Abstract
Advances in neuroimaging techniques have provided insight into the role of the brain in the regulation of food intake and weight. Growing evidence demonstrate that energy dense, palatable foods elicit similar responses in reward-related brain regions that mimic those of addictive substances. Currently, various models of obesity’s relation to reward from food have been theorized. There is evidence to support a theory of hypo-responsivity of reward regions to food, where individuals consume excess amounts to overcome this reward deficit. There is also data to support a theory of hyper-responsivity of reward regions, where individuals who experience greater reward from food intake are at risk for overeating. However, these seemingly discordant theories are static in nature and do not account for the possible effects of repeated overeating on brain responsivity to food and initial vulnerability factors. Here we review data that support these theories and propose a dynamic vulnerability model of obesity that appears to offer a parsimonious theory that accommodates extant findings.
Keywords: neuroimaging, functional MRI, food reward, obesity, addiction, striatum, dopamine, TaqIA A1 allele
Obesity is associated with increased risk for atherosclerotic cerebrovascular disease, coronary heart disease, colorectal cancer, hyperlipidemia, hypertension, gallbladder disease, and diabetes mellitus, resulting in over 111,000 deaths annually in the US [1]. In the US 65% of adults are overweight or obese [2]. Unfortunately, virtually all treatments result in only transient weight loss and most prevention programs do not significantly reduce risk for future weight gain [3, 4]. The limited success of treatment and prevention interventions may be due to an incomplete understanding of the processes that increase risk for obesity. Brain imaging techniques, including functional magnetic resonance imaging (fMRI) and positron emission tomography (PET), are increasingly being employed to investigate the neural basis of eating behavior, weight regulation and the development of obesity. Results from these neuroimaging studies often point toward variability in reward responsivity to food, frequently dovetailing with traditional addiction literature. Indeed, mounting evidence suggests similarities between subjective reward from food and psychoactive substances. Food deprivation increases the reinforcement value of food and psychoactive substances [5, 6] and produces improved dopamine (DA) receptor functioning [7]. Elevated sucrose preference in animals is associated with greater self-administration of cocaine [8] and sucrose intake reduces cocaine’s reinforcing value [9].
The parallels food and drug reward are increasingly evident, yet there remain numerous differences. The foremost differences between the two are: 1) physiological need to consume food to sustain life and 2) the low rate at which drug addiction occurs relative the prevalence of obesity. Individuals are born with an innate preference for sweet as a mechanism to maintain adequate energy for growth [10], and develop a preference for fat before the introduction solid foods if not in early childhood [11]. This preference for sweet and fat in combination with the current environment that presents large portions of energy-dense foods [12] creates a scenario primed for overeating and obesity. Yet it is important to note that current drugs of abuse capitalize on reward circuitry that evolved to encourage adequate intake of food for the survival of the species (as well as reproductive success). Despite this knowledge, the neural basis of reward responsivity to food and its relation to overconsumption and excess weight remains largely unclear. The purpose of this review is to examine current models of reward responsivity contributing to overeating and present an integrated reward-based model of obesity vulnerability.
Hypo-responsivity of reward circuitry & obesity
Some theorists posit that obese individuals show hypo-responsivity of reward circuitry, which leads them to repeatedly overeat (or use psychoactive drugs) to compensate for this deficiency [13, 14]. Brain imaging studies have identified regions that respond to food receipt and encode the relative perceived pleasantness of foods. Consumption of palatable food activates the midbrain, insula, dorsal striatum, subcallosal cingulate, and prefrontal cortex and these responses decrease as a function of satiety and declines in food pleasantness [15, 16]. Consumption of a pleasant meal is also associated with DA release in the dorsal striatum and the magnitude of release correlates with ratings of meal pleasantness [17].
In line with the reward deficiency model, obese versus lean individuals have lower basal DA levels and D2 receptor availability [18, 19], implying that they show reduced DA receptor binding in reward circuitry. Further, overweight obesity prone rats versus non-obesity prone rats show lower basal DA levels and ex vivo DA release in response to electrical stimulation in nucleus accumbens, dorsal striatum, and medial prefrontal cortex tissue [20]. However, Yang and Meguid reported that obese versus lean rats show more phasic release of DA during feeding [21]. Additionally, in three separate samples, obese versus lean adolescents show less activation in the dorsal striatum in response to consumption of chocolate milkshake (vs tasteless solution) [22, 23].
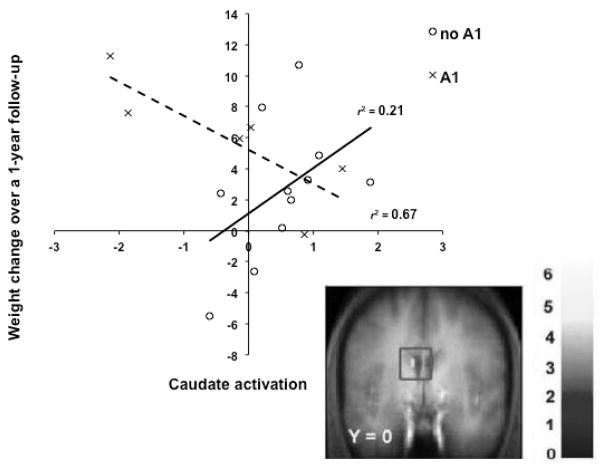
Findings from the neuroimaging literature also point to an interaction between hypo-responsivity to reward from food and the TaqIA polymorphism (rs1800497; GenBank accession number NP848605.1). Critically, humans who have a TaqIA A1 allele exhibit less striatal activation in response to food intake and show elevated future weight gain (Fig 1; [22]). The TaqIA A1 is also associated with lower D2 striatal receptor availability and reduced striatal resting metabolism [24, 25]. Additionally, those with the TaqIA A1 allele have significantly less gray matter volume in the dopaminergic midbrain than those with the A2 allele [26]. While these are results from studies with relatively small sample sizes that are predominately Caucasian, they echo evidence that substance abuse is associated with low D2 receptor density and blunted sensitivity of reward circuitry [27].
