Abstract
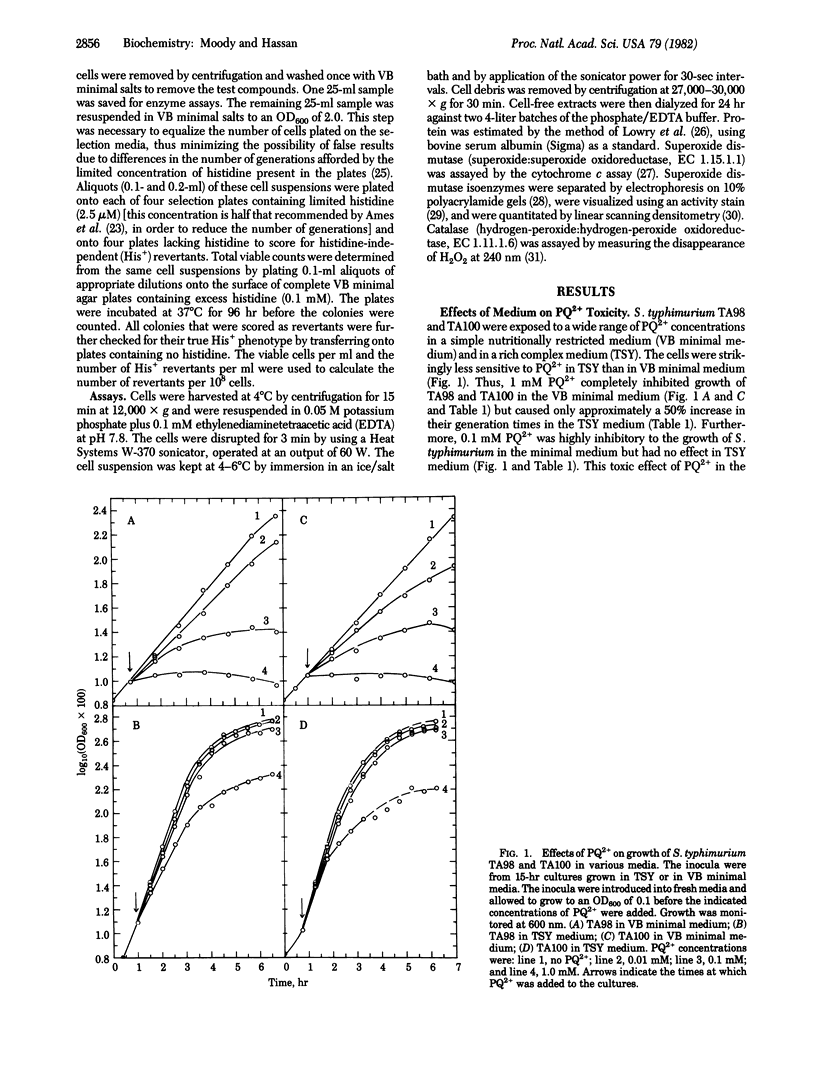
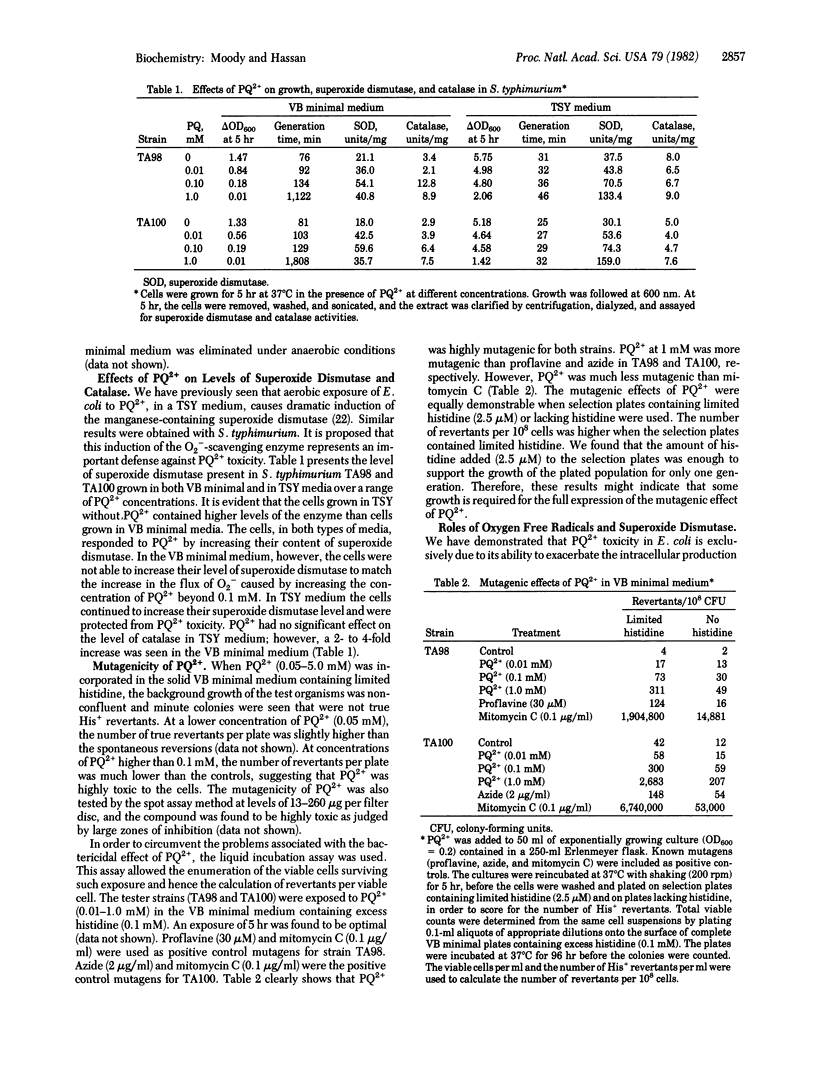
Paraquat (1,1'-dimethyl-4,4'-bipyridinium dichloride) was used as an intracellular generator of oxygen free radicals and was found to be highly mutagenic for Salmonella typhimurium. It caused both base-pair substitution and frameshift mutations. Paraquat was much more toxic and mutagenic in a simple nutritionally restricted medium than in a rich complex medium. The mutagenicity of paraquat was dependent upon the presence of a supply of both electrons and oxygen. Cells containing high levels of superoxide dismutase (superoxide:superoxide oxidoreductase, EC 1.15.1.1) were more resistant to the toxicity and the mutagenicity of paraquat than were cells containing normal levels of this enzyme. The mutagenicity of paraquat thus appears to be due to its ability to exacerbate the intracellular production of superoxide radicals.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Mccann J., Yamasaki E. Methods for detecting carcinogens and mutagens with the Salmonella/mammalian-microsome mutagenicity test. Mutat Res. 1975 Dec;31(6):347–364. doi: 10.1016/0165-1161(75)90046-1. [DOI] [PubMed] [Google Scholar]
- Armel P. R., Strniste G. F., Wallace S. S. Studies on Escherichia coli x-ray endonuclease specificity. Roles of hydroxyl and reducing radicals in the production of DNA lesions. Radiat Res. 1977 Feb;69(2):328–338. [PubMed] [Google Scholar]
- BEERS R. F., Jr, SIZER I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952 Mar;195(1):133–140. [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Brawn K., Fridovich I. DNA strand scission by enzymically generated oxygen radicals. Arch Biochem Biophys. 1981 Feb;206(2):414–419. doi: 10.1016/0003-9861(81)90108-9. [DOI] [PubMed] [Google Scholar]
- Bruyninckx W. J., Mason H. S., Morse S. A. Are physiological oxygen concentrations mutagenic? Nature. 1978 Aug 10;274(5671):606–607. doi: 10.1038/274606a0. [DOI] [PubMed] [Google Scholar]
- Cone R., Hasan S. K., Lown J. W., Morgan A. R. The mechanism of the degradation of DNA by streptonigrin. Can J Biochem. 1976 Mar;54(3):219–223. doi: 10.1139/o76-034. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dodge A. D. The mode of action of the bipyridylium herbicides, paraquat and diquat. Endeavour. 1971 Sep;30(111):130–135. doi: 10.1016/0160-9327(71)90039-1. [DOI] [PubMed] [Google Scholar]
