Abstract
Objective: Health-related quality of life (HRQOL) is often much reduced among individuals with multiple sclerosis (MS), and incidences of depression, fatigue, and anxiety are high. We examined effects of a mindfulness-based intervention (MBI) compared to usual care (UC) upon HRQOL, depression, and fatigue among adults with relapsing-remitting or secondary progressive MS.
Methods: A total of 150 patients were randomly assigned to the intervention (n = 76) or to UC (n = 74). MBI consisted of a structured 8-week program of mindfulness training. Assessments were made at baseline, postintervention, and 6 months follow-up. Primary outcomes included disease-specific and disease-aspecific HRQOL, depression, and fatigue. Anxiety, personal goal attainment, and adherence to homework were secondary outcomes.
Results: Attrition was low in the intervention group (5%) and attendance rate high (92%). Employing intention-to-treat analysis, MBI, compared with UC, improved nonphysical dimensions of primary outcomes at postintervention and follow-up (p < 0.002); effect sizes, 0.4–0.9 posttreatment and 0.3–0.5 at follow-up. When analyses were repeated among subgroups with clinically relevant levels of preintervention depression, fatigue, or anxiety, postintervention and follow-up effects remained significant and effect sizes were larger than for the total sample.
Conclusions: In addition to evidence of improved HRQOL and well-being, these findings demonstrate broad feasibility and acceptance of, as well as satisfaction and adherence with, a program of mindfulness training for patients with MS. The results may also have treatment implications for other chronic disorders that diminish HRQOL.
Classification of evidence: This trial provides Class III evidence that MBI compared with UC improved HRQOL, fatigue, and depression up to 6 months postintervention.
Keywords: ANCOVA = analysis of covariance; CES-D = Center for Epidemiologic Studies Depression Scale; CI = confidence interval; EDSS = Expanded Disability Status Scale; ES = effect size; HAQUAMS = Hamburg Quality of Life Questionnaire in Multiple Sclerosis; HRQOL = health-related quality of life; MBI = mindfulness-based intervention; MFIS = Modified Fatigue Impact Scale; MS = multiple sclerosis; NNT = number needed to treat; PQOLC = Profile of Health-Related Quality of Life in Chronic Disorders; PRO = patient-reported outcome; STAI = Spielberger Trait Anxiety Inventory; UC = usual care.
Multiple sclerosis (MS) is the most common nontraumatic neurologic disease among young adults, with a prevalence in Europe and the United States of 50–200 per 100,000.1 Alongside physical complaints, many patients with MS have substantially impaired health-related quality of life (HRQOL), depression, fatigue, and anxiety.2 Lifetime prevalence of depression among patients with MS is about 50%.3 At least 65% of patients with MS complain of fatigue, 15%–50% considering fatigue their most disabling symptom.4 Anxiety disorder is also common, with a point prevalence of 25%.5
HRQOL psychosocial impairment shows great variation among patients with MS and is, to an important degree, independent of extent of disease.6–10 Additionally, many disease-modifying drug regimens appear to produce modest or no improvements in HRQOL7,11–14 or even negative changes.15 Consequently, complementary treatments that may improve HRQOL are highly relevant to issues of overall efficacy of MS treatment.
Controlled trials of behavioral interventions among patients with MS have focused upon treatment of depression, fatigue, or anxiety in patients selected for high symptom severity.16–18 Improvements in well-being or HRQOL19 as primary aims among more broadly representative samples of patients with MS have been neglected, although HRQOL has gained increasing status as a metric of treatment effectiveness.11–14,20
We report a randomized controlled trial of a mindfulness-based, group intervention for enhancing HRQOL and alleviating symptoms of depression and fatigue among patients with MS, examining postintervention and 6-month follow-up effects. Evidence suggests the feasibility of positively influencing HRQOL in MS and other serious chronic disorders that impair well-being.
METHODS
Study sample.
A total of 164 patients with MS referred themselves after having received information via the outpatient neurology clinic of the University Hospital Basel, other physicians, or advertisements posted in the Swiss Multiple Sclerosis Society Bulletin. The study was conducted from February 2007 to March 2009.
Neurologists verified all patients for the following inclusion criteria: 1) diagnosis of relapsing-remitting (but no more than 2 exacerbations within the last year) or secondary progressive MS21 and 2) an Expanded Disability Status Scale (EDSS)22 score of ≤6 (no to moderately severe disability), with ≤1 step increase within the last year. Excluded were patients with reported or medically recorded diagnoses of current serious psychological disorders other than depression and anxiety syndromes, evidence of dementia as indicated by testing below the fifth percentile in at least 3 of 6 dimensions of neuropsychological functioning (i.e., attention and concentration, processing speed, executive function, verbal memory, and verbal processing), other currently life-threatening or severely disabling physical disorders, current MS exacerbation, symptomatic MS medication altered in the last 3 months, other disorders of the CNS, pregnancy, or inability to speak or read German.
