Abstract
In this study, we report experimental evidence of the thioautotrophic activity of the epibiotic microbial community associated with the setae of Shinkaia crosnieri, a galatheid crab that is endemic to deep-sea hydrothermal systems in the Okinawa Trough in Japan. Microbial consumption of reduced sulfur compounds under in situ hydrostatic and atmospheric pressure provided evidence of sulfur-oxidizing activity by the epibiotic microbial community; the rate of sulfur oxidation was similar under in situ and decompressed conditions. Results of the microbial consumption of reduced sulfur compounds and tracer experiments using 13C-labeled bicarbonate in the presence and absence of thiosulfate (used as a thioautotrophic substrate) convincingly demonstrated that the epibiotic microbial community on S. crosnieri drove primary production via an energy metabolism that was coupled with the oxidation of reductive sulfur compounds. A combination of tracer experiments, fluorescence in situ hybridization (FISH) and nano-scale secondary ion mass spectrometry (Nano-SIMS) indicated that the filamentous cells of the genus Sulfurovum belonging to the class Epsilonproteobacteria were thioautotrophs in the epibiotic community of S. crosnieri. In conclusion, our results strongly suggest that thioautotrophic production by Sulfurovum members present as the epibiotic microbial community play a predominant role in a probable nutritional ectosymbiosis with S. crosnieri.
Introduction
Many species of invertebrates that dwell in deep-sea hydrothermal vents are known to host bacteria (epibionts) that adhere to the surface of specialized tissues such as the dorsal setae of the polychaete Alvinella pompejana, the gill chamber of the shrimp Rimicaris exoculata, the setae of the crabs Shinkaia crosnieri and Kiwa hirsuta, and the iron-sulfide-coated scales of the scaly-foot snail [1]–[5]. The epibiotic microbial community associated with the host animals commonly includes major phylotypes belonging to the genus Sulfurovum within the class Epsilonproteobacteria [1]–[5]. Microscopic fluorescence in situ hybridization (FISH) has revealed that members of the genus Sulfurovum dominate epibiotic communities and have similar morphological features, such as long and thick filaments [1], [4], [5].
Potential molecular evidences of chemolithotrophic primary production by the epibionts have been obtained by metagenomic characterization of the epibiotic A. pompejana community, which is dominated by typical filamentous Sulfurovum epibionts [1], [6]. The genes that are involved in the complete reductive tricarboxylic acid (rTCA) cycle as well as in the oxidation of reduced sulfur compounds and hydrogen have been identified in gene assemblages of epsilonproteobacterial epibionts [6]. Similarly, genes that are involved in the rTCA and Calvin cycles as well as in the oxidation of reduced sulfur compounds and hydrogen have been identified in genomic DNA extracted from the epibiotic R. exoculata community [7]. These results suggest that the epibiotic microbial community associated with A. pompejana and R. exoculata mediates functionally active chemoautotrophy via sulfur and/or hydrogen oxidation. In our previous study, we reported clear evidence of autotrophy among the epibiotic microbial community associated with S. crosnieri, which predominately comprised morphotypes and phylotypes of members of the genus Sulfurovum [5]. We showed that 13C-labeled bicarbonate was incorporated in the epibiotic microbial community, and that the primary production by the epibionts was enhanced by the addition of potential thioautotrophic substrates such as sulfide and thiosulfate, but not hydrogen. Although these findings strongly suggest that the epibiotic microbial community associated with S. crosnieri harbors functionally active thioautotrophic populations, the thioautotrophic phylotypes have not yet been specified and the expected sulfur-oxidizing activity has not been confirmed [5]. Physiological, genomic, and biochemical characterizations of several Sulfurovum isolates have pointed that most of Sulfurovum members are chemolithoautotrophs that are sustained by various energy metabolisms that use reduced sulfur compounds [8]–[10]. However, it is not entirely clear whether the long and thick filamentous Sulfurovum epibionts associated with these deep-sea vent invertebrates serve as thioautotrophic primary producers.
Therefore, in this study, we attempted to obtain direct evidence of the sulfur-oxidizing activity of the epibionts by investigating the consumption of reduced sulfur compounds by the epibiotic microbial community on S. crosnieri. The sulfur-oxidizing activity of the epibiotic microbial community on living S. crosnieri individuals and cut setae was characterized under atmospheric and in situ hydrostatic pressure to consider the effect of hydrostatic pressure on epibiotic microbial functioning. Moreover, we determined the cell-level-chemoautotrophic productivity of the filamentous Sulfurovum epibionts of S. crosnieri using a combination of FISH and Nano-SIMS to obtain direct evidence of thioautotrophic activity of the Sulfurovum epibionts.
