Abstract
Prevalence of cirrhosis among older adults is expected to increase; therefore, we studied the health status, functional disability, and need for supportive care in a large national sample of individuals with cirrhosis. A prospective cohort of individuals with cirrhosis was identified within the longitudinal, nationally representative Health and Retirement Study (HRS). Cirrhosis cases were identified in linked Medicare data via ICD-9-CM codes, and compared to an age-matched cohort without cirrhosis. Two primary outcome domains were assessed: 1) patients’ health status (perceived health status, comorbidities, healthcare utilization, and functional disability as determined by activities of daily living [ADLs] and instrumental activities of daily living [IADLs]), and 2) informal caregiving (hours of caregiving provided by a primary informal caregiver and associated cost). Adjusted negative binomial regression was used to assess the association between cirrhosis and functional disability. 317 individuals with cirrhosis and 951 age-matched comparators were identified. Relative to the comparison group, individuals with cirrhosis had worse self-reported health status, more comorbidities, and used significantly more health care services (hospitalizations, nursing home stays, physician visits; p<0.001 for all bivariable comparisons). They also had greater functional disability (p<0.001 for ADLs and IADLs), despite adjustment for covariates such as comorbidities and healthcare utilization. Individuals with cirrhosis received over twice the number of informal caregiving hours per week (p<0.001), at an annual cost of $4,700 per person.
Conclusion
Older Americans with cirrhosis have high rates of disability, health care utilization, and need for informal caregiving. Improved care coordination and caregiver support is necessary to optimize management of this frail population.
Keywords: Elderly, Chronic liver disease, Disability, Informal Caregiving, Healthcare utilization
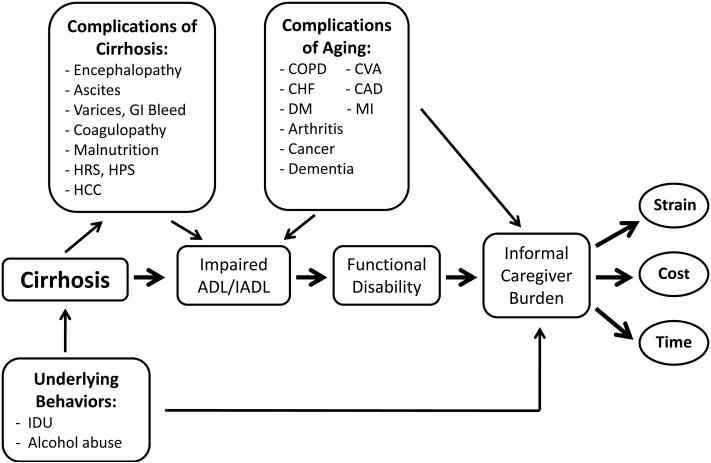
The prevalence of cirrhosis among older adults is expected to increase (1), in part due to the rising incidence of nonalcoholic fatty liver disease and the aging of the hepatitis C population (2,3). Patients with cirrhosis, especially those with age-related comorbidities, experience several potentially debilitating complications which can have a significant impact on activities of daily living (ADLs, such as the ability to dress oneself) and instrumental activities of daily living (IADLs, such as the ability to manage shopping or housework). These impairments-- combined with the associated complex regimen of dietary restrictions, medications, laboratory testing and clinic visits-- make management of cirrhosis in the elderly very complex (4). Furthermore, optimal home-based care is limited without caregivers who can help supplement the care that clinicians provide (5). Figure 1 presents a conceptual framework demonstrating how cirrhosis-related complications, underlying psychosocial/behavioral issues, and aging might contribute to increased caregiver time and burden.
Figure 1.
Conceptual framework demonstrating how cirrhosis-related complications, underlying psychosocial/behavioral issues, and aging contribute to increased informal caregiver burden. Abbreviations: GI, gastrointestinal; HRS, hepatorenal syndrome; HPS, hepatopulmonary syndrome; COPD, chronic obstructive pulmonary disease; CHF, congestive heart failure; DM, diabetes mellitus; CVA, cerebrovascular accident; CAD, coronary artery disease; MI, myocardial infarction, ADL, activity of daily living; IADL, instrumental activity of daily living, IDU; intravenous drug use.
The importance of informal caregiving by family members has been well described for patients with other chronic diseases such as diabetes, congestive heart failure, and stroke. Caregiver involvement improves patient outcomes (6), and interventions can increase caregiver effectiveness (7-9). Informal caregiving for these conditions has also been shown to cause significant economic and health burdens for the caregivers (10-16). For older adults with cirrhosis, the degree of functional impairment and involvement of informal caregivers has not been well described. The aim of the current study was to use a unique, large national dataset to assess health status and functional disability of older individuals with cirrhosis and its complications, as well as estimate the burden and cost of informal caregiving in this population.
