Abstract
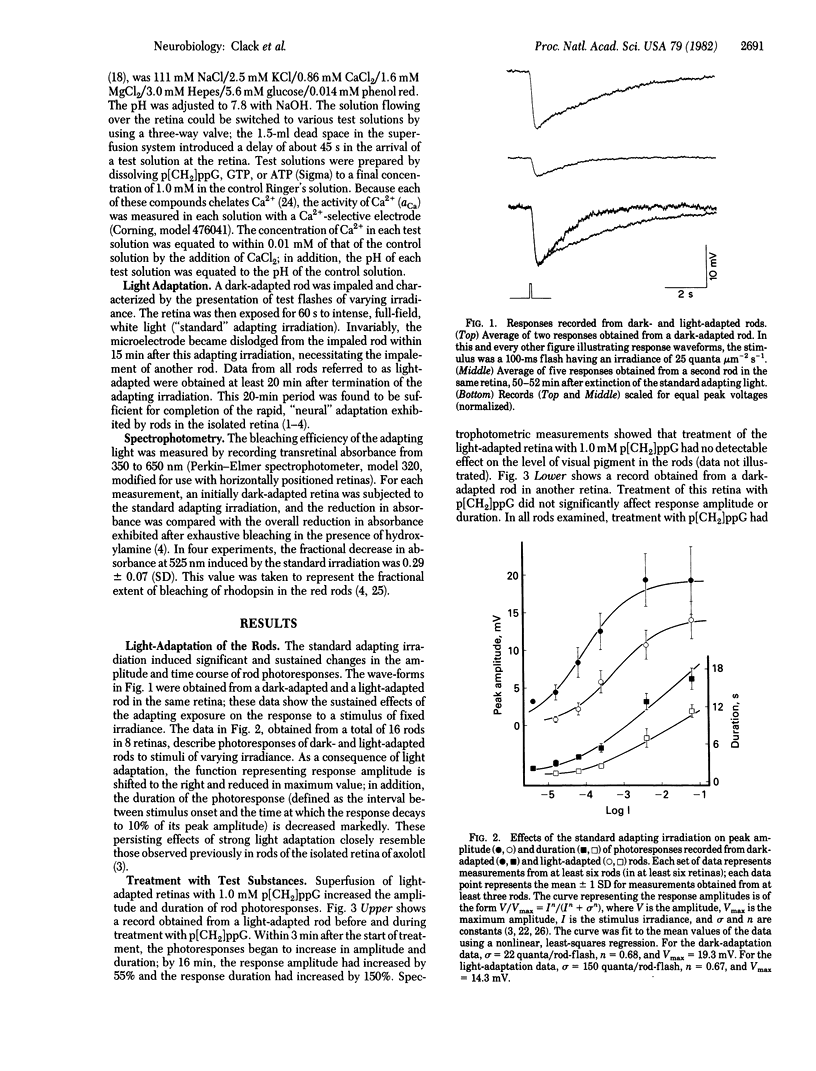
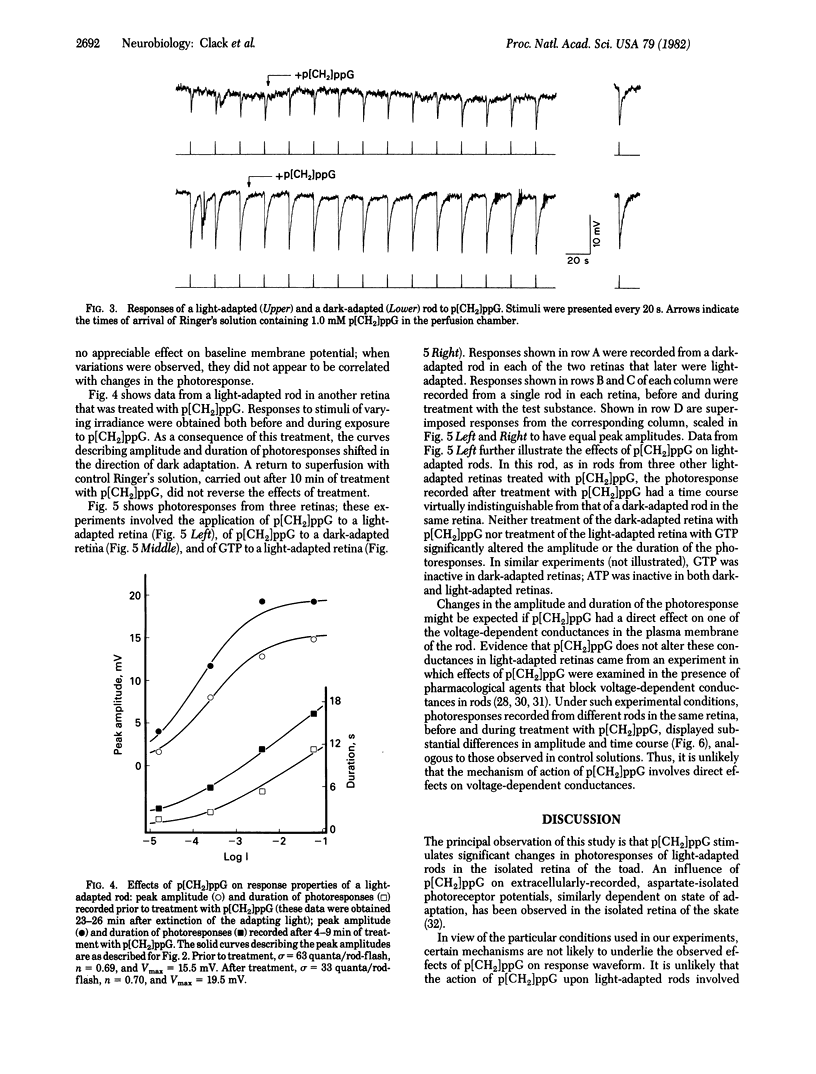
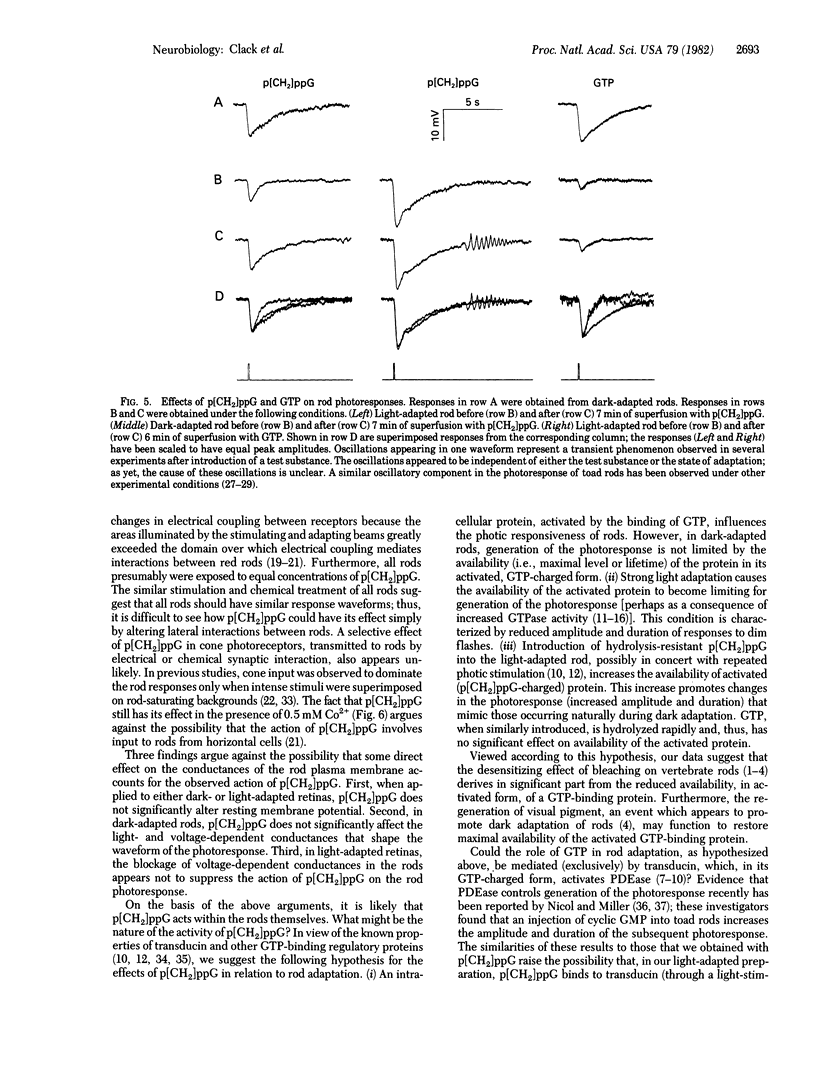
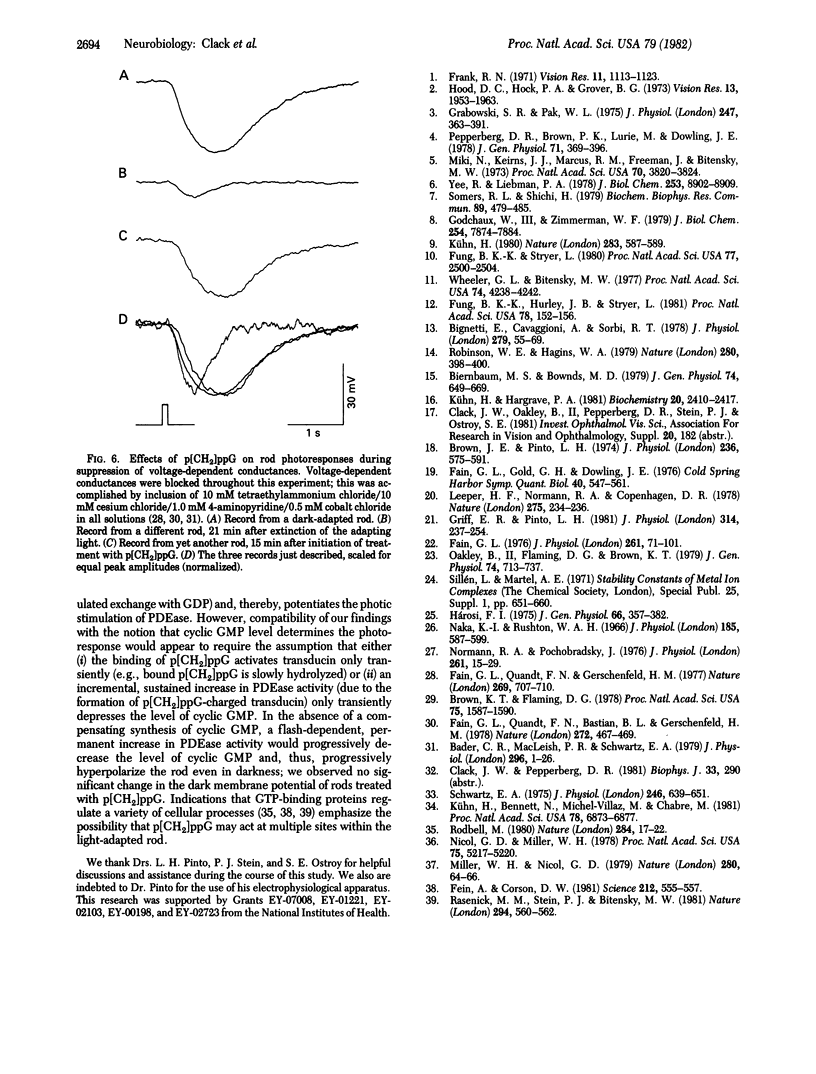
Responses to 100-ms flashes were recorded intracellularly from dark- and light-adapted rod photoreceptors in the isolated retina of the toad, Bufo marinus. Properties of photoresponses were analyzed under each condition of adaptation when retinas were superfused with 1.0 mM guanosine 5'-[beta, gamma-methylene]triphosphate (p[CH2]ppG), a hydrolysis-resistant analog od GTP. When applied to retinas that previously had been subjected to intense light (approximately 30% bleach), p[CH2]ppG increased both the amplitude and duration of photoresponses. By contrast, treatment of dark-adapted retinas with p[CH2]ppG did not alter these response parameters. When similarly applied to either dark- or light-adapted retinas, GTP had no effects on amplitude or duration of photoresponses. These results are discussed in terms of GTP-dependent mechanisms for rod adaptation.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader C. R., Macleish P. R., Schwartz E. A. A voltage-clamp study of the light response in solitary rods of the tiger salamander. J Physiol. 1979 Nov;296:1–26. doi: 10.1113/jphysiol.1979.sp012988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biernbaum M. S., Bownds M. D. Influence of light and calcium on guanosine 5'-triphosphate in isolated frog rod outer segments. J Gen Physiol. 1979 Dec;74(6):649–669. doi: 10.1085/jgp.74.6.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bignetti E., Cavaggioni A., Sorbi R. T. Light-activated hydrolysis of GTP and cyclic GMP in the rod outer segments. J Physiol. 1978 Jun;279:55–69. doi: 10.1113/jphysiol.1978.sp012330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Pinto L. H. Ionic mechanism for the photoreceptor potential of the retina of Bufo marinus. J Physiol. 