Results and Discussion
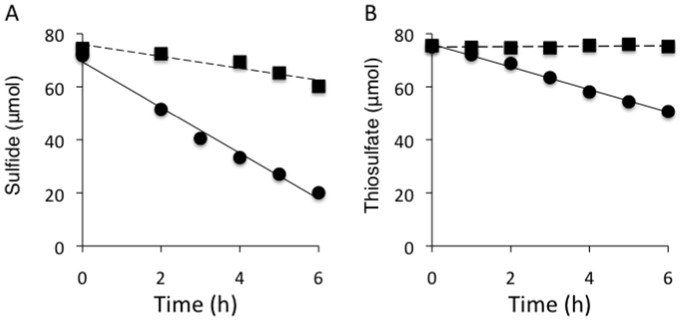
The sulfur-oxidizing activity of mixed setae samples was directly investigated by a time course evaluation of the consumption of sulfide or thiosulfate by the epibionts (Figure 1). The concentration of sulfide decreased under aerobic conditions both in the presence and absence of setae sample, and this was probably due to chemical oxidation by oxygen (Figure 1A). However, a greater concentration of sulfide was consumed during incubation with the mixed setae sample, and the net sulfide consumption rate of the sample was estimated to be 374 µmol· h−1·g−1 dry weight of setae (Figure 1A). The concentration of thiosulfate decreased only on incubation with the setae sample (Figure 1B). The estimated net rate of consumption of thiosulfate in the mixed setae sample was 99 µmol·h−1·g−1 dry weight of setae (Figure 1B). Our results clearly indicated that epibiotic microbial community of the S. crosnieri setae contained sulfur-oxidizing populations although it is unclear whether the populations were autotrophic and/or heterotrophic. This is the first direct evidence for the existence of functionally active sulfur-oxidizing populations in the epibiotic microbial community associated with invertebrates that inhabit deep-sea hydrothermal vents.
Figure 1. Time course of the consumption of reduced sulfur compounds by epibiotic microbial community associated with S. crosnieri setae.
The concentration of hydrogen sulfide was examined at the indicated intervals in the absence (black square) and presence (black circle) of the mixed setae samples (A). The unbroken line indicates linear regression analysis for the absence of the mixed setae (y = −2.236x+75.9, R2 = 0.89) and the solid line indicates linear regression analysis for the presence of the mixed setae (y = −8.602x+69.4, R2 = 0.99) (A). The concentration of thiosulfate was determined at indicated intervals in the absence (black square) and presence (black circle) of mixed setae samples (B). The unbroken line indicates linear regression analysis for the absence of the mixed setae (y = 0.079x+75.0, R2 = 0.11) and the solid line indicates linear regression analysis for the presence of the mixed setae (y = −4.264x+76.0, R2 = 0.99) (B). Statistically significant differences in consumption in the presence and absence of the setae samples at individual time points were determined by unpaired t-test (A, P = 2×10−7, B, P = 4×10−10).
The sulfur-oxidizing activity of the epibionts associated with living S. crosnieri individuals was investigated under atmospheric and elevated hydrostatic pressure using a newly developed high-pressure continuous-flow apparatus (Figure S1). The hydrostatic pressure was increased to 12.0 MPa, since this value corresponds to the hydrostatic pressure at a water depth of approximately 1200 m. The depth of the major S. crosnieri habitats, such as the Iheya North and Hatoma Knoll fields, ranges from approximately 1000 to 1500 m [5], [11]. The S. crosnieri individuals were confirmed to be alive during incubation in both piezophilic and non-piezophilic conditions, since we were able to observe them moving and performing a number of behaviors. A decrease in the sulfide concentration in the effluent seawater was detected under all conditions, and this was probably due to chemical oxidation by oxygen (Table 1). However, greater sulfide consumption rates were obtained in the incubation experiments performed with living S. crosnieri individuals (Table 1). The net sulfide consumption rate of the living individuals was estimated to be 576 and 557 µmol·h−1·g−1 dry weight of setae under 0.1 and 12.0 MPa, respectively (Table 1). Although we had expected that the sulfide consumption rate of the S. crosnieri epibionts would potentially be affected by hydrostatic pressure, these results show that the sulfur-oxidizing activity of the epibionts was similar under conditions mimicking in situ hydrostatic pressure and ambient surface pressure (Table 1). Several piezophilic thioautotrophs have been isolated from deep-sea hydrothermal environments, and their growth rates have been shown to be strongly affected by hydrostatic pressure [12]. Since growth rate is regulated by whole cellular functions, it may be much more responsive to differences in hydrostatic pressure than the energy and carbon metabolism pathways that involve sulfide oxidation. This is the first study to demonstrate that the sulfur-oxidizing activity of an epibiotic microbial community is not significantly affected by change in hydrostatic pressure. These results can be interpreted as indicating that the metabolic rates of epibiotic microbial communities associated with invertebrates from deep-sea vents are not strongly influenced by depressurization, and that the rate constants determined under non-pressurized conditions can be applied to estimations of function and metabolism in situ in deep-sea habitats.
Table 1. Net consumption rates of sulfide during continuous supply of sulfide to a living S. crosnieri individual at a hydrostatic pressure of 0.1 and 12.0 MPa.
| Pressure | Sulfide consumption rate (µmol/h) | Net sulfide consumption rate | ||
| (MPa) | with individual | without individual | (µmol/h/individual) | (µmol/h/g of setae) |
| 0.1 | 51.7 | 12.7 | 39.0 | 576 |
| 12.0 | 52.1 | 12.9 | 39.2 | 557 |
The comparison of the sulfide consumption rates of the epibionts associated with living individual and dissected setae showed similar levels of slightly different sulfur-oxidizing activities (Figure 1 and Table 1). The net sulfide consumption rate of the epibionts associated with living S. crosnieri individual (576 µmol·h−1·g−1 setae) was 1.5-fold higher than that associated with dissected setae (383 µmol·h−1·g−1 setae; Figure 1A and Table 1). The difference may simply be explained by the subsidiary functions of microbial communities populating the gut and body surface or as an analytical deviation resulting from our limited number of measurement points. However, the rate of sulfide consumption may actually differ between the epibionts associated with living individual and those associated with dissected setae. It is likely that the convective transport of sulfide to the epibionts associated with living S. crosnieri individuals differs from those associated with dissected setae. In our experiments investigating sulfur-oxidizing activity, living S. crosnieri individuals were frequently observed to move in glass vessels during incubation whereas dissected setae remained in a static position in artificial seawater in a glass bottle. It is difficult to assume that there were significant differences in the physiological state and abundance of the epibiotic microbial community associated with living individual and dissected setae because the sulfide consumption experiments were conducted using fresh S. crosnieri individuals and setae are always covered with the numerous epibionts. Therefore, the different consumption rates suggest that the epibiotic microbial community associated with living S. crosnieri is advantageous for ensuring accessibility to sulfide. This may be an important insight into how epibiotic microbial communities on deep-sea vent invertebrates benefit from their hosts.
