The water permeability of the cell plasma membrane is regulated by the presence of active aquaporins. This work demonstrates that the syntaxin SNARE SYP121 acts as a selective modulator of the maize aquaporin (PIP2;5) plasma membrane delivery and activity through physical interaction.
Abstract
Plasma membrane intrinsic proteins (PIPs) are aquaporins facilitating the diffusion of water through the cell membrane. We previously showed that the traffic of the maize (Zea mays) PIP2;5 to the plasma membrane is dependent on the endoplasmic reticulum diacidic export motif. Here, we report that the post-Golgi traffic and water channel activity of PIP2;5 are regulated by the SNARE (for soluble N-ethylmaleimide-sensitive factor protein attachment protein receptor) SYP121, a plasma membrane resident syntaxin involved in vesicle traffic, signaling, and regulation of K+ channels. We demonstrate that the expression of the dominant-negative SYP121-Sp2 fragment in maize mesophyll protoplasts or epidermal cells leads to a decrease in the delivery of PIP2;5 to the plasma membrane. Protoplast and oocyte swelling assays showed that PIP2;5 water channel activity is negatively affected by SYP121-Sp2. A combination of in vitro (copurification assays) and in vivo (bimolecular fluorescence complementation, Förster resonance energy transfer, and yeast split-ubiquitin) approaches allowed us to demonstrate that SYP121 and PIP2;5 physically interact. Together with previous data demonstrating the role of SYP121 in regulating K+ channel trafficking and activity, these results suggest that SYP121 SNARE contributes to the regulation of the cell osmotic homeostasis.
INTRODUCTION
Water uptake and maintenance of the cell osmotic homeostasis are major constraints encountered by living organisms. One of the protein families involved in the regulation of these processes is the aquaporin family. Aquaporins are small (28 to 34 kD) membrane proteins that form tetrameric channels facilitating the movement of water and/or small neutral solutes through cellular membranes (Gomes et al., 2009). Compared with mammals, plants express a high number of aquaporin isoforms, underlining their fundamental roles in growth, development, and abiotic stress tolerance (Maurel et al., 2008; Gomes et al., 2009; Heinen et al., 2009). Among the plant aquaporins, the plasma membrane intrinsic proteins (PIPs) are subdivided into two sequence-related groups, PIP1s and PIP2s, the members of which exhibit different water channel activities when expressed in Xenopus laevis oocytes (Chaumont et al., 2000; Temmei et al., 2005; Vandeleur et al., 2009; Bellati et al., 2010; Otto et al., 2010). When expressed alone, maize (Zea mays) PIP1s are inactive, whereas Zm-PIP2s cause a marked increase in the osmotic water permeability coefficient, Pf. However, when coexpressed, Zm-PIP1s and Zm-PIP2s physically interact to increase the Pf compared with oocytes expressing Zm-PIP2s alone (Fetter et al., 2004).
As integral membrane proteins, PIPs are synthesized in the endoplasmic reticulum (ER) and then transported along the secretory pathway to reach the plasma membrane. We previously showed that PIP trafficking is regulated by a heterooligomerization process (Fetter et al., 2004; Zelazny et al., 2007). When transiently expressed alone in maize cells, Zm-PIP1s and Zm-PIP2s differ in their subcellular localization. Zm-PIP1s are retained in the ER, whereas Zm-PIP2s are targeted to the plasma membrane (Zelazny et al., 2007). However, upon coexpression, Zm-PIP1s are relocalized from the ER to the plasma membrane as a result of their physical interaction with Zm-PIP2s, demonstrated by Förster resonance energy transfer (FRET) and fluorescence lifetime imaging microscopy experiments. A diacidic motif, DIE (Asp-Ile-Glu) at position 4 to 6 in the N terminus of Zm-PIP2;5 is essential for ER export (Zelazny et al., 2009). This motif is conserved and functional in Zm-PIP2;4 but is absent in Zm-PIP2;1. However, the replacement of the N terminus of Zm-PIP1;2 by the N terminus of Zm-PIP2;5 containing the diacidic motif is not sufficient to cause the export of Zm-PIP1;2 from the ER. A functional diacidic motif has also been identified in the N terminus of Arabidopsis thaliana PIP2;1 (Sorieul et al., 2011). In addition, the traffic and plasma membrane abundance of the At-PIP2;1 is regulated by different posttranslational modifications, including phosphorylation and ubiquitylation (Prak et al., 2008; Lee et al., 2009). Once located in the plasma membrane, At-PIP2;1 can move into or out of membrane microdomains and undergoes constitutive cycling, involving clathrin-dependent and raft-associated endocytosis (Paciorek et al., 2005; Dhonukshe et al., 2007; Li et al., 2011). The presence of PIPs in endosomes reflects a dynamic regulation of their density at the plasma membrane and their sorting between storage or degradation paths (Wudick et al., 2009; Luu et al., 2012). In this process, the trans-Golgi network (TGN) could act as a late sorting platform in the secretory pathway (Robinson et al., 2008; Richter et al., 2009), but the molecular actors regulating the post-Golgi trafficking of PIPs and their insertion in the plasma membrane remain elusive.
In mammals, the proper trafficking and anchoring of aquaporin2 (AQP2) in the apical membrane of collecting duct epithelial cells are specifically regulated by SNARE (for soluble N-ethylmaleimide-sensitive factor protein attachment protein receptor) complexes (Mistry et al., 2009; Wang et al., 2010). SNAREs are membrane proteins that facilitate vesicular trafficking in eukaryotes. They are subdivided into Q- and R-SNAREs, according to their conserved residues within the SNARE motif (Fasshauer et al., 1998). SNAREs are present in the membranes of all subcellular compartments and play a major role in vesicular fusion and membrane biosynthesis. Genome analysis showed that the SNARE family is larger in plants than in other eukaryotes (Sanderfoot, 2007). Indeed, more than 60 SNARE isoforms have been identified in the Arabidopsis genome, and orthologs are found in rice (Oryza sativa) for most of them, supporting the idea that they are conserved between monocots and dicots (Sutter et al., 2006a). Syntaxins belong to the Q-SNARE subfamily and, in Arabidopsis, seven of them localize to the plasma membrane and are involved in traffic at this membrane (Grefen et al., 2011). Among these plasma membrane resident syntaxins, SYP121 has been the most intensively studied and is known to mediate vesicle traffic between the Golgi complex and plasma membrane (Geelen et al., 2002). Disrupting its function by overexpressing a dominant-negative cytosolic (so-called Sp2) fragment specifically affected the trafficking to the plasma membrane (Tyrrell et al., 2007). In tobacco (Nicotiana tabacum), SYP121-Sp2 suppressed the mobility and delivery of the potassium channel KAT1 to the plasma membrane, but not of the H+-ATPase (Sutter et al., 2006b). Interestingly, SYP121 was recently shown to regulate the gating of the AKT1/KC1 K+ channel complex through a direct interaction involving an FxRF motif located within the first 12 residues of the protein (Honsbein et al., 2009; Grefen et al., 2010). Altogether, these data raise the hypothesis that SYP121 acts as a molecular governor, coordinating the gating of ion channels in parallel with membrane traffic and, therefore, ion uptake with increased membrane surface area through vesicle fusion during cell expansion (Grefen and Blatt, 2008; Honsbein et al., 2011).
As water movement is a key element of the mechanism that regulates cell osmotic homeostasis, we wondered if SYP121 could also regulate the traffic and activity of PIP aquaporins. In barley (Hordeum vulgare), PIP2 colocalized in ROR2/SYP121 vesicle-like compartments, supporting this hypothesis (Kwaaitaal et al., 2010). We report here that the SYP121-Sp2 from Arabidopsis and its putative ortholog from maize affect the delivery of Zm-PIP2;5 to the plasma membrane in maize mesophyll protoplasts and epidermal cells. Furthermore, the traffic inhibition is correlated with a decrease in the protoplasts Pf. As Zm-PIP2;5 water channel activity is also reduced in the presence of the ZmSYP121-Sp2 fragment in Xenopus oocytes, we tested the possibility that both proteins directly interact. Physical interaction between both proteins was demonstrated in vitro and in vivo by different technical approaches. These data indicate that SNARE might play an important role in the regulation and maintenance of osmolarity in the cytosol through a coordinated regulation of aquaporins and K+ channels.
RESULTS
The AtSYP121-Sp2 Fragment Reduces Plasma Membrane Delivery of Zm-PIP2;5
When expressed in maize mesophyll protoplasts, monomeric yellow fluorescent protein (mYFP):ZmPIP2;5 accumulates in the plasma membrane (Zelazny et al., 2007). To determine whether this localization is dependent on the activity of the syntaxin SYP121 or not, we coexpressed mYFP:ZmPIP2;5 and the Arabidopsis dominant-negative AtSYP121-Sp2 fragment fused to the C terminus of monomeric cyan fluorescent protein (mCFP) and analyzed the cellular localization and intensity of the fluorescent proteins using confocal laser scanning microscopy (CLSM). Fusions of the fluorescent proteins to the N terminus of At-SYP121 and Zm-PIP2;5 have been previously shown not to affect the subcellular localization of both proteins in protoplasts (Uemura et al., 2004; Zelazny et al., 2007). When expressed alone, mYFP:ZmPIP2;5 mainly accumulated in the cell periphery (Figure 1A). The fluorescent signal colocalized with the styryl dye FM4-64 (Figures 1Aa to 1Ac) and mCFP:tagged Np-PMA2 H+-ATPase (Lefebvre et al., 2004) (Figures 1Ad to 1Af), demonstrating that ZmPIP2;5 was mainly present in the plasma membrane. When coexpressed with mCFP:AtSYP121-Sp2, mYFP:ZmPIP2;5 was still present in the plasma membrane, but the fluorescent signal intensity was much weaker than in cells expressing mYFP:PIP2;5 alone (Figures 1Ag to 1Ai). mYFP:ZmPIP2;5 also labeled internal structures. To quantify the plasma membrane fluorescence intensity, an image analysis procedure was developed. Briefly, the average thickness of the plasma membrane was determined using the colocalized mYFP:ZmPIP2;5 and FM4-64 fluorescent signals, and the difference between the overall and intracellular fluorescent signals was used to calculate the plasma membrane fluorescence (for more details, see Methods). The mYFP plasma membrane fluorescence intensity was significantly lower (P < 0.05) in protoplasts coexpressing the mYFP:ZmPIP2;5 and mCFP:AtSYP121-Sp2 fragment than in protoplasts expressing mYFP:ZmPIP2;5 alone (Figure 1B), indicating that AtSYP121-Sp2 interferes with Zm-PIP2;5 delivery to the plasma membrane.
Figure 1.
Both AtSYP121-Sp2 and ZmSYP121-Sp2 Fragments Selectively Reduce the Accumulation of Zm-PIP2;5 in the Plasma Membrane of Maize Mesophyll Protoplasts.
(A) Plasma membrane abundance of mYFP:ZmPIP2;5 in maize mesophyll protoplasts. (a) to (c) Protoplast expressing mYFP:ZmPIP2;5 (a). (b) Channel showing the FM4-64 fluorescence and chlorophyll a autofluorescence. (c) Merged image. (d) to (f) Protoplast coexpressing mYFP:PIP2;5 (d) and mCFP:PMA2 (e). (f) Merged image. (g) to (i) Protoplast coexpressing mYFP:PIP2;5 (g) and mCFP:AtSYP121-Sp2 (h). (i) Merged image. Bars = 5 µm.
(B) Quantification of mYFP:ZmPIP2;5 fluorescence intensity in the protoplast plasma membrane (PM). Acquisitions were performed with the same settings for all samples. Each bar represents the mean of five independent experiments (10 protoplasts per experiment). Error bars show the 95% confidence interval. Student’s t test, P < 0.05 was used to compare the data. Means that are significantly different (P < 0.05) are indicated by different letters. a.u., arbitrary units.
(C) Plasma membrane abundance of Zm-PIP2;5 in maize mesophyll protoplasts. (a) and (b) Protoplast expressing mYFP:ZmPIP2;5 (b). (a) Chlorophyll a autofluorescence. (c) and (d) Protoplast coexpressing the soluble mCFP (c) and mYFP:ZmPIP2;5 (d). (e) and (f) Protoplast coexpressing mCFP:ZmSYP121 (e) and mYFP:ZmPIP2;5 (f). (g) and (h) Protoplast coexpressing mCFP:ZmSYP121-Sp2 (g) and mYFP:ZmPIP2;5 (h). All the pictures were acquired with the same settings. In panel (a), the chloroplast autofluorescence is shown, whereas, in panels (c), (e), and (f), it is subtracted. Bars = 5µm.
(D) Quantification of mYFP:ZmPIP2;5 fluorescence intensity in the protoplast plasma membrane. Each bar represents the mean of three independent experiments (10 protoplasts by experiments). Error bars show 95% confidence interval. Statistical differences were inferred by analysis of variance (ANOVA) followed by Tukey's honestly significant difference test. Means that are significantly different (P < 0.05) are indicated by different letters.