1974 Feb;236(3):575–591. doi: 10.1113/jphysiol.1974.sp010453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. T., Flaming D. G. Opposing effects of calcium and barium in vertebrate rod photoreceptors. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1587–1590. doi: 10.1073/pnas.75.3.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain G. L., Gold G. H., Dowling J. E. Receptor coupling in the toad retina. Cold Spring Harb Symp Quant Biol. 1976;40:547–561. doi: 10.1101/sqb.1976.040.01.051. [DOI] [PubMed] [Google Scholar]
- Fain G. L., Quandt F. N., Bastian B. L., Gerschenfeld H. M. Contribution of a caesium-sensitive conductance increase to the rod photoresponse. Nature. 1978 Mar 30;272(5652):466–469. doi: 10.1038/272467a0. [DOI] [PubMed] [Google Scholar]
- Fain G. L., Quandt F. N., Gerschenfeld H. M. Calcium-dependent regenerative responses in rods. Nature. 1977 Oct 20;269(5630):707–710. doi: 10.1038/269707a0. [DOI] [PubMed] [Google Scholar]
- Fain G. L. Sensitivity of toad rods: Dependence on wave-length and background illumination. J Physiol. 1976 Sep;261(1):71–101. doi: 10.1113/jphysiol.1976.sp011549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein A., Corson D. W. Excitation of Limulus photoreceptors by vanadate and by a hydrolysis-resistant analog of guanosine triphosphate. Science. 1981 May 1;212(4494):555–557. doi: 10.1126/science.6782676. [DOI] [PubMed] [Google Scholar]
- Frank R. N. Properties of "neural" adaptation in components of the frog electroretinogram. Vision Res. 1971 Oct;11(10):1113–1123. doi: 10.1016/0042-6989(71)90115-5. [DOI] [PubMed] [Google Scholar]