The autotrophic productivity of the epibiotic microbial community associated with the mixed setae was determined by incubation with NaH13CO3 in the presence and absence of thiosulfate (used as a sulfur-oxidizing compound) (Table 2). After 48 h of incubation, the bulk organic carbon of the epibiotic microbial community was found to be enriched with 13C during incubation in the presence and absence of thiosulfate (Table 2). The 13C enrichment of bulk organic carbon in the epibiotic microbial community was 2.4-fold higher (unpaired t-test, P = 4×10−5) during incubation with thiosulfate compared with during incubation without thiosulfate (Table 2). Although previous uptake experiments using 13C-labeled tracers and a previous analysis of potentially functional genes suggested that sulfur-oxidizing chemoautotrophic production occurs in such epibiotic microbial communities [5]–[7], the result of a combination of thiosulfate-enhanced enrichment of 13C-labeled bicarbonate and sulfur-oxidizing activity in similar subsamples of mixed setae convincingly demonstrated presence of a thioautotrophic population in the S. crosnieri epibionts (Figure 1 and Table 2).
Table 2. Stable carbon isotope analysis of the incorporation of 13C-labeled bicarbonate by the epibiotic microbial community on S. crosnieri setae.
| Incubation Time (hours) | Tracer and Additional Energy Source | δ13C (‰)* | Average 13C/12C | % Enrichment |
| 0 | – | −41.3±0.2 | 0.01077±0.00002 | – |
| 48 | 13CO2 | 778±34 | 0.0200±0.0004 | 74±3 |
| 13CO2 + thiosulfate | 1851±68 | 0.0320±0.0008 | 176±7 |
δ 13C values were measured in triplicate and were expressed as the mean ± SD.
The 13C enrichment E (%) was calculated using the following equation: E = (Fsample/Fcontrol − 1) ×100 (%). Fsample and Fcontrol represent the isotopic abundance of 13C atoms of the epibionts before and after 13C labeling [F = (13C/12C)/(1+13C/12C)].
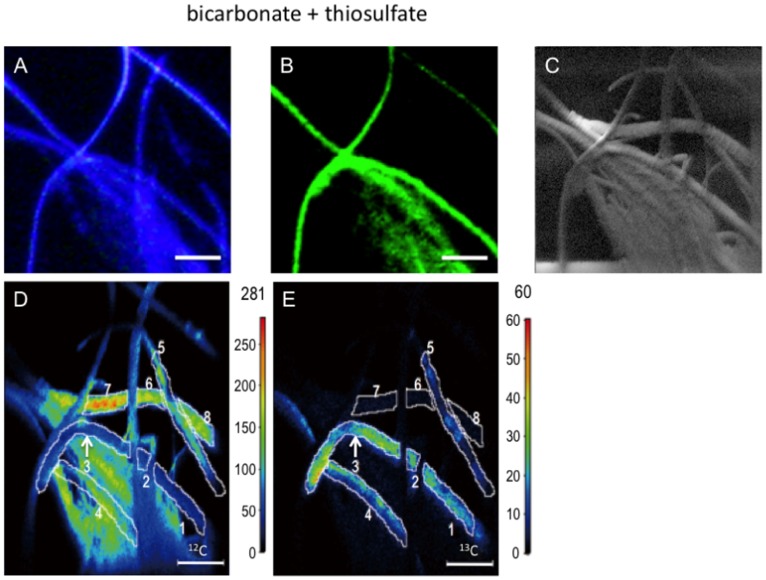
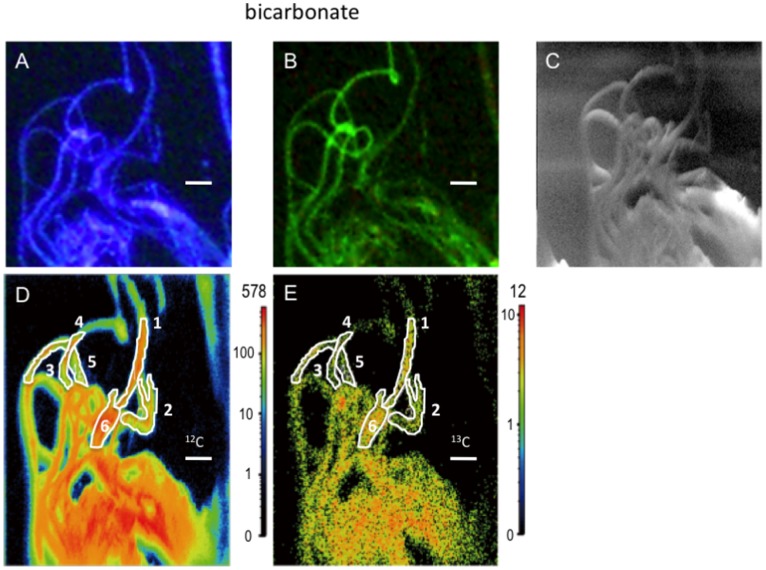
After 13C-bicarbonate tracer experiments in the presence and absence of thiosulfate, subsamples of mixed setae were used for FISH and Nano-SIMS to clarify the cell-specific trophic nature of the Sulfurovum epibionts (Figures 2 and 3). FISH using a Sulfurovum-specific probe demonstrated that the predominant morphotype of the epibionts exhibited long and thick filaments, and these were identified as the Sulfurovum members within the Epsilonproteobacteria (Figures 2B and 3B). FISH–Nano-SIMS of the epibiotic microbial community after 13C-bicarbonate incubation with additional thiosulfate indicated that a Sulfurovum species that contributed to 13C enrichment of cellular carbon was present (Figure 2B, 2D and 2E). The carbon isotope abundance in representative locations of the epibiont cells was quantified, and the estimated average 13C/12C ratios were compared to the value of the bulk organic carbon of the epibiotic microbial community before the tracer experiments (control epibiont cells) (Figure 2 and Tables 2 and 3). The 13C/12C ratios of the filamentous Sulfurovum cells were enriched up to 2713% compared to the average 13C/12C ratio of the control epibiont cells (Figure 2 and Table 3). Nano-SIMS carbon isotope mappings (12C and 13C) of the epibionts incubated in the absence of thiosulfate showed very similar distribution patterns of 12C and 13C, indicating that all epibionts were either enriched or not enriched with 13C (Figure 3). Comparison with epibiont cells before tracer experiments indicated that the representative Sulfurovum epibionts displayed no cellular carbon 13C enrichment during the tracer experiments performed in the absence of thiosulfate (Table 3). These results indicate that filamentous Sulfurovum cells assimilated inorganic carbon only