Figure 1.
Differential activation in the caudate in response to milkshake receipt (contrasted with tasteless receipt) across weight change over one year by TaqIA A1 status. Those with the A1 allele (dashed line) show decreases in activation as weight increases, whereas those without the A1 allele (solid line) show increases in activation as weight increases [22].
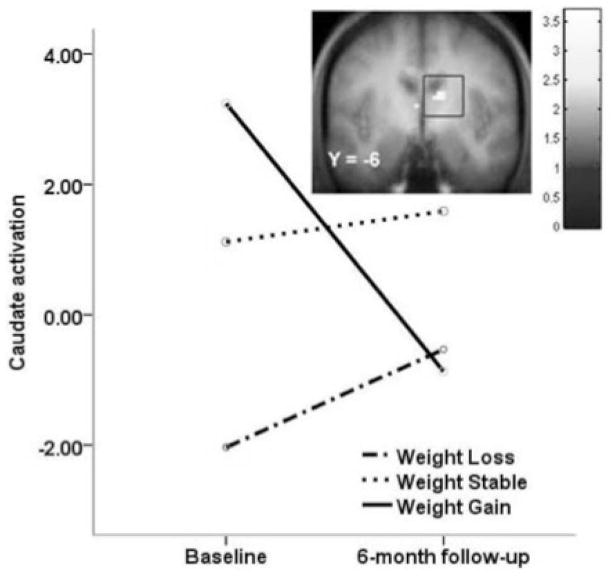
Although these findings suggest that hypo-responsive DA-reward circuitry increases risk for future weight gain, it is possible that consumption of a high-sugar, high-fat foods leads to down-regulation of D2 receptors [28], paralleling neural response to chronic use of psychoactive drugs [29]. Animal studies suggest that repeated intake of sweet and fatty foods results in down-regulation of post-synaptic D2 receptors, increased D1 receptor binding, decreased D2 sensitivity, and reduced reward sensitivity [30–33]. Given that most participants were initially overweight in our prospective study showing differential patterns of caudate activation associated with weight gain by A1 allele status [22], it is possible that a history of overeating contributed to the observed predictive effect. Thus, we tested whether overeating was associated with an attenuated striatal response to palatable food in humans; we found that women who gained weight over a 6-month period (n=8) showed a reduced striatal response to chocolate milkshake relative to baseline and women who did not gain weight (n=12; Fig 2; [34]). This finding dovetails with evidence that weight loss increases D2 receptor availability [35], is associated with increased striatal responsivity to food pictures [36], and suggests that reduced responsivity of DA-based reward circuitry may be a consequence of overeating, rather than an initial vulnerability factor. Unfortunately in this case, the sample was not large enough to reliably investigate the influence of TaqIA A1 allele status.
Figure 2.
Decreased activation in the caudate in response to milkshake receipt (contrasted with tasteless receipt) by weight change group over a 6-month period. Those that gained weight (solid line) showed decreases in activation, whereas those that lost weight (dashed line) or were weight stable (dotted line) showed slight increases in activation in this region [34].
Hyper-responsivity of reward circuitry & obesity
Other theorists posit that individuals who experience greater reward from food intake are at risk for overeating [23, 28, 37]. This is akin to the reinforcement sensitivity model of substance abuse, which posits that certain people show greater reactivity of brain reward systems to reinforcing drugs [37]. In line with this thesis, in response to pictures of palatable foods, obese versus lean humans show greater activation in the striatum, insula, orbitofrontal cortex (OFC), and amygdala [38–40], which are regions that encode the reward value of stimuli [41–43]. In response to anticipated receipt of palatable foods, obese versus lean humans also show greater activation in the primary taste cortex (anterior insula, frontal operculum), which encodes tastes such as sweetness, and in oral somatosensory regions (Rolandic operculum, operculum) [23] which encodes properties such as viscosity and fat texture that signal the caloric density of food [44].