Standard protocol approvals, registration, and patient consents.
The study was approved by the Ethics Commission of Both Basels and is registered at www.controlled-trials.com, ISRCTN21643919. All participants completed written informed consent.
Randomization.
Eligible patients completed all preintervention assessments before randomization. Randomization was conducted by the principal investigator who had no prior contact with patients and was fully blinded to all patient information, except study identification number of patients. A random-event generator (www.randomizer.org) was employed, using blocks of 4–6. A list was then prepared and sent to the coordinator who informed all patients in writing of their assignment. The allocation procedure was subsequently checked by the principal investigator to ensure accurate assignment, and no deviations were found. All patient-reported outcome (PRO) measures were entered into a database by personnel blinded to group assignment.
Intervention.
MBI is based upon concepts of mental training that propose that nonjudgmental awareness of moment-to-moment experience (i.e., mindfulness) may positively affect accuracy of perception, acceptance of intractable health-related changes, realistic sense of control, and appreciation of available life experiences.23,24
MBI closely followed the program of mindfulness-based stress reduction23 and included 1) a personal intake interview to define realistic goals of participants and establish personal rapport; 2) 8 weekly 2.5-hour classes in mindfulness practices (10–15 participants/group; exercises did not exceed patients' level of functioning); 3) one Saturday, 7-hour session at week 6; 4) homework assignments (approximately 40 minutes/day), emphasized as essential to success of the program; 5) a postintervention interview to evaluate personal experiences, goal attainment, and future maintenance of acquired skills. Each class covered specific exercises and topics within the context of mindfulness training, i.e., practices during lying, sitting, and dynamic yoga postures, as well as during everyday life, e.g., stressful situations and social interactions. Mindfulness exercises included observation of sensory, affective, and cognitive domains of perceptible experience. The all-day retreat integrated familiar exercises and presented new ones. In all, 6 MBI courses for the experimental arm of the study were individually conducted by 2 experienced, certified teachers, each with >9 years teaching experience.
Usual care group.
All patients in the usual care (UC) group (and in the MBI group) received regular, currently optimal medical care during the duration of the study, as provided by the neurology department of the hospital. This included one medical examination at preintervention and another at 6 months postintervention, with additional measures as individually required. UC patients were offered the MBI 6 months after conclusion of the treatment period.
Assessment.
Primary outcomes of HRQOL, depression, and fatigue were validated PRO measures, administered at preintervention, postintervention, and 6-month follow-up. Secondary measures included anxiety, perceived personal goal attainment after MBI, and self-reported homework adherence. Reported outcome measurements occurred within 2 weeks before and after intervention and 6 months postintervention.
The following instruments were used (see appendix e-1 on the Neurology® Web site at www.neurology.org for details).
HRQOL.
HRQOL was assessed with 2 inventories, one developed for patients with chronic disorders (i.e., disease-aspecific) and the other specifically for patients with MS. The German-language Profile of Health-Related Quality of Life in Chronic Disorders (PQOLC)25 was employed as disease-aspecific measure (6 subscales: functional status, ability to relax and enjoy life, negative affect, positive affect, social functioning, and sense of belonging). It shows high sensitivity to change.25,26
Disease-specific HRQOL was assessed with the German version of the Hamburg Quality of Life Questionnaire in Multiple Sclerosis (HAQUAMS; 5 subscales: fatigue/thinking, lower limb mobility, upper limb mobility, social communication, and mood, also published in English).27 Well-validated, it exhibits low to moderate sensitivity to change.27,28 According to the a priori analysis plan, composite averages of subscales of each of the 2 HRQOL inventories were employed as primary outcome measures (see Analysis). Two subscales specifically evaluated disease-related physical limb mobility impairment. Because we did not hypothesize that MBI would influence disease, we employed the sum of the other subscales as the measure of HRQOL, and separately report findings for the limb mobility subscales.
Depression, fatigue, and anxiety.
Depression was measured with the Center for Epidemiologic Studies Depression Scale (CES-D)29; 3) fatigue, with the Modified Fatigue Impact Scale (MFIS)30; and 4) anxiety, with the Spielberger Trait Anxiety Inventory (STAI).31 Internal reliabilities of all scales were similar at preintervention (Cronbach α, 0.89–0.96).