(E) Plasma membrane abundance of mCFP:PMA2 in maize mesophyll protoplasts. (a) to (c) Protoplast expressing mCFP:PMA2 (a). (b) YFP channel. (c) Chlorophyll a channel. (d) to (f) Protoplast coexpressing mCFP:PMA2 (d) and mYFP:ZmSYP121-Sp2 (e). (f) Chlorophyll a channel. All the images were acquired with the same settings. Bars = 5 µm.
(F) Quantification of mCFP:PMA2 fluorescence intensity in the protoplast plasma membrane. Each bar represents the mean of three independent experiments (10 protoplasts per experiment). Error bars show the 95% confidence interval. Means are not significantly different (Student’s t test, P = 0.90).
Zm-SYP121 Is the Functional Ortholog of At-SYP121
Maize database analysis allowed the identification of a maize SYP121 protein that shared 54% amino acid identity with At-SYP121. Both proteins cluster in the same phylogenetic group as shown by the neighbor-joining tree (see Supplemental Figure 1 and Supplemental Data Set 1 online). To test if this sequence similarity could reflect a conservation in the regulation of Zm-PIP2;5 delivery to the plasma membrane, the SYP121 cDNA was cloned from total RNA extracted from maize roots and fused to mCFP and mYFP sequences in plant expression vectors. A truncated version of the cDNA was also prepared to produce the ZmSYP121-Sp2 fragment. These constructs were then transfected in maize mesophyll protoplasts. Figures 1C and 1D show the subcellular localization of mYFP:ZmPIP2;5 and the plasma membrane fluorescence signal intensity, respectively, in protoplasts transiently expressing mYFP:ZmPIP2;5 alone (Figures 1Ca and 1Cb) and coexpressing mYFP:ZmPIP2;5 and mCFP:ZmSYP121 (Figures 1Ce and 1Cf) or mCFP:ZmSYP121-Sp2 (Figures 1Cg and 1Ch). The soluble mCFP was coexpressed with mYFP:ZmPIP2;5 as a control (Figures 1Cc and 1Cd). When expressed alone or coexpressed with mCFP or mCFP:ZmSYP121, mYFP:ZmPIP2;5 accumulated in the cell plasma membrane (Figures 1Cb, 1Cd, and 1Cf). Quantification of the plasma membrane fluorescence intensities in the mYFP channel showed that the mYFP:ZmPIP2;5 and mCFP:ZmSYP121 coexpressing protoplasts had a slightly weaker plasma membrane fluorescence than protoplasts expressing mYFP:ZmPIP2;5 alone, but the intensities were not significantly different (Figure 1D). By contrast, upon coexpression with the mCFP:ZmSYP121-Sp2 fragment, the mYFP:ZmPIP2;5 plasma membrane signal intensity was strongly decreased (Figures 1Ch and 2Af), indicating that Zm-SYP121 is a functional ortholog of AtSYP121. In protoplasts cotransfected with equal amounts of plasmids encoding mCFP:ZmSYP121-Sp2 or mYFP:ZmPIP2;5, mYFP:ZmPIP2;5 only poorly accumulated in internal structures. However, when protoplasts were transfected with a 3:1 ratio of mCFP:ZmSYP121-Sp2 and mYFP:ZmPIP2;5 plasmids, a significant accumulation of mYFP:ZmPIP2;5 in internal structures was observed (see Supplemental Figure 2 online).
For comparison, we examined the fluorescence distribution of mCFP:NpPMA2 expressed in maize protoplasts. It was previously shown that the delivery of Np-PMA2 to the plasma membrane was not affected by AtSYP121-Sp2 in tobacco epidermal cells (Sutter et al., 2006a). When coexpressed with mYFP:ZmSYP121-Sp2 in maize protoplasts, no modification in mCFP:NpPMA2 plasma membrane localization and intensity was detected (Figures 1E and 1F).
To analyze the dynamics of the effect of ZmSYP121-Sp2 on newly synthesized mYFP:ZmPIP2;5 that reaches the plasma membrane via anterograde transport, we compared the intensity of mYFP signal according to time in mesophyll protoplasts expressing mYFP:ZmPIP2;5 alone or coexpressing mYFP:ZmPIP2;5 with mCFP:ZmSYP121-Sp2. One image acquisition was performed every 30 min during 16 h after cell transfection (see Supplemental Figure 3 online with associated Supplemental Movie 1 and Supplemental Movie Legend 1 online). The quantification of the global (plasma membrane and intracellular) mYFP fluorescent signals in protoplasts expressing mYFP:ZmPIP2;5 alone or in combination with mCFP:ZmSYP121-Sp2 is shown in Supplemental Figure 4 online. The mYFP and mCFP signals were detected from 5 to 8 h after transfection. During the first 8 h, the increase in mYFP signal intensity (integrated density) was slightly lower but not significantly different in cells coexpressing mYFP:ZmPIP2;5 and mCFP:ZmSYP121-Sp2 compared with protoplasts expressing mYFP:ZmPIP2;5 alone, indicating that the protein synthesis rate was not affected. However, 12 h after transfection, while the mYFP signal was still increasing in protoplasts expressing mYFP:ZmPIP2;5 alone, it plateaued in cells coexpressing mCFP:ZmSYP121-Sp2 and mYFP:ZmPIP2;5. During this time range, the signal of mCFP:ZmSYP121-Sp2 was still rising, demonstrating that protein synthesis was not affected by mCFP:ZmSYP121-Sp2 expression.
To monitor the cycling of Zm-PIP2;5 from intracellular compartments to the plasma membrane or within the membrane surface, we performed fluorescence recovery after photobleaching (FRAP) experiments in tobacco epidermal cells transiently expressing mYFP:ZmPIP2;5 alone or together with mCFP:ZmSYP121-Sp2 (see Supplemental Figure 5 online). In cells expressing mYFP:ZmPIP2;5 alone, a recovery of 20% of the initial plasma membrane fluorescence was detected 150 s after photobleaching. These data are in agreement with previous findings on the KAT1 K+ channel in Arabidopsis (Honsbein et al., 2009) and on At-PIP2;1 (Sorieul et al., 2011; Luu et al., 2012). In cells coexpressing mCFP:ZmSYP121-Sp2 and mYFP:ZmPIP2;5, the recovery reached only 5% of the initial plasma membrane signal.
The Inhibition of Zm-PIP2;5 Delivery to the Plasma Membrane Is SYP121-Sp2 Specific
To test whether the delivery of Zm-PIP2;5 to the plasma membrane was specifically dependent on SYP121, mYFP:ZmPIP2;5 was coexpressed in maize protoplasts with the Sp2 truncated forms of At-SYP71, At-SYP122, or At-SYP21 syntaxins, and its abundance in the plasma membrane was measured (Figure 2). SYP71 and SYP122 are syntaxins localized in the plasma membrane (Leyman et al., 2000; Borner et al., 2005; Suwastika et al., 2008), while SYP21 was found in the prevacuolar compartment membrane (da Silva Conceição et al., 1997; Sanderfoot et al., 2001; Tse et al., 2004). None of these Sp2 fragments affected the plasma membrane accumulation of mYFP:ZmPIP2;5 (Figures 2A and 2B), although AtSYP21-Sp2 has been shown to be active in suppressing traffic to the (pre)vacuolar compartment (Tyrrell et al., 2007). The plasma membrane fluorescent signal intensity detected in cells coexpressing mCFP-tagged Sp2 fragments of At-SYP71, At-SYP122, or At-SYP21 with mYFP:ZmPIP2;5 was similar to the intensity found in protoplasts expressing mYFP:ZmPIP2;5 alone or in combination with mCFP:ZmSYP121. As previously mentioned, protoplasts coexpressing mYFP:ZmPIP2;5 and mCFP:ZmSYP121-Sp2 showed a strong reduction of the plasma membrane signal. These data demonstrate that the regulation of Zm-PIP2;5 trafficking to the plasma membrane is specifically dependent on Zm-SYP121.
Figure 2.
Inhibition of Zm-PIP2;5 Delivery to the Plasma Membrane Is Specific for ZmSYP121-Sp2.
(A) Plasma membrane abundance of mYFP:ZmPIP2;5 in maize mesophyll protoplasts. (a) and (b) Protoplast expressing mYFP:ZmPIP2;5 (b). (a) Chlorophyll a autofluorescence. (c) and (d) Protoplast coexpressing mCFP:ZmSYP121 (c) and mYFP:ZmPIP2;5 (d). (e) and (f) Protoplast coexpressing mCFP:ZmSYP121-Sp2 (e) and mYFP:ZmPIP2;5 (f). (g) and (h) Protoplast coexpressing mCFP:AtSYP71-Sp2 (g) and mYFP:ZmPIP2;5 (h). (i) and (j) Protoplast coexpressing mCFP:AtSYP21-Sp2 (i) and mYFP:ZmPIP2;5 (j). (k) and (l) Protoplast coexpressing mCFP:AtSYP122-Sp2 (k) and mYFP:ZmPIP2;5 (l). All images were acquired with the same settings. In panel (a), autofluorescence of the chlorophyll a is shown, whereas in panels (c) to (k), chlorophyll a autofluorescence is subtracted. Bars = 5 µm.
(B) Quantification of mYFP:ZmPIP2;5 fluorescence signal intensity in the protoplast plasma membrane (PM). Each bar represents the mean of three independent experiments (10 protoplasts per experiment). Error bars show the 95% confidence interval. Statistical differences were inferred by ANOVA, followed by the Tukey's honestly significant difference test. Means that are significantly different (P < 0.05) are indicated by different letters. a.u., arbitrary units.
Coexpression of Zm-PIP2;5 and the ZmSYP121-Sp2 Fragment Leads to a Decrease in Maize Protoplast Pf and Reproduces the Cell Water Permeability Phenotype of the syp121-1 Arabidopsis Mutant
To determine whether the reduction in Zm-PIP2;5 delivery to the plasma membrane was correlated with a decrease in the membrane water permeability coefficient, we analyzed the swelling of protoplasts expressing mYFP:ZmPIP2;5 or mCFP:ZmSYP121 singly or coexpressing mYFP:ZmPIP2;5 with mCFP:SYP121 or mCFP:ZmSYP121-Sp2. Mock protoplasts were used as a negative control. For each protoplast population, the Pf values were obtained as described (Moshelion et al., 2004; Volkov et al., 2007), plotted on a frequency diagram (Figure 3A), and the medians were calculated (Figure 3B). Expression of mYFP:ZmPIP2;5 alone induced an important increase in the Pf values compared with the mock protoplasts. Its coexpression with mCFP:ZmSYP121 did not alter this high Pf. For both protoplast populations, the Pf distributions were similar, with the presence of cells having a Pf higher than 10 µm s−1. By contrast, protoplasts coexpressing mYFP:ZmPIP2;5 and mCFP:ZmSYP121-Sp2 exhibited similar Pf values as mock or mCFP:ZmSYP121 expressing protoplasts.
Figure 3.
Maize Mesophyll Protoplasts Coexpressing mCFP:ZmSYP121-Sp2 and mYFP:ZmPIP2;5 Phenocopies the Lower Plasma Membrane Water Permeability (Pf) of Arabidopsis syp121-1 Mesophyll Protoplasts.
(A) Relative distribution of Pf values of maize mesophyll protoplasts untransfected or (co)expressing mYFP:ZmPIP2;5, mYFP:ZmPIP2;5 and mCFP:ZmSYP121, mYFP:ZmPIP2;5 and mCFP:SYP121-Sp2, or mCFP:ZmSYP121.
(B) Median Pf values for each protoplast subpopulation shown in (A).
(C) Relative distribution of Pf values of mesophyll protoplasts from wild-type (WT) and syp121 Arabidopsis plants.
(D) Median Pf values for both Arabidopsis protoplast subpopulations.
Each bar represents the mean of 20 measurements. Error bars show 95% confidence interval. Statistical differences were inferred by the Kruskall-Wallis test followed by a Dunns test or Mann-Whitney U test. Medians that are significantly different (P < 0.05) are shown with different letters.
A detailed analysis of the swelling curves obtained with protoplasts expressing mYFP:ZmPIP2;5 or mCFP:ZmSYP121-Sp2 and mYFP:ZmPIP2;5 was performed as previously reported (Moshelion et al., 2004; Volkov et al., 2007; Moshelion et al., 2009). While no difference in the lag phase preceding the swelling event was detected, the Pf values determined 15, 30, and 45 s after the solution exchange increased during the swelling event but were always significantly lower for protoplasts coexpressing mYFP:ZmPIP2;5 and mCFP:ZmSYP121-Sp2 compared with their respective controls (see Supplemental Figure 6 online). In addition, the Pf differences between these two cell populations increased with the swelling time, probably reflecting differences in the amount of active aquaporins reaching the plasma membrane (Moshelion et al., 2004).
To further characterize the role of SYP121 in regulating the membrane water permeability, leaf protoplasts isolated from the wild type (Columbia-0) and syp121-1 Arabidopsis mutated line (Collins et al., 2003) were isolated and subjected to an osmotic challenge, and the Pf was determined. Interestingly, syp121-1 protoplasts had a lower Pf than wild-type ones (Figures 3C and 3D), phenocopying the permeability of maize protoplasts expressing the SYP121-Sp2 fragment. These data demonstrate a clear link between SYP121 function in Arabidopsis and the regulation of the plasma membrane water permeability controlled by aquaporins.