- Fung B. K., Hurley J. B., Stryer L. Flow of information in the light-triggered cyclic nucleotide cascade of vision. Proc Natl Acad Sci U S A. 1981 Jan;78(1):152–156. doi: 10.1073/pnas.78.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godchaux W., 3rd, Zimmerman W. F. Membrane-dependent guanine nucleotide binding and GTPase activities of soluble protein from bovine rod cell outer segments. J Biol Chem. 1979 Aug 25;254(16):7874–7884. [PubMed] [Google Scholar]
- Grabowski S. R., Pak W. L. Intracellular recordings of rod responses during dark-adaptation. J Physiol. 1975 May;247(2):363–391. doi: 10.1113/jphysiol.1975.sp010936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griff E. R., Pinto L. H. Interactions among rods in the isolated retina of Bufo marinus. J Physiol. 1981 May;314:237–254. doi: 10.1113/jphysiol.1981.sp013704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood D. C., Hock P. A., Grover B. G. Dark adaptation of the frog's rods. Vision Res. 1973 Oct;13(10):1953–1963. doi: 10.1016/0042-6989(73)90066-7. [DOI] [PubMed] [Google Scholar]
- Hárosi F. I. Absorption spectra and linear dichroism of some amphibian photoreceptors. J Gen Physiol. 1975 Sep;66(3):357–382. doi: 10.1085/jgp.66.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok-Keung Fung B., Stryer L. Photolyzed rhodopsin catalyzes the exchange of GTP for bound GDP in retinal rod outer segments. Proc Natl Acad Sci U S A. 1980 May;77(5):2500–2504. doi: 10.1073/pnas.77.5.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn H., Bennett N., Michel-Villaz M., Chabre M. Interactions between photoexcited rhodopsin and GTP-binding protein: kinetic and stoichiometric analyses from light-scattering changes. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6873–6877. doi: 10.1073/pnas.78.11.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn H., Hargrave P. A. Light-induced binding of guanosinetriphosphatase to bovine photoreceptor membranes: effect of limited proteolysis of the membranes. Biochemistry. 1981 Apr 28;20(9):2410–2417. doi: 10.1021/bi00512a007. [DOI] [PubMed] [Google Scholar]