Neuropsychological assessment (see appendix e-1)32 was administered at preintervention and 6-month follow-up by the attending neurologist, and employed a battery of tests to assess 1) short-term verbal memory and delayed recall, 2) attention, 3) information processing speed, 4) verbal fluency, and 5) cognitive interference and inhibitory control.
At postintervention interview, MBI participants completed a personal goal-attainment questionnaire that assessed the degree to which preintervention goals had been achieved on an 11-point scale (−5 [unmet] to + 5 [far beyond expectation]); personal goals varied from general (e.g., cope better with daily life) to specific (e.g., sleep better). MBI participants also completed a postintervention questionnaire regarding adherence to type, frequency, and duration of homework exercises performed during the previous 4 weeks.
Analysis.
Intention-to-treat analyses are reported. Data of missing study patients were imputed by linear multiple regression that adjusted for age, gender, and disease progression (entry EDSS score; STATISTICA 6.0). Statistical power calculation of this study was based on earlier studies.24,26 With 4 primary outcome measures (the 2 HRQOL scales, depression, and fatigue), a minimal sample size of 70 patients/group was required, based on an effect size (ES) of 0.53 and power = 0.8.
HRQOL measures were derived by averaging PQOLC and HAQUAMS subscale scores at each timepoint. Seven patients began psychopharmacologic treatment between postintervention and follow-up, and this variable (presence or absence) was included as covariate in all analyses.
Because preintervention primary outcome measures were intercorrelated (absolute r = 0.64–0.87), a repeated-measures multivariate analysis of covariance (ANCOVA) was first performed with a grouping factor (MBI vs UC) and 2 repeated measures (3 outcomes and 2 timepoints) for change from preintervention to postintervention and preintervention to 6-month-follow-up.
To examine effects upon individual outcomes, planned contrasts were performed for each dependent variable within and between each timepoint. For individual outcomes that showed tendencies to differ at preintervention (p < 0.2), ANCOVAs were performed, with preintervention values entered as additional covariates, and adjusted mean changes presented for each group. Significance level was α = 0.05.
Secondary analyses of variance or ANCOVAs were performed on all subscales of the respective HRQOL inventories to evaluate which dimensions of HRQOL were influenced by the intervention. ES was also calculated for each outcome (group difference between preintervention to postintervention score divided by pooled preintervention SD).33 Number needed to treat (NNT)34 was calculated from the criteria of minimally important differences achieving ≥10% improvement of outcome scale range35 (e.g., ≥10-point improvement on outcome measure with a 100-point range). It represents the number of patients needed to treat in order for one patient to show improvement.
Possible influences of gender, disease-modifying drugs, or EDSS disease stage were examined by creating a second grouping variable and repeating all analyses. These grouping variables were made by dichotomizing patients into males vs females, patients with vs without current disease-modifying medication, or patients with less vs more MS disability, defined as EDSS ≤3.0 vs those with EDSS >3.0. Also, correlational analyses within the MBI group examined associations between outcome change and preintervention neuropsychological status.
Depending upon the measure, 25%–30% of participants showed very low scores on depression, fatigue, or anxiety, as well as high levels of HRQOL. Consequently, floor or ceiling effects were present that serve to blunt extent of effects for the total group. Therefore, secondary analyses were also repeated employing subgroups in which established clinical cutoff points were employed for depression (score ≥16),29 fatigue (score ≥37),36 and anxiety (score ≥43).37 These may be informative to examine effects upon clinically relevant subgroups. Subgroups for the HRQOL outcomes reported, on average, being at least somewhat impaired.
Findings are also presented for homework adherence and goal attainment for the MBI group.
Classification of evidence.
This trial provides Class III evidence whether MBI, compared with UC, improves HRQOL, fatigue, and depression up to 6 months postintervention.
RESULTS
Sample selection and attrition.
A total of 164 patients self-referred to the study. Eleven were excluded and 3 declined to participate (figure 1). The remaining 150 patients were randomized. Due to the small preintervention refusal rate, no comparisons were made between refusers and participants. Female: male ratio was 119:31, indicating oversampling of women given the 70:30 population estimate of MS.