Finally, we note that no significant increase in Pf was measured in maize protoplasts coexpressing mYFP:ZmPIP2;5 and mCFP:ZmSYP121-Sp2 compared with mock cells (Figure 3B), even if some mYFP fluorescence was detected in the plasma membrane (Figures 1Ch and 2Af). These observations led us to question whether ZmSYP121-Sp2 might regulate Zm-PIP2;5 activity independently of any effect mediated through trafficking.
The Water Channel Activity of Zm-PIP2;5 Is Regulated by ZmSYP121-Sp2 in Xenopus Oocytes
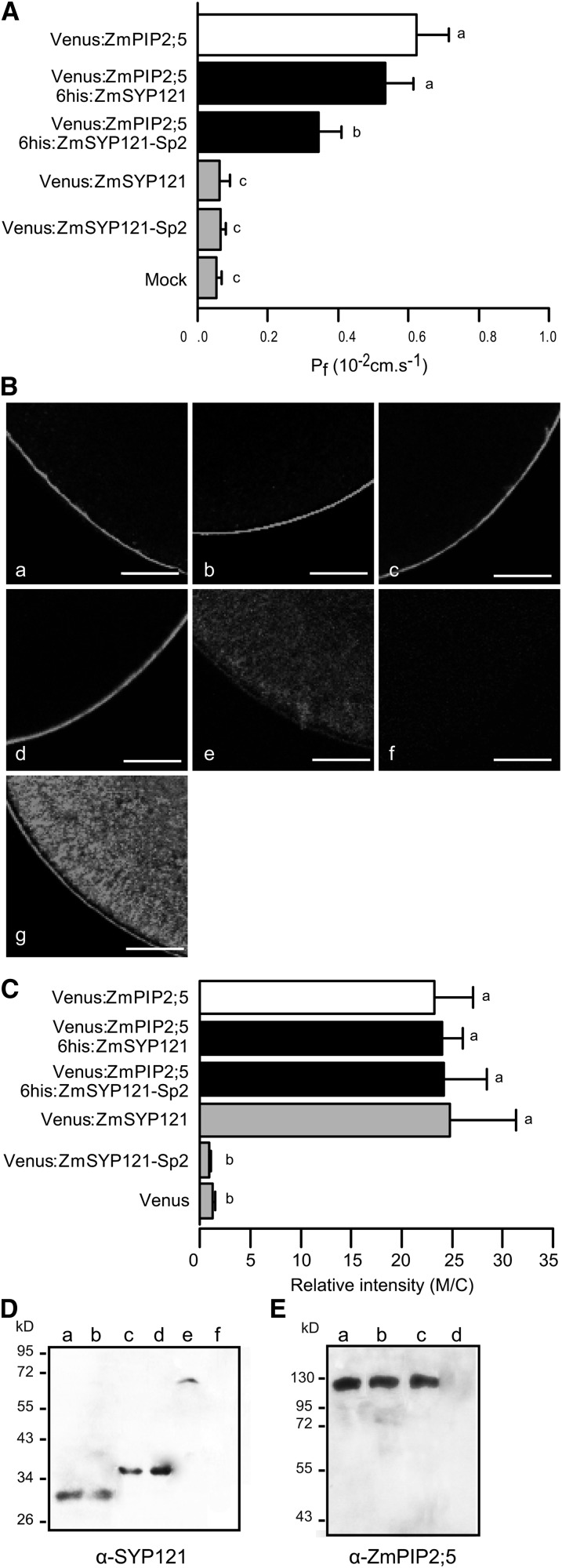
We tested the impact of Zm-SYP121 and ZmSYP121-Sp2 on the water channel activity of Zm-PIP2;5 expressed in Xenopus oocytes. Venus:ZmPIP2;5 was expressed alone or together with Zm-SYP121 or the ZmSYP121-Sp2 fragment fused to a 6 His tag at the N terminus. The Pf values calculated from oocyte swelling experiments were plotted in Figure 4A. In contrast with 6His:ZmSYP121, 6His:ZmSYP121-Sp2 induced a significant decrease in the Pf of Zm-PIP2;5 expressing oocytes, but the Pf was significantly higher than the Pf of mock oocytes and oocytes expressing 6His:ZmSYP121 or 6His:ZmSYP121-Sp2 alone. The Pf decrease might be due to impairment of the delivery of Venus:ZmPIP2;5 into the oocyte plasma membrane. To determine the amount of protein in the cell membrane, the Venus fluorescence was detected in fixed oocytes using CLSM and quantified (Figures 4B and 4C). When coexpressed with either 6His:ZmSYP121 or 6His:ZmSYP121-Sp2, Venus:ZmPIP2;5 was still localized in the plasma membrane (Figures 4Ba, 4Bb, and 4Bc) and the plasma membrane Venus fluorescence intensity was similar to the intensity measured in the plasma membrane of oocytes expressing Venus:ZmPIP2;5 alone (Figure 4C). Immunodetection analysis of the microsomal fraction showed that the Zm-PIP2;5 amount was similar in oocytes expressing Zm-PIP2;5 alone or together with Zm-SYP121 or Zm-SYP121-Sp2 (Figures 4D and 4E). As the Pf values measured in these experiments were around 0.4 to 0.6 µm s−1, far below the maximum values reported when higher amounts of Zm-PIP cRNAs were injected, we can exclude the possibility that the protein expression was saturated (Chaumont et al., 2000; Fetter et al., 2004). Altogether, these data are consistent with a direct effect of Sp2 on Zm-PIP2;5 activity that is independent of any action on Zm-PIP2;5 trafficking to the plasma membrane.
Figure 4.
ZmSYP121-Sp2 Decreases the Pf of Xenopus Oocytes When Coexpressed with Zm-PIP2;5.
(A) Pf of Xenopus oocytes (co)expressing Venus:ZmPIP2;5, Venus:ZmPIP2;5 and 6His:ZmSYP121, Venus:ZmPIP2;5 and 6His:ZmSYP121-Sp2, or 6His:ZmSYP121 and 6His:ZmSYP121-Sp2 or oocytes injected with water (Mock). Each bar is the mean of 10 replicates. Error bars are 95% confidence intervals. Values were subjected to ANOVA and Tukey posthoc analysis. Means that are significantly different (P < 0.05) are indicated by different letters.
(B) Confocal images of oocytes (co)expressing Venus:ZmPIP2;5 (a), Venus:ZmPIP2;5 and 6His:ZmSYP121 (b), (c) Venus:ZmPIP2;5 and 6His:ZmSYP121-Sp2, Venus:ZmSYP121 (d), or Venus:ZmSYP121-Sp2 (e), water-injected oocytes (f), or oocytes expressing Venus (g). The oocytes were fixed in 4% (w/v) paraformaldehyde and analyzed by CLSM. Note that all proteins mainly accumulated in the plasma membrane, except Venus:ZmSYP121-Sp2, which showed a localization pattern similar to that of Venus. Bars = 100 µm.
(C) Relative Venus fluorescence signal intensity in the plasma membrane (M) compared with the cytoplasm (C) of oocytes expressing Venus:ZmPIP2;5, Venus:ZmPIP2;5, and 6His:ZmSYP121, Venus:ZmPIP2;5 and 6His:ZmSYP121-Sp2, Venus:ZmSYP121, Venus:ZmSYP121-Sp2, or Venus. Each bar is the mean of 20 replicates. Error bars are 95% confidence intervals. Values were subjected to ANOVA and Tukey posthoc analysis. Means that are significantly different (P < 0.05) are indicated by different letters.
(D) Oocyte total protein extracts were probed with antibodies raised against AtSYP121. Protein extracts from oocytes expressing Venus:ZmPIP2;5 and 6His:ZmSYP121-Sp2 (a), 6His:ZmSYP121-Sp2 (b), Venus:ZmPIP2;5 and 6His:ZmSYP121 (c), 6His:ZmSYP121 (d), Venus:ZmSYP121 (e), or mock-injected sample (f).
(E) Oocyte total protein extracts were probed with antibodies raised against Zm-PIP2;5. Protein extracts from oocytes expressing Venus:ZmPIP2;5 (a), Venus:ZmPIP2;5 and 6His:ZmSYP121 (b), and Venus:ZmPIP2;5 and 6His:ZmSYP121-Sp2 (c), or mock-injected sample (d). No DTT was added to the samples, meaning that only dimers of Venus:ZmPIP2;5 (∼125 kD) are detected.
Zm-PIP2;5 and Zm-SYP121 Physically Interact in Xenopus Oocytes and in Plant Cells
The regulation of the water channel activity of Zm-PIP2;5 by the ZmSYP121-Sp2 fragment might be due to a direct physical interaction between both proteins. To evaluate this possibility, we extracted the microsomes of oocytes expressing 6His-tagged Zm-PIP2;5 alone or together with Venus:ZmSYP121 and purified 6His:ZmPIP2;5 on a nickel–nitrilotriacetic acid affinity column. The presence of 6 His:ZmPIP2;5 and Venus:ZmSYP121 in the elution fraction was checked by immunodetection using antibodies raised against ZmPIP2;5 and green fluorescent protein (GFP), respectively (Chaumont et al., 2000; Hachez et al., 2006) (Figures 5A and 5B). A signal corresponding to the Zm-PIP2;5 dimer was detected in the eluted fractions coming from oocytes expressing 6His:ZmPIP2;5 alone or together with Venus:ZmSYP121 (Figure 5A, lanes a and b). No signal was observed in eluted fractions from mock oocytes or oocytes expressing Venus:ZmSYP121 alone (Figure 5A, lanes c and d). The 6His:ZmPIP2;5 dimeric form was due to the presence of a disulfide bond linking two Zm-PIP2;5 monomers (Bienert et al., 2012) and was detected as no reducing agent was added in the solubilization buffer. Interestingly, a YFP signal corresponding to Venus:ZmSYP121 was observed in the fraction coming from oocytes coexpressing 6His:ZmPIP2;5 and Venus:ZmSYP121 (Figure 5B, lane b), demonstrating that both proteins were copurified.
Figure 5.
Zm-SYP121 and Zm-PIP2;5 Interact Both in Oocytes and in Plant Cells.
(A) and (B) Copurification of solubilized proteins extracted from Xenopus oocyte microsomal fractions on nickel–nitrilotriacetic acid resin. Elution fractions were probed with antibodies raised against Zm-PIP2;5 in (A) and against GFP in (B). Eluted fractions containing proteins extracted from oocytes expressing 6His:ZmPIP2;5 (a), 6His:ZmPIP2;5 and Venus:ZmSYP121 (b), and Venus:ZmSYP121 (c) or water-injected oocytes (d).
(C) BiFC in maize epidermal cells and mesophyll protoplasts. (a) Confocal images of maize epidermal cells transiently expressing mYFP:ZmPIP2;5 and (b) stained with the plasma membrane stain, styryl dye FM4-64. (c) Colocalization was determined as the relative fluorescence intensities of mYFP and FM4-64 along the white dotted line. The black dotted line above the spectra represents the white dotted lines shown in (a) and (b). Note the matches between peaks for the two channels corresponding to plasma membranes. (d) to (g) Maize epidermal cells coexpressing mCFP:ZmSYP121 (d) and mYFP:ZmPIP2;5 (e). The plasma membrane was stained with FM4-64 (f). Merged image of mCFP:ZmSYP121, mYFP:ZmPIP2;5, and FM4-64 (g). (h) to (k) BiFC experiments in maize epidermal cells transfected by biolistic bombardment. Confocal images of epidermal cells expressing VenusN:ZmPIP2;5 and VenusC:ZmPIP1;2 (h), and VenusN:ZmPIP2;5 and VenusC:ZmSYP121 (i). (j) Bright field. (k) YFP channel of cells expressing VenusN:PMA2 and VenusC:ZmSYP121. (l) to (r) BiFC experiments in maize mesophyll protoplasts. Confocal images of protoplasts transiently coexpressing VenusN:ZmPIP2;5 and VenusC:ZmPIP1;2 ([l] and [m]) or VenusN:ZmPIP2;5 and VenusC:ZmSYP121 ([n] and [o]). (p) to (r) Protoplasts coexpressing VenusN:PMA2 and VenusC:ZmSYP121. (p) Chlorophyll a autofluorescence. (q) YFP channel. (r) Bright field. Bars = 20 μm in (a) to (k) and 5 μm in (l) to (r). a.u., arbitrary units.
(D) Emission spectra after excitation at 514 nm of mYFP:ZmPIP2;5 (black solid line) and reconstituted Venus (gray dashed line). Fluorescent signal was collected from 517 to 683 nm with an interval of 9.8 nm. mYFP and reconstituted Venus share a similar emission pattern.