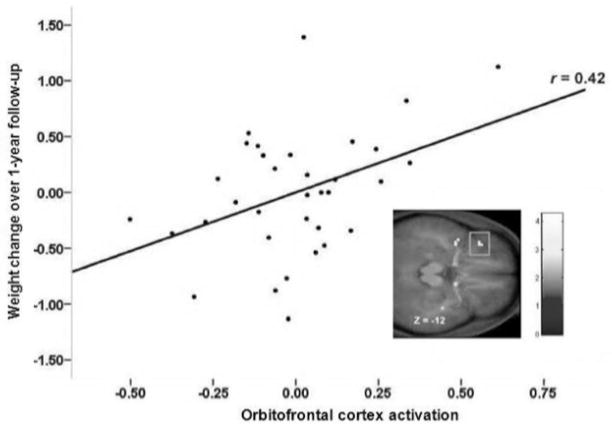
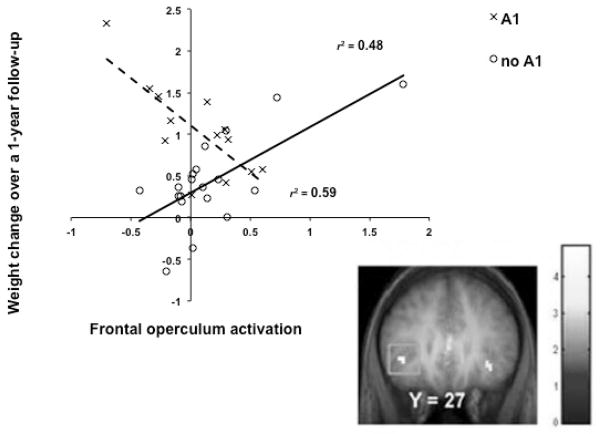
The overall pattern of findings suggest a dissociation between reward from food receipt and the incentive salience of food cues, wherein obese humans show less activation of reward regions to food receipt, but greater activation in regions that encode the reward value of food cues. Incentive salience theory posits that reward from receipt and anticipation operate in tandem with the development of the reinforcing value of food, but after repeated presentations of food, hedonics decrease, while anticipatory reward increases [45]. This is supported by animal literature where, after repeated exposure to sugar, DA release was deceased during sugar receipt [46]. Additionally, hyper-responsivity to anticipation could be a function of altered reward and behavioral control brain networks, as chronic heroin users show greater resting-state functional connectivity between the nucleus accumbens and the anterior cingulate (ACC) and the OFC and decreased connectivity between the prefrontal cortex and OFC and ACC relative to matched controls [47]. Importantly, a prospective study revealed that individuals who showed greater activation in the OFC in response to cues for appetizing versus unappetizing food images showed elevated future weight gain (Fig 3; [48]). Mirroring the moderating effects of the TaqIA A1 allele noted above, blunted response of the dorsal striatum and frontal operculum to food images predicted future increases in BMI for those with an A1 allele, but elevated activation in these regions predicted weight gain for those without the A1 allele (Fig 4; [40]). Findings suggest that individuals who show hyper-responsivity of regions that encode the incentive salience of food cues are at increased risk for future weight gain and that these predictive effects are moderated by A1 allele status.
Figure 3.
Activation in the orbitofrontal cortex in response to initial orientation to appetizing food images (contrasted with pictures of glasses of water) related to weight change over a one-year period [48].
Figure 4.
Differential activation in the frontal operculum in response to appetizing food pictures (contrasted with pictures of glasses of water) across weight change over one year by TaqIA A1 status. Those with the A1 allele (dashed line) show increases in activation as weight decreases, whereas those without the A1 allele (solid line) show increases in activation as weight increases [40].
However, it is not clear whether hyper-responsivity of reward regions to food receipt or anticipated receipt represent initial vulnerability factors for overeating. Animal experiments indicate that, after conditioning, the reward value of food shifts from food intake and to anticipated food. For example, naïve monkeys showed activation of mesotelencephalic DA neurons only in response to a rewarding food taste; however, after conditioning, activation began to precede reward delivery and eventually maximal activity was elicited by the conditioned stimuli that predicted food reward rather than by actual food receipt [49]. Additionally, Kiyatkin and Gratton (1994) found that the greatest dopaminergic activation occurred in an anticipatory fashion as rats approached and pressed the bar that produced food reward, with activation decreasing as the rat received and ate the food. Blackburn et al. (1989) found that DA activity was greater in the nucleus accumbens of rats after presentation of a conditioned stimulus that usually signaled food receipt than after delivery of an unexpected meal. There is also evidence that a history of elevated sugar intake may contribute to excess elevations in anticipatory reward from food [50]. Rats exposed to intermittent sugar availability show signs of dependence (escalation in sugar binging, mu-opioid and DA receptor changes, and deprivation-induced sugar binges) and somatic, neurochemical, and behavioral signs of opioid withdrawal [50]. This withdrawal can be precipitated by naloxone, suggesting that opioids play a major role in sugar dependence [50]. Additionally, a diet of intermittent excessive sugar consumption is associated with cross-sensitization of amphetamine, suggesting an alteration in the DA system [51]. Collectively these data indicate that repeated exposure of a rewarding food (i.e., conditioning of food receipt and its preceding cues) generates in a shift in the reward responsivity, suggesting a more complex model leading to overeating and subsequent obesity.
Dynamic theories of reward circuitry & obesity
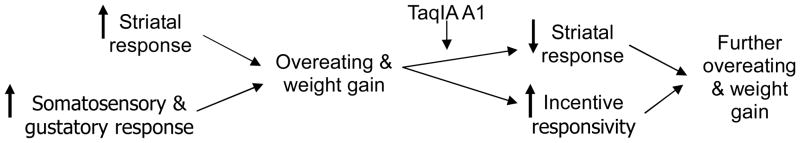
Thus, at present it is unclear whether the initial vulnerability for obesity is the hypo-responsivity of the striatum to food receipt or the hyper-responsivity of regions that encode the incentive salience of food cues, given the evidence that overeating may lead to the development of both of these anomalies and the fact that most participants in our prior prospective studies were already overweight at baseline. Extant findings are generally consistent with three theories regarding initial vulnerability and the etiologic process that causes obesity. One theory is that individuals at risk for obesity experience: 1) initial weaker dorsal striatal activation from food intake, leading them to overeat palatable foods to compensate for this reward deficit and also 2) the emergence of hyper-responsivity of regions that encode incentive salience of food cues through conditioning that occurs after repeated overeating episodes (initial hypo-reward model). A second theory is that individuals at risk for obesity show: 1) initial hyper-responsivity of regions that encode the incentive salience of food cues, leading to overeating and 2) a consequent reduction in D2 receptor density in the striatum and blunted DA signaling in response to food intake (hyper-incentive sensitization model). A third theory is that individuals at risk for obesity experience: 1) initial hyper-reward responsivity from food intake, leading to overeating which 2) reduces striatal D2 receptor density and DA signaling in response to food intake, as well as the emergence of 3) hyper-responsivity of regions that encode the incentive salience of food cues through conditioning during repeated bouts of overeating, both of which may drive further overeating in a feed-forward fashion (dynamic vulnerability model). This latter model accords with the thesis that hyper-responsivity of reward circuitry increases risk for overeating [23, 28, 37], though it adds the notion of plasticity in reward circuitry’s response to food after overeating occurs.