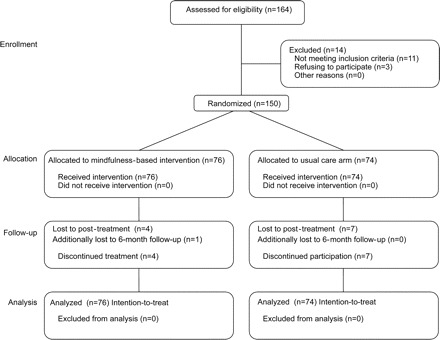
Figure 1 Flow of participants
Four of 76 patients dropped out of the MBI course and did not complete postintervention inventories (5%). Two reported a loss of interest, one disease-related problems, and one no reason. All remaining MBI participants (72 patients) were present for at least 6 of the 9 sessions (average attendance rate, 92% of all sessions) and completed all outcome measures. Seven patients in the UC group did not complete the postintervention phase inventories (9%) for unknown reasons. Due to the small attrition rate, no comparisons were made between dropouts and completers.
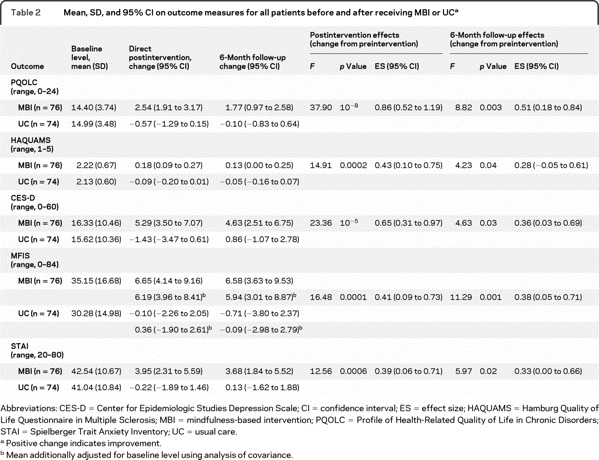
Table 1 presents baseline sociodemographic and clinical characteristics. There were no significant differences between groups. There were no baseline differences; only MFIS showed a tendency to differ between groups (p = 0.06; see table 2).
Table 1 Baseline sociodemographic and clinical characteristics by intervention status
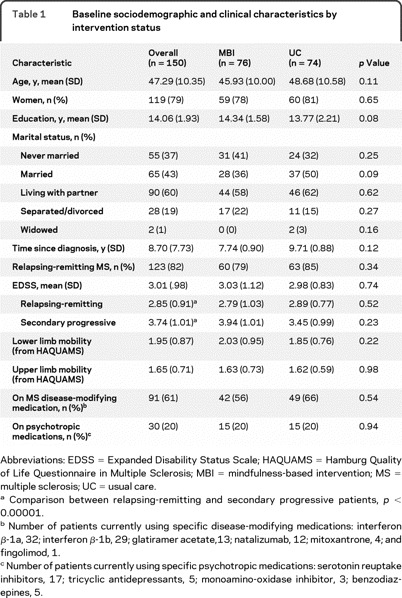
Table 2 Mean, SD, and 95% CI on outcome measures for all patients before and after receiving MBI or UC
Outcomes.
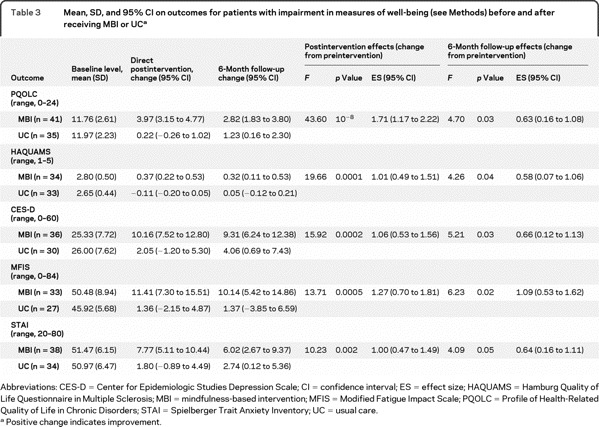
Preintervention scores are presented for the entire sample in table 2 and for impaired subgroups in table 3. Imputed means and 95% confidence intervals (CIs) were not substantially or significantly different from completer analyses (results not presented).
Table 3 Mean, SD, and 95% CI on outcomes for patients with impairment in measures of well-being (see Methods) before and after receiving MBI or UC
Primary outcomes.
Multivariate ANCOVA revealed differences between groups for primary outcomes; the MBI group showed greater improvement across outcomes (F1,147 = 27.07, p < 10−5). A group × timepoint effect was also found (Wilks [1,147] = 0.96, p < 0.02), indicating that group differences were larger at postintervention than follow-up (figure 2). Planned comparisons showed significant differences between groups for all primary outcomes at both timepoints (figure 2). However, benefits in PQOLC and CES-D were reduced from postintervention to 6-month follow-up (p < 0.03). Univariate outcomes are presented in table 2. Parallel to ESs, NNT ranged from 2.5 to 6.4, postintervention; 5.8 to 8.2, 6-month follow-up. At postintervention, all subscales of the PQOLC showed greater improvements among MBI participants in contrast to the UC group (p < 0.001), whereas negative affect and sense of belonging were no longer significant at 6-month follow-up (other p < 0.05). MBI participants manifested greater improvements on HAQUAMS subscales of fatigue/thinking and mood (p ≤ 0.05) at both endpoints. Change in HAQUAMS subscales of lower and upper limb mobility did not differ between groups (p > 0.60).