The interaction between Zm-PIP2;5 and Zm-SYP121 was also checked in plant cells using bimolecular fluorescence complementation (BiFC) experiments in maize epidermal cells transfected by biolistic bombardment. We used split Venus (ADE48838.1) fusion constructs (Nour-Eldin et al., 2006; Shyu et al., 2006). First, the plasma membrane localization of transiently expressed mYFP:ZmPIP2;5 was confirmed by colocalization with the styryl dye FM4-64 (Figures 5Ca to 5Cc). When mCFP:ZmSYP121 and mYFP:ZmPIP2;5 were coexpressed, both proteins colocalized at the cell periphery (Figures 5Cd to 5Cg), as previously observed in maize protoplasts (Figures 1Ce to 1Cf). As we previously showed that Zm-PIP2;5 and Zm-PIP1;2 interact in maize cells (Zelazny et al., 2007), we used them as a positive control in the BiFC experiments. When VenusN:ZmPIP2;5 and VenusC:ZmPIP1;2 were coexpressed, a fluorescent signal was detected mostly in the cell periphery (Figure 5Ch), validating the method. When VenusN:ZmPIP2;5 and VenusC:ZmSYP121 were coexpressed, a Venus signal was observed in the cell plasma membrane (Figure 5Ci), demonstrating an interaction between the two proteins. Fluorescent signals were also detected in the plasma membrane and/or internal membranes when these proteins were coexpressed in maize mesophyll protoplasts (Figures 5Cl to 5Co). The spectral specificity of the signal was validated by determination of its emission spectrum (Figure 5D). Cells expressing VenusN:PMA2 and VenusC:ZmSYP121 were used as negative controls for interaction (Figures 5Cj and 5Ck or 5Cp and 5Cr). Similar BiFC results were obtained when the proteins were expressed in tobacco epidermal cells (see Supplemental Figure 7 online).
To further validate the direct interaction between Zm-PIP2;5 and Zm-SYP121, FRET experiments were performed on maize mesophyll protoplasts and tobacco epidermal cells coexpressing mCFP:ZmPIP1;2 and mYFP:ZmPIP2;5 or mCFP:ZmSYP121 and mYFP:ZmPIP2;5. The FRET efficiency was determined by measuring the fluorescence intensity of the donor fluorochrome before and after acceptor photobleaching (Bhat et al., 2005). A representative experiment is given in Supplemental Figure 8 online for each cell type, and quantitative results are reported in Table 1. Coexpression of mCFP:ZmPIP1;2 and mYFP:ZmPIP2;5 or mCFP:ZmSYP121 and mYFP:ZmPIP2;5 in maize protoplasts gave a FRET efficiency of ∼26 and 15%, respectively, values significantly higher than those of the negative controls constituted by the coexpression of mCFP:ZmPIP2;5 and mYFP:ROP6 (Zelazny et al., 2007). These values were validated by assessing FRET efficiency by acceptor-sensitized emission on the same cells. Data obtained by the two methods showed 3% divergence, which is comparable to the stochastic variations observed in the acceptor photobleaching experiments (see Supplemental Figure 9 online).
Table 1. FRET Efficiencies for the PIP/SYP121 Interaction Studies.
| Protein | FRET Efficiency ± sd (%) | No. of Measurements | Cell Type |
|---|---|---|---|
| mCFP:ZmPIP1;2 + mYFP:ZmPIP2;5 | 25.7 ± 9.56 | 69 | Maize protoplasts |
| mCFP:ZmSYP121 + mYFP:ZmPIP2;5 | 14.8 ± 3.12 | 35 | Maize protoplasts |
| mCFP:ZmPIP2;5 + mYFP:ROP6 | 6.86 ± 9.26 | 20 | Maize protoplasts |
| mCFP:ZmPIP1;2 + mYFP:ZmPIP2;5 | 23.9 ± 5.42 | 35 | Tobacco epidermal cells |
| mCFP:ZmSYP121 + mYFP:ZmPIP2;5 | 14.1 ± 7.96 | 50 | Tobacco epidermal cells |
For each analysis, the average value of the FRET efficiency was determined as described in Methods.
Additionally, we tested the potential for SYP121 interaction with Zm-PIP2;5 using a mating-based, split-ubiquitin assay, much as previously described in the analysis of the Arabidopsis SYP121 interaction with the K+ channels KC1 and KAT1 (Honsbein et al., 2009, 2011; Grefen et al., 2010). In this case, we used a ZmPIP2;5-Cub-PLV fusion protein as bait and tested its ability to rescue growth of diploid yeast carrying the prey fusions of Zm-SYP121 and At-SYP121 with Nub, the N-terminal half of ubiquitin. For purposes of comparison, we tested for interactions with a homolog of Zm-PIP2;5, the Arabidopsis aquaporin At-PIP2;2, and we performed each assay with both the maize SNARE and the Arabidopsis SNARE SYP121. Figures 6A and 6B summarize the results of these experiments, with the column on the left in each frame showing the control for growth on mating at 24 h and the subsequent three columns showing growth on increasing concentrations of Met that repress the expression of the bait. Figure 6C shows the immunoblot analysis for the two experiments, confirming the expression of all four proteins and the presence of bands at the correct molecular weights. From these results, it is clear that Zm-PIP2;5 interacts with the maize SYP121, and even more strongly with At-SYP121, in high concentrations of Met. The Arabidopsis aquaporin also interacted with At-SYP121 but showed only a weak association with its maize homolog. We note that the negative control (NubG) also showed a low level of growth rescue with the ZmPIP2;5-Cub-PLV fusion, which may suggest some leakage, possibly a low level of proteolytic turnover in this case sufficient to promote growth under the nonstringent, low Met conditions. Nonetheless, a comparison shows a clear interaction of Zm-PIP2;5 with Zm-SYP121, consistent with our copurification data. Taken together, these data demonstrate a direct interaction between Zm-SYP121 and Zm-PIP2;5, consistent with a regulation of the water channel activity of Zm-PIP2;5 by SYP121.
Figure 6.
PIP2 Proteins Interact with SYP121 in Mating-Based Spilt-Ubiquitin Assay.
(A) and (B) Mating-based split-ubiquitin assays were performed using At-PIP2;2 (A) and Zm-PIP2;5 (B) as baits, in each case with Zm-SYP121 and the Arabidopsis ortholog At-SYP121 as the prey. Diploid yeast containing the water channels as bait and the SNAREs as prey or Nub-moiety peptides (NubG = negative; NubI = positive control) were dropped in a dilution series (OD 1.0 and 0.1) onto the yeast synthetic media CSM-L-W- to verify mating and on CSM-L-, W-, Ura-, H-, M-, and Ade- containing increasing Met levels to repress expression of the bait. Yeast growth was recorded after incubation for 24 h (mating test) and for 48 h (interaction test) at 30°C.
(C) Expression of bait and prey fusion proteins was verified using anti-SYP121 antibody (Tyrrell et al., 2007) and VP16 (Abcam) antibodies. Molecular masses of the primary bands in the protein gel blots are indicated below. Ponceau S staining (above) was recorded to monitor equal loading. The double bands are characteristic of the SNAREs (Geelen et al., 2002).
DISCUSSION
Aquaporins constitute an important selective pathway for water and small neutral solutes to move across cellular membranes. However, to exert their function, aquaporins have to reach their target membrane and be active. PIP aquaporins are synthesized at the ER and traffic through the secretory pathway before reaching the plasma membrane (Paciorek et al., 2005; Dhonukshe et al., 2007; Jaillais et al., 2007; Zelazny et al., 2007; Sorieul et al., 2011). The fusion of vesicles coming from the endomembrane compartments to the plasma membrane is generally dependent on the activities of SNARE complexes in all eukaryotic cells (Pratelli et al., 2004). Among the SNARE proteins, the plasma membrane syntaxin SYP121 was demonstrated to regulate the delivery but also the gating of plant K+ channels through direct physical interaction, indicating that this protein may play an important role in the control of ion transport and cellular volume (Sutter et al., 2006b; Honsbein et al., 2009).
As aquaporin-facilitated water movement through the plasma membrane is an inherent process controlling the cell water homeostasis, we questioned whether SNAREs also regulate the traffic and activity of these channels. Our results now address this question using Zm-PIP2;5 as a model aquaporin and the plasma membrane–resident syntaxin SYP121. We demonstrate that the plasma membrane delivery of ZmPIP2;5, but not the H+-ATPase PMA2, is reduced by dominant-negative Sp2 fragments of SYP121 from Arabidopsis and maize. This regulation is SYP121 specific, as Sp2 fragments of At-SYP71, At-SYP122, and At-SYP21 syntaxins had no effect on Zm-PIP2;5 traffic. We also show that the membrane osmotic water permeability coefficient of plant cells and oocytes coexpressing Zm-PIP2;5 and SYP121-Sp2 is significantly reduced. However, in contrast with what is observed in plant cells, the Sp2 fragment did not affect the plasma membrane localization of Zm-PIP2;5 in oocytes, suggesting a role of SYP121 in regulating aquaporin activity in specific conditions. We now also demonstrate a direct interaction of maize SYP121 with Zm-PIP2;5. These findings suggest an important role for SNAREs in the regulation of the traffic and activity of plasma membrane aquaporins.
The Syntaxin SYP121 Is a Specific Regulator of Zm-PIP2;5 Plasma Membrane Trafficking
Coexpression of mYFP:ZmPIP2;5 with the soluble ZmSYP121-Sp2 fragment (cotransfected plasmid ratio of 1:1) leads to a decrease in its plasma membrane abundance and, to an extent, its accumulation in intracellular structures. This intracellular accumulation is further increased in cells cotransfected with the plasmids encoding mYFP:ZmPIP2;5 and mCFP:ZmSYP121-Sp2 at a ratio of 1:3 (see Supplemental Figure 4 online). The total amount of mYFP:ZmPIP2;5 fluorescence (plasma membrane and internal signals) in these protoplasts was similar to the one found in cells expressing mYFP:ZmPIP2;5 alone. These data are in accordance with the observation that the soluble SYP121-Sp2 fragment competes with the endogenous full-length SYP121 and behaves as a dominant-negative (competitor) protein (Geelen et al., 2002). From the time-lapse experiments, we found that mYFP:ZmPIP2;5 accumulates in the plasma membrane within 6 h of transfection (see Supplemental Figure 3, Supplemental Movie 1, and Supplemental Movie Legend 1 online). However, in cells coexpressing mYFP:ZmPIP2;5 and ZmSYP121-Sp2, the quantification of fluorescence intensity provides unequivocal evidence that the traffic dynamics of mYFP:ZmPIP2;5 are affected primarily at time points after the protein had reached the plasma membrane, suggesting an action of the Sp2 fragments on the vesicles containing newly synthesized and recycling PIPs from the plasma membrane. This is in agreement with the results obtained using the vesicle-trafficking inhibitor brefeldin A, which prevents the traffic from the Golgi apparatus to the plasma membrane and inhibits the recycling of membrane protein to the plasma membrane (Geldner et al., 2001; Dhonukshe et al., 2007). Brefeldin A blocks the plasma membrane trafficking of At-PIP2;1 in Arabidopsis roots (Paciorek et al., 2005) and phenocopies the KAT1 K+ channel trafficking phenotype in the syp121 mutant (Eisenach et al., 2012). Indeed, SYP121 cycles actively between the TGN and the plasma membrane (Reichardt et al., 2011). In addition, the mCFP:ZmSYP121-Sp2 signal continuously rose during our experiments, demonstrating that the global protein synthesis is not inhibited and did not constitute the origin of the decrease in mYFP:ZmPIP2;5 plasma membrane fluorescence (see Supplemental Figure 4 online).
The short-term FRAP kinetic experiments showed that only 20% ± 4.48% of YFP:ZmPIP2;5 is mobile at the cell periphery. Similar results were obtained in Arabidopsis plants expressing AtPIP2;1:GFP (Li et al., 2011; Sorieul et al., 2011; Luu et al., 2012). Interestingly, coexpression with the dominant-negative SYP121-Sp2 fragment reduced the mobile fraction to 7.02% ± 3.62% but the fitted time constants were not affected (20.7 ± 7.69 s and 19.64 ± 6.58 s, respectively). We interpret this result as an inhibition of mYFP:ZmPIP2;5 recycling to the plasma membrane in these cells. We did not detect any fluorescence loss in the neighborhood of the bleached area, suggesting that little lateral diffusion of ZmPIP2;5 occurred (see Supplemental Figure 4 online). While very low lateral membrane mobility was observed by FRAP in normal conditions for several fluorescent tagged channels, including Arabidopsis KAT1, PIP2;1, and Nodulin 26-like intrinsic protein NIP5;1, a boric acid channel, the mobility of At-KAT1 in the presence of the Sp2 fragment increased significantly, indicating a role of the SYP121 in KAT1 distribution and behavior at the membrane surface (Sutter et al., 2006b; Takano et al., 2010; Sorieul et al., 2011; Luu et al., 2012). This was not observed for Zm-PIP2;5, suggesting either that SYP121 is required for docking this channel in the plasma membrane but not for its anchoring or that in vivo interactions are more transient than they are for the K+ channel and therefore do not constitute the overriding factor determining mobility.