To evaluate these three models, we tested whether lean adolescents at high versus low risk for obesity – by virtue of dual parental obesity – showed greater or weaker activation of DA-based reward circuitry in response to receipt and anticipated receipt of palatable food (N=60). We also contrasted activation in response to receipt and anticipated receipt of money to determine whether any anomalies in reward-related regions were specific to food or generalized. High-risk versus low-risk youth showed greater caudate, parietal operculum, and frontal operculum activation in response to food receipt, as well as evidence of greater caudate, putamen, insula, and OFC activation in response to money receipt (Fig 5; [52]). However, high-risk versus low-risk youth did not show a differential response to anticipated food or monetary receipt [52]. Importantly, the enhanced opercular responses, observed selectively to milkshake receipt, correspond to oral somatosensory cortex, which represents the viscosity of oral stimuli [44]. These preliminary findings align with evidence that obese versus lean individuals show elevated responsivity in somatosensory regions in response to anticipated receipt of palatable foods [23] and images of palatable foods [40], greater regional blood flow in somatosensory regions in response to images of palatable foods [53], and greater resting glucose metabolism in the oral somatosensory cortex [54]. Moreover, since oral viscosity is a primary sensory signal of the fat content of foods, these findings also accord with evidence that obese versus lean humans rate high-fat foods as more pleasant and consume more of such foods [55–58], obese prone rats showed greater sensitivity to oral fat [59], and children at risk for obesity by virtue of parental obesity prefer the taste of high-fat foods and show a more avid feeding style than children of lean parents [60–62]. Research also indicates that preferences for high-fat foods predict elevated future weight gain [61, 63–65]. These preliminary data suggest that youth at risk for obesity initially show greater responsivity of reward circuitry, coupled with elevated responsivity of oral somatosensory regions to food, but no differences in anticipation of reward supporting the first stage of the dynamic vulnerability model. Previous prospective data, specifically the inverse relation between weight gain and striatum activity in response to food receipt (Fig 2; [34]) and the positive relation of weight gain and OFC activity in response to palatable food images (Fig 3; [48]) support the later stages of the dynamic vulnerability model. However, extent studies have not provide a test of all aspects of the dynamic vulnerability model, which would require repeated fMRI scans during a period of time in which some subjects develop obesity and others do not.
Figure 5.
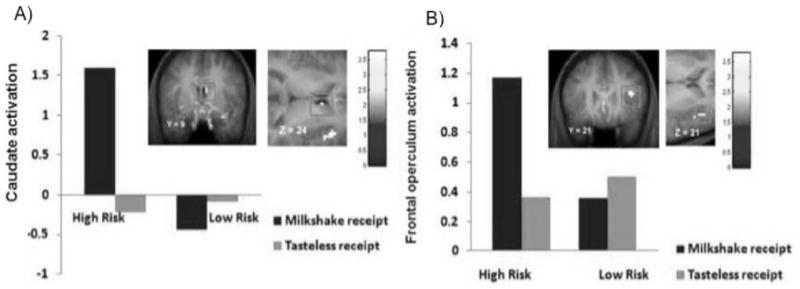
Greater activation in the A) caudate and B) frontal operculum in adolescents at a high risk for obesity vs. adolescents at a low risk for obesity in response to milkshake receipt (contrasted with tasteless receipt). Despite no difference in current BMI, adolescents at high risk for obesity show greater activation in these reward and gustatory related regions to a palatable food [52].
Additionally, we compared genetic data of the youth at high-risk versus low-risk for obesity. Although the prevalence of the TaqIA A1 allele was three times greater in youth at high-risk versus low-risk for obesity (35% vs. 12%; t(58) = 2.00, p = .05), this genotype was not significantly related to striatal response to food receipt or anticipated receipt. This is in contrast to results from overweight samples, wherein A1 allele status correlated with reduced response to palatable food intake in DA source and target regions [22, 66]. This hints at a possible gene-environment interaction in which A1 allele status only increases risk for a blunted striatal response if paired with overeating or elevated adiposity. This possibility is in line with the thesis of plasticity of striatal response to food intake secondary to overeating. Further, the TaqIA polymorphism is located on the novel ANKK1 (ankyrin repeat and protein kinase domain-containing progestin 1) just downstream from the DRD2 receptor [67] and is thought to regulate this receptor via inflammatory processes [68]. Given that obesity is associated with chronic low-grade inflammation, an intriguing, though speculative hypothesis is that overeating or elevated adiposity is associated with an inflammatory process that reduces striatal D2 receptor expression. This notion is supported by reports that weight-associated inflammation is related to the reduction of the microstructural integrity of brain regions involved with food reward and eating behavior [69]. This working hypothesis aligns with evidence that blunted dorsal striatal response to food and blunted dorsal striatal, OFC, and frontal operculum response to food images only increases risk for future weight gain among individuals with the A1 allele [22, 40, 66]. This pattern of findings suggests that TaqIA A1 allele status may amplify the predictive effects of the vulnerability factors to be examined herein.