- Farrington J. A., Ebert M., Land E. J., Fletcher K. Bipyridylium quaternary salts and related compounds. V. Pulse radiolysis studies of the reaction of paraquat radical with oxygen. Implications for the mode of action of bipyridyl herbicides. Biochim Biophys Acta. 1973 Sep 26;314(3):372–381. doi: 10.1016/0005-2728(73)90121-7. [DOI] [PubMed] [Google Scholar]
- Fenn W. O., Gerschman R., Gilbert D. L., Terwilliger D. E., Cothran F. V. MUTAGENIC EFFECTS OF HIGH OXYGEN TENSIONS ON ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1957 Dec 15;43(12):1027–1032. doi: 10.1073/pnas.43.12.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Annu Rev Biochem. 1975;44:147–159. doi: 10.1146/annurev.bi.44.070175.001051. [DOI] [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978 Sep 8;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- Gifford G. D. Mutation of an auxotrophic strain of Escherichia coli by high pressure oxygen. Biochem Biophys Res Commun. 1968 Oct 24;33(2):294–298. doi: 10.1016/0006-291x(68)90783-3. [DOI] [PubMed] [Google Scholar]
- Green M. H., Muriel W. J. Mutagen testing using TRP+ reversion in Escherichia coli. Mutat Res. 1976 Feb;38(1):3–32. doi: 10.1016/0165-1161(76)90076-5. [DOI] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Enzymatic defenses against the toxicity of oxygen and of streptonigrin in Escherichia coli. J Bacteriol. 1977 Mar;129(3):1574–1583. doi: 10.1128/jb.129.3.1574-1583.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Intracellular production of superoxide radical and of hydrogen peroxide by redox active compounds. Arch Biochem Biophys. 1979 Sep;196(2):385–395. doi: 10.1016/0003-9861(79)90289-3. [DOI] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Paraquat and Escherichia coli. Mechanism of production of extracellular superoxide radical. J Biol Chem. 1979 Nov 10;254(21):10846–10852. [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Physiological function of superoxide dismutase in glucose-limited chemostat cultures of Escherichia coli. J Bacteriol. 1977 May;130(2):805–811. doi: 10.1128/jb.130.2.805-811.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Regulation of the synthesis of superoxide dismutase in Escherichia coli. Induction by methyl viologen. J Biol Chem. 1977 Nov 10;252(21):7667–7672. [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Superoxide radical and the oxygen enhancement of the toxicity of paraquat in Escherichia coli. J Biol Chem. 1978 Nov 25;253(22):8143–8148. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lesko S. A., Lorentzen R. J., Ts'o P. O. Role of superoxide in deoxyribonucleic acid strand scission. Biochemistry. 1980 Jun 24;19(13):3023–3028. doi: 10.1021/bi00554a029. [DOI] [PubMed] [Google Scholar]