after incubation in the presence of thiosulfate (unpaired t-test, P = 3×10−6), whereas our previous study revealed that the epibiotic microbial communities on S. crosnieri assimilated inorganic carbon both in the presence and absence of reduced sulfur compounds [5]. In conclusion, it became evident that thiosulfate served as an energy source for autotrophic production by the epibionts. Therefore, FISH–Nano-SIMS of the epibiotic microbial community during 13C-bicarbonate incubation provided clear evidence of the thioautotrophic productivity of the Sulfurovum-affiliated epibionts, and strongly suggested that the predominant epibiotic population comprised sulfur-oxidizing chemoautotrophs that were capable of using reduced sulfur compounds as an energy sources. This study is the first report of cell-specific thioautotrophic metabolism in epibiotic microbial communities associated with deep-sea vent invertebrates. Autotrophy of the microbial communities associated with deep-sea and terrestrial invertebrates has been demonstrated previously by RuBisCO activity, gene expression of ATP citrate lyase (a key enzyme in the rTCA cycle), and incorporation of labeled bicarbonate [13]–[15]. In addition, our previous study indicated the possible nutritional transportation from the organic carbons produced by chemoautotrophic and methanotrophic epibionts on S. crosnieri to the host body because 13C-labeled bicarbonate and methane were incorporated into the S. crosnieri body as well as the epibiotic microbial community [5]. From our previous and present studies, it is concluded that primary production by sulfur-oxidizing chemoautotrophs as well as methanotrophs in the epibiotic microbial community associated with S. crosnieri provide nutritional support for the host, and that Sulfurovum members, which constitute a large proportion of the epibiotic community, play a predominant role in the probable nutritional ectosymbiosis with S. crosnieri.
Figure 2. Analyses of epibiotic microbial cells after 13C bicarbonate tracer experiments.
Microscopic image of DAPI-stained epibiont cells in a specimen (A). Microscopic image of epibiont cells specifically bound to the EPI653 probe, which indicates that members of the genus Sulfurovum belonging to the class Epsilonproteobacteria were present in the same specimen (B). Scanning electron micrograph of epibiont cells in the same specimen (C). 12C-mapping image of epibiont cells in the same specimen performed using Nano-SIMS (D). 13C-mapping image of epibiont cells in the same specimen performed using Nano-SIMS (E). The values (secondary ion counts) corresponding to each of the colors are shown in the scale to the right of each map (D and E). The estimated 13C/12C ratios of the cells that are enclosed by the white lines are indicated in Table 3. The scale bar is 10 µm.
Figure 3. Analyses of epibiont cells after 13C bicarbonate tracer experiments performed in the presence of potential thioautotrophic substrates.
Microscopic image of DAPI-stained epibiont cells in a specimen (A). Microscopic image of epibiont cells specifically bound to the EPI653 probe, which indicates that members of the genus Sulfurovum belonging to the class Epsilonproteobacteria were present in the same specimen (B). Scanning electron micrograph of epibiont cells in the same specimen (C). 12C-mapping image of epibiont cells in the same specimen performed using Nano-SIMS (D). 13C-mapping image of epibiont cells in the same specimen performed using Nano-SIMS (E). The values (secondary ion counts) corresponding to each of the colors are shown in the scale to the right of each map (D and E). The estimated 13C/12C ratios of the cells that are enclosed by the white lines are indicated in Table 3. The scale bar is 20 µm.