Conclusion
Collectively, extant findings suggest the possibility of a dynamic vulnerability model for obesity that may evolve and change over time in response to overeating and/or fat accumulation (Fig 6). We submit that individuals at risk for obesity initially show hyper-responsivity of the striatum to general reward and somatosensory regions in response to palatable, energy dense foods, which increases risk for overeating. We posit that the oral somatosensory responses reflect altered sensitivity for fat and/or enhanced preference for high-fat foods. We further submit that overeating, especially in individuals with an A1 allele polymorphism, may in turn result in a down-regulation of DA-based reward regions, producing a blunted striatal response to food intake, which may lead people to overeat in an effort to achieve the same subjective reward from palatable food they initially felt in a feed forward manner. The overeating may also result in greater responsivity of regions that encode the incentive salience of food cues, which might be moderated by TaqIA A1 allele status, making people more vulnerable to food cues in our obesogenic environment, which also may increase risk for escalation of overeating in a feed forward fashion. Although this working dynamic vulnerability model holds promise, it rests on data from prospective fMRI with relatively small, ethnically similar samples.
Figure 6.
Dynamic vulnerability model of obesity
An additional construct to this model that has been theorized to interact with reward sensitivity is impulsivity. For example, Dawe and Loxton posit in addition to initial hyper-responsivity of the reward pathways those that exhibit impulsive behaviors are likely to overeat [37]. Research has found that both obesity and eating pathology are associated with elevated impulsivity. Both self-reported and laboratory measures of impulsivity correlate positively with caloric intake [70, 71] and body mass index [72–74]. Despite these observed relations, few data are available to elucidate the possible interaction of impulsivity and reward responsivity to food on a neural level, specifically whether impulsive behavior is predisposing or consequential factor of overeating, making this an important priority for future research. It is reasonable to hypothesize that impulsivity is an initial vulnerability to overeating that parallels initial hyper-responsivity to food receipt. However, it is also reasonable to suggest that impulsivity develops through conditioning in a similar matter to the regions that encode the incentive salience of food cues.
Currently studies have investigated individual components of these models with limited reach to capture a more complete picture. It is a possibility that these theories each contribute to overeating and the development of obesity and are more prevalent in certain specific populations (e.g., sex, ethnicity, additional genetic polymorphisms) or specific to components of food (e.g., fat, sugar, caffeine). Thus, it will be important for future repeated-measures brain imaging studies to use both fMRI and PET techniques designed to capture the vulnerability factors that initially give rise to overeating among lean youth at high versus low risk for future weight gain and the changes in neural responsivity that appear to emerge to sustain overeating.
Key Learning Objectives.
Evaluate current theories of obesity stemming from variability in reward-related brain responsivity to food.
Identify a dynamic theory of brain responsivity and obesity.
Future Research Questions.
How does brain responsivity to food cues and consumption change as a result of overeating?
Are the changes in food-related reward processing a result of repeated consumption of rewarding foods or a function physiological change (e.g., excess fat mass; regional changes in brain volumes)?
Are initial vulnerability factors of obesity evident on a neural level? If so, can they be addressed to prevent excess weight gain?
ABBREVATIONS
- fMRI
Functional neuroimaging
- PET
Positron emission tomography
- DA
Dopamine
- OFC
Orbitofrontal cortex
- ACC
Anterior cingulate cortex
References
- 1.Flegal KM, Graubard BI, Williamson DF, et al. Excess deaths associated with underweight, overweight, and obesity. Jama-Journal of the American Medical Association. 2005;293(15):1861–1867. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- 2.Hedley AA, Ogden CL, Johnson CL, et al. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. Jama-Journal of the American Medical Association. 2004;291(23):2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 3.Jeffery RW, Drewnowski A, Epstein LH, et al. Long-term maintenance of weight loss: Current status. Health Psychology. 2000;19(1):5–16. doi: 10.1037/0278-6133.19.suppl1.5. [DOI] [PubMed] [Google Scholar]
- 4.Stice E, Shaw H, Marti CN. A meta-analytic review of obesity prevention programs for children and adolescents: the skinny on interventions that work. Psychol Bull. 2006;132(5):667–91. doi: 10.1037/0033-2909.132.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carr KD, Kim GY, de Vaca SC. Rewarding and locomotor-activating effects of direct dopamine receptor agonists are augmented by chronic food restriction in rats. Psychopharmacology. 2001;154(4):420–428. doi: 10.1007/s002130000674. [DOI] [PubMed] [Google Scholar]