- Lorentzen R. J., Lesko S. A., McDonald K., Ts'o P. O. Toxicity of metabolic benzo(a)pyrenediones to cultured cells and the dependence upon molecular oxygen. Cancer Res. 1979 Aug;39(8):3194–3198. [PubMed] [Google Scholar]
- Lorentzen R. J., Ts'o P. O. Benzo[a]yrenedione/benzo[a]pyrenediol oxidation-reduction couples and the generation of reactive reduced molecular oxygen. Biochemistry. 1977 Apr 5;16(7):1467–1473. doi: 10.1021/bi00626a035. [DOI] [PubMed] [Google Scholar]
- Lown J. W., Begleiter A., Johnson D., Morgan A. R. Studies related to antitumor antibiotics. Part V. Reactions of mitomycin C with DNA examined by ethidium fluorescence assay. Can J Biochem. 1976 Feb;54(2):110–119. doi: 10.1139/o76-018. [DOI] [PubMed] [Google Scholar]
- Massie H. R., Samis H. V., Baird M. B. The kinetics of degradation of DNA and RNA by H 2 O 2 . Biochim Biophys Acta. 1972 Jul 31;272(4):539–548. doi: 10.1016/0005-2787(72)90509-6. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Day E. D., Jr Superoxide-dependent production of hydroxyl radical catalyzed by iron-EDTA complex. FEBS Lett. 1978 Feb 1;86(1):139–142. doi: 10.1016/0014-5793(78)80116-1. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Rhaese H. J., Freese E. Chemical analysis of DNA alterations. I. Base liberation and backbone breakage of DNA and oligodeoxyadenylic acid induced by hydrogen peroxide and hydroxylamine. Biochim Biophys Acta. 1968 Feb 26;155(2):476–490. [PubMed] [Google Scholar]
- Sharma R. C., Yamamoto O. Base modification in adult animal liver DNA and similarity to radiation-induced base modification. Biochem Biophys Res Commun. 1980 Sep 30;96(2):662–671. doi: 10.1016/0006-291x(80)91406-0. [DOI] [PubMed] [Google Scholar]
- Smith K. C., Hays J. E. The response of uracil-2-14C to x-irradiation under nitrogen and oxygen and to treatment with ascorbic acid. Radiat Res. 1968 Jan;33(1):129–141. [PubMed] [Google Scholar]
- Taube H. Mechanisms of oxidation with oxygen. J Gen Physiol. 1965 Sep;49(1 Suppl):29–52. doi: 10.1085/jgp.49.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzman S. A., Stossel T. P. Mutation caused by human phagocytes. Science. 1981 May 1;212(4494):546–547. doi: 10.1126/science.6259738. [DOI] [PubMed] [Google Scholar]
- van Hemmen J. J. 6-amino-8-hydroxy-7,8-dihydro-purine: radiation product of adenine. Nat New Biol. 1971 May 19;231(20):79–80. doi: 10.1038/newbio231079a0. [DOI] [PubMed] [Google Scholar]