EXPERIMENTAL PROCEDURES
Human Subjects
This study was conducted using prospectively collected data from the Health and Retirement Study (HRS) linked to the Center for Medicare and Medicaid Services (CMS) standard analytic files. The HRS is a biennial, longitudinal survey of a nationally representative cohort of U.S. adults over 50 years of age. The HRS includes more than 22,000 Americans, with interviews performed every two years, providing detailed information on participants’ functional condition, health status, and caregiver assistance. The HRS has been used previously to characterize the functioning and caregiver support for individuals with chronic diseases such as congestive heart failure and diabetes (10,17). HRS respondents that met the following criteria were included in the study population: 1) community-dwelling (i.e. those living in skilled nursing facilities or nursing homes were excluded), 2) completed an interview some time between 1998-2008, and 3) age ≥ 65 years at the time of the interview. Because HRS surveys may not accurately identify patients with cirrhosis, we linked surveys to Medicare claims using International Classification of Diseases (ICD) codes, as described below. The first CMS claim date on which a cirrhosis diagnosis was identified is referred to as the “index date.” The HRS interview following the index date is referred to as the “index HRS interview,” and was the source of information for the current study. Median time from “index date” to “index HRS interview” was 370 days (range 1-1,090 days) – cases without an interview within three years of the index date were excluded from the analysis.
Cirrhosis Cases
A set of ICD-9-CM codes were utilized to identify cases with cirrhosis and its complications. Individuals with cirrhosis were identified from all available Medicare claims files (Carrier, inpatient, outpatient, skilled nursing, home health, and hospice) between 1995-2007 as those individuals having at least one of the following ICD-9-CM claims for 1) cirrhosis: Alcoholic cirrhosis 571.2; Cirrhosis not due to alcohol 571.5; or 2) complications of cirrhosis: Hepatic encephalopathy 572.2; Ascites (789.5 until 2007, then 789.59); Hepatorenal syndrome 572.4; Esophageal varices with bleeding 456.0, 456.2; Esophageal varices without bleeding 456.1, 456.2; Portal hypertension 572.3; Hepatocellular carcinoma 155.0; and Spontaneous bacterial peritonitis 567.23. Individuals identified solely by ascites code were included only if they had two or more ascites claims on different days in a one year period.
This algorithm was then externally validated by reviewing patient charts for a sample of patients identified by ICD-9-CM codes and receiving care at the University of Michigan in 2008. Positive predictive value (PPV) of the ICD-9-CM codes was determined using a random sample of 100 outpatients and inpatients with these billing codes seen at the University of Michigan in 2008. A diagnosis of cirrhosis was determined by chart audit performed by a hepatologist based on a) compatible histology, b) imaging showing a cirrhotic liver with splenomegaly and a platelet count of <120,000/mm3, or c) evidence of decompensated cirrhosis with hepatic encephalopathy, hepatorenal syndrome, ascites, or variceal bleeding. Of those with a verified diagnosis of cirrhosis, further chart review was performed to determine if cirrhosis was compensated or decompensated at the time that the ICD-9-CM code was billed. A second cohort of patients was used to determine sensitivity of the ICD-9-CM codes. Inpatient and outpatient billing codes were assessed over the past two years for a random sample of 100 patients from another study for which cirrhosis patients had been enrolled prospectively. The above validity tests showed that our algorithm of ICD-9-CM codes had a positive predictive value of 88% and a sensitivity of 67%. Of the patients with a diagnosis of cirrhosis verified by chart review, 43% had compensated and 57% had decompensated cirrhosis at the time of coding, indicating that our algorithm of ICD-9-CM codes identified patients with both compensated and decompensated cirrhosis.
Comparison Group
An age-matched cohort of HRS respondents who did not have cirrhosis served as a comparison group. Each cirrhosis case was matched by age with three comparators, drawn from the pool of HRS respondents completing surveys during the same period and enrolled in Medicare Parts A&B FFS (Fee-For-Service) in the month of the index date, but without any Medicare claims indicating cirrhosis.
Data Analysis
Two primary outcome domains were assessed: 1) patients’ health status (perceived health status, comorbidities, healthcare utilization, and functional disability), and 2) informal caregiving (hours of caregiving provided by a primary informal caregiver and associated cost). In order to determine degree of functional decline over time, change in functional disability and hours of informal caregiving was measured over the time period between the HRS interview before and after the index date (first date of cirrhosis detection by ICD-9-CM code).
Self-reported comorbid medical illnesses included hypertension, diabetes, cancer, chronic lung disease (asthma, chronic obstructive lung disease), heart disease, stroke, and arthritis. Cognitive function was measured using a validated screening test for cognitive function (35-point scale including tests of memory, serial 7 subtractions, naming, and orientation) (18). Although objective testing was used for cognitive assessment, it is important to note that these tests do not differentiate between impairment due to hepatic encephalopathy or competing etiologies such as Alzheimers or alcohol-related dementia. Based on these tests, cognitive function was categorized using accepted cut-points into three levels of functioning: normal, mild-moderate impairment, and severe impairment. Healthcare utilization was measured by the number of subjects that required hospitalization, nursing home stay, or home health services during the two years prior to the index HRS survey. Number of physician visits as well as outof-pocket medical expenses over two years was also recorded.
Subjects’ ability to perform tasks of daily living was assessed within the two commonly recognized domains of activities of daily living (ADLs) and instrumental activities of daily living (IADLs). ADLs include the following 6 activities: eating, dressing, bathing, toileting, getting in and out of bed, and mobility inside own residence. ADLs were considered impaired if the subject reported difficulty performing or receiving help with any of the above 6 activities. IADLs include the following 5 activities: cooking, grocery shopping, taking medications, managing money, and using the telephone. IADLs were considered impaired if the subject reported difficulty performing or receiving help with any of the above 5 activities.