- 6.Raynor HA, Epstein LH. The relative-reinforcing value of food under differing levels of food deprivation and restriction. Appetite. 2003;40(1):15–24. doi: 10.1016/s0195-6663(02)00161-7. [DOI] [PubMed] [Google Scholar]
- 7.Wilson C, Nomikos GG, Collu M, et al. Dopaminergic Correlates of Motivated Behavior - Importance of Drive. Journal of Neuroscience. 1995;15(7):5169–5178. doi: 10.1523/JNEUROSCI.15-07-05169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levine AS, Kotz CM, Gosnell BA. Sugars: hedonic aspects, neuroregulation, and energy balance. American Journal of Clinical Nutrition. 2003;78(4):834S–842S. doi: 10.1093/ajcn/78.4.834S. [DOI] [PubMed] [Google Scholar]
- 9.Comer SD, Lac ST, Wyvell CL, et al. Combined effects of buprenorphine and a nondrug alternative reinforcer on IV cocaine self-administration in rats maintained under FR schedules. Psychopharmacology. 1996;125:355–360. doi: 10.1007/BF02246018. [DOI] [PubMed] [Google Scholar]
- 10.Beidler LM. Bioloigcal basis of food selection. In: Barker LM, editor. The Psychobiology of Human Food Selection. England Ellis Horwood Limited; Chichester, United Kingdom: 1982. pp. 3–15. [Google Scholar]
- 11.Drewnowski A. Taste preferences and food intake. Annual Review of Nutrition. 1997;17:237–253. doi: 10.1146/annurev.nutr.17.1.237. [DOI] [PubMed] [Google Scholar]
- 12.Hill JO, Peters JC. Environmental contributions to the obesity epidemic. Science. 1998;280(5368):1371–1374. doi: 10.1126/science.280.5368.1371. [DOI] [PubMed] [Google Scholar]
- 13.Wang GJ, Volkow ND, Fowler JS. The role of dopamine in motivation for food in humans: implications for obesity. Expert Opin Ther Targets. 2002;6(5):601–9. doi: 10.1517/14728222.6.5.601. [DOI] [PubMed] [Google Scholar]
- 14.Blum K, Braverman ER, Holder JM, et al. Reward deficiency syndrome: A biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. Journal of Psychoactive Drugs. 2000:32. doi: 10.1080/02791072.2000.10736099. [DOI] [PubMed] [Google Scholar]
- 15.Small DM, Zatorre RJ, Dagher A, et al. Changes in brain activity related to eating chocolate - From pleasure to aversion. Brain. 2001;124:1720–1733. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- 16.Kringelbach ML, O’Doherty J, Rolls ET, et al. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cerebral Cortex. 2003;13(10):1064–1071. doi: 10.1093/cercor/13.10.1064. [DOI] [PubMed] [Google Scholar]
- 17.Small DM, Jones-Gotman M, Dagher A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage. 2003;19(4):1709–15. doi: 10.1016/s1053-8119(03)00253-2. [DOI] [PubMed] [Google Scholar]
- 18.Volkow ND, Wang GJ, Telang F, et al. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. Neuroimage. 2008;42(4):1537–43. doi: 10.1016/j.neuroimage.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang GJ, Volkow ND, Logan J, et al. Brain dopamine and obesity. Lancet. 2001;357(9253):354–7. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- 20.Geiger BM, Behr GG, Frank LE, et al. Evidence for defective mesolimbic dopamine exocytosis in obesity-prone rats. Faseb Journal. 2008;22(8):2740–2746. doi: 10.1096/fj.08-110759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang ZJ, Meguid MM. LHA dopaminergic activity in obese and lean Zucker rats. Neuroreport. 1995;6(8):1191–4. doi: 10.1097/00001756-199505300-00029. [DOI] [PubMed] [Google Scholar]
- 22.Stice E, Spoor S, Bohon C, et al. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science. 2008;322(5900):449–452. doi: 10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stice E, Spoor S, Bohon C, et al. Relation of Reward From Food Intake and Anticipated Food Intake to Obesity: A Functional Magnetic Resonance Imaging Study. Journal of Abnormal Psychology. 2008;117(4):924–935. doi: 10.1037/a0013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noble EP, Gottschalk LA, Fallon JH, et al. D-2 dopamine receptor polymorphism and brain regional glucose metabolism. American Journal of Medical Genetics. 1997;74(2):162–166. [PubMed] [Google Scholar]
- 25.Tupala E, Hall H, Bergstrom K, et al. Dopamine D2 receptors and transporters in type 1 and 2 alcoholics measured with human whole hemisphere autoradiography. Hum Brain Mapp. 2003;20(2):91–102. doi: 10.1002/hbm.10129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cerasa A, Gioia MC, Tarantino P, et al. The DRD2 TaqIA polymorphism associated with changed midbrain volumes in healthy individuals. Genes Brain and Behavior. 2009;8(4):459–463. doi: 10.1111/j.1601-183X.2009.00492.x. [DOI] [PubMed] [Google Scholar]
- 27.Goldstein RZ, Alia-Klein N, Tomasi D, et al. Is decreased prefrontal cortical sensitivity to monetary reward associated with impaired motivation and self-control in cocaine addiction? American Journal of Psychiatry. 2007;164(1):43–51. doi: 10.1176/appi.ajp.164.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis C, Strachan S, Berkson M. Sensitivity to reward: implications for overeating and overweight. Appetite. 2004;42(2):131–8. doi: 10.1016/j.appet.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Volkow ND, Fowler JS, Wang GJ. Role of dopamine in drug reinforcement and addiction in humans: results from imaging studies. Behavioural Pharmacology. 2002;13(5–6):355–366. doi: 10.1097/00008877-200209000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Colantuoni C, Schwenker J, McCarthy J, et al. Excessive sugar intake alters binding to dopamine and mu-opioid receptors in the brain. Neuroreport. 2001;12(16):3549–52. doi: 10.1097/00001756-200111160-00035. [DOI] [PubMed] [Google Scholar]