The syntaxin SNAREs are well conserved among all eukaryotes (Yoshizawa et al., 2006). In plants, sequence comparison allowed the identification of rice orthologs for most of the Arabidopsis Q-SNAREs (Sutter et al., 2006a). Our observation that SYP121-Sp2 fragments from Arabidopsis and maize decreased the plasma membrane trafficking of mYFP:ZmPIP2;5 in tobacco epidermal cells and maize mesophyll protoplasts demonstrates that the mechanism of SYP121-mediated plasma membrane anchoring of PIP2 aquaporins is conserved between monocots and dicots. In addition, the localization of the human AQP2 at the apical membrane of epithelial cells of the collecting duct is also specifically regulated by the syntaxin-3, SNARE-associated protein Snapin and SNAP33 complex (Procino et al., 2008; Mistry et al., 2009), suggesting that the relative abundance of plasma membrane aquaporins is regulated by SNARE complexes in all eukaryotes.
The specificity of SYP121 in regulating plasma membrane Zm-PIP2;5 trafficking is indicated by the fact that AtSYP122-Sp2, AtSYP71-Sp2, and AtSYP21-Sp2 do not significantly alter the plasma membrane Zm-PIP2;5 abundance. In silico search in the Gramene database (http://www.gramene.org/) showed that At-SYP122, At-SYP71, and At-SYP21 have close homologs in maize, indicating a possible conservation of the trafficking regulation. Protein trafficking to the plasma membrane in Arabidopsis is defined by at least seven of the nine Qa-SNAREs of the SYP1 subfamily (Grefen et al., 2011). AtSYP121 and its close homolog At-SYP122 localize to the plasma membrane and their function is partially redundant (Leyman et al., 2000; Collins et al., 2003; Uemura et al., 2004). However, a coexpression study with a dominant-negative mutant of Rab11, a GTPase required for plasma membrane and cell plate trafficking, indicate that SYP121 and SYP122 drive two independent secretory events (Rehman et al., 2008). Interestingly, similarly to what has been observed for AtSYP121-Sp2 in tobacco (Sutter et al., 2006b), ZmSYP121-Sp2 does not affect the plasma membrane delivery of the mCFP:NpPMA2 H+ATPase in maize protoplasts.
SYP121 Affects the Membrane Osmotic Water Permeability by Direct Interaction with Zm-PIP2;5
A first link between the trafficking and the regulation of Zm-PIP2;5 activity by Zm-SYP121 derives from protoplast swelling assays. A significant decrease in Pf was measured in protoplasts coexpressing mYFP:ZmPIP2;5 and the ZmSYP121-Sp2 fragment. The most plausible hypothesis is that this Pf alteration results from a decrease in mYFP:ZmPIP2;5 abundance in the plasma membrane. However, this interpretation does not preclude additional effects of ZmSYP121-Sp2 in directly modifying the water channel activity of ZmPIP2;5. We observed that oocytes coexpressing mYFP:ZmPIP2;5 and ZmSYP121-Sp2 were characterized by a lower Pf compared with oocytes expressing mYFP:ZmPIP2;5 singly or in combination with Zm-SYP121. Significantly, in these oocytes, no difference in the mYFP:ZmPIP2;5 expression level and plasma membrane fluorescence intensity was detected, suggesting that in this heterologous system, mYFP:ZmPIP2;5 trafficking could not explain the difference in activity.
In mammalian cells, the plasma membrane delivery and gating of the cAMP-gated chloride channel and the inward rectifier potassium channels (Kir) are regulated by syntaxin and other exocytotic proteins (Naren et al., 1997; Leung et al., 2007). In plants, At-SYP121 directly interacts with the KC1 K+ regulatory subunit and modulates the activity of the AKT1/KC1 potassium channel (Honsbein et al., 2009; Grefen et al., 2010). Similarly, a direct interaction between Zm-PIP2;5 and Zm-SYP121 is demonstrated by affinity chromatography copurification after their expression in oocytes, yeast mating-based split-ubiquitin assays, and microscopy-based techniques (BiFC and FRET) in plant cells. These imaging approaches showed an interaction between Zm-PIP2;5 and Zm-SYP121 in both the plasma membrane and ER. This latter localization was predominant in maize protoplasts. Considering that Zm-PIP2;5 and Zm-SYP121 use the secretory pathway to reach the plasma membrane, this localization may simply reflect the lack of sufficient coordinate partners necessary to correctly process and target the proteins to the plasma membrane. Validation of in vivo interactions comes from FRET measurements by acceptor photobleaching. In our hands, this FRET methodology produces similar results to the FRET measured by acceptor-sensitized emission. Interactions between mYFP:ZmPIP2;5 and mCFP:ZmSYP121 are detected in the plasma membrane, but the FRET efficiency was 10% weaker than the efficiency measured in cells coexpressing mCFP:ZmPIP1;2 and mYFP:ZmPIP2;5. Since no FRET was detected in protoplasts coexpressing mCFP:ZmPIP2;5 and mYFP:ROP6, a plasma membrane–localized GTPase, which does not interact with Zm-PIP2;5 (Bischoff et al., 2000; Zelazny et al., 2007), we concluded that FRET by acceptor photobleaching could be used to investigate membrane protein interactions in tobacco epidermal cells and maize protoplasts. Altogether, the microscopy-based interaction data demonstrate that SYP121 physically interacts with Zm-PIP2;5 in plant cells. These observations were further supported by yeast split-ubiquitin assays that demonstrated an interaction of Zm-PIP2;5 with Zm-SYP121 or At-SYP121 in the rescue of diploid yeast growth.
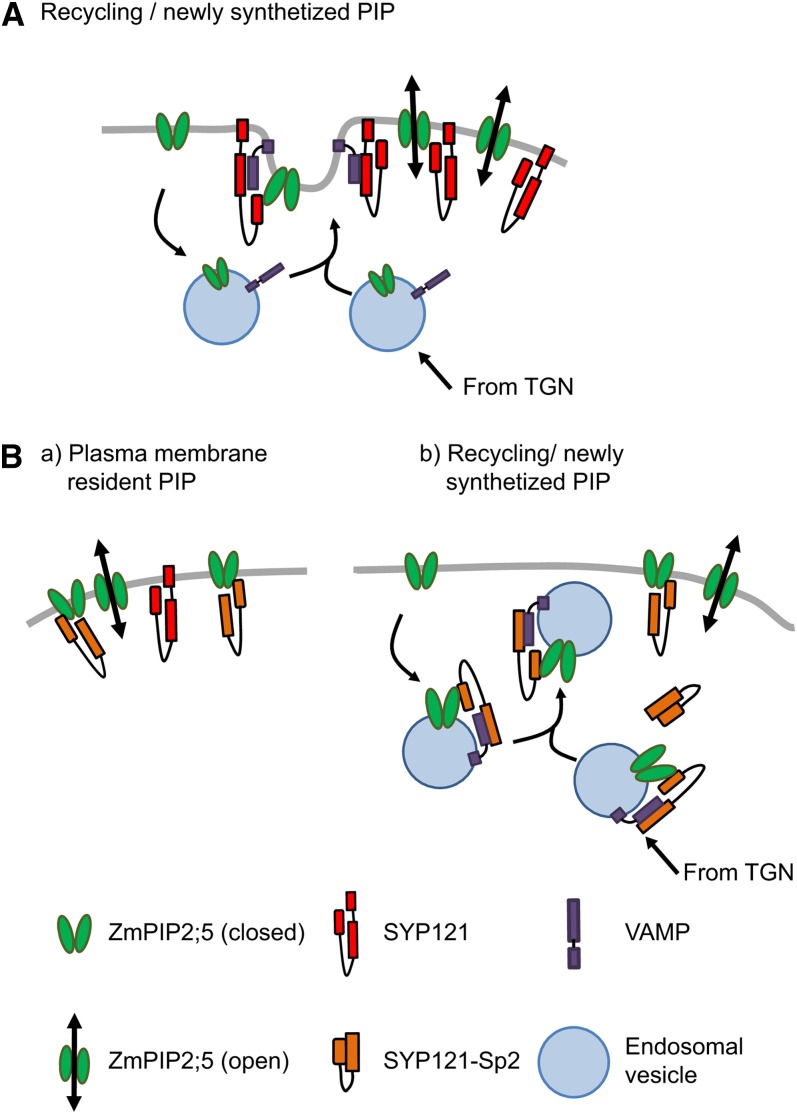
These data allow us to propose hypothetical models (Figure 7). In plant cells, the plasma membrane delivery of Zm-PIP2;5 during both constitutive recycling and anterograde transport from the TGN vesicles is mediated by SYP121, either by a Qa-SNARE/VAMP vesicle recognition independent of Zm-PIP2;5 and/or by a direct docking interaction between Zm-PIP2;5, SYP121, and VAMP (Figure 7A). In cells coexpressing Zm-PIP2;5 and ZmSYP121-Sp2, the truncated SNARE interacts with Zm-PIP2;5 and VAMP, preventing the formation of the SNARE complex with native Zm-SYP121 and, therefore, Zm-PIP2;5 plasma membrane delivery (Figure 7B). In addition, experiments in Xenopus oocytes indicate that SYP121-Sp2 can directly affect the activity of Zm-PIP2;5. In this system, the vesicular secretion of heterologously expressed protein appears not to be specific (Mohun et al., 1981), explaining the fact that SYP121-Sp2 does not affect Zm-PIP2;5 trafficking to the plasma membrane. In cells (co)expressing Zm-PIP2;5 and Zm-SYP121, the two partners interact at the plasma membrane and possibly in the vesicle without affecting the trafficking or water channel activity of Zm-PIP2;5. By contrast, in oocytes coexpressing Zm-PIP2;5 and ZmSYP121-Sp2, the plasma membrane delivery of ZmPIP2;5 is not affected, but its water channel activity is significantly decreased. The observation that no significant increase in Pf was measured in maize protoplasts coexpressing mYFP:ZmPIP2;5 and mCFP:ZmSYP121-Sp2 compared with mock cells, even if some mYFP fluorescence was detected in the plasma membrane, suggest that, in plant cells, the Sp2 soluble fragment also inhibits Zm-PIP2;5 activity (Figure 7B). One hypothesis is that the Sp2-soluble fragment interacts with Zm-PIP2;5 cytosolic loops or extremities and modifies its conformation and gating (Törnroth-Horsefield et al., 2006). A modification of AQP1-mediated water entry in pancreatic zymogen granules was shown to be blocked after incubation with antibodies raised against the C-terminal end of the protein (Cho et al., 2002). Because of steric constraint for accessibility, this kind of conformational change would not occur with membrane-anchored SYP121, which would rather act to increase PIP stability in the plasma membrane.
Figure 7.
Hypothetical Models Summarizing the Regulation of Zm-PIP2;5 Trafficking and Activity by Zm-SYP121.
(A) Regulation of Zm-PIP2;5 trafficking in plant cells coexpressing Zm-PIP2;5 and full-length Zm-SYP121. Constitutive recycling and delivery of newly synthesized Zm-PIP2;5 to the plasma membrane are regulated by SYP121 through Qa-SNARE/VAMP and direct interactions. SYP121 allows a proper docking of Zm-PIP2;5 at the plasma membrane and regulates its amount.
(B) Regulation of Zm-PIP2;5 trafficking in plant cells coexpressing Zm-PIP2;5 and the ZmSYP121-Sp2 fragment. (a) The dominant-negative mutant SYP121-Sp2 might bind to Zm-PIP2;5 proteins, altering their water channel activity and preventing their interaction with full-length SYP121. (b) Constitutive recycling and delivery of newly synthesized Zm-PIP2;5 to the plasma membrane are altered by the binding of SYP121-Sp2 to VAMP and Zm-PIP2;5. This interaction does not allow SNARE complex formation and the fusion of vesicles carrying Zm-PIP2;5 cargo to the plasma membrane. Additionally, SYP121-Sp2 might bind resident ZmPIP2;5 proteins and inhibit their water transport activity. See text for additional explanations.
Is SYP121 a Stress-Responsive SNARE Involved in the Maintenance of the Cell Turgor and Water Balance in Plants?
It is striking to observe that the SNARE SYP121 regulates the trafficking and activity of plasma membrane K+ channels and aquaporins, transporters involved in the regulation of cell water homeostasis. In addition, all of these components are regulated by the stress hormone abscisic acid (Leyman et al., 1999; Geelen et al., 2002; Parent et al., 2009). An attractive hypothesis is that SYP121 acts as an essential component to coregulate ion and water movement through the cell membrane.