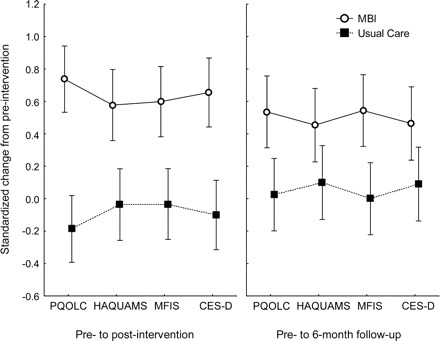
Figure 2 Primary outcome changes from preintervention
Standardized change scores (adjusted means) for primary outcomes from preintervention levels; whiskers, ± 95% confidence intervals. Positive scores indicate better well-being for all measures. A constant was added to each point (0.3) in order to enhance interpretation of the finding (i.e., so that the zero level approximately reflects no change). CES-D = Center for Epidemiologic Studies Depression Scale; HAQUAMS = Hamburg Quality of Life Questionnaire in Multiple Sclerosis; MBI = mindfulness-based intervention; MFIS = Modified Fatigue Impact Scale; PQOLC = Profile of Health-Related Quality of Life in Chronic Disorders.
Indication of clinically significant levels of depression, fatigue, or anxiety was found for 40%–44% of patients; 62% of all participants showed evidence for at least one. When subgroups with evidence of impaired well-being were analyzed (table 3), differences between intervention groups remained significant despite reduced sample size (NNTs ranged from 1.6 to 3.3 at postintervention; 3.8 to 5.6 at 6-month follow-up).
Secondary outcomes.
Improvements in anxiety scores (STAI) showed similar results as primary outcomes (table 2). MBI participants significantly improved more than the UC group at both timepoints. When subgroup analysis of clinically elevated anxiety was performed (table 3), MBI participants showed improvement at both timepoints.
Analyses of primary and secondary outcomes were also performed entering gender, treatment vs nontreatment of disease-modifying agents, or EDSS level (0–3 vs >3) as a second grouping variable (in addition to intervention group). There were no main or interaction effects upon any measure for gender, disease-modifying drug, or EDSS level, indicating no moderating influence of any of these factors (data not shown). Baseline neuropsychological scores did not correlate with MBI or UC changes from preintervention at either timepoint.
MBI participants reported practicing, on average, 5.11 times/week (SD 2.24; CI 4.59–5.64 times), a total time average of 76% of assigned homework (SD 13.80; CI 72.76%–79.25%). Amount of practice correlated with improvements in PQOLC, depression, and fatigue (r = 0.25–0.31; p < 0.04).
Mean postintervention goal attainment scale score was 1.78 (SD 1.2; CI 1.49–2.07 points), indicating that MBI participants generally perceived greater benefits than expected. Only 2 participants scored slightly in the negative range (≥−0.67 points). No adverse events or side effects were reported.
DISCUSSION
This randomized trial provides evidence that MBI may improve HRQOL and other measures of well-being for at least 8 months among mild to moderately severely impaired patients with MS not preselected for depression, fatigue, or other psychosocial problems. Effects of intervention were unrelated to gender, degree of impairment (i.e., EDSS level), or presence or absence of disease-modifying medication. Also, baseline neuropsychological status was not related to outcome, suggesting that MBI is appropriate for many patients with MS. Furthermore, attrition particularly in the intervention arm was extremely low, and MBI attendance rate high, although many patients traveled substantial distances to attend sessions (often hours), and mobility problems frequently made travel difficult. Furthermore, almost all MBI patients reported high goal satisfaction. In addition to efficacy of MBI, such positive response may reflect a strong desire among patients for treatments complementary to medical management, to enhance coping with consequences of MS.
Patients with MS must endure unpredictability of disease throughout their lives, in terms of exacerbations or worsening of symptoms, and the emotional, social, professional, recreational, and physical costs of the disease.6–10 Although 6-month follow-up benefits remained significant, especially for fatigue, slippage of effects did occur for disease-aspecific HRQOL and depressive symptoms and may indicate that the 2-month intervention requires supplementation by regular booster sessions in order to maintain gains. Future research should address this issue.