Table 3. Cell-specific incorporation of 13C-labeled bicarbonate by the Sulfurovum epibionts on S. crosnieri seta in the presence and the absence of thiosulfate according to FISH–Nano-SIMS.
| Tracer | Location | Average 13C/12C | Enrichment (%) |
| 1* | 0.428±0.086 | 2713±588 | |
| 2* | 0.292±0.058 | 2019±435 | |
| NaH13CO3 | 3* | 0.394±0.079 | 2551±552 |
| + | 4† | 0.135±0.027 | 1012±225 |
| thiosulfate | 5† | 0.082±0.016 | 608±143 |
| 6† | 0.010±0.002 | −10±20 | |
| 7† | 0.011±0.002 | −5±20 | |
| 8† | 0.010±0.002 | −10±20 | |
| 1* | 0.009±0.002 | −15±20 | |
| 2* | 0.010±0.002 | −10±20 | |
| NaH13CO3 | 3* | 0.011±0.002 | −5±20 |
| 4* | 0.009±0.002 | −15±20 | |
| 5* | 0.009±0.002 | −15±20 | |
| 6* | 0.010±0.002 | −10±20 | |
| – | Control epibiont cells | 0.01077 | – |
The percent enrichment was estimated using the average 13C/12C ratio of bulk organic carbon of the epibiotic microbial community before the experiment shown in Table 2.
The filamentous cells were identified as Sulfurovum members by FISH.
The filamentous cells were not identified as the Sulfurovum members by FISH.
FISH–Nano-SIMS indicated that cellular 13C enrichment did not occur among the Sulfurovum epibionts during the tracer experiments performed in the absence of thiosulfate (Figure 2 and Table 3), whereas 13C enrichment of bulk organic carbon in the epibiotic microbial community was detected after incubation in the absence of thiosulfate (Table 2). In our previous study [5], it was hypothesized that 13C-labeled bicarbonate enrichment of the epibiotic community without any additional energy source was attributed to the potential metabolic activities of epibionts using the remaining reduced sulfur compounds that might be preserved inside and/or outside the epibiont cells. Indeed, in the microbial epibiotic community associated with R. exoculata, sulfur granules are only observed inside thin filamentous epibiont cells, while they are absent from thick filamentous epibionts [16]. Therefore, it was inferred that 13C-labeled bicarbonate incorporation that was independent of any additional energy source might be promoted by chemoautotrophic populations of epibionts (e.g., potentially thioautotrophic gamma-proteobacterial populations with thin filamentous morphotypes) other than thick filamentous Sulfurovum epibionts [5]. Since the short and thin filamentous morphotypes of gamma-proteobacterial epibionts are usually located deep within aggregates of the long filamentous morphotypes of Sulfurovum epibionts [5] and the primary beam did not reach the interior populations of epibionts in Nano-SIMS analysis, FISH–Nano-SIMS did not examine the potential metabolic activities of gamma-proteobacterial epibionts using the remaining reduced sulfur compounds.
In this study, the thioautotrophic productivity of the predominant Sulfurovum epibionts was verified using cell-specific FISH–Nano-SIMS. However, the thioautotrophic activity of the epibiotic microbial community requires further investigation. In cell-specific FISH–Nano-SIMS, the filamentous epibionts that did not bind to the Sulfurovum-specific FISH probe showed considerable 13C enrichment of cellular carbon during incubation in the presence of thiosulfate compared with Sulfurovum epibionts incubated in the absence of thiosulfate (unpaired t-test, P = 0.0002; Figure 2A, 2B, 2E and Table 3). These results show that the epibiotic microbial community of S. crosnieri setae includes the Sulfurovum members and currently unidentified thioautotrophs. In addition, not all of the Sulfurovum filamentous epibionts showed significant enrichment of 13C-carbon during incubation in the presence of thiosulfate (Figure 2B, 2E). This may indicate that not all the Sulfurovum filamentous epibionts possess the dissimilatory thiosulfate oxidation pathway coupled with carbon fixation or express the enzymes that are involved in dissimilatory thiosulfate oxidation. This is also one possible explanation for why the consumption rate of sulfide was greater (4-fold) than that of thiosulfate (Figure 1). Whole genome analysis [9] and biochemical characterization [17] have indicated that the quinone reductase (Sqr) and epsilon-type Sox system are potentially responsible for sulfide, polysulfide, and thiosulfate oxidation to sulfate in the chemolithotrophic energy metabolism of Sulfurovum sp. strain NBC37-1. Although Sulfurovum sp. strain NBC37-1 constitutively expresses these sulfur-oxidizing enzymes, it remains unclear whether other Sulfurovum epibionts always express the enzymes that are involved in thioautotrophic metabolism. A previous study revealed that the Sulfurovum members and their relatives are not necessarily thioautotrophic chemoautotrophs, and that some represent strictly hydrogenotrophic chemoautotrophs [8]. The long and thick filamentous Sulfurovum epibionts on S. crosnieri setae morphologically appear homogenous, but considerable genetic heterogeneity is perceived in the 16S rRNA gene phylotype composition [5]. Although the Sulfurovum epibionts seem to be primarily thioautotrophs, the long and thick filamentous Sulfurovum epibionts may consist of metabolically heterogeneous components. These points will be the focus of further investigations.
Materials and Methods
Ethics Statement
The locations for sample collection were not privately owned or protected in any way and no specific permits were required for the described field studies and sample collection. The field studies did not involve any endangered or protected species.
