control slope: r = 0.62, p < 0.0001; patients in PVS slope: r = 0.12, p = not significant; difference between slopes: F = 16.4, p < 0.0001) and (B) contralateral BA 40 (controls slope: r = 0.71, p < 0.0001; patients in PVS slope: r = 0.40, p < 0.005; difference between slopes: F = 27, p < 0.0001). rCBF = regional cerebral blood flow.
DISCUSSION
Consciousness would come from multiple long-distance connections according to the global neuronal workspace theory, which predicts that damage to these long-distance white matter tracts would impair the access to consciousness.23 Our work made a particular focus on the main areas of 2 competing systems, the ARAS and the precuneus, because both were altered in our group of patients in PVS, in an opposite way. Moreover, they rely on 2 main processes, external vs self-referential processes. Basal resting activity was first measured in patients and controls to detect abnormalities in these structures before assessing a putative impaired interaction, interaction that is involved in sensory perception.
Consciousness can be divided into 2 main components: arousal (i.e., wakefulness or vigilance) and awareness (e.g., awareness of the environment and of the self; figure 2).20 Conscious perception of external sensory stimuli relates to the intensity of activation of ARAS in particular.3,24 Identifying the neural correlates of PVS, using functional brain imaging devices, offers 2 potential benefits. First, it could contribute to an assessment of the level and content of cognitive processing in noncommunicative patients.21,25–27 Clinical practice shows that recognizing unambiguous signs of conscious perception of the environment and of the self in some patients with brain damage can be very challenging28,29 because it depends on the subject's residual communication capacity and on the reproducibility of the behavioral tests that are performed. Second, a study of the brain function of this state could contribute to the identification of the neural substrates of perceptual awareness.20,30,31
There has been growing interest in the study of regions of the brain with an intense basal metabolic activity in a neural network referred to as the DMN. Compared with healthy subjects, patients in PVS showed a smaller degree of deactivation in the precuneus. This region of the brain would represent a “critical node in the DMN.”11 Studies using functional brain imaging have identified the functional correlate of this structure: visuospatial imaging, episodic memory, and development of a concept of self from a first-person perspective, which is essential to the phenomenon of agency attribution.32 These highly associative functions have been linked to the emergence of self-awareness within an individual reference framework. This hypothesis is supported by research that demonstrates selective hypometabolism in this posteromedial cortex during states of altered consciousness, such as sleep,33 general anesthesia,34 or PVS.21 Furthermore, the functional recovery of these patients seems to be linked with normalization of the metabolic activity in this region of the brain.19
Our first hypothesis was that the ARAS, which deals with wakefulness, would display a preserved metabolism. We effectively found an activity in this area; however, it was partly abnormal because it was hyperintense. Higher spontaneous activity in the vigilance system and in areas involved in external stimuli perception has a facilitatory effect on external stimuli perception.35 Thus, we wondered whether these patients would display a hypersensitivity to external stimuli. Because no hyperactivation was seen in the primary sensory cortex, this hypothesis does not seem reliable. However, this result strengthens our second hypothesis of an impaired functional connectivity between ARAS and upper structures such as the thalamus or precuneus in patients in PVS. Regarding this second hypothesis, we did not find the thalamus, but the precuneus (figure 2). Not finding the thalamus is not surprising, because we know that the ascending sensory pathway ending in the primary sensory cortex S1 is preserved in PVS. We suggest that the functional connectivity found in controls between the ARAS and the precuneus arises from a direct or indirect anatomic link: precuneus toward ARAS32 or ARAS–thalamus,36 and then thalamus–precuneus.32 It is likely that an impaired metabolism in the precuneus modulates the activity of the ARAS as a top-down process and induces the abnormal hyperactivation. These data are in accord with neural models of the emergence of consciousness within a “global workspace” divided into 2 competing systems: one allowing conscious access to external stimuli (modulated by the ascending systems) and another allowing self-referential processes (DMN).23,30 This model predicts a facilitatory effect of a vigilance-related increase in cerebral spontaneous activity on external stimuli processing.35 Impaired functional connectivity between both structures in PVS highlights functional dysfunction which may underlie altered conscious perception.