Several previous controlled trials of cognitive-behavioral interventions have shown benefits upon depression, fatigue, or anxiety in patients with MS.19,38,39 However, these and other studies have often used individual therapy, and findings have mainly focused upon single dimensions of well-being, e.g., depression or fatigue. Our results provide evidence of benefits across a broad range of parameters of well-being, achievable by means of a cost-effective and relatively brief group intervention. Additionally, programs successful at maintaining long-term psychosocial improvement may help to evaluate whether parameters of well-being meaningfully contribute to disease-related physiologic processes (e.g., immune function) in MS and other diseases involving immunologic dysfunction.40
Disease-modifying treatments do not necessarily have beneficial impact upon HRQOL or coping responses.7,11–13 Consequently there is increasing recognition that assessment of HRQOL, in addition to measurement of disease progress, should play a role in efficacy trials.2 Behavioral treatments are available that enhance the well-being of patients with MS and others with debilitating long-lasting conditions. Therefore, such interventions might importantly serve to complement disease-modifying benefits of medical therapies. MBI may have particular potential, because it is not aimed at a single disease or specific dimensions of well-being.
Several limitations of this investigation require mention. First, UC was the control arm and did not include an active intervention intended to improve primary outcome measures; thus the specificity of MBI was not evaluated. Effects may have been influenced by nonspecific aspects, e.g., general social support, motivational factors, or placebo effects. Secondly, our findings are based upon PROs, because no validated objective measures exist for well-being. Self-report assessments are susceptible to various response biases, e.g., social desirability and memory effects. Nevertheless, absence of group differences in HAQUAMS subscales of physical mobility mitigates the likelihood of serious response bias in this study. Along another line, the mobility findings indicate that MBI did not seem to benefit physical functioning. However, future investigation should address more objective indices of well-being, e.g., independent ratings, real-life event-sampling of PROs, or daily activity. Additionally, if mindfulness training is specifically responsible for the effects of treatment, the mechanisms by which MBI achieves these benefits remain unclear, whether enhancement of sense of control and accuracy of perception, or increased tolerance, acceptance, patience, and courage to deal with unpredictable life events.
This investigation suggests the significance and potential success of interventions aimed toward ameliorating fundamental aspects of psychosocial and existential distress of chronically ill patients, which may be incompletely addressed by currently available medical management programs.
ACKNOWLEDGMENT
The authors thank the patients with MS for their participation and Barbara Dietz-Waschkowski, Ulrike Kesper-Grossman, and Susanne Pueschel for their instruction of MBI.
DISCLOSURE
Dr. Grossman has received research support from the Swiss National Science Foundation, the Swiss Multiple Sclerosis Foundation, the Stanley T. Johnson Foundation, and the Swiss Cancer League, Sanofi-Aventis, Merck Serono, and Biogen Dompé AG. Dr Grossman had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Kappos serves on the editorial board of the International MS Journal; receives research support from the Swiss National Research Foundation, the Swiss MS Society, and the Gianni Rubatto Foundation (Zurich); has served on scientific advisory boards and his Department at the University Hospital Basel; and has received research support from Acorda Therapeutics Inc., Actelion Pharmaceuticals Ltd., Abbott, AstraZeneca, Bayhill Therapeutics, Bayer Schering Pharma, Biogen Idec, Boehringer Ingelheim, Centocor Ortho Biotech Inc., Eisai Inc., Genzyme Corporation, GlaxoSmithKline, The Immune Response Corporation, MediciNova, Inc., Neurocrine Biosciences, Novartis, Sanofi-Aventis, Merck Serono, Roche, Teva Pharmaceutical Industries Ltd., UCB, and Wyeth. Dr. Gensicke reports no disclosures. Dr. D'Souza has received funding for travel from Bayer Schering Pharma. Dr. Mohr receives research support from the NIH (R01-MH059708 [PI], R01-HD043323 [Co-PI], and R34 MH078922 [PI]) and from the US Veterans Administration HSR&D. Dr. Penner has served on scientific advisory boards for Novartis and Bayer Schering Pharma; and has received funding for travel and speaker honoraria from Bayer Schering Pharma, Novartis, Biogen Idec, Merck Serono, and Teva Pharmaceutical Industries Ltd./Sanofi-Aventis. C. Steiner reports no disclosures.
Supplementary Material
Footnotes
Editorial, page 1130
Supplemental data at www.neurology.org
Study funding: Supported by the Swiss National Science Foundation (3200B0-112604), the Stanley T. Johnson Foundation, the Swiss Multiple Sclerosis Foundation, Sanofi-Aventis, Merck Serono, and Biogen-Dompé AG (all to P.G.).