Collection of S. crosnieri from the Deep-sea Hydrothermal Field
S. crosnieri individuals were collected from the Iheya North field in the Okinawa Trough, Japan, during dive#1062 on October 9, 2009 (27° 47.46′N, 126° 53.01′E, depth 1004 m) and dive#1355 on March 19, 2012 (27° 47.46′N, 126° 53.81′E, depth 986 m) using the JAMSTEC remotely operated vehicle (ROV) “HyperDolphin”. Individuals were collected using a suction sampler and were stored in a box of chilled water in the ROV. Immediately after onboard recovery, the individuals were thoroughly washed using sterile artificial seawater. After washing, setae from the three individuals collected during dive#1062 were cut and mixed together. These procedures were performed below 5°C because the ambient temperature of S. crosnieri in their natural habitat ranges from 4 to 6°C. Mixed setae collected during dive#1355 were used for measuring sulfur-oxidizing activity and tracer experiments with 13C-labeled bicarbonate. Two living S. crosnieri individuals (37-mm carapace length) collected during dive#1355 were used for measuring sulfur-oxidizing activity under both in situ hydrostatic (12.0 MPa) and atmospheric pressure (0.1 MPa).
Sulfur-oxidizing Activity Measurements
Mixed setae samples from the three individuals were used for measuring sulfur-oxidizing activity; measurement was performed onboard. The mixed setae were incubated at 5°C in 295-mL glass bottles that were sealed with butyl rubber stoppers and contained 200 mL of artificial seawater (25 g·L−1 NaCl, 4.2 g·L−1 MgCl2·6H2O, 3.4 g·L−1 MgSO4·7H2O, 0.5 g·L−1 KCl, 0.7 g·L−1 CaCl2·2H2O, 14 mg·L−1 K2HPO4, 2.1 mg·L−1 NH4Cl and 6.8 mg·L−1 NaNO3; adjusted to pH 7.3) filtered using a 0.22 µm-pore membrane under air. The artificial seawater was supplemented with sodium sulfide and sodium thiosulfate (used as reduced sulfur compounds) in the presence of 1 mM sodium bicarbonate at final concentrations of 375 µM. The artificial seawater in the bottles was subsampled at the indicated intervals. The dry weight of the mixed setae incubated with sodium sulfide and sodium thiosulfate was 17 and 43 mg, respectively.
The sulfur-oxidizing activity of S. crosnieri setae was examined using a high-pressure continuous-flow apparatus at 5°C; this supplied a certain concentration of sodium sulfide and oxygen to a living S. crosnieri individual confined in a glass incubation vessel under a given hydrostatic pressure (up to 25 MPa) (Figure S1). The apparatus comprised a plunger pump (LC-10Ai, Shimadzu Co., Japan), a stainless steel pressure chamber with two observation windows (Horiguchi Ironworks Co., Japan), and glass incubation vessels (Horiguchi Ironworks Co., Japan) directly connected with hydraulic tubes to the liquid supplied by the plunger pump and discharged through a pressure relief valve (Figure S1). All the hydraulic tubing for transportation of the liquid was manufactured using PEEK or silica-coated stainless steel (Swegelok). The hydrostatic pressure (up to 25 MPa) and the continuous flow (up to 9.99 mL·min−1) in the glass incubation vessels were regulated by the plunger pump and the pressure relief valve when the experiments were conducted under high hydrostatic pressure (Figure S1). If the experiments were conducted under atmospheric pressure (0.1 MPa), the pressure relief valve was disconnected. The hydrostatic pressure (up to 25 MPa) of the tap water used to fill the stainless steel pressure chamber was adjusted by a hydraulic pump (HP-150, Syn Co., Kyoto, Japan). The cylinder glass incubation vessel used as a specimen holder (diameter, 50 mm; length, 80 mm; volume, 182 mL) had a stainless steel cover with inlet and outlet ports that were sealed by a butyl rubber gasket (thickness, 5 mm) at the top and butyl rubber bellows (thickness, 5 mm) at the bottom (Figure S2). The butyl rubber bellows served as a pressure buffer for the liquid between the inside of the glass vessel and the pressure chamber. During the incubation experiments in which living animals were used, the motility and behaviors of the animals were monitored through the observation windows in the pressure chamber as the specimen holder was made of cylinder glass (Figure S2).
In experiments measuring the sulfur-oxidizing activity of epibionts associated with living S. crosnieri individuals, artificial seawater supplemented with 270 µM sodium sulfide and 1 mM sodium bicarbonate was supplied at a flow rate of 9.0 mL·min−1 for 60 min at 5°C under hydrostatic pressure of 0.1 or 12.0 MPa. Pressurization to 12.0 MPa required 1–2 min. The influent and effluent seawater were sampled at 10-min intervals, and the sulfide concentration was determined as described below. In both the piezophilic and non-piezophilic experiments, the sulfide concentration became constant after 40 min of incubation. We used the sulfide concentrations of the influent and effluent seawater at 50 and 60 min after the initiation of continuous flow to determine the average consumption rate of sulfide. The dry weight of S. crosnieri setae used for sulfur-oxidizing activity experiments under 0.1 and 12.0 MPa was 68 and 70 mg, respectively.
The concentration of sulfide in the seawater was determined using the methylene blue method [18]. The concentration of thiosulfate was determined using ion chromatography using the Shim-pack IC-A3 column (Shimadzu Co., Japan) and a buffer system consisting of 8 mM p-hydroxybenzoic acid, 3.2 mM bis(2-hydroxyethyl)imino-tris(hydroxymethyl)methane, and 50 mM boric acid.