- 31.Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nature Neuroscience. 2010;13(5):635–U156. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelley AE, Will MJ, Steininger TL, et al. Restricted daily consumption of a highly palatable food (chocolate Ensure (R)) alters striatal enkephalin gene expression. European Journal of Neuroscience. 2003;18(9):2592–2598. doi: 10.1046/j.1460-9568.2003.02991.x. [DOI] [PubMed] [Google Scholar]
- 33.Bello NT, Lucas LR, Hajnal A. Repeated sucrose access influences dopamine D2 receptor density in the striatum. Neuroreport. 2002;13(12):1575–8. doi: 10.1097/00001756-200208270-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stice E, Yokum S, Blum K, et al. Weight Gain Is Associated with Reduced Striatal Response to Palatable Food. Journal of Neuroscience. 2010;30(39):13105–13109. doi: 10.1523/JNEUROSCI.2105-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steele KE, Prokopowicz GP, Schweitzer MA, et al. Alterations of Central Dopamine Receptors Before and After Gastric Bypass Surgery. Obesity Surgery. 2010;20(3):369–374. doi: 10.1007/s11695-009-0015-4. [DOI] [PubMed] [Google Scholar]
- 36.DelParigi A, Chen K, Salbe AD, et al. Successful dieters have increased neural activity in cortical areas involved in the control of behavior. International Journal of Obesity. 2007;31(3):440–448. doi: 10.1038/sj.ijo.0803431. [DOI] [PubMed] [Google Scholar]
- 37.Dawe S, Loxton NJ. The role of impulsivity in the development of substance use and eating disorders. Neuroscience & Biobehavioral Reviews. 2004;28(3):343–351. doi: 10.1016/j.neubiorev.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 38.Rothemund Y, Preuschhof C, Bohner G, et al. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage. 2007;37(2):410–421. doi: 10.1016/j.neuroimage.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 39.Stoeckel LE, Weller RE, Cook EW, et al. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage. 2008;41(2):636–647. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 40.Stice E, Yokum S, Bohon C, et al. Reward circuitry responsivity to food predicts future increases in body mass: Moderating effects of DRD2 and DRD4. Neuroimage. 2010;50(4):1618–1625. doi: 10.1016/j.neuroimage.2010.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beaver JD, Lawrence AD, Van Ditzhuijzen J, et al. Individual differences in reward drive predict neural responses to images of food. Journal of Neuroscience. 2006;26(19):5160–5166. doi: 10.1523/JNEUROSCI.0350-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Doherty JP, Deichmann R, Critchley HD, et al. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33(5):815–826. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- 43.Pelchat ML, Johnson A, Chan R, et al. Images of desire: food-craving activation during fMRI. Neuroimage. 2004;23(4):1486–1493. doi: 10.1016/j.neuroimage.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 44.de Araujo IE, Rolls ET. Representation in the human brain of food texture and oral fat. Journal of Neuroscience. 2004;24(12):3086–3093. doi: 10.1523/JNEUROSCI.0130-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95(8):S91–S117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- 46.Colantuoni C, Rada P, McCarthy J, et al. Evidence that intermittent, excessive sugar intake causes endogenous opioid dependence. Obesity Research. 2002;10(6):478–488. doi: 10.1038/oby.2002.66. [DOI] [PubMed] [Google Scholar]
- 47.Ma N, Liu Y, Li N, et al. Addiction related alteration in resting-state brain connectivity. Neuroimage. 2010;49(1):738–744. doi: 10.1016/j.neuroimage.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yokum S, Ng J, Stice E. Attentional Bias for Food Images Associated with Elevated Weight and Future Weight Gain: An fMRI Study. Obesity. doi: 10.1038/oby.2011.168. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schultz W, Apicella P, Ljungberg T. Responses of monkey dopamine neurons to reward and conditioned stimuli during successive steps of learning a delayed response task. J Neurosci. 1993;13(3):900–13. doi: 10.1523/JNEUROSCI.13-03-00900.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Avena NM, Long KA, Hoebel BG. Sugar-dependent rats show enhanced responding for sugar after abstinence: Evidence of a sugar deprivation effect. Physiology & Behavior. 2005;84(3):359–362. doi: 10.1016/j.physbeh.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 51.Avena NM, Hoebel BG. A diet promoting sugar dependency causes behavioral cross-sensitization to a low dose of amphetamine. Neuroscience. 2003;122(1):17–20. doi: 10.1016/s0306-4522(03)00502-5. [DOI] [PubMed] [Google Scholar]