Disclosure: Author disclosures are provided at the end of the article.
Received January 4, 2010. Accepted in final form May 17, 2010.
REFERENCES
- 1.Compston A, Confavreux C. The distribution of multiple sclerosis. In: Compston A, ed. McAlpine's Multiple Sclerosis, 4th ed. London: Churchill Livingstone Elsevier; 2005:71–112.
- 2.Foley JF, Brandes DW. Redefining functionality and treatment efficacy in multiple sclerosis. Neurology 2009;72:S1–S11. [DOI] [PubMed] [Google Scholar]
- 3.Siegert RJ, Abernethy DA. Depression in multiple sclerosis: a review. J Neurol Neurosurg Psychiatry 2005;76:469–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hadjimichael O, Vollmer T, Oleen-Burkey M. Fatigue characteristics in multiple sclerosis: the North American Research Committee on Multiple Sclerosis (NARCOMS) survey. Health Qual Life Outcomes 2008;6:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feinstein A, O'Connor P, Gray T, Feinstein K. The effects of anxiety on psychiatric morbidity in patients with multiple sclerosis. Mult Scler 1999;5:323–326. [DOI] [PubMed] [Google Scholar]
- 6.Ford HL, Gerry E, Johnson MH, Tennant A. Health status and quality of life of people with multiple sclerosis. Disabil Rehabil 2001;23:516–521. [DOI] [PubMed] [Google Scholar]
- 7.Haupts M, Elias G, Hardt C, et al. [Quality of life in patients with remitting-relapsing multiple sclerosis in Germany.] Nervenarzt 2003;74:144–150. [DOI] [PubMed] [Google Scholar]
- 8.Murphy N, Confavreux C, Haas J, et al. Quality of life in multiple sclerosis in France, Germany, and the United Kingdom: Cost of Multiple Sclerosis Study Group. J Neurol Neurosurg Psychiatry 1998;65:460–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koch M, Uyttenboogaart M, van Harten A, Heerings M, De Keyser J. Fatigue, depression and progression in multiple sclerosis. Mult Scler 2008;14:815–822. [DOI] [PubMed] [Google Scholar]
- 10.Kern S, Schrempf W, Schneider H, Schultheiss T, Reichmann H, Ziemssen T. Neurological disability, psychological distress, and health-related quality of life in MS patients within the first three years after diagnosis. Mult Scler 2009;15:752–758. [DOI] [PubMed] [Google Scholar]
- 11.Putzki N, Fischer J, Gottwald K, et al. Quality of life in 1000 patients with early relapsing-remitting multiple sclerosis. Eur J Neurol 2009;16:713–720. [DOI] [PubMed] [Google Scholar]
- 12.Vermersch P, de Seze J, Delisse B, Lemaire S, Stojkovic T. Quality of life in multiple sclerosis: influence of interferon-beta1a (Avonex) treatment. Mult Scler 2002;8:377–381. [DOI] [PubMed] [Google Scholar]
- 13.Zivadinov R, Zorzon M, Tommasi MA, et al. A longitudinal study of quality of life and side effects in patients with multiple sclerosis treated with interferon beta-1a. J Neurol Sci 2003;216:113–118. [DOI] [PubMed] [Google Scholar]
- 14.Rudick RA, Miller D, Hass S, et al. Health-related quality of life in multiple sclerosis: effects of natalizumab. Ann Neurol 2007;62:335–346. [DOI] [PubMed] [Google Scholar]
- 15.Simone IL, Ceccarelli A, Tortorella C, et al. Influence of Interferon beta treatment on quality of life in multiple sclerosis patients. Health Qual Life Outcomes 2006;4:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohr DC, Boudewyn AC, Goodkin DE, Bostrom A, Epstein L. Comparative outcomes for individual cognitive-behavior therapy, supportive-expressive group psychotherapy, and sertraline for the treatment of depression in multiple sclerosis. J Consult Clin Psychol 2001;69:942–949. [PubMed] [Google Scholar]
- 17.Crawford JD, McIvor GP. Group psychotherapy: benefits in multiple sclerosis. Arch Phys Med Rehabil 1985;66:810–813. [PubMed] [Google Scholar]
- 18.Tesar N, Baumhackl U, Kopp M, Gunther V. Effects of psychological group therapy in patients with multiple sclerosis. Acta Neurol Scand 2003;107:394–399. [DOI] [PubMed] [Google Scholar]