The ARAS is located at a critical juncture in the inflow of sensory information and can modulate conscious states.22 Assessing dynamic changes of brain function between a relaxed awake resting state and an attention-demanding task showed a significant increase of rCBF in the ARAS36 and an opposite pattern in the precuneus.11–13 In healthy subjects, the fluctuations of activity in these 2 structures (precuneus and ARAS) in the basal state are opposite and are thought to predict whether a somatosensory stimulus will be consciously perceived.3 Our study shows that these 2 structures interact also during a sensory stimulus. Circadian promotion of alertness is associated with increased relative metabolism in the ARAS and decreased relative metabolism in posterior cortical regions, including the precuneus.37 For example, several works suggest that a synchronized transition between the waking state and non-REM sleep can be established only if these regions interact in a well-balanced way.38 Finally, it should be mentioned that, in patients with multiple sclerosis, some studies have shown the involvement of ARAS in the impairment of attentional processes39 and conscious perception of external stimuli.24
Using H2O15 PET while electrically stimulating the median nerve, a significantly smaller degree of activation has been identified in brain associative areas in patients in PVS compared with control subjects.10 In addition, it seems that an impaired functional connectivity between primary cortex and associative structures is characteristic of the patient group. The authors interpreted these results as being linked to the functional isolation of high-level integrative structures which would be essential for accessing consciousness.1,18,20
Our experiment, based on a different and more ecologic stimulation (proprioceptive stimulation), seems to confirm this hypothesis. For the stimulation (passive movement), a special device was used that could selectively activate brain regions related to proprioception.7 It is important to point out that, in our case, the stimulus was nonpainful, which obviates concerns associated with whether such patients, who cannot communicate, experience pain.1 We identified a smaller degree of activation of high-level associative structures (BA 40 on both sides) in patients in PVS despite a comparable recruitment of the primary cortex (contralateral S1M1). Interestingly, in the PVS group, an impaired functional connectivity was found between the contralateral S1M1 and bilateral BA 40 which was different between the ipsilateral and contralateral sides in patients in PVS, preserved in the contralateral hemisphere and abolished in the ipsilateral one (figure 3). This discrepancy with previous work1 may account for the difference between stimuli (noxious vs nonnoxious). Thus, preserved functional connectivity between S1M1 and BA 40 in the case of an intermediate level of consciousness, as in a minimally conscious state,40 must be interpreted with caution and not as an objective evidence of a putative pain perception capacity.
At present, we have several proofs of the existence of a link between the development of perceptual awareness and the interaction of the sensory cortices with a high-level frontoparietal network. However, the role of the sensory cortices in relation to high-level structures is still a controversial issue. Some patients in PVS do seem to have “islands of brain activation” within the associative structures.27 Future studies in this area will need to address the following questions: What is the minimal degree of complexity of the underlying neural network? What are the precise roles of functional connectivity in the corticocortical and corticosubcortical connections during altered states of consciousness? What is the diagnostic and prognostic value of the functional lesions that are identified?
AUTHOR CONTRIBUTIONS
Statistical analysis was performed by Dr. Isabelle Loubinoux, INSERM U825, Purpan CHU Hospital, Toulouse, France.
ACKNOWLEDGMENT
The authors thank G. Viallard, PhD, S. Balduick, PhD, and H. Gros, PhD, from INSERM UMR 825, Toulouse, France, for technical assistance.
DISCLOSURE
Dr. Silva and Dr. Alacoque report no disclosures. Dr. Woods receives research support from the NIH [NCRR 5 P41 RR013642 (Coinvestigator), NICHD, 1R01HD46740-01A1 (Coinvestigator), U54 RR21813 (Coinvestigator), U54 RR21813 (Co-PI), NIMH P01 EB001955 (Coinvestigator), and NCRR 2R01RR016300-03 (Coinvestigator)]. Dr. Fourcade serves as Associate Redactor for Annales Françaises Anesthésie Réanimation. Dr. Samii and Dr. Marque report no disclosures. Dr. Mazziotta has served/serves on scientific advisory boards for Cure Alzheimer's Fund, Investment Company of America, Pierson Lovelace Foundation, Austin Hospital University of Melbourne, USC Alzheimer's Disease Research Center, University of Michigan, Parkinson's Disease Research Center, UCSF Memory & Aging Center, University of Miami, Medical Imaging Center for Experimental Neuroscience, UCLA Alzheimer's Disease Center, Cure Autism Now, Hereditary Disease Foundation, John Douglas French Alzheimer's Foundation, National Foundation for Brain Research, Organization for Human Brain Mapping, ABC News, AstraZeneca, BioBarrier, Inc., Brainsonix, and Partners in Discovery; has received funding for travel from Investment Company of America and AstraZeneca; serves as Deputy Editor of Current Opinions in Neurology and on the editorial boards of the American Journal of Bioethics–Neuroscience, Brain Structure & Function, Clinical Neuroscience Research, Experimental Neurology, Human Brain Mapping, Neurobiology of Disease, Neurology Today, and The Neuroscientist; has received license fee payments and receives royalties on US Patent 5,008,546, issued 1991: Intraoperative High Energy Beta Probe for Tumor Detection; receives royalties from publishing Brain Mapping: The Methods (Academic Press, 1996), Human Brain Function (Academic Press, 1997), Brain Mapping: The Systems (Academic Press, 2000), Brain Mapping: The Disorders (Academic Press, 2000), and Brain Mapping: The Methods (Academic Press, Elsevier Sciences, Amsterdam, 2nd edition, 2002); has received honoraria for lectures or educational activities not funded by industry; reports that 100% of his clinical effort is spent on imaging (PET, MRI, TMS, and EEG); and receives Board of Directors compensation and stock options in Brain Mapping Technologies, Inc. Dr. Chollet and Dr. Loubinoux report no disclosures.