- Kühn H. Light- and GTP-regulated interaction of GTPase and other proteins with bovine photoreceptor membranes. Nature. 1980 Feb 7;283(5747):587–589. doi: 10.1038/283587a0. [DOI] [PubMed] [Google Scholar]
- Leeper H. F., Normann R. A., Copenhagen D. R. Evidence for passive electrotonic interactions in red rods of toad retina. Nature. 1978 Sep 21;275(5677):234–236. doi: 10.1038/275234b0. [DOI] [PubMed] [Google Scholar]
- Miki N., Keirns J. J., Marcus F. R., Freeman J., Bitensky M. W. Regulation of cyclic nucleotide concentrations in photoreceptors: an ATP-dependent stimulation of cyclic nucleotide phosphodiesterase by light. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3820–3824. doi: 10.1073/pnas.70.12.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka K. I., Rushton W. A. S-potentials from luminosity units in the retina of fish (Cyprinidae). J Physiol. 1966 Aug;185(3):587–599. doi: 10.1113/jphysiol.1966.sp008003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol G. D., Miller W. H. Cyclic GMP injected into retinal rod outer segments increases latency and amplitude of response to illumination. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5217–5220. doi: 10.1073/pnas.75.10.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normann R. A., Pochobradský J. Oscillations in rod and horizontal cell membrane potential: evidence for feed-back to rods in the vertebrate retina. J Physiol. 1976 Sep;261(1):15–29. doi: 10.1113/jphysiol.1976.sp011546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B., 2nd, Flaming D. G., Brown K. T. Effects of the rod receptor potential upon retinal extracellular potassium concentration. J Gen Physiol. 1979 Dec;74(6):713–737. doi: 10.1085/jgp.74.