Besides modulating trafficking and anchoring of KAT1, SYP121 can directly modulate K+ channel activity. Split-ubiquitin, BiFC, and coimmunoprecipitation experiments demonstrated that SYP121, but not its closest homolog SYP122, directly interacts with the KC1 regulatory subunit to form a tripartite SYP121-KC1-AKT1 complex. Mutations in any of the three proteins selectively suppressed the inward-rectifying K+ current in Arabidopsis root epidermal protoplasts as well as K+ acquisition and growth in seedlings when channel-mediated K+ uptake was limiting (Honsbein et al., 2009). Further characterization showed that SYP121 can also interact with KAT1 (Grefen et al., 2010) and that the FxRF motif located in the first 12 amino acid residues of SYP121 was required for the selective interaction with the K+ channels (Grefen et al., 2010). These data indicate an essential role of SYP121 in the connection between ion transport and membrane traffic (Grefen et al., 2011; Honsbein et al., 2011). Our results suggest that SYP121 could also regulate water homeostasis through modulation of aquaporin docking and possibly activity. We showed here that SYP121 can physically interact with Zm-PIP2;5 and acts as a regulator of its plasma membrane delivery. The absence of direct modulation of Zm-PIP2;5 water channel activity by full-length SYP121 led us to suggest that it can rather play a role in coordinating water and K+ ion fluxes. We propose that SYP121 acts to coordinate the plasma membrane density of both PIP and K+ channels by regulating their membrane delivery and recycling. The next challenge will be to monitor the dynamics of PIPs and K+ channels in the plasma membrane of cells subjected to osmotic stress. However, as SYP121 physically interacts with both PIPs and KC1 subunits, we cannot exclude at this stage that SYP121 also plays a role as a physical bridge between both proteins, resulting in the formation of a higher complex. It was suggested that PIPs could act as turgor sensors in the plasma membrane that modulate the conductance of K+ ion channels (Hill et al., 2004). The PIP/SYP121/K+ channel tripartite regulation could coordinate the turgor sensing with a tight tuning of ions and water movement and content in growing cells, guard cells, or cells submitted to drought stress. A direct link between ion transport, stomatal aperture, and optimization of water use efficiency has recently been established in the Arabidopsis syp121 loss-of-function mutant growing in high light intensity and low relative humidity (Eisenach et al., 2012). Importantly, we showed that protoplasts isolated from syp121 leaves have a lower membrane water permeability than do wild-type protoplasts, indicating a role of SYP121 in aquaporin regulation. Altogether, our data add evidence to the concept of SNAREs being a molecular governor (Grefen and Blatt, 2008; Honsbein et al., 2011) that coordinates membrane transporter traffic and activity with the regulation of water and solute transport in plant cells.
METHODS
Isolation and Transfection of Maize and Arabidopsis Protoplasts
Maize (Zea mays) B73 seedlings were grown, and mesophyll protoplast isolation and transfection were performed as described previously (Zelazny et al., 2007). Arabidopsis thaliana Columbia-0 wild type and syp121 (syp121-1/pen1-1) mutants (Collins et al., 2003) were grown in soil under 250 μmol m−2 s−1 in long days (16:8 light [L]:dark [D]) at 22:21°C (L:D) with a relative humidity of 70%. Mesophyll protoplasts were isolated as described previously (Ramahaleo et al., 1999).
Protoplast Swelling Assay
Maize and Arabidopsis protoplast swelling experiments were performed as described previously (Moshelion et al., 2004; Volkov et al., 2007). The solutions used for Arabidopsis protoplast swelling have been described (Postaire et al., 2010). The selection of protoplasts (co)expressing mYFP:ZmPIP2;5 and mCFP:ZmSYP121 or mCFP:ZmSYP121-Sp2 was achieved due to their fluorescence emission.
Phylogenetic Analysis
The multiple alignment in Supplemental Figure 1 online was obtained using the ClustalW algorithm with a Blosum62 scoring matrix, open gap penalty of 10, and gap extension penalty of 0.5. The neighbor-joining method was used to build the phylogenetic tree. Nodes’ significance was inferred by 1000 bootstrap iterations.
Molecular Biology
PCR products encoding Np-PMA2, Zm-PIP2;5, Zm-SYP121, ZmSYP121-Sp2, AtSYP121-Sp2, AtSYP122-Sp2, AtSYP21-Sp2, and AtSYP71-Sp2 cDNAs were directionally subcloned using a uracil excision-based improved high-throughput USER cloning technique (Nour-Eldin et al., 2006) into the USER-compatible plant expression vectors pCAMBIA2300 35Su Nterm mYFP and pCAMBIA2300 35Su Nterm mCFP (Bienert et al., 2011). This allowed the expression and in vivo visualization of the protein of interest tagged with either mCFP or mYFP at the N terminus. For BiFC experiments, new vectors were generated by adding a linker coding for GSGGSGGS before the USER cassette in the pCAMBIA2300 35Su Nterm Y155N and pCAMBIA2300 35Su Nterm Y155C vectors and after the USER cassette in the pCAMBIA2300 35Su Cterm Y155N and pCAMBIA2300 35Su Nterm C155C vectors. This vector set was used to express Zm-PIP2;5, Zm-PIP1;2, Zm-SYP121, and Np-PMA2 fused to the split Venus. All cloning products were checked by sequencing. cDNAs encoding Zm-PIP2;5, Zm-SYP121, Zm-SYP121-Sp2, and Zm-PIP1;2 were subcloned into the USER-compatible Xenopus laevis expression pNB1u, pNBRGS-HISu, and pNB1YFPu vectors containing the YFP gene or a sequence encoding a RGS-6 His tag 5′ of the insertion site (Nour-Eldin et al., 2006).
Particle Bombardment Transfection
Gold beads (0.6-µm diameter, 480 µg) were coated with 1 µg of plasmid DNA. The median parts of the third leaf of 7-d-old etiolated maize seedlings were placed in a Petri dish, which was positioned 3 cm below the microprojectile stopping plate. Samples were bombarded once under 948 kPa partial vacuum at a rupture pressure of 1100 p.s.i. (7600 kPa) using a PDS-1000/He Biolistic device (Bio-Rad). After transfection, leaf explants were incubated overnight at 25°C in the dark on solid half-strength Hoagland medium.
Confocal Microscopy
Detection of mCFP and mYFP fusion proteins was performed using a Zeiss LSM710 confocal microscope equipped with a spectral detector. Imaging of the maize mesophyll protoplasts was achieved with a Plan-Apochromat ×63/1.40 oil immersion objective. mCFP and mYFP were excited with the 445- and 514-nm laser lines, respectively. Emitted light was collected through a dichroic mirror on detectors 450 to 510 nm (mCFP) and 520 to 600 nm (mYFP). In each experiment, calibration of the laser beam intensity, gain, and offset parameters were achieved on cells expressing mYFP:ZmPIP2;5. The same parameters were then used in image acquisitions performed on coexpressing cells, allowing a subsequent calculation of the plasma membrane fluorescence intensity. The fluorescence intensity in the protoplast plasma membrane and intracellular compartments was quantified using an in-house developed macro for the ImageJ software. First, the average thickness of the plasma membrane was determined using the colocalized signals arising from mYFP:ZmPIP2;5 and the steryl dye FM4-64 by averaging 100 measurements (10 measurements performed on 10 independent cells). The mean value of the optical thickness of the protoplast plasma membrane was 0.35 μm. Computation of the images was then performed in three steps. First, the image was binarized, smoothed by the application of a median filter, and, finally, converted into traces using the outline function of the software. Second, the periphery trace was converted into the region of interest (ROI-1), which thus contains the overall cell fluorescence. Third, the ROI-1 was narrowed 0.35 μm in diameter to obtain ROI-2 that reflected the intracellular fluorescence. The fluorescence in the plasma membrane is given by the difference between the integrated signal densities (product of the surface area and the intensity of the signal) measured in ROI-1 and ROI-2.
Time-lapse acquisitions were performed using a combination of the time-lapse, tile scan, position, and Z-stack modules of the Zen 2009 software (Carl Zeiss MicroImaging). Image acquisition was done every 30 min for 16 h, using a C-Apochromat ×40/1.20 water immersion objective to maximize the size of the acquisition field.
FRAP experiments were performed as follows. Photobleaching (80 iterations, 514-nm laser beam at 100% [i.e., 7.5 mW]) was performed in a defined ROI and recovery of fluorescence was monitored by acquisition of one image every 5 s for 200 s. FRAP data were analyzed as previously described (Sprague et al., 2004). The best fitting curve was obtained according to a single exponential association using the “bottom to span” algorithm in Prism software (GraphPad Software). The following equation was used: y = span × (1 − e-kt) + bottom, where the bottom is defined by the minimal fluorescence intensity recorded after photobleaching, span is the difference between the bottom and the calculated plateau, and k is the binding constant defined by the half time of recovery, such as t1/2 = 0.69/k.
The BiFC assay was performed in tobacco (Nicotiana tabacum) epidermal cells transformed by Agrobacterium tumefaciens infiltration (Batoko et al., 2000) or in polyethylene glycol–transfected maize protoplasts. In cells exhibiting a fluorescence signal after excitation at 514 nm, an emission spectrum was determined to validate the signal specificity. The subcellular localization was assessed by Z-stack acquisition.
FRET was measured using either the acceptor photobleaching or acceptor-sensitized emission methods. Acquisitions were performed on two optical tracks, in sequential line mode. For the photobleaching method, three images were acquired for both the mCFP and mYFP channels, and then the mYFP was bleached by 80 iterations with full-power laser illumination, while the fluorescence intensity of the mCFP was simultaneously monitored along time. The FRET efficiency (E) was determined according the equation E = 1− IDA/ID, where IDA is the intensity of the donor fluorescence before photobleaching and ID the fluorescence intensity of the donor after photobleaching, as described elsewhere (Kwaaitaal et al., 2010). The sensitized emission method was used on a subpopulation of tobacco epidermal cells and protoplasts to confirm the data obtained by acceptor photobleaching. One acquisition track was composed of two acquisition channels to detect the mCFP and FRET signals. mCFP was excited using the 445-nm laser beam, and the emitted light was collected between 447 and 517 nm and between 523 nm and 630 nm for mCFP and FRET detection, respectively. The acceptor was excited at 514 nm, and emitted light was collected between 523 and 628 nm. FRET efficiency was calculated according to the Youvan method (Youvan et al., 1997) using the FRET module of Zen 2009 software (Carl Zeiss MicroImaging).
Heterologous Expression in Xenopus Oocytes
In vitro cRNA synthesis, oocyte isolation, microinjection, and measurement of Pf and microsome isolation were performed as described by Fetter et al. (2004). Oocytes were injected with 2 or 4 ng of cRNA encoding the 6 His-tagged or YFP-tagged proteins, respectively. The cRNA was quantified with a Nanodrop 1000 spectrophotometer (NanoDrop Technologies) and its integrity checked on an agarose gel. Oocytes expressing the YFP-tagged constructs were fixed and sliced as described previously (Sayers et al., 1997). Fluorescence was visualized by CLSM using a Plan-Neofluar ×10/0.30 objective as described above and quantified with ImageJ software.
Mating-Based Split-Ubiquitin Assays
The haploid yeast strains THY.AP4 and THY.AP5 (Obrdlik et al., 2004; Grefen et al., 2007) were transformed as described previously (Grefen et al., 2009). Approximately 15 single colonies of bait (Cub-PLV) and prey (Nub) constructs were selected following 3 d of growth on plates and inoculated in vector-selective media for overnight growth. Liquid cultures were harvested at an OD600 of 2 to 3 and resuspended in yeast-extract peptone dextrose medium. Equal volumes (20 µL) of each bait and prey were mixed and dropped on YPD plates. After 6 to 8 h incubation at 30°C, yeast was scraped off and streaked on vector-selective media (CSM-L-, W-, and Ura-). After ∼1 to 2 d of growth, the diploid yeast was used to inoculate liquid vector-selective media and grown overnight. The next day, serial dilutions at OD600 1.0 and 0.1 in water were dropped, 7 μL per spot, onto plates without and with the addition of 5, 50, and 500 µM Met on interaction-selective media (CSM-L-, W-, Ura-, Ade-, H-, and M-). Growth was monitored after 2 or 3 d at 30°C. Control plates on vector-selective media were incubated for 24 h only to verify that an equal amount of yeast had been dropped. To verify expression, haploid yeast was harvested prior to mating and analyzed via immunoblotting using polyclonal antibodies against SYP121 and VP16, as described previously (Grefen et al., 2009; Honsbein et al., 2009).
Solubilization and Purification of His:ZmPIP2;5 and Immunodetection
Microsomes were resuspended in 1× PBS buffer. Three hundred micrograms of proteins were solubilized with 1% (w/v) octyl-β-d-glucopyranoside in a final volume of 400 μL and incubated for 2 h on a rotary wheel at room temperature. Unsolubilized material was removed by centrifugation at 169,000g for 20 min, and the supernatant was added to 100 μL of Ni2+-nitriloacetic acid agarose matrix (Qiagen) preequilibrated with 1× PBS buffer containing 1% [w/v] octyl-β-d-glucopyranoside and 10 mM imidazole. The mixture was incubated for 2 h on a rotary wheel at room temperature, loaded on a column, and washed three times with 1 mL of 1× PBS buffer containing 10 mM imidazole and 0.05% Tween 20. Bound proteins were eluted with 100 μL Laemmli buffer. Proteins (50 µL/well) were electrophoresed by SDS-PAGE, transferred to a nitrocellulose membrane, and immunodetected with antibodies directed against GFP (Duby et al., 2001), SYP121 (Tyrrell et al., 2007), and Zm-PIP2;5 (Hachez et al., 2006) by the enhanced bioluminescence method.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL and maize sequencing project data libraries (http://www.maizesequence.org/index.html; Schnable et al., 2009) under the following accession numbers: At-SYP111, NP_172332; At-SYP112, NP_179418.2; At-SYP121, NP_187788; At-SYP122, NP_190808; At-SYP123, NP_192242; At-SYP124, NP_176324; At-SYP125, NP_172591; At-SYP131, NP_187030; At-SYP132, NP_568187; Os-SYP111, ABB22783; Os-SYP112, ABA96047; Os-SYP121, ABF99241; Os-SYP124, NP_001172852; Os-SYP131, NP_001058959; Os-SYP132, NP_001056925; Zm-SYP111, NP_001149999; Zm-SYP112, GRMZM2G168017; Zm-SYP121, NP_001150776, Zm-SYP124, GRMZM2G411561; Zm-SYP123, GRMZM2G416733; Zm-SYP132, NP_001149376; Zm-SYP131, GRMZM2G330772; Np-PMA2, AAA34052; and Zm-PIP2;5, AF130975.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Unrooted Phylogenetic Tree of the Plasma Membrane–Localized Qa SNAREs.