Tracer Experiments
The mixed setae samples were also used for the tracer experiments. The mixed setae were incubated for 48 h at 5°C in 295-mL glass bottles sealed with butyl rubber stoppers and containing 200 mL of artificial seawater under air. Supplemental 13C-labeled bicarbonate (NaH13CO3) was added to the seawater at a final concentration of 1 mM in the presence or absence of 300 µM sodium thiosulfate, which was used as a reduced sulfur compound. NaH13CO3 was purchased from Cambridge Isotope Laboratories, Inc. (Andover, MA, USA). After 48 h of incubation onboard, the mixed setae samples were harvested and frozen at −80°C. At the onshore laboratory, the setae samples were lyophilized and the epibiont cells were used for 13C composition analysis.
FISH
After the tracer experiments, a portion of the mixed setae samples was fixed using 4% paraformaldehyde in phosphate-buffered saline overnight at 4°C and stored in 50% ethanol in phosphate-buffered saline at −30°C until FISH. The fixed samples were then spotted onto glass slides and air dried before dehydration by sequential washes in 50%, 80%, and 100% (v/v) ethanol for 3 min each. Hybridization with the probe EPI653, which was specifically designed for detecting Sulfurovum phylotypes within the class Epsilonproteobacteria [5], was performed as previously described [5]. The probe was labeled with Alexa 488. After hybridization, the epibiotic microbial community associated with the setae was transferred to a brass pedestal (Vector Laboratories, Burlingame, CA, USA) and viewed using a Nikon E800 epifluorescence microscope (Nikon Instruments, Inc., Melville, NY, USA). The images were collected and analyzed using NIS Elements AR 2.30 and Hotfix (Build 312) image analysis software (Nikon Instruments, Inc.).
Nano-scale Secondary Ion Mass Spectrometry
Cell-specific carbon isotope ratios of the epibiotic microbial community associated with S. crosnieri setae were measured using the NanoSIMS 50 ion microprobe (AMETEK Co., CAMECA, Courbevoie, France) at the University of Tokyo. The lateral resolution of Nano-SIMS is up to 50 nm, and it has been successful in determining the isotopic distributions of carbon in cell and mineral samples at the submicron scale and at diameters of a few microns [19], [20]. Following incubation with NaH13CO3 in the presence or absence of thiosulfate, the subsamples were prepared for FISH and placed on 10-nm thick brass plates that were gold coated for charge compensation. Before carbon isotopic measurement, Cs+ ions were implanted on the sample surface under a high current beam (15 pA·s·µm−2). Carbon isotopic measurement was performed using a 6-pA Cs+ primary beam that was stepped over the 100×100 µm field of a 256×256 pixel raster with a counting time of 1 or 5 ms per pixel.
Negative secondary ions (12C−, 13C−, 12C12C−, and 12C13C− ions) were accelerated by the application of 8 kV at the sample surface and were simultaneously detected using four electron multipliers in the ion-counting mode at a mass resolution that was sufficient to separate 13C− from 12CH− and 12C13C− from 12C12CH−. The entrance slit width was set to approximately 30 µm, and the exit slit width of each EM detector was set to 50 µm.
Recorded images and data were processed using CAMECA WinImage software. The different scans of each image were aligned to correct for image drift during acquisition. 13C enrichment of a microbial cell was calculated by drawing a region of interest (ROI) on C− images and calculating the 13C/12C ratio from 13C−/12C− ratio of the ROI by correcting for the effect of instrumental mass fractionation. The instrumental mass fractionation of C isotopes was estimated to be −35‰ by measuring the 13C−/12C− ratios of epibionts for which the 13C/12C ratios were determined by the conventional EA-IRMS method. The reproducibility of the 13C−/12C− ratio of an in-house standard (Escherichia coli) was 20% (1SD, n = 10). 13C enrichment E (%) was calculated with the following equation: E = (Fsample/Fcontrol–1)×100 (%) where Fsample and Fcontrol show the isotopic abundance of 13C atoms of the epibionts before and after 13C labeling [F = (13C/12C)/(1+13C/12C)]. The data were expressed relative to the PDB (Peedee belemnite) standard.
Stable Carbon Isotopic Analyses of 13C-labeled and Non-labeled Setae
The mixed setae samples obtained from the three S. crosnieri individuals before and after the 13C-labeled tracer experiments were thoroughly washed in sterile artificial seawater without carbonates, frozen, and stored at −80°C. Contaminating carbonates in the samples were removed as previously described [5]. The 13C composition of the samples was determined using a mass spectrometer (Delta Plus XP; Thermo Finnigan, Bremen, Germany) that was coupled online via a Finnigan ConFlo III interface with an elemental analyzer (FlashEA 1112; ThermoQuest, Milan, Italy). All samples were measured in triplicate.
Supporting Information
High-pressure continuous-flow system.
(TIF)
Structure of the specimen holder.
(TIF)
Acknowledgments
We are grateful to the crews of RV/Natsushima and the operation teams of DSV/HyperDolphin for helping us collect the deep-sea hydrothermal samples.