Supplementary Material
Glossary
- ARAS
ascending reticular activating system
- BA
Brodmann area
- DMN
default-mode network
- FWE
family-wise error
- IPL
inferior parietal lobule
- PVS
persistent vegetative state
- rCBF
regional cerebral blood flow
- S1M1
primary sensorimotor cortex
- SVC
small volume correction.
Footnotes
Supplemental data at www.neurology.org
Study funding: Supported by INSERM, which had no role in the study design, data collection, data analysis, data interpretation, or writing of this report.
Disclosure: Author disclosures are provided at the end of the article.
Received July 11, 2009. Accepted in final form November 5, 2009.
REFERENCES
- 1.Laureys S, Faymonville ME, Peigneux P, et al. Cortical processing of noxious somatosensory stimuli in the persistent vegetative state. Neuroimage 2002;17:732–741. [PubMed] [Google Scholar]
- 2.Laureys S, Goldman S, Phillips C, et al. Impaired effective cortical connectivity in vegetative state: preliminary investigation using PET. Neuroimage 1999;9:377–382. [DOI] [PubMed]
- 3.Boly M, Balteau E, Schnakers C, et al. Baseline brain activity fluctuations predict somatosensory perception in humans. Proc Natl Acad Sci USA 2007;104:12187–12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Medical aspects of the persistent vegetative state (2). The Multi-Society Task Force on PVS. N Engl J Med 1994;330:1572–1579. [DOI] [PubMed] [Google Scholar]
- 5.Medical aspects of the persistent vegetative state (1). The Multi-Society Task Force on PVS. N Engl J Med 1994;330:1499–1508. [DOI] [PubMed] [Google Scholar]
- 6.Wade DT, Johnston C. The permanent vegetative state: practical guidance on diagnosis and management. BMJ 1999;319:841–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alary F, Doyon B, Loubinoux I, et al. Event-related potentials elicited by passive movements in humans: characterization, source analysis, and comparison to fMRI. Neuroimage 1998;8:377–390. [DOI] [PubMed]
- 8.Carel C, Loubinoux I, Boulanouar K, et al. Neural substrate for the effects of passive training on sensorimotor cortical representation: a study with functional magnetic resonance imaging in healthy subjects. J Cereb Blood Flow Metab 2000;20:478–484. [DOI] [PubMed]
- 9.Mima T, Sadato N, Yazawa S, et al. Brain structures related to active and passive finger movements in man. Brain 1999;122(pt 10):1989–1997. [DOI] [PubMed] [Google Scholar]
- 10.Weiller C, Jueptner M, Fellows S, et al. Brain representation of active and passive movements. Neuroimage 1996;4:105–110. [DOI] [PubMed] [Google Scholar]
- 11.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA 2003;100:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci 2001;2:685–694. [DOI] [PubMed]
- 13.McKiernan KA, D'Angelo BR, Kaufman JN, Binder JR. Interrupting the “stream of consciousness”: an fMRI investigation. Neuroimage 2006;29:1185–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friston K, Worsley KJ, Frackowiak RSJ, Mazziotta JC, Evans AC. Assessing the significance of focal activations using their spatial extent. Hum Brain Mapp 1994;1:214–220. [DOI] [PubMed]
- 15.Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab 1992;12:900–918. [DOI] [PubMed] [Google Scholar]
- 16.Conchou F, Loubinoux I, Castel-Lacanal E, et al. Neural substrates of low-frequency repetitive transcranial magnetic stimulation during movement in healthy subjects and acute stroke patients: a PET study. Hum Brain Mapp 2009;30:2542–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage 1997;6:218–229. [DOI] [PubMed] [Google Scholar]
- 18.Laureys S, Boly M. What is it like to be vegetative or minimally conscious? Curr Opin Neurol 2007;20:609–613. [DOI] [PubMed]
- 19.Laureys S, Faymonville ME, Moonen G, Luxen A, Maquet P. PET scanning and neuronal loss in acute vegetative state. Lancet 2000;355:1825–1826; author reply 1827. [DOI] [PubMed] [Google Scholar]