Supplemental Figure 2. Intracellular Accumulation of mYFP:ZmPIP2;5 in Protoplasts Transfected with the Plasmids Encoding mCFP:ZmSYP121-Sp2 and mYFP:ZmPIP2;5 at a 3:1 Ratio.
Supplemental Figure 3. Still Images of Supplemental Movie 1, Showing Time-Lapse Acquisition of mCFP:ZmSYP121-Sp2 and mYFP:ZmPIP2;5 Accumulation in Maize Mesophyll Protoplasts.
Supplemental Figure 4. ZmSYP121-Sp2 Affects Plasma Membrane Delivery but Not Synthesis of mYFP:ZmPIP2;5.
Supplemental Figure 5. Fluorescence Recovery after Photobleaching.
Supplemental Figure 6. Pf Values Measured after 15, 30, and 45 s of Protoplast Swelling.
Supplemental Figure 7. Zm-SYP121 and Zm-PIP2;5 Interact in Tobacco Epidermal Cells.
Supplemental Figure 8. FRET Experiments.
Supplemental Figure 9. Comparison of Sensitized Emission and Acceptor Photobleaching FRET Efficiencies.
Supplemental Data Set 1. Multiple Alignments of the Full-Length Protein Sequences of Qa SNARE Used to Build the Phylogenetic Tree of Supplemental Figure 2 Online.
Supplemental Movie 1. Movie of Time-Lapse Acquisition of mCFP:ZmSYP121-Sp2 and mYFP:ZmPIP2;5 Accumulation in Maize Mesophyll Protoplasts.
Supplemental Movie Legend 1. Legend for Supplemental Movie 1.
Supplementary Material
Acknowledgments
We thank the imaging platform IMABIOL. This work was supported by grants from the Belgian National Fund for Scientific Research (FNRS), the Interuniversity Attraction Poles Programme–Belgian Science Policy, the “Communauté française de Belgique–Actions de Recherches Concertées”, and by grants BB/F001630/1 and BB/H0009817/1 to M.R.B. from the UK Biotechnology and Biological Sciences Research Council. A.B. was a postdoctoral research fellow at the FNRS. G.P.B. was supported by an individual Marie Curie European fellowship and by the FNRS. A.S.C. was a research fellow at the “Fonds pour la Formation à la Recherche dans l’Industrie et dans l’Agriculture.”
AUTHOR CONTRIBUTIONS
A.B. designed and performed research, analyzed data, developed new analytic tools, and wrote the article. E.B. performed research and analyzed data. G.P.B. designed the research, provided molecular tools, and analyzed data. A.S.C. provided molecular tools, performed research, and analyzed the data. A.E. provided technical assistance. C.G. performed research, analyzed data, and wrote the article. F.C. designed the research and, together with M.R.B., analyzed data and wrote the article.
Glossary
- PIP
plasma membrane intrinsic proteins
- ER
endoplasmic reticulum
- FRET
Förster resonance energy transfer
- TGN
trans-Golgi network
- SNARE
soluble N-ethylmaleimide-sensitive factor protein attachment protein receptor
- mCFP
monomeric cyan fluorescent protein
- mYFP
monomeric yellow fluorescent protein
- FRAP
fluorescence recovery after photobleaching
- Pf
osmotic water permeability coefficient
- CLSM
confocal laser scanning microscopy
- GFP
green fluorescent protein
- BiFC
bimolecular fluorescence complementation
- ROI
region of interest
- ANOVA
analysis of variance
References
- Batoko H., Zheng H.Q., Hawes C., Moore I. (2000). A rab1 GTPase is required for transport between the endoplasmic reticulum and Golgi apparatus and for normal Golgi movement in plants. Plant Cell 12: 2201–2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellati J., Alleva K., Soto G., Vitali V., Jozefkowicz C., Amodeo G. (2010). Intracellular pH sensing is altered by plasma membrane PIP aquaporin co-expression. Plant Mol. Biol. 74: 105–118 [DOI] [PubMed] [Google Scholar]
- Bhat R.A., Miklis M., Schmelzer E., Schulze-Lefert P., Panstruga R. (2005). Recruitment and interaction dynamics of plant penetration resistance components in a plasma membrane microdomain. Proc. Natl. Acad. Sci. USA 102: 3135–3140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienert G.P., Bienert M.D., Jahn T.P., Boutry M., Chaumont F. (2011). Solanaceae XIPs are plasma membrane aquaporins that facilitate the transport of many uncharged substrates. Plant J. 66: 306–317 [DOI] [PubMed] [Google Scholar]
- Bienert G.P., Cavez D., Besserer A., Berny M.C., Gilis D., Rooman M., Chaumont F. (2012). A conserved cysteine residue is involved in disulfide bond formation between plant plasma membrane aquaporin monomers. Biochem. J. 445: 101–111 [DOI] [PubMed] [Google Scholar]
- Bischoff F., Vahlkamp L., Molendijk A., Palme K. (2000). Localization of AtROP4 and AtROP6 and interaction with the guanine nucleotide dissociation inhibitor AtRhoGDI1 from Arabidopsis. Plant Mol. Biol. 42: 515–530 [DOI] [PubMed] [Google Scholar]
- Borner G.H., Sherrier D.J., Weimar T., Michaelson L.V., Hawkins N.D., Macaskill A., Napier J.A., Beale M.H., Lilley K.S., Dupree P. (2005). Analysis of detergent-resistant membranes in Arabidopsis. Evidence for plasma membrane lipid rafts. Plant Physiol. 137: 104–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaumont F., Barrieu F., Jung R., Chrispeels M.J. (2000). Plasma membrane intrinsic proteins from maize cluster in two sequence subgroups with differential aquaporin activity. Plant Physiol. 122: 1025–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S.J., Sattar A.K., Jeong E.H., Satchi M., Cho J.A., Dash S., Mayes M.S., Stromer M.H., Jena B.P. (2002). Aquaporin 1 regulates GTP-induced rapid gating of water in secretory vesicles. Proc. Natl. Acad. Sci. USA 99: 4720–4724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins N.C., Thordal-Christensen H., Lipka V., Bau S., Kombrink E., Qiu J.L., Hückelhoven R., Stein M., Freialdenhoven A., Somerville S.C., Schulze-Lefert P. (2003). SNARE-protein-mediated disease resistance at the plant cell wall. Nature 425: 973–977 [DOI] [PubMed] [Google Scholar]
- da Silva Conceição A., Marty-Mazars D., Bassham D.C., Sanderfoot A.A., Marty F., Raikhel N.V. (1997). The syntaxin homolog AtPEP12p resides on a late post-Golgi compartment in plants. Plant Cell 9: 571–582 [PMC free article] [PubMed] [Google Scholar]
- Dhonukshe P., Aniento F., Hwang I., Robinson D.G., Mravec J., Stierhof Y.-D., Friml J. (2007). Clathrin-mediated constitutive endocytosis of PIN auxin efflux carriers in Arabidopsis. Curr. Biol. 17: 520–527 [DOI] [PubMed] [Google Scholar]
- Duby G., Oufattole M., Boutry M. (2001). Hydrophobic residues within the predicted N-terminal amphiphilic alpha-helix of a plant mitochondrial targeting presequence play a major role in in vivo import. Plant J. 27: 539–549 [DOI] [PubMed] [Google Scholar]
- Eisenach C., Chen Z.H., Grefen C., Blatt M.R. (2012). The trafficking protein SYP121 of Arabidopsis connects programmed stomatal closure and K(+) channel activity with vegetative growth. Plant J. 69: 241–251. [DOI] [PubMed]
- Fasshauer D., Sutton R.B., Brunger A.T., Jahn R. (1998). Conserved structural features of the synaptic fusion complex: SNARE proteins reclassified as Q- and R-SNAREs. Proc. Natl. Acad. Sci. USA 95: 15781–15786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetter K., Van Wilder V., Moshelion M., Chaumont F. (2004). Interactions between plasma membrane aquaporins modulate their water channel activity. Plant Cell 16: 215–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geelen D., Leyman B., Batoko H., Di Sansebastiano G.P., Moore I., Blatt M.R. (2002). The abscisic acid-related SNARE homolog NtSyr1 contributes to secretion and growth: Evidence from competition with its cytosolic domain. Plant Cell 14: 387–406 Erratum. Plant Cell 14: 963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldner N., Friml J., Stierhof Y.D., Jürgens G., Palme K. (2001). Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 413: 425–428 [DOI] [PubMed] [Google Scholar]
- Gomes D., Agasse A., Thiébaud P., Delrot S., Gerós H., Chaumont F. (2009). Aquaporins are multifunctional water and solute transporters highly divergent in living organisms. Biochim. Biophys. Acta 1788: 1213–1228 [DOI] [PubMed] [Google Scholar]
- Grefen C., Blatt M.R. (2008). SNAREs—Molecular governors in signalling and development. Curr. Opin. Plant Biol. 11: 600–609 [DOI] [PubMed] [Google Scholar]
- Grefen C., Chen Z., Honsbein A., Donald N., Hills A., Blatt M.R. (2010). A novel motif essential for SNARE interaction with the K(+) channel KC1 and channel gating in Arabidopsis. Plant Cell 22: 3076–3092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grefen C., Honsbein A., Blatt M.R. (2011). Ion transport, membrane traffic and cellular volume control. Curr. Opin. Plant Biol. 14: 332–339 [DOI] [PubMed] [Google Scholar]
- Grefen C., Lalonde S., Obrdlik P. (2007). Split-ubiquitin system for identifying protein-protein interactions in membrane and full-length proteins. Curr. Protoc. Neurosci. 5: 27. [DOI] [PubMed]
- Grefen C., Obrdlik P., Harter K. (2009). The determination of protein-protein interactions by the mating-based split-ubiquitin system (mbSUS). Methods Mol. Biol. 479: 217–233 [DOI] [PubMed] [Google Scholar]
- Hachez C., Moshelion M., Zelazny E., Cavez D., Chaumont F. (2006). Localization and quantification of plasma membrane aquaporin expression in maize primary root: A clue to understanding their role as cellular plumbers. Plant Mol. Biol. 62: 305–323 [DOI] [PubMed] [Google Scholar]
- Heinen R.B., Ye Q., Chaumont F. (2009). Role of aquaporins in leaf physiology. J. Exp. Bot. 60: 2971–2985 [DOI] [PubMed] [Google Scholar]
- Hill A.E., Shachar-Hill B., Shachar-Hill Y. (2004). What are aquaporins for? J. Membr. Biol. 197: 1–32 [DOI] [PubMed] [Google Scholar]
- Honsbein A., Blatt M.R., Grefen C. (2011). A molecular framework for coupling cellular volume and osmotic solute transport control. J. Exp. Bot. 62: 2363–2370 [DOI] [PubMed] [Google Scholar]
- Honsbein A., Sokolovski S., Grefen C., Campanoni P., Pratelli R., Paneque M., Chen Z., Johansson I., Blatt M.R. (2009). A tripartite SNARE-K+ channel complex mediates in channel-dependent K+ nutrition in Arabidopsis. Plant Cell 21: 2859–2877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaillais Y., Santambrogio M., Rozier F., Fobis-Loisy I., Miège C., Gaude T. (2007). The retromer protein VPS29 links cell polarity and organ initiation in plants. Cell 130: 1057–1070 [DOI] [PubMed] [Google Scholar]
- Kwaaitaal M., Keinath N.F., Pajonk S., Biskup C., Panstruga R. (2010). Combined bimolecular fluorescence complementation and Forster resonance energy transfer reveals ternary SNARE complex formation in living plant cells. Plant Physiol. 152: 1135–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.K., Cho S.K., Son O., Xu Z., Hwang I., Kim W.T. (2009). Drought stress-induced Rma1H1, a RING membrane-anchor E3 ubiquitin ligase homolog, regulates aquaporin levels via ubiquitination in transgenic Arabidopsis plants. Plant Cell 21: 622–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre B., Batoko H., Duby G., Boutry M. (2004). Targeting of a Nicotiana plumbaginifolia H+-ATPase to the plasma membrane is not by default and requires cytosolic structural determinants. Plant Cell 16: 1772–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung Y.M., Kwan E.P., Ng B., Kang Y., Gaisano H.Y. (2007). SNAREing voltage-gated K+ and ATP-sensitive K+ channels: Tuning beta-cell excitability with syntaxin-1A and other exocytotic proteins. Endocr. Rev. 28: 653–663 [DOI] [PubMed] [Google Scholar]
- Leyman B., Geelen D., Blatt M.R. (2000). Localization and control of expression of Nt-Syr1, a tobacco SNARE protein. Plant J. 24: 369–381 [DOI] [PubMed] [Google Scholar]
- Leyman B., Geelen D., Quintero F.J., Blatt M.R. (1999). A tobacco syntaxin with a role in hormonal control of guard cell ion channels. Science 283: 537–540 [DOI] [PubMed] [Google Scholar]