An informal caregiver was defined as a family member or unpaid relative with no organizational affiliation who provided in-home care to the respondent. Data recorded for the caregiver included gender and relationship to the respondent. The number of hours per week of informal caregiving was calculated using the average number of days per week and the average number of hours per day that the subject reported receiving informal care in the past month. The hours of caregiving were averaged across all subjects, including those who did not receive any caregiving.
Demographic data included gender, age, race, ethnicity, living situation (married, unmarried living with others, unmarried living alone), presence of children that live within 10 miles, years of education, insurance other than Medicare/Medicaid, and household net worth.
Cost of informal care
The annual cost of informal caregiving can be estimated using opportunity cost, which accounts for the cost of hours sacrificed by the informal caregiver in order to care for a patient by applying a market wage rate to caregiving activities (19,20). Opportunity cost can be estimated using the wage of an equivalent service, such as that of a home health aide. The yearly opportunity cost of informal caregiving for individuals with cirrhosis was estimated by multiplying the median hourly national wage for a home health aide ($9.85) (21) × the weekly hours of informal caregiving × 52 (weeks per year). Upper and lower bound cost estimates for informal caregiving were created using 10th and 90th percentile hourly wage for a home health aide, respectively (10th percentile: $7.67; 90th percentile: $14.13). Cost estimates were averaged across all subjects, including those who did not receive any caregiving.
Statistical Analysis
Descriptive bivariate statistics were analyzed using chi squared and F-tests for categorical and continuous variables respectively. Negative binomial regression was used to estimate the independent effect of cirrhosis on number of impaired ADLs (and IADLs). Based on regression results we calculated incident rate ratios (IRR), which is the ratio of the incidence rate of impaired ADLs (or IADLs) in individuals with cirrhosis relative to the rate of impairment among individuals without cirrhosis. An IRR >1 indicates that cirrhosis is associated with increased impairment in functional status compared to their age-matched controls. The model was adjusted for potential confounders known to be associated with cirrhosis and independently associated with poor functional status (age, gender, race, ethnicity, schooling, net worth, living arrangement, comorbidities, and insurance other than Medicare/Medicaid). Comorbidities were entered into the model as seven separate binary indicators, one for each comorbid condition. Cognitive impairment was intentionally excluded from this model since neurocognitive dysfunction may directly result from cirrhosis and thus be a pathway to disability rather than a confounder. To determine whether health care utilization confounded the association between cirrhosis and disability, a sensitivity analysis was performed by creating an interaction variable between presence of cirrhosis and number of physician visits (over two years) and including it as a covariate in the regression model. All analyses were carried out using SAS 9.1.3 (Cary, NC) and were adjusted for the matched case-comparator design. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the appropriate institutional review committee.
RESULTS
Study Population Characteristics
We identified 317 cirrhosis cases and 951 comparators in the linked HRS-Medicare data. Relative to the comparison group, individuals with cirrhosis were more likely to be Hispanic (p<0.001), have less education (p=0.001), and have lower net worth (p=0.040) (Table 1). The two groups were similar with respect to the proportion with insurance other than Medicare/Medicaid (p=0.091).
Table 1.
Demographic characteristics of patients with cirrhosis and comparison group1
| Variable | Cirrhosis Cases (n=317) | Comparison group (n=951) | P-value |
|---|---|---|---|
| Age | 74.7 ± 0.4 | 74.7 ± 0.2 | 0.68 |
| Gender (Male) | 143 (45.1) | 393 (41.3) | 0.24 |
| Race/Ethnicity | |||
| White | 227 (71.6) | 797 (83.8) | <0.001 |
| Black | 45 (14.2) | 98 (10.3) | |
| Hispanic | 40 (12.6) | 49 (5.2) | |
| Other | 5 (1.6) | 7 (0.7) | |
| Education (years) | 11.0 ± 0.2 | 11.7 ± 0.1 | 0.001 |
| Living Arrangement | |||
| Married, living with spouse | 172 (54.3) | 553 (58.2) | 0.35 |
| Unmarried, living with others | 50 (15.8) | 117 (12.3) | |
| Unmarried, living alone | 95 (30.0) | 281 (29.6) | |
| Children within 10 miles | 203 (65.1) | 555 (59.7) | 0.14 |
| Household net worth2 ($) | 102,439 (27,600-292,500) | 181,513 (43,926-493,178) | 0.04 |
Data presented as (mean ± S.E.) for continuous variables, n (%) for categorical variables
Median (interquartile range)
Health Status and Functional Disability
Individuals with cirrhosis had a greater number of medical comorbidities (p<0.001) than comparison group, worse perceived health status (p<0.001) and more severe cognitive impairment (p=0.001) (Table 2). They also required more than double the health care services (hospitalizations, nursing home stays, and physician visits; p<0.001 for all) and had significantly higher out of pocket medical expenses (p=0.001) compared to those without cirrhosis, yet only 25% reported receiving home health services. One quarter of individuals with cirrhosis reported their health status as “poor,” compared to only 11% of those without cirrhosis (p<0.001 for the trend, Table 2).
Table 2.