- 19.Hart S, Fonareva I, Merluzzi N, Mohr DC. Treatment for depression and its relationship to improvement in quality of life and psychological well-being in multiple sclerosis patients. Qual Life Res 2005;14:695–703. [DOI] [PubMed] [Google Scholar]
- 20.Seid M, Varni JW, Segall D, Kurtin PS. Health-related quality of life as a predictor of pediatric healthcare costs: a two-year prospective cohort analysis. Health Qual Life Outcomes 2004;2:48. [DOI] [PMC free article] [PubMed]
- 21.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol 2001;50:121–127. [DOI] [PubMed] [Google Scholar]
- 22.Kurtzke JF. Disability rating scales in multiple sclerosis. Ann NY Acad Sci 1984;436:347–360. [DOI] [PubMed] [Google Scholar]
- 23.Kabat-Zinn J. Full Catastrophe Living: Using the Wisdom of Your Body and Mind to Face Stress, Pain and Illness. New York: Delacorte; 1990
- 24.Grossman P, Niemann L, Schmidt S, Walach H. Mindfulness-based stress reduction and health benefits: a meta-analysis. J Psychosom Res 2004;57:35–43. [DOI] [PubMed] [Google Scholar]
- 25.Siegrist J, Broer M, Junge A. [Profile of Quality of Life for the Chronically Ill: Manual.] Göttingen: Beltz Test; 1996
- 26.Grossman P, Tiefenthaler U, Raysz A, Kesper U. Mindfulness training as an intervention for fibromyalgia: evidence of postintervention and 3-year follow-up benefits in well-being. Psychother Psychosom 2007;76:226–233. [DOI] [PubMed] [Google Scholar]
- 27.Gold SM, Heesen C, Schulz H, et al. Disease specific quality of life instruments in multiple sclerosis: validation of the Hamburg Quality of Life Questionnaire in Multiple Sclerosis (HAQUAMS). Mult Scler 2001;7:119–130. [DOI] [PubMed] [Google Scholar]
- 28.Gold SM, Schulz H, Stein H, Solf K, Schulz KH, Heesen C. Responsiveness of patient-based and external rating scales in multiple sclerosis: head-to-head comparison in three clinical settings. J Neurol Sci 2009;16:16. [DOI] [PubMed] [Google Scholar]
- 29.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401.
- 30.Ritvo PG, Fischer JS, Miller DM, Andrews H, Paty DW, LaRocca NG. Multiple Sclerosis Quality of Life Inventory: A User's Manual. New York: National Multiple Sclerosis Society; 1997
- 31.Spielberger CD, Gorsuch RL. Manual for the State-Trait Anxiety Inventory. New York: Consulting Psychologists Press; 1970
- 32.Calabrese P, Kalbe EJK. The Multiple Sclerosis Inventarium Cognition (MUSIC). Psychoneurol 2004;30:384–388. [Google Scholar]
- 33.Cohen J. Statistical Power Analysis for the Behavioral Sciences. New York: Academic Press; 1988
- 34.Walter S. Number needed to treat (NNT): estimation of a measure of clinical benefit. Stat Med 2001;20:3947–3962. [DOI] [PubMed]
- 35.Osoba D, Bezjak A, Brundage M, Zee B, Tu D, Pater J. Analysis and interpretation of health-related quality-of-life data from clinical trials: basic approach of The National Cancer Institute of Canada Clinical Trials Group. Eur J Cancer 2005;41:280–287. [DOI] [PubMed] [Google Scholar]
- 36.Flachenecker P, Kumpfel T, Kallmann B, et al. Fatigue in multiple sclerosis: a comparison of different rating scales and correlation to clinical parameters. Mult Scler 2002;8:523–526. [DOI] [PubMed] [Google Scholar]
- 37.Stark D, Kiely M, Smith A, Velikova G, House A, Selby P. Anxiety disorders in cancer patients: their nature, associations, and relation to quality of life. J Clin Oncol 2002;20:3137–3148. [DOI] [PubMed]
- 38.Mohr DC, Hart S, Vella L. Reduction in disability in a randomized controlled trial of telephone-administered cognitive-behavioral therapy. Health Psychol 2007;26:554–563. [DOI] [PubMed] [Google Scholar]
- 39.van Kessel K, Moss-Morris R, Willoughby E, Chalder T, Johnson MH, Robinson E. A randomized controlled trial of cognitive behavior therapy for multiple sclerosis fatigue. Psychosom Med 2008;70:205–213. [DOI] [PubMed] [Google Scholar]
- 40.Kern S, Ziemssen T. Brain-immune communication psychoneuroimmunology of multiple sclerosis. Mult Scler 2008;14:6–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