Funding Statement
This work was supported by a Grant-in-Aid for Science Research (Nos. 20109005 and 21244077 to K. Takai) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan and by a grant from the Japan Agency for Marine-Earth Science and Technology (JAMSTEC). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Cary SC, Cottrell MT, Stein JL, Camacho F, Desbruyeres D (1997) Molecular identification and localization of filamentous symbiotic bacteria associated with the hydrothermal vent annelid Alvinella pompejana . Appl Environ Microbiol 63: 1124–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goffredi SK, Jones WJ, Erhlich H, Springer A, Vrijenhoek RC (2008) Epibiotic bacteria associated with the recently discovered Yeti crab, Kiwa hirsuta . Environ Microbiol 10: 2623–2634. [DOI] [PubMed] [Google Scholar]
- 3. Goffredi SK, Waren A, Orphan VJ, Van Dover CL, Vrijenhoek RC (2004) Novel forms of structural integration between microbes and a hydrothermal vent gastropod from the Indian Ocean. Appl Environ Microbiol 70: 3082–3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Polz MF, Cavanaugh CM (1995) Dominance of one bacterial phylotype at a Mid-Atlantic Ridge hydrothermal vent site. Proc Natl Acad Sci USA 92: 7232–7236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Watsuji T, Nakagawa S, Tsuchida S, Toki T, Hirota A, et al. (2010) Diversity and function of epibiotic microbial communities on the galatheid crab, Shinkaia crosnieri . Microb Environ 25: 288–294. [DOI] [PubMed] [Google Scholar]
- 6. Grzymski JJ, Murray AE, Campbell BJ, Kaplarevic M, Gao GR, et al. (2008) Metagenome analysis of an extreme microbial symbiosis reveals eurythermal adaptation and metabolic flexibility. Proc Natl Acad Sci USA 105: 17516–17521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hugler M, Petersen JM, Dubilier N, Imhoff JF, Sievert SM (2011) Pathways of carbon and energy metabolism of the epibiotic community associated with the deep-sea hydrothermal vent shrimp Rimicaris exoculata . PLoS One 6: e16018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nakagawa S, Takai K, Inagaki F, Hirayama H, Nunoura T, et al. (2005) Distribution, phylogenetic diversity and physiological characteristics of epsilon-Proteobacteria in a deep-sea hydrothermal field. Environ Microbiol 7: 1619–1632. [DOI] [PubMed] [Google Scholar]
- 9. Nakagawa S, Takaki Y, Shimamura S, Reysenbach AL, Takai K, et al. (2007) Deep-sea vent epsilon-proteobacterial genomes provide insights into emergence of pathogens. Proc Natl Acad Sci USA 104: 12146–12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamamoto M, Takai K (2011) Sulfur metabolisms in epsilon-and gamma-Proteobacteria in deep-sea hydrothermal fields. Front Microbio 2: doi: 10.3389/fmicb.2011.00192. [DOI] [PMC free article] [PubMed]
- 11. Kawagucci S, Chiba H, Ishibashi J, Yamanaka T, Toki T, et al. (2011) Hydrothermal fluid geochemistry at the Iheya North field in the mid-Okinawa Trough: Implication for origin of methane in subseafloor fluid circulation systems. Geochem J 45: 109. [Google Scholar]
- 12. Takai K, Miyazaki M, Hirayama H, Nakagawa S, Querellou J, et al. (2009) Isolation and physiological characterization of two novel, piezophilic, thermophilic chemolithoautotrophs from a deep-sea hydrothermal vent chimney. Environ microbiol 11: 1983–1997. [DOI] [PubMed] [Google Scholar]
- 13. Campbell BJ, Stein JL, Cary SC (2003) Evidence of chemolithoautotrophy in the bacterial community associated with Alvinella pompejana, a hydrothermal vent polychaete. Appl Environ Microbiol 69: 5070–5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wirsen CO, Jannasch HW, Molyneaux SJ (1993) Chemosynthetic microbial activity at Mid-Atlantic Ridge hydrothermal vent sites. J Geophys Res 98: 9693–9703. [Google Scholar]
- 15. Dattagupta S, Schaperdoth I, Montanari A, Mariani S, Kita N, et al. (2009) A novel symbiosis between chemoautotrophic bacteria and a freshwater cave amphipod. ISME J 3: 935–943. [DOI] [PubMed] [Google Scholar]
- 16. Zbinden M, Shillito B, Le Bris N, de Villardi de Montlaur C, Roussel E, et al. (2008) New insigths on the metabolic diversity among the epibiotic microbial communitiy of the hydrothermal shrimp Rimicaris exoculata . J Exp Mar Biol Ecol 359: 131–140. [Google Scholar]
- 17. Yamamoto M, Nakagawa S, Shimamura S, Takai K, Horikoshi K (2010) Molecular characterization of inorganic sulfur-compound metabolism in the deep-sea epsilonproteobacterium Sulfurovum sp. NBC37–1. Environ Microbiol 12: 1144–1153. [DOI] [PubMed] [Google Scholar]
- 18. Fogo JK, Popowsky M (1949) Spectrophotometric determination of hydrogen sulfide. Methylene blue method. Anal Chem 21: 732–734. [Google Scholar]
- 19. Morono Y, Terada T, Nishizawa M, Ito M, Hillion F, et al. (2011) Carbon and nitrogen assimilation in deep subseafloor microbial cells. Proc Natl Acad Sci USA 108: 18295–18300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Floss C, Stadermann FJ, Bradley J, Bajt S, Graham G (2004) Carbon and nitrogen isotopic anomalies in an anhydrous interplanetary dust particle. Science 303: 1355–1358. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
High-pressure continuous-flow system.
(TIF)
Structure of the specimen holder.
(TIF)