Self reported health status and medical conditions of patients with cirrhosis and comparison group1
| Variable | Cirrhosis Cases (n=317) | Comparison group (n=951) | P-value |
|---|---|---|---|
| Perceived health status | |||
| Excellent | 16 (5.1) | 96 (10.1) | <0.001 |
| Very good/good/fair | 223 (70.6) | 747 (78.6) | |
| Poor | 77 (24.3) | 107 (11.3) | |
| Perceived pain | |||
| None | 187 (59.0) | 669 (70.7) | <0.001 |
| Mild/moderate | 96 (30.3) | 212 (22.4) | |
| Severe | 34 (10.7) | 65 (6.9) | |
| Medical comorbidities | |||
| Hypertension | 185 (58.4) | 534 (56.3) | 0.55 |
| Diabetes | 97 (30.6) | 169 (17.8) | <0.001 |
| Cancer | 74 (23.4) | 153 (16.1) | 0.005 |
| Lung disease | 56 (17.7) | 96 (10.1) | <0.001 |
| Heart disease | 133 (42.1) | 290 (30.6) | <0.001 |
| Stroke | 51 (16.1) | 105 (11.1) | 0.02 |
| Arthritis | 212 (66.9) | 589 (62.0) | 0.10 |
| # Medical comorbidities | 2.6 ± 0.08 | 2.0 ± 0.04 | <0.001 |
| Cognitive impairment | |||
| None | 175 (55.4) | 635 (66.8) | 0.001 |
| Mild-Moderate | 92 (29.1) | 204 (21.5) | |
| Severe | 49 (15.5) | 112 (11.8) | |
| Hospital stay 2 | 212 (66.9) | 311 (32.7) | <0.001 |
| Nursing home stay 2 | 55 (17.4) | 53 (5.6) | <0.001 |
| Home health services 2 | 74 (24.8) | 98 (10.6) | <0.001 |
| # Physician visits 2 | 20.5 ± 2.4 | 10.8 ± 0.5 | <0.001 |
| Out of pocket medical expenses2,3 ($) | 2,150 (526-5,379) | 1,459 (433-3,709) | 0.001 |
Data presented as (mean ± S.E.) for continuous variables, n (%) for categorical variables
Over the past 2 years, self-reported
Median (interquartile range)
Individuals with cirrhosis had greater impairment of ADLs compared to the comparison group (p<0.001), with 38% indicating at least one impaired ADL (Table 3). Overall, 14% of individuals with cirrhosis could perform only 0-2 of their ADLs (i.e. 4-6 impaired ADLs). IADLs were similarly impaired, with 10% of individuals with cirrhosis able to perform only 0-1 IADLs (i.e. 4-5 impaired IADLs). The most common ADL and IADL impairments among those with cirrhosis were “dressing” and “grocery shopping” respectively. After adjusting for covariates associated with functional disability, having cirrhosis was independently associated with impaired ADLs (adjusted IRR=1.50, p=0.004) and impaired IADLs (adjusted IRR=1.72, p<0.001) (Table 4). In other words, after adjustment those with cirrhosis experienced 1.50 times more ADL impairments and 1.72 times more IADL impairments compared to those without cirrhosis. Sensitivity analysis using an interaction variable between cirrhosis and number of physicians visits revealed no significant interaction between cirrhosis and healthcare utilization (ADL model: p=0.33; IADL model: p=0.80). In fact, greater use of health care services correlated more strongly with disability among the comparison group than the cirrhosis group (data not shown). Thus, healthcare utilization does not confound the independent association between cirrhosis and disability.
Table 3.
Functional status of patients with cirrhosis and comparison group1
| Variable | Cirrhosis Cases (n=317) | Comparison group (n=951) | P-value |
|---|---|---|---|
| # of impaired ADLs | |||
| 0 | 195 (61.5) | 722 (75.9) | <0.001 |
| 1-3 | 79 (24.9) | 166 (17.5) | |
| 4-6 | 43 (13.6) | 63 (6.6) | |
| Type of ADL impairment | |||
| Walking across room | 63 (19.9) | 110 (11.6) | 0.001 |
| Dressing | 76 (24.0) | 139 (14.6) | <0.001 |
| Bathing | 71 (22.4) | 114 (12.0) | <0.001 |
| Transferring | 46 (14.6) | 86 (9.1) | 0.005 |
| Toileting | 52 (16.5) | 91 (9.6) | <0.001 |
| Eating | 36 (11.4) | 58 (6.1) | 0.002 |
| Loss of ADLs over time 2,3 | |||
| < 0 | 25 (8.6) | 66 (7.7) | 0.001 |
| 0 | 180 (62.1) | 641 (74.7) | |
| 1 | 32 (11.0) | 76 (8.9) | |
| >2 | 53 (18.3) | 75 (8.7) | |
| # of impaired IADLs | |||
| 0 | 204 (64.4) | 753 (79.3) | <0.001 |
| 1-3 | 80 (25.2) | 146 (15.5) | |
| 4-5 | 33 (10.4) | 50 (5.3) | |
| Type of IADL impairment | |||
| Grocery shopping | 91 (30.3) | 135 (14.8) | <0.001 |
| Managing money | 65 (21.5) | 96 (10.5) | <0.001 |
| Cooking | 63 (21.7) | 102 (11.6) | <0.001 |
| Taking medication | 41 (13.1) | 47 (5.1) | <0.001 |
| Using the telephone | 38 (12.1) | 77 (8.2) | 0.026 |
| Loss of lADLs over time 2,3 | |||
| < 0 | 13 (4.5) | 42 (5.0) | <0.001 |
| 0 | 187 (64.9) | 673 (79.3) | |
| 1 | 35 (12.2) | 68 (8.0) | |
| >2 | 53 (18.4) | 66 (7.8) | |
Data presented as (mean ± S.E.) for continuous variables, n (%) for categorical variables
< 0 indicates gain in ADLs (functional improvement); >0 indicates loss of ADLs (functional decline)
Median = 2.1 years (time between pre-index date survey and post-index date survey).
