Abstract
Lens regeneration among vertebrates is basically restricted to some amphibians. The most notable cases are the ones that occur in premetamorphic frogs and in adult newts. Frogs and newts regenerate their lens in very different ways. In frogs the lens is regenerated by transdifferentiation of the cornea and is limited only to a time before metamorphosis. On the other hand, regeneration in newts is mediated by transdifferentiation of the pigment epithelial cells of the dorsal iris and is possible in adult animals as well. Thus, the study of both systems could provide important information about the process. Molecular tools have been developed in frogs and recently also in newts. Thus, the process has been studied at the molecular and cellular levels. A synthesis describing both systems was long due. In this review we describe the process in both Xenopus and the newt. The known molecular mechanisms are described and compared.
1. Background
Among vertebrates the ability of regenerating the eye lens is restricted only to frogs and newts. Such regeneration is also remarkable because the lens is regenerated by transdifferentiation of terminally differentiated somatic cells. Regeneration of the lens in adult newts was observed first by Collucci in 1891 and independently by Wolff in 1895, after whom the process is often called Wolffian regeneration. The process of lens regeneration in frogs was characterized more recently by Freeman (1963). There are, however, some basic differences between regeneration in frogs and newts. Frogs can normally regenerate the lens during a certain period before metamorphosis and the source of the regenerated lens is the cornea. On the other hand, lens regeneration in newts occurs throughout their adult life and is mediated via transdifferentiation of the dorsal iris pigment epithelial cells (PECs). A few other organisms can apparently replace the lens via Wollfian lens regeneration, including cobitid fish (Sato, 1961; Mitashov, 1966). In this review we will outline and compare these processes mainly in both amphibians with a focus on the molecular and cellular aspects. In recent years, advances in molecular technology and manipulation in these animals has led to important understanding of the process and its relevance to regenerative biology in general, and positions these models as excellent systems in which to undertake further studies.
2. LENS REGENERATION IN Xenopus
2.1. Morphological events
Though not as extensive as the forms of regeneration displayed by certain urodele amphibians (e.g., newts and salamanders), some species of anurans (frogs) exhibit the capacity to regenerate certain structures such as the intestine, larval tail (including the spinal cord, and muscles), limb structures, and even parts of the eye (including the retina, and lens; reviewed by Henry, 2003; Slack et al., 2004, 2008; Callery, 2006; Tseng and Levin, 2008; Henry et al., 2008; Beck et al., 2009; Filoni, 2009). The most widely studied species are African frogs of the genus Xenopus. One species in particular, Xenopus laevis, has been used for over 60 years as an experimental model system in studies of development and regeneration (Kay and Peng, 1991; Tinsley and Kobel, 1996; Gurdon and Hopwood, 2000; Beck and Slack, 2001; Henry, 2003; Callery 2006; Henry et al., 2008). More recently, many researchers have adopted a related species, X. tropicalis. The latter has certain advantages that include a shorter generation time and X. tropicalis is a true diploid, in contrast to the allotetraploid condition found in X. laevis. These features and the smaller genome size favored the selection of X. tropicalis for genome sequencing, which was been completed by the U.S. Department of Energy’s Joint Genome Institute (http://genome.jgi-psf.org/Xentr4/Xentra4.home.html). Given the recent advances in high-throughput sequencing technology, an effort is being mounted to sequence the genome of X. laevis (http://www.xenbase.org/common/), which will serve as an additional resource for molecular level studies, like those described below.
Species in the genus Xenopus are the only frogs known to regenerate lenses (reviewed by Henry, 2003; Filoni 2009). Lens regeneration in X. laevis was first described by Freeman (1963) and differs significantly from that of Wolffian lens regeneration, which has been well described for newts and salamanders (discussed below). Following removal of the original lens in Xenopus tadpoles, the inner layer of the outer corneal epithelium undergoes a series of transformations to re-form a new lens. Freeman sub-divided this process into 5 stages, which are illustrated in Figure 1. This process has been described as one of transdifferentiation (metaplasia) of the corneal epithelium (though see further discussion, below), and shares many similarities to that of embryonic lens formation, but there are also some interesting differences (Henry, 2003; discussed below). Lens formation typically occurs in the cornea epithelium that lies directly over the pupillary opening and requires secreted factors provided by the neural retina (Freeman, 1963; Filoni et al., 1981, 1982, 1983). Under normal circumstances, the physical presence of the inner cornea endothelium (derived from neural crest) and the lens prevent these factors from reaching the cornea epithelium (Freeman, 1963; Filoni et al., 1997; Henry and Elkins, 2001). Removal of either barrier alone will not initiate lens regeneration (Bosco et al., 1980; Filoni et al., 1980; Cioni et al., 1982). Furthermore, experiments reveal