Abstract
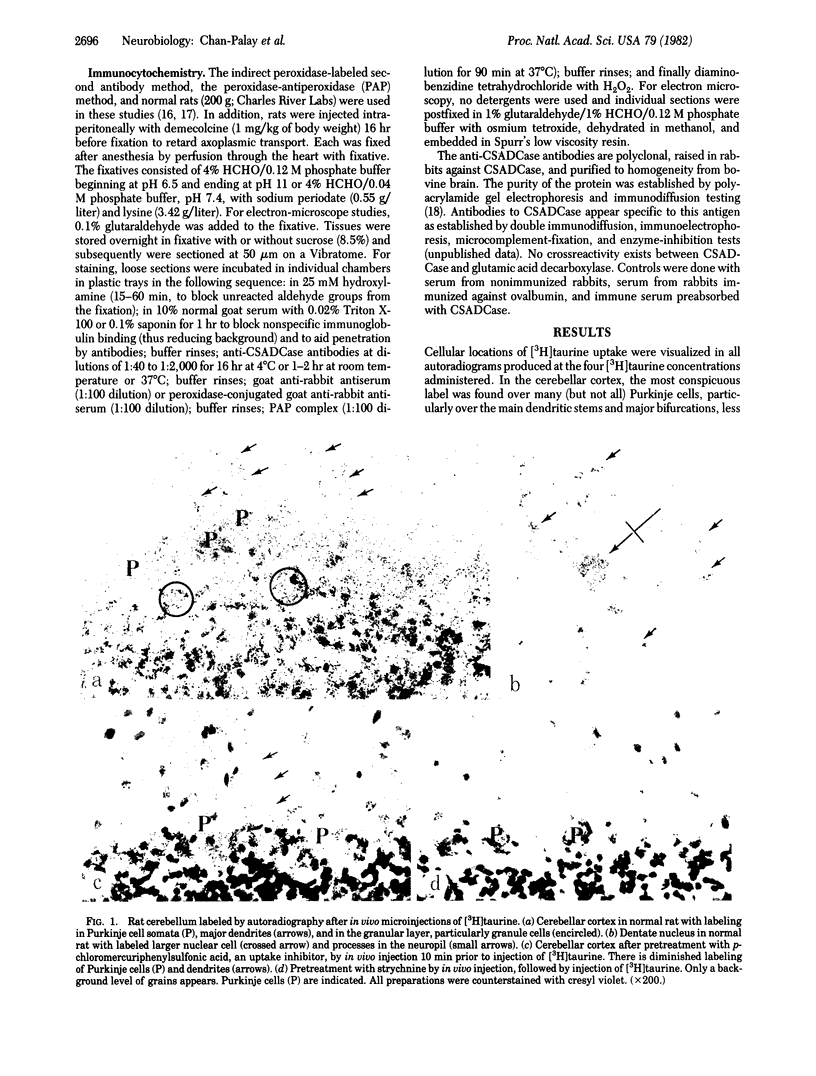
Taurine neurons and their dendrites and axons were visualized in the mammalian cerebellum by autoradiography, after in vivo injections of [3H]taurine directly into the cerebellar cortex or deep cerebellar nuclei, and by immunocytochemistry at the light- and electron-microscope levels with antibodies against cysteine-sulfinic acid decarboxylase (CSADCase; L-cysteine-sulfinate carboxylyase, EC 4.1.1.29). Uptake and sequestration of [3H]taurine labeled numerous Purkinje cell somata, primary dendrites, and axons; many granule cell somata, dendrites, and parallel fibers; stellate, basket, and Golgi cells; the larger neurons in all deep cerebellar nuclei; the largest neurons in the lateral vestibular nucleus; and, more rarely, Purkinje cell axonal terminals in the neuropil. The label at all sites was diminished by preinjection into the cerebellum of hypotaurine, p-chloromercuriphenylsulfonic acid, or β-alanine, and was virtually eliminated by strychnine. Immunocytochemical labeling with polyclonal antibodies directed against CSADCase, the enzyme responsible for the synthesis of hypotaurine from cysteine sulfinic acid and taurine from cysteic acid, had a similar distribution. In electron micrographs, immunoreactivity within Purkinje cell somata and dendrites was localized to the Golgi apparatus, the inner plasma membrane, and condensed nonmembranous foci (120 nm in diameter) marked by clumps of peroxidase reaction product. Large Nissl bodies were usually not CSADCase immunoreactive. Numerous immunoreactive granule cells, dendrites, and parallel fibers were recognized. Pretreatment of the animals with colchicine increased the intensity of CSADCase immunoreactivity but did not change the number or distribution of labeled cells. These experiments indicate that taurine is synthesized and involved in a specific uptake process by cerebellar neurons. Neuroglial cells do not synthesize taurine but some neuroglia take up [3H]taurine. These findings call for a reexamination of the physiological function of taurine in the cerebellum. A hypothesis is proposed that taurine may be involved in the regulation of calcium, in dendritic spike generation, and in the inhibition of impulse propagation in major Purkinje cell dendrites.