Cirrhosis cases: n=290, Comparison group: n=858
Table 4.
Incident rate ratio (IRR) of the association between cirrhosis and functional disability.
| IRR 1 | Cirrhosis | No cirrhosis | P-value |
|---|---|---|---|
| Impaired ADLs | |||
| Unadjusted | 1.73 (1.38-2.15) | Ref. | <0.001 |
| Adjusted2 | 1.50 (1.14-1.97) | Ref. | 0.004 |
| Impaired IADLs | |||
| Unadjusted | 1.95 (1.55-2.46) | Ref. | <0.001 |
| Adjusted2 | 1.72 (1.30-2.29) | Ref. | <0.001 |
IRR >1 indicates that cirrhosis is associated with higher incidence rate of functional disability (impaired ADL or IADL) compared to no cirrhosis.
Adjusted for age, gender, race, ethnicity, schooling, net worth, living arrangement, medical comorbidities, and insurance other than Medicare/Medicaid.
Receipt of Informal Care
One-third of individuals with cirrhosis identified a caregiver (formal or informal), with less than 10% of patients receiving formal (paid) care. Individuals with cirrhosis received over twice the informal caregiving hours relative to the comparison group (p<0.001), with informal care most often provided by the subjects’ children (Table 5).
Table 5.
Patterns of receiving care for patients with cirrhosis and comparison group1
| Variable | Cirrhosis Cases (n=317) | Comparison group (n=951) | P-value |
|---|---|---|---|
| Receiving care | |||
| Informal | 99 (31.2) | 157 (16.5) | <0.001 |
| Formal | 29 (9.2) | 47 (4.9) | 0.006 |
| Any (formal or informal) | 105 (33.1) | 170 (17.9) | <0.001 |
| Gender of informal caregiver 2 | |||
| Male | 38 (38.4) | 66 (42.0) | 0.56 |
| Female | 73 (73.7) | 114 (72.6) | 0.84 |
| Relationship of informal caregiver to patient 2 | |||
| Child/children | 57 (57.6) | 93 (59.2) | 0.79 |
| Spouse | 41 (41.4) | 55 (35.0) | 0.30 |
| Grandchild | 6 (6.1) | 14 (8.9) | 0.41 |
| Other caregiver | 20 (20.2) | 31 (19.8) | 0.93 |
| Informal care received (hours per week) | 9.14 ± 1.42 | 4.00 ± 0.59 | <0.001 |
| Increase in informal care received over time3 (hours per week) | 6.78 ± 1.32 | 2.75 ± 0.56 | <0.001 |
Data presented as (mean ± S.E.) for continuous variables, n (%) for categorical variables
Patient was able to indicate more than one informal caregiver; Among those with at least one informal caregiver
Median = 2.1 years (time between pre-index date survey and post-index date survey).
Cirrhosis cases: n=290, Comparison group: n=858
Change in Functional Status and Caregiving Over Time
Change in functional status and caregiving was determined using data from the HRS interview before and after the index date (first date of cirrhosis detection by ICD-9-CM code). Median time from the pre-index date interview until the post-index date interview was 775 days (2.1 years), range 474-1,853 days. Cases without an interview within three years prior to the index date were excluded from this pre-post analysis. All cirrhosis cases and comparators completed an HRS survey after the index date, however some did not have an HRS survey performed prior to the index date. Therefore, 9% of cirrhosis patients and 10% of controls were excluded from these analyses leaving a sample of 290 cirrhosis cases and 858 comparators. Nearly 30% of patients with cirrhosis demonstrated functional decline over the pre-post time period (median 2.1 years), as defined by loss of at least one or more ADL. Moreover, 18% of individuals with cirrhosis had severe functional decline (loss of two or more ADLs), doubling that of the age-matched comparison group (Table 3). A similar rate of functional decline was seen for IADLs (Table 3). Over the pre-post time period, individuals with cirrhosis received 6.8 additional hours of informal caregiving per week, more than twice as much as the increase in the age-matched comparison group (Table 5).
Annual Cost of Informal Caregiving
Using the 2009 median national wage for a home health aide ($9.85 per hour20), the annual cost of informal caregiving for elderly individuals with cirrhosis was $4700 per person, compared to $2100 for age-matched elderly individuals without cirrhosis. Using the 10th and 90th percentile hourly national wage for a home health aide, annual cost of informal caregiving ranged $3700-$6700 for patients with cirrhosis and $1600-$2900 for patients without cirrhosis.
DISCUSSION
In this nationally representative sample of older Americans, we found that individuals with cirrhosis had significantly worse health status and greater functional disability compared to those without cirrhosis, requiring nearly twice the amount of informal caregiving at an annual societal cost of approximately $4,700 per individual. Nearly 20% of subjects with cirrhosis experienced severe functional decline (loss of two or more ADLs) over a median of approximately two years, more than doubling that of age-matched individuals without cirrhosis. As the incidence of nonalcoholic fatty liver disease increases and the hepatitis C population ages, cirrhosis among the elderly will become increasingly prevalent and imposes a significant burden to patients and their caregivers.