- 52.Stice E, Yokum S, Burger KS, et al. Youth at Risk for Obesity Show Greater Activation of Striatal and Somatosensory Regions to Food. Journal of Neuroscience. doi: 10.1523/JNEUROSCI.6604-10.2011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karhunen LJ, Lappalainen RI, Tammela L, et al. Subjective and physiological cephalic phase responses to food in obese binge-eating women. International Journal of Eating Disorders. 1997;21(4):321–328. doi: 10.1002/(sici)1098-108x(1997)21:4<321::aid-eat3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 54.Wang GJ, Volkow ND, Fowler JS. The role of dopamine in motivation for food in humans: implications for obesity. Expert Opin Ther Targets. 2002;6(5):601–609. doi: 10.1517/14728222.6.5.601. [DOI] [PubMed] [Google Scholar]
- 55.Drewnowski A, Brunzell JD, Sande K, et al. Sweet tooth reconsidered - taste responsiveness in human obesity. Physiology & Behavior. 1985;35(4):617–622. doi: 10.1016/0031-9384(85)90150-7. [DOI] [PubMed] [Google Scholar]
- 56.McGloin AF, Livingstone MBE, Greene LC, et al. Energy and fat intake in obese and lean children at varying risk of obesity. International Journal of Obesity. 2002;26(2):200–207. doi: 10.1038/sj.ijo.0801883. [DOI] [PubMed] [Google Scholar]
- 57.Nicklas TA, Yang SJ, Baranowski T, et al. Eating patterns and obesity in children. The Bogalusa Heart Study Am J Prev Med. 2003;25(1):9–16. doi: 10.1016/s0749-3797(03)00098-9. [DOI] [PubMed] [Google Scholar]
- 58.Rissanen A, Hakala P, Lissner L, et al. Acquired preference especially for dietary fat and obesity: a study of weight-discordant monozygotic twin pairs. International Journal of Obesity. 2002;26(7):973–977. doi: 10.1038/sj.ijo.0802014. [DOI] [PubMed] [Google Scholar]
- 59.Pittman DW, Smith KR, Crawley ME, et al. Orosensory Detection of Fatty Acids by Obesity-Prone and Obesity-Resistant Rats: Strain and Sex Differences. Chemical Senses. 2008;33(5):449–460. doi: 10.1093/chemse/bjn012. [DOI] [PubMed] [Google Scholar]
- 60.Fisher JO, Birch LL. Fat preferences and fat consumption of 3-year-old to 5-year-old children are related to parental adiposity. Journal of the American Dietetic Association. 1995;95(7):759–764. doi: 10.1016/S0002-8223(95)00212-X. [DOI] [PubMed] [Google Scholar]
- 61.Stunkard AJ, Berkowitz RI, Stallings VA, et al. Energy intake, not energy output, is a determinant of body size in infants. Am J Clin Nutr. 1999;69(3):524–30. doi: 10.1093/ajcn/69.3.524. [DOI] [PubMed] [Google Scholar]
- 62.Wardle J, Guthrie C, Sanderson S, et al. Food and activity preferences in children of lean and obese parents. Int J Obes Relat Metab Disord. 2001;25(7):971–7. doi: 10.1038/sj.ijo.0801661. [DOI] [PubMed] [Google Scholar]
- 63.Berkey CS, Rockett HRH, Field AE, et al. Sugar-added beverages and adolescent weight change. Obesity Research. 2004;12(5):778–788. doi: 10.1038/oby.2004.94. [DOI] [PubMed] [Google Scholar]
- 64.Ludwig DS, Peterson KE, Gortmaker SL. Relation between consumption of sugar-sweetened drinks and childhood obesity: a prospective, observational analysis. The Lancet. 2001;357(9255):505–508. doi: 10.1016/S0140-6736(00)04041-1. [DOI] [PubMed] [Google Scholar]
- 65.Salbe AD, DelParigi A, Pratley RE, et al. Taste preferences and body weight changes in an obesity-prone population. American Journal of Clinical Nutrition. 2004;79(3):372–378. doi: 10.1093/ajcn/79.3.372. [DOI] [PubMed] [Google Scholar]
- 66.Felsted JA, Ren XY, Chouinard-Decorte F, et al. Genetically Determined Differences in Brain Response to a Primary Food Reward. Journal of Neuroscience. 2010;30(7):2428–2432. doi: 10.1523/JNEUROSCI.5483-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Neville MJ, Johnstone EC, Walton RT. Identification and characterization of ANKK1: A novel kinase gene closely linked to DRD2 on chromosome band 11q23.1. Human Mutation. 2004;23(6):540–545. doi: 10.1002/humu.20039. [DOI] [PubMed] [Google Scholar]
- 68.Huang WH, Payne TJ, Ma JZ, et al. Significant Association of ANKK1 and Detection of a Functional Polymorphism with Nicotine Dependence in an African-American Sample. Neuropsychopharmacology. 2009;34(2):319–330. doi: 10.1038/npp.2008.37. [DOI] [PubMed] [Google Scholar]
- 69.Cazettes F, Cohen JI, Yau PL, et al. Obesity-mediated inflammation may damage the brain circuit that regulates food intake. Brain Research. 2011;1373:101–109. doi: 10.1016/j.brainres.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guerrieri R, Nederkoorn C, Jansen A. How impulsiveness and variety influence food intake in a sample of healthy women. Appetite. 2007;48(1):119–122. doi: 10.1016/j.appet.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 71.Guerrieri R, Nederkoorn C, Stankiewicz K, et al. The influence of trait and induced state impulsivity on food intake in normal-weight healthy women. Appetite. 2007;49(1):66–73. doi: 10.1016/j.appet.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 72.Bonato DP, Boland FJ. Delay of gratification in obese children. Addictive Behaviors. 1983;8(1):71–74. doi: 10.1016/0306-4603(83)90059-x. [DOI] [PubMed] [Google Scholar]
- 73.Nederkoorn C, Braet C, Van Eijs Y, et al. Why obese children cannot resist food: the role of impulsivity. Eat Behav. 2006;7(4):315–22. doi: 10.1016/j.eatbeh.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 74.Nederkoorn C, Smulders FTY, Havermans RC, et al. Impulsivity in obese women. Appetite. 2006;47(2):253–256. doi: 10.1016/j.appet.2006.05.008. [DOI] [PubMed] [Google Scholar]