Keywords: Purkinje cell, transmitters, cerebellar nuclei
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CURTIS D. R., WATKINS J. C. The excitation and depression of spinal neurones by structurally related amino acids. J Neurochem. 1960 Sep;6:117–141. doi: 10.1111/j.1471-4159.1960.tb13458.x. [DOI] [PubMed] [Google Scholar]
- Chan-Palay V., Nilaver G., Palay S. L., Beinfeld M. C., Zimmerman E. A., Wu J. Y., O'Donohue T. L. Chemical heterogeneity in cerebellar Purkinje cells: existence and coexistence of glutamic acid decarboxylase-like and motilin-like immunoreactivities. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7787–7791. doi: 10.1073/pnas.78.12.7787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan-Palay V., Palay S. L. Interrelations of basket cell axons and climbing fibers in the cerebellar cortex of the rat. Z Anat Entwicklungsgesch. 1970;132(3):191–227. doi: 10.1007/BF00523377. [DOI] [PubMed] [Google Scholar]
- Chan-Palay V., Wu J. Y., Palay S. L. Immunocytochemical localization of gamma-aminobutyric acid transaminase at cellular and ultrastructural levels. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2067–2071. doi: 10.1073/pnas.76.4.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis D. R., Watkins J. C. The pharmacology of amino acids related to gamma-aminobutyric acid. Pharmacol Rev. 1965 Dec;17(4):347–391. [PubMed] [Google Scholar]
- Davison A. N., Kaczmarek L. K. Taurine--a possible neurotransmitter? Nature. 1971 Nov 12;234(5324):107–108. doi: 10.1038/234107a0. [DOI] [PubMed] [Google Scholar]
- GAITONDE M. K., RICHTER D. The metabolic activity of the proteins of the brain. Proc R Soc Lond B Biol Sci. 1956 Mar 27;144(918):83–99. doi: 10.1098/rspb.1956.0019. [DOI] [PubMed] [Google Scholar]
- Haas H. L., Hösli L. The depression of brain stem neurones by taurine and its interaction with strychnine and bicuculline. Brain Res. 1973 Mar 30;52:399–402. doi: 10.1016/0006-8993(73)90680-x. [DOI] [PubMed] [Google Scholar]
- Haas H. L., Hösli L. The depression of brain stem neurones by taurine and its interaction with strychnine and bicuculline. Brain Res. 1973 Mar 30;52:399–402. doi: 10.1016/0006-8993(73)90680-x. [DOI] [PubMed] [Google Scholar]
- Jasper H. H., Koyama I. Rate of release of amino acids from the cerebral cortex in the cat as affected by brainstem and thalamic stimulation. Can J Physiol Pharmacol. 1969 Oct;47(10):889–905. doi: 10.1139/y69-146. [DOI] [PubMed] [Google Scholar]
- Kaczmarek L. K., Davison A. N. Uptake and release of taurine from rat brain slices. J Neurochem. 1972 Oct;19(10):2355–2362. doi: 10.1111/j.1471-4159.1972.tb01289.x. [DOI] [PubMed] [Google Scholar]
- Llinás R., Nicholson C., Freeman J. A., Hillman D. E. Dendritic spikes and their inhibition in alligator Purkinje cells. Science. 1968 Jun 7;160(3832):1132–1135. doi: 10.1126/science.160.3832.1132. [DOI] [PubMed] [Google Scholar]
- Lähdesmäki P., Oja S. S. Effect of electrical stimulation on the influx and efflux of taurine in brain slices of newborn and adult rats. Exp Brain Res. 1972;15(4):430–438. doi: 10.1007/BF00234128. [DOI] [PubMed] [Google Scholar]
- Nilaver G., Defendini R., Zimmerman E. A., Beinfeld M. C., O'Donohue T. L. Motilin in the Purkinje cell of the cerebellum. Nature. 1982 Feb 18;295(5850):597–598. doi: 10.1038/295597a0. [DOI] [PubMed] [Google Scholar]
- Obata K., Ito M., Ochi R., Sato N. Pharmacological properties of the postsynaptic inhibition by Purkinje cell axons and the action of gamma-aminobutyric acid on deiters NEURONES. Exp Brain Res. 1967;4(1):43–57. doi: 10.1007/BF00235216. [DOI] [PubMed] [Google Scholar]
- Pasantes-Morales H., Klethi J., Urban P. F., Mandel P. The effect of electrical stimulation, light and amino acids on the efflux of 35S-taurine from the retina of domestic fowl. Exp Brain Res. 1974 Jan 31;19(2):131–141. doi: 10.1007/BF00238530. [DOI] [PubMed] [Google Scholar]
- Schmid R., Sieghart W., Karobath M. Taurine uptake in synaptosomal fractions of rat cerebral cortex. J Neurochem. 1975 Jul;25(1):5–9. doi: 10.1111/j.1471-4159.1975.tb07686.x. [DOI] [PubMed] [Google Scholar]
- Wu J. Y., Moss L. G., Chen M. S. Tissue and regional distribution of cysteic acid decarboxylase. A new assay method. Neurochem Res. 1979 Apr;4(2):201–212. doi: 10.1007/BF00964144. [DOI] [PubMed] [Google Scholar]