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepperberg D. R., Brown P. K., Lurie M., Dowling J. E. Visual pigment and photoreceptor sensitivity in the isolated skate retina. J Gen Physiol. 1978 Apr;71(4):369–396. doi: 10.1085/jgp.71.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasenick M. M., Stein P. J., Bitensky M. W. The regulatory subunit of adenylate cyclase interacts with cytoskeletal components. Nature. 1981 Dec 10;294(5841):560–562. doi: 10.1038/294560a0. [DOI] [PubMed] [Google Scholar]
- Robinson W. E., Hagins W. A. GTP hydrolysis in intact rod outer segments and the transmitter cycle in visual excitation. Nature. 1979 Aug 2;280(5721):398–400. doi: 10.1038/280398a0. [DOI] [PubMed] [Google Scholar]
- Rodbell M. The role of hormone receptors and GTP-regulatory proteins in membrane transduction. Nature. 1980 Mar 6;284(5751):17–22. doi: 10.1038/284017a0. [DOI] [PubMed] [Google Scholar]
- Schwartz E. A. Cones excite rods in the retina of the turtle. J Physiol. 1975 Apr;246(3):639–651. doi: 10.1113/jphysiol.1975.sp010908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers R. L., Shichi H. Light-stimulated GTP binding to a membrane protein in rod outer segments. Biochem Biophys Res Commun. 1979 Jul 27;89(2):479–485. doi: 10.1016/0006-291x(79)90654-5. [DOI] [PubMed] [Google Scholar]
- Wheeler G. L., Bitensky M. W. A light-activated GTPase in vertebrate photoreceptors: regulation of light-activated cyclic GMP phosphodiesterase. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4238–4242. doi: 10.1073/pnas.74.10.4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee R., Liebman P. A. Light-activated phosphodiesterase of the rod outer segment. Kinetics and parameters of activation and deactivation. J Biol Chem. 1978 Dec 25;253(24):8902–8909. [PubMed] [Google Scholar]