In addition to the potential burden to caregivers, individuals with cirrhosis also strain the health care system. Annually, cirrhosis results in 50,000 hospitalizations (22); of those who survive hospitalization, approximately 20% are readmitted within 30 days (23). Our findings show that over two thirds of individuals with cirrhosis report being admitted to the hospital within the prior 2 years, which is twice that of age-matched individuals without cirrhosis. Moreover, less than one quarter of individuals with cirrhosis received home health care services after hospital discharge, indicating a potentially lost opportunity for improved care transitions. In other diseases, improving patient and caregiver knowledge about chronic disease management and integrating caregivers in the healthcare process have been shown to significantly decrease hospital admission rates (24,25). Our findings suggest that applying these concepts and services among patients with cirrhosis has the potential to result in significant cost savings.
It is important to emphasize this study compared subjects with cirrhosis to age-matched individuals, not healthy controls. As expected with advancing age, individuals in the comparison group had several comorbidities (e.g. arthritis 62%, cardiac disease 31%, diabetes 18%, cancer 16%; Table 2), all of which can be independently associated with significant functional decline and cost. Thus, the current study highlights the incremental disability, cost, and caregiver burden of cirrhosis, even relative to other serious chronic illnesses. Future research is needed to examine the role that specific cirrhosis-related complications (such as muscle wasting, hepatic encephalopathy, ascites) contribute to the overall disability found in this study.
To our knowledge, this is the first study to use a population-based sample to quantify functional disability and the impact on formal and informal care of individuals with cirrhosis. For comparison, a similar study of the HRS dataset showed that individuals with congestive heart failure require an average of 6.7 hours of informal care per week – 2.5 fewer hours per week than those with cirrhosis (10). Data such as these have been used to demonstrate the need and potential efficacy of innovative programs that provide caregiver training and education (26,27), improve communication between provider and patients or caregivers (e.g. telemedicine) (8,28), and create infrastructure for comprehensive chronic disease management (29) and post-discharge transitional care (30,31). As evidenced by our findings, patients with cirrhosis require similar support for basic activities such as bathing and taking medications, thereby necessitating the intervention of informal caregivers to help prevent potential poor outcomes (e.g. falls, missed appointments, medication noncompliance). Moreover, the significantly lower education level found in our study emphasizes that cirrhotics may have poor knowledge and coping strategies for managing their chronic disease, further contributing to functional disability. Currently there are few structured services that promote patient education and self-care or caregiver support for the cirrhosis population.
Our study has some limitations that warrant comment. While there are several studies that have defined cirrhosis using ICD-9 codes (32-25), prior methods have not been validated. In order to maximize specificity, we selected a narrow spectrum of ICD-9-CM codes, therefore may have excluded patients with well compensated cirrhosis that are either unaware of diagnosis, asymptomatic with no prior history of decompensation, or who have limited interaction with the healthcare system. Similarly, it is possible that a small percent of the comparison group may undiagnosed cirrhosis. Additionally, our study population may have excluded patients that lack comorbidities that would prompt medical care for reasons other than cirrhosis. However, we would expect a similar phenomenon in the comparison group, therefore both groups may equally consist of “sicker” patients. Also, the current study lacked histological, laboratory or imaging data to confirm cirrhosis diagnosis. Although data such as medical comorbidities and health care utilization (hospitalization, nursing home, physician visits) were self-reported, several studies have demonstrated the accuracy of self-reported diagnoses (36-39). Finally, because cases were identified via linkage with the CMS database, our findings are limited to individuals with cirrhosis who are age 65 or greater.
Other limitations of our study involve our method for estimating cost, which likely resulted in an underestimate of the cost of informal caregiving. Estimates were based on caregiving from family members only, and did not include costs associated with caregiving from non-family members (e.g. friends, neighbors). Moreover, cost estimates were based only on assistance with ADLs and IADLs and did not include other time-consuming caregiving activities such as transportation to a hospital for clinic visits, laboratory tests, paracenteses or variceal banding. Similarly, our cost estimates do not include other significant caregiving costs such as out-of-pocket expenses related to medications, medical supplies, or lost wages (patient or caregiver) related to cirrhosis. A recent study by Bajaj et al highlighted these other cirrhosis-related expenses and the detrimental effect they can have on patients’ ability to adhere to medical recommendations (4).
This population-based study confirms the significant burden and cost that cirrhosis and its complications places upon the patient, caregiver, as well as the health care system. Clinicians should be aware of the increased need for informal caregiving among patients with cirrhosis, especially older individuals with other age-related comorbidities. Additionally, health care economists and policy makers should consider the significant functional limitations of this population as well as the substantial hours of informal caregiving required to help avert preventable poor outcomes related to the patients’ inability to independently manage their disease. Greater focus on a comprehensive delivery of care for patients with cirrhosis, including involvement of caregivers and improved care coordination, is necessary to optimize management of this frail population.
Acknowledgments
Financial Support: NIH T32 training grant DK62708 (MOR); NIH K23DK085204 and American Gastroenterology Research Scholar Award (MLV); NIH K08HL091249 (TJI); NIH R01 AG030155 (KML). John Piette is a VA Senior Research Career Scientist. The Health and Retirement Study is sponsored by the National Institute on Aging (U01 AG09740) and performed at the Institute for Social Research, University of Michigan.