- Li X., Wang X., Yang Y., Li R., He Q., Fang X., Luu D.T., Maurel C., Lin J. (2011). Single-molecule analysis of PIP2;1 dynamics and partitioning reveals multiple modes of Arabidopsis plasma membrane aquaporin regulation. Plant Cell 23: 3780–3797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu D.T., Martinière A., Sorieul M., Runions J., Maurel C. (2012). Fluorescence recovery after photobleaching reveals high cycling dynamics of plasma membrane aquaporins in Arabidopsis roots under salt stress. Plant J. 69: 894–905 [DOI] [PubMed] [Google Scholar]
- Maurel C., Verdoucq L., Luu D.T., Santoni V. (2008). Plant aquaporins: Membrane channels with multiple integrated functions. Annu. Rev. Plant Biol. 59: 595–624 [DOI] [PubMed] [Google Scholar]
- Mistry A.C., Mallick R., Klein J.D., Weimbs T., Sands J.M., Fröhlich O. (2009). Syntaxin specificity of aquaporins in the inner medullary collecting duct. Am. J. Physiol. Renal Physiol. 297: F292–F300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohun T.J., Lane C.D., Colman A., Wylie C.C. (1981). The secretion of proteins in vitro from Xenopus oocytes and their accessory cells: A biochemical and morphological study. J. Embryol. Exp. Morphol. 61: 367–383 [PubMed] [Google Scholar]
- Moshelion M., Hachez C., Ye Q., Cavez D., Bajji M., Jung R., Chaumont F. (2009). Membrane water permeability and aquaporin expression increase during growth of maize suspension cultured cells. Plant Cell Environ. 32: 1334–1345 [DOI] [PubMed] [Google Scholar]
- Moshelion M., Moran N., Chaumont F. (2004). Dynamic changes in the osmotic water permeability of protoplast plasma membrane. Plant Physiol. 135: 2301–2317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naren A.P., Nelson D.J., Xie W., Jovov B., Pevsner J., Bennett M.K., Benos D.J., Quick M.W., Kirk K.L. (1997). Regulation of CFTR chloride channels by syntaxin and Munc18 isoforms. Nature 390: 302–305 [DOI] [PubMed] [Google Scholar]
- Nour-Eldin H.H., Hansen B.G., Nørholm M.H., Jensen J.K., Halkier B.A. (2006). Advancing uracil-excision based cloning towards an ideal technique for cloning PCR fragments. Nucleic Acids Res. 34: e122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrdlik P., et al. (2004). K+ channel interactions detected by a genetic system optimized for systematic studies of membrane protein interactions. Proc. Natl. Acad. Sci. USA 101: 12242–12247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto B., et al. (2010). Aquaporin tetramer composition modifies the function of tobacco aquaporins. J. Biol. Chem. 285: 31253–31260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paciorek T., Zazímalová E., Ruthardt N., Petrásek J., Stierhof Y.-D., Kleine-Vehn J., Morris D.A., Emans N., Jürgens G., Geldner N., Friml J. (2005). Auxin inhibits endocytosis and promotes its own efflux from cells. Nature 435: 1251–1256 [DOI] [PubMed] [Google Scholar]
- Parent B., Hachez C., Redondo E., Simonneau T., Chaumont F., Tardieu F. (2009). Drought and abscisic acid effects on aquaporin content translate into changes in hydraulic conductivity and leaf growth rate: A trans-scale approach. Plant Physiol. 149: 2000–2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postaire O., Tournaire-Roux C., Grondin A., Boursiac Y., Morillon R., Schäffner A.R., Maurel C. (2010). A PIP1 aquaporin contributes to hydrostatic pressure-induced water transport in both the root and rosette of Arabidopsis. Plant Physiol. 152: 1418–1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prak S., Hem S., Boudet J., Viennois G., Sommerer N., Rossignol M., Maurel C., Santoni V. (2008). Multiple phosphorylations in the C-terminal tail of plant plasma membrane aquaporins: Role in subcellular trafficking of AtPIP2;1 in response to salt stress. Mol. Cell. Proteomics 7: 1019–1030 [DOI] [PubMed] [Google Scholar]
- Pratelli R., Sutter J.U., Blatt M.R. (2004). A new catch in the SNARE. Trends Plant Sci. 9: 187–195 [DOI] [PubMed] [Google Scholar]
- Procino G., Barbieri C., Tamma G., De Benedictis L., Pessin J.E., Svelto M., Valenti G. (2008). AQP2 exocytosis in the renal collecting duct — Involvement of SNARE isoforms and the regulatory role of Munc18b. J. Cell Sci. 121: 2097–2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramahaleo T., Morillon R., Alexandre J., Lassalles J.P. (1999). Osmotic water permeability of isolated protoplasts. Modifications during development. Plant Physiol. 119: 885–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman R.U., Stigliano E., Lycett G.W., Sticher L., Sbano F., Faraco M., Dalessandro G., Di Sansebastiano G.P. (2008). Tomato Rab11a characterization evidenced a difference between SYP121-dependent and SYP122-dependent exocytosis. Plant Cell Physiol. 49: 751–766 [DOI] [PubMed] [Google Scholar]
- Reichardt I., Slane D., El Kasmi F., Knöll C., Fuchs R., Mayer U., Lipka V., Jürgens G. (2011). Mechanisms of functional specificity among plasma-membrane syntaxins in Arabidopsis. Traffic 12: 1269–1280 [DOI] [PubMed] [Google Scholar]
- Richter S., Voss U., Jürgens G. (2009). Post-Golgi traffic in plants. Traffic 10: 819–828 [DOI] [PubMed] [Google Scholar]
- Robinson D.G., Jiang L., Schumacher K. (2008). The endosomal system of plants: Charting new and familiar territories. Plant Physiol. 147: 1482–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderfoot A. (2007). Increases in the number of SNARE genes parallels the rise of multicellularity among the green plants. Plant Physiol. 144: 6–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderfoot A.A., Kovaleva V., Bassham D.C., Raikhel N.V. (2001). Interactions between syntaxins identify at least five SNARE complexes within the Golgi/prevacuolar system of the Arabidopsis cell. Mol. Biol. Cell 12: 3733–3743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayers L.G., Miyawaki A., Muto A., Takeshita H., Yamamoto A., Michikawa T., Furuichi T., Mikoshiba K. (1997). Intracellular targeting and homotetramer formation of a truncated inositol 1,4,5-trisphosphate receptor-green fluorescent protein chimera in Xenopus laevis oocytes: Evidence for the involvement of the transmembrane spanning domain in endoplasmic reticulum targeting and homotetramer complex formation. Biochem. J. 323: 273–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyu Y.J., Liu H., Deng X., Hu C.D. (2006). Identification of new fluorescent protein fragments for bimolecular fluorescence complementation analysis under physiological conditions. Biotechniques 40: 61–66 [DOI] [PubMed] [Google Scholar]
- Schnable P.S., et al. (2009) The B73 maize genome: Complexity, diversity, and dynamics. Science 326: 1112–1115 [DOI] [PubMed] [Google Scholar]
- Sorieul M., Santoni V., Maurel C., Luu D.T. (2011). Mechanisms and effects of retention of over-expressed aquaporin AtPIP2;1 in the endoplasmic reticulum. Traffic 12: 473–482 [DOI] [PubMed] [Google Scholar]
- Sprague B.L., Pego R.L., Stavreva D.A., McNally J.G. (2004). Analysis of binding reactions by fluorescence recovery after photobleaching. Biophys. J. 86: 3473–3495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter J.U., Campanoni P., Blatt M.R., Paneque M. (2006a). Setting SNAREs in a different wood. Traffic 7: 627–638 [DOI] [PubMed] [Google Scholar]
- Sutter J.U., Campanoni P., Tyrrell M., Blatt M.R. (2006b). Selective mobility and sensitivity to SNAREs is exhibited by the Arabidopsis KAT1 K+ channel at the plasma membrane. Plant Cell 18: 935–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwastika I.N., Uemura T., Shiina T., H Sato M., Takeyasu K. (2008). SYP71, a plant-specific Qc-SNARE protein, reveals dual localization to the plasma membrane and the endoplasmic reticulum in Arabidopsis. Cell Struct. Funct. 33: 185–192 [DOI] [PubMed] [Google Scholar]
- Takano J., Tanaka M., Toyoda A., Miwa K., Kasai K., Fuji K., Onouchi H., Naito S., Fujiwara T. (2010). Polar localization and degradation of Arabidopsis boron transporters through distinct trafficking pathways. Proc. Natl. Acad. Sci. USA 107: 5220–5225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temmei Y., Uchida S., Hoshino D., Kanzawa N., Kuwahara M., Sasaki S., Tsuchiya T. (2005). Water channel activities of Mimosa pudica plasma membrane intrinsic proteins are regulated by direct interaction and phosphorylation. FEBS Lett. 579: 4417–4422 [DOI] [PubMed] [Google Scholar]
- Törnroth-Horsefield S., Wang Y., Hedfalk K., Johanson U., Karlsson M., Tajkhorshid E., Neutze R., Kjellbom P. (2006). Structural mechanism of plant aquaporin gating. Nature 439: 688–694 [DOI] [PubMed] [Google Scholar]
- Tse Y.C., Mo B., Hillmer S., Zhao M., Lo S.W., Robinson D.G., Jiang L. (2004). Identification of multivesicular bodies as prevacuolar compartments in Nicotiana tabacum BY-2 cells. Plant Cell 16: 672–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrrell M., Campanoni P., Sutter J.U., Pratelli R., Paneque M., Sokolovski S., Blatt M.R. (2007). Selective targeting of plasma membrane and tonoplast traffic by inhibitory (dominant-negative) SNARE fragments. Plant J. 51: 1099–1115 [DOI] [PubMed] [Google Scholar]
- Uemura T., Ueda T., Ohniwa R.L., Nakano A., Takeyasu K., Sato M.H. (2004). Systematic analysis of SNARE molecules in Arabidopsis: Dissection of the post-Golgi network in plant cells. Cell Struct. Funct. 29: 49–65 [DOI] [PubMed] [Google Scholar]
- Vandeleur R.K., Mayo G., Shelden M.C., Gilliham M., Kaiser B.N., Tyerman S.D. (2009). The role of plasma membrane intrinsic protein aquaporins in water transport through roots: Diurnal and drought stress responses reveal different strategies between isohydric and anisohydric cultivars of grapevine. Plant Physiol. 149: 445–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkov V., Hachez C., Moshelion M., Draye X., Chaumont F., Fricke W. (2007). Water permeability differs between growing and non-growing barley leaf tissues. J. Exp. Bot. 58: 377–390 [DOI] [PubMed] [Google Scholar]
- Wang C.C., Ng C.P., Shi H., Liew H.C., Guo K., Zeng Q., Hong W. (2010). A role for VAMP8/endobrevin in surface deployment of the water channel aquaporin 2. Mol. Cell. Biol. 30: 333–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wudick M.M., Luu D.T., Maurel C. (2009). A look inside: Localization patterns and functions of intracellular plant aquaporins. New Phytol. 184: 289–302 [DOI] [PubMed] [Google Scholar]
- Yoshizawa A.C., Kawashima S., Okuda S., Fujita M., Itoh M., Moriya Y., Hattori M., Kanehisa M. (2006). Extracting sequence motifs and the phylogenetic features of SNARE-dependent membrane traffic. Traffic 7: 1104–1118 [DOI] [PubMed] [Google Scholar]
- Youvan D.C., Silva C.M., Bylina E.J., Coleman W.J., Dilworth M.R., Yang M.M. (1997). Calibration of fluorescence resonance energy transfer in microscopy using genetically engineered GFP derivatives on nickel chelating beads. Biotechnol. et alia 3: 1–18 [Google Scholar]
- Zelazny E., Borst J.W., Muylaert M., Batoko H., Hemminga M.A., Chaumont F. (2007). FRET imaging in living maize cells reveals that plasma membrane aquaporins interact to regulate their subcellular localization. Proc. Natl. Acad. Sci. USA 104: 12359–12364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelazny E., Miecielica U., Borst J.W., Hemminga M.A., Chaumont F. (2009). An N-terminal diacidic motif is required for the trafficking of maize aquaporins ZmPIP2;4 and ZmPIP2;5 to the plasma membrane. Plant J. 57: 346–355 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.