- 20.Laureys S, Owen AM, Schiff ND. Brain function in coma, vegetative state, and related disorders. Lancet Neurol 2004;3:537–546. [DOI] [PubMed] [Google Scholar]
- 21.Laureys S, Lemaire C, Maquet P, Phillips C, Franck G. Cerebral metabolism during vegetative state and after recovery to consciousness. J Neurol Neurosurg Psychiatry 1999;67:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steriade M. Arousal: revisiting the reticular activating system. Science 1996;272:225–226. [DOI] [PubMed] [Google Scholar]
- 23.Dehaene S, Naccache L. Towards a cognitive neuroscience of consciousness: basic evidence and a workspace framework. Cognition 2001;79:1–37. [DOI] [PubMed] [Google Scholar]
- 24.Reuter F, Del Cul A, Malikova I, et al. White matter damage impairs access to consciousness in multiple sclerosis. Neuroimage 2009;44:590–599. [DOI] [PubMed] [Google Scholar]
- 25.Laureys S, Faymonville ME, Luxen A, Lamy M, Franck G, Maquet P. Restoration of thalamocortical connectivity after recovery from persistent vegetative state. Lancet 2000;355:1790–1791. [DOI] [PubMed] [Google Scholar]
- 26.Owen AM, Coleman MR, Boly M, Davis MH, Laureys S, Pickard JD. Using functional magnetic resonance imaging to detect covert awareness in the vegetative state. Arch Neurol 2007;64:1098–1102. [DOI] [PubMed] [Google Scholar]
- 27.Qiu J. Probing islands of consciousness in the damaged brain. Lancet Neurol 2007;6:946–947. [DOI] [PubMed] [Google Scholar]
- 28.Andrews K, Murphy L, Munday R, Littlewood C. Misdiagnosis of the vegetative state: retrospective study in a rehabilitation unit. BMJ 1996;313:13–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Childs NL, Mercer WN, Childs HW. Accuracy of diagnosis of persistent vegetative state. Neurology 1993;43:1465–1467. [DOI] [PubMed] [Google Scholar]
- 30.Baars BJ, Ramsoy TZ, Laureys S. Brain, conscious experience and the observing self. Trends Neurosci 2003;26:671–675. [DOI] [PubMed] [Google Scholar]
- 31.Naccache L. Psychology: is she conscious? Science 2006;313:1395–1396. [DOI] [PubMed] [Google Scholar]
- 32.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain 2006;129:564–583. [DOI] [PubMed] [Google Scholar]
- 33.Maquet P. Functional neuroimaging of normal human sleep by positron emission tomography. J Sleep Res 2000;9:207–231. [DOI] [PubMed] [Google Scholar]
- 34.Fiset P, Paus T, Daloze T, et al. Brain mechanisms of propofol-induced loss of consciousness in humans: a positron emission tomographic study. J Neurosci 1999;19:5506–5513. [DOI] [PMC free article] [PubMed]
- 35.Dehaene S, Changeux JP. Ongoing spontaneous activity controls access to consciousness: a neuronal model for inattentional blindness. PLoS Biol 2005;3:e141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kinomura S, Larsson J, Gulyas B, Roland PE. Activation by attention of the human reticular formation and thalamic intralaminar nuclei. Science 1996;271:512–515. [DOI] [PubMed] [Google Scholar]
- 37.Buysse DJ, Nofzinger EA, Germain A, et al. Regional brain glucose metabolism during morning and evening wakefulness in humans: preliminary findings. Sleep 2004;27:1245–1254. [DOI] [PubMed] [Google Scholar]
- 38.Nofzinger EA, Buysse DJ, Miewald JM, et al. Human regional cerebral glucose metabolism during non-rapid eye movement sleep in relation to waking. Brain 2002;125:1105–1115. [DOI] [PubMed] [Google Scholar]
- 39.Gadea M, Martinez-Bisbal MC, Marti-Bonmati L, et al. Spectroscopic axonal damage of the right locus coeruleus relates to selective attention impairment in early stage relapsing-remitting multiple sclerosis. Brain 2004;127:89–98. [DOI] [PubMed] [Google Scholar]
- 40.Boly M, Faymonville ME, Schnakers C, et al. Perception of pain in the minimally conscious state with PET activation: an observational study. Lancet Neurol 2008;7:1013–1020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