Abbreviations
- ADL
activities of daily living
- IADL
instrumental activities of daily living
- PPV
positive predictive value
- CMS
Center for Medicare and Medicaid Services
- HRS
Health and Retirement Study
- IRR
incident rate ratio
Footnotes
Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the US government.
Disclosures: None. No conflicts of interest exist for all authors
Writing Assistance: not applicable
Author contributions: Each author was actively involved with the study concept and design, interpretation of data, and critical revision of the manuscript. Additionally, MOR was responsible for drafting the manuscript, KML was responsible for data acquisition and RJM was responsible for statistical analysis. All authors approved the final version of the manuscript.
REFERENCES
- 1.Goldacre MJ. Demography of aging and the epidemiology of gastrointestinal disorders in the elderly. Best Pract Res Clin Gastroenterol. 2009;23:793–804. doi: 10.1016/j.bpg.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–21. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 3.Davis GL, Later MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138:513–521. doi: 10.1053/j.gastro.2009.09.067. [DOI] [PubMed] [Google Scholar]
- 4.Bajaj JS, Wade JB, Gibson DP, Heuman DM, Thacker LR, Sterling RK, et al. The Multi-Dimensional Burden of Cirrhosis and Hepatic Encephalopathy on Patients and Caregivers. Am J Gastroenterol. 2011 May 10; doi: 10.1038/ajg.2011.157. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Volk ML, Piette JD, Singal AS, Lok AS. Chronic disease management for patients with cirrhosis. Gastroenterology. 2010;139:14–6.e1. doi: 10.1053/j.gastro.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith CE, Piamjariyakul U, Yadrich DM, Ross VM, Gajewski B, Williams AR. Complex home care: part III--economic impact on family caregiver quality of life and patients’ clinical outcomes. Nurs Econ. 2010;28:393–9. 414. [PMC free article] [PubMed] [Google Scholar]
- 7.Hepburn K, Lewis M, Tornatore J, Sherman CW, Bremer KL. The Savvy Caregiver program: the demonstrated effectiveness of a transportable dementia caregiver psychoeducation program. J Gerontol Nurs. 2007;33:30–6. doi: 10.3928/00989134-20070301-06. [DOI] [PubMed] [Google Scholar]
- 8.Grant JS, Elliott TR, Weaver M, Bartolucci AA, Giger JN. Telephone intervention with family caregivers of stroke survivors after rehabilitation. Stroke. 2002;33:2060–5. doi: 10.1161/01.str.0000020711.38824.e3. [DOI] [PubMed] [Google Scholar]
- 9.Chien LY, Chu H, Guo JL, Liao YM, Chang LI, Chen CH, Chou KR. Caregiver support groups in patients with dementia: a meta-analysis. Int J Geriatr Psychiatry. 2011 Feb. doi: 10.1002/gps.2660. doi: 10.1002/gps.2660. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Gure TR, Kabeto MU, Blaum CS, Langa KM. Degree of disability and patterns of caregiving among older Americans with congestive heart failure. J Gen Intern Med. 2007;23:70–76. doi: 10.1007/s11606-007-0456-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langa KM, Vijan S, Hayward RA, Chernew ME, Blaum CS, Kabeto MU, et al. Informal caregiving for diabetes and diabetic complications among elderly Americans. J Gerontol. 2002;57B:S177–S186. doi: 10.1093/geronb/57.3.s177. [DOI] [PubMed] [Google Scholar]
- 12.Rosland AM, Piette JD. Emerging models for mobilizing family support for chronic disease management: a structured review. Chronic Illness. 2010;6:7–21. doi: 10.1177/1742395309352254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hooley PJ, Butler G, Howlett JG. The relationship of quality of life, depression, and caregiver burden in outpatients with congestive heart failure. CHF. 2005;11:303–310. doi: 10.1111/j.1527-5299.2005.03620.x. [DOI] [PubMed] [Google Scholar]
- 14.Saunders MM. Factors associated with caregiver burden in heart failure family caregivers. West J Nurs Res. 2008;30(8):943–959. doi: 10.1177/0193945908319990. [DOI] [PubMed] [Google Scholar]
- 15.McCullagh E, Brigstocke G, Donaldson N, Kalra L. Determinants of caregiving burden and quality of life in caregivers of stroke patients. Stroke. 2005;36:2181–2186. doi: 10.1161/01.STR.0000181755.23914.53. [DOI] [PubMed] [Google Scholar]
- 16.Piette JD, Rosland AM, Silveira M, Kabeto M, Langa KM. The case for involving adult children outside of the household in the self-management support of older adults with chronic illness. Chronic Illness. 2010;6:34–45. doi: 10.1177/1742395309347804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vijan S, Hayward RA, Langa KM. The impact of diabetes on workforce participation: results from a national household sample. Health Serv Res. 2004;39:6:1653–1669. doi: 10.1111/j.1475-6773.2004.00311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–94. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arno PS, Levine C, Memmott MM. The economic value of informal caregiving. Health Affairs. 1999;18:182–188. doi: 10.1377/hlthaff.18.2.182. [DOI] [PubMed] [Google Scholar]
- 20.van den Berg B, Brouwer WB, Koopmanschap MA. Economic valuation of informal care. An overview of methods and applications. Eur J Health Econ. 2004;5:36–45. doi: 10.1007/s10198-003-0189-y. [DOI] [PubMed] [Google Scholar]
- 21.Bureau of Labor Statistics: Occupational Employment Statistics, Occupational Employment and Wages. 2009 May; http://www.bls.gov/oes/current/oes311011.htm.
- 22.Nguyen GC. Nationwide increase in hospitalizations and hepatitis C among inpatients with cirrhosis and sequelae of portal hypertension. Clin Gastroenterol Hepatol. 2007;5:1092–1099. doi: 10.1016/j.cgh.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 23.Berman K, Tandra S, Forssell K, Vuppalanch R, Burton JR, Jr, Nguyen J, et al. Incidence and predictors of 30-day readmission among patients hospitalized for advanced liver disease. Clin Gastroenterol Hepatol. 2011;9:254–9. doi: 10.1016/j.cgh.2010.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coleman EA, Williams MV. Executing high-quality care transitions: a call to do it right. J Hosp Med. 2007;2:287–9. doi: 10.1002/jhm.276. [DOI] [PubMed] [Google Scholar]
- 25.Gleckman H. Coordinating care: an elusive but critical goal. Managing chronic illnesses during transitions presents tough challenges. Health Prog. 2009;90:32–5. [PubMed] [Google Scholar]
- 26.Hickenbottom SL, Fendrick AM, Kutcher JS, Kabeto MU, Katz SJ, Langa KM. A national study of the quantity and cost of informal caregiving for the elderly with stroke. Neurology. 2002;58:1754–1759. doi: 10.1212/wnl.58.12.1754. [DOI] [PubMed] [Google Scholar]
- 27.Kalra L, Evans A, Perez I, Melbourn A, Patel A, Knapp M, et al. Training carers of stroke patients: randomized controlled trial. BMJ. 2004;328:1099–1103. doi: 10.1136/bmj.328.7448.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Evans RL, Matlock AL, Bishop DS, Stranahan A, Pederson C. Family intervention after stroke: does counseling or education help? Stroke. 1998;19:1243–1249. doi: 10.1161/01.str.19.10.1243. [DOI] [PubMed] [Google Scholar]
- 29.Demaerschalk BM. Telemedicine or Telephone Consultation in Patients with Acute Stroke. Curr Neurol Neurosci Rep. 2010;11:42–51. doi: 10.1007/s11910-010-0147-x. [DOI] [PubMed] [Google Scholar]
- 30.Dennis M, O'Rourke S, Slattery J, Staniforth T, Warlow C. Evaluation of a stroke family care worker: results of a randomized controlled trial. Br Med J. 1997;314:1071–1076. doi: 10.1136/bmj.314.7087.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson CS, Rubenach S, Mhurchu CN, Clark MS, Spencer C, Winsor A. Home or hospital for stroke rehabilitation? Results of a randomized controlled trail: Health outcomes at 6 months. Stroke. 2000;31:1024–1031. doi: 10.1161/01.str.31.5.1024. [DOI] [PubMed] [Google Scholar]
- 32.Bialke SR. Chronic liver disease among two American Indian patient populations in the southwestern United States, 2000-2003. J Clin Gastroenterol. 2008;42:949–54. doi: 10.1097/mcg.0b013e318054492a. [DOI] [PubMed] [Google Scholar]
- 33.Dangleben DA. Impact of cirrhosis on outcomes in trauma. J Am Coll Surg. 2006;203:908–13. doi: 10.1016/j.jamcollsurg.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 34.Ruhl CE, Everhart JE. Coffee and tea consumption are associated with a lower incidence of chronic liver disease in the United States. Gastroenterology. 2005;129:192836. doi: 10.1053/j.gastro.2005.08.056. [DOI] [PubMed] [Google Scholar]
- 35.Shim YK, Perper JA, Kuller LH. Factors associated with the decline in cirrhosis death rates among young adults in Allegheny County, Pennsylvania, 1973-1985. Am J Epidemiol. 1993;138:531–43. doi: 10.1093/oxfordjournals.aje.a116887. [DOI] [PubMed] [Google Scholar]
- 36.McAdams MA, Maynard JW, Baer AN, Köttgen A, Clipp S, Coresh J, Gelber AC. Reliability and sensitivity of the self-report of physician-diagnosed gout in the campaign against cancer and heart disease and the atherosclerosis risk in the community cohorts. J Rheumatol. 2011;38:135–41. doi: 10.3899/jrheum.100418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta V, Gu K, Chen Z, Lu W, Shu XO, Zheng Y. Concordance of self-reported and medical chart information on cancer diagnosis and treatment. BMC Med Res Methodol. 2011;11:72. doi: 10.1186/1471-2288-11-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dominguez FJ, Lawrence C, Halpern EF, Drohan B, Grinstein G, Black DM, Smith BL, Gadd MA, Specht M, Kopans DB, Moore RH, Hughes SS, Roche CA, Hughes KS. Accuracy of self-reported personal history of cancer in an outpatient breast center. J Genet Couns. 2007;16:341–5. doi: 10.1007/s10897-006-9067-y. [DOI] [PubMed] [Google Scholar]
- 39.Coolman M, de Groot CJ, Jaddoe VW, Hofman A, Raat H, Steegers EA. Medical record validation of maternally reported history of preeclampsia. J Clin Epidemiol. 2010;63:932–7. doi: 10.1016/j.jclinepi.2009.10.010. [DOI] [PubMed] [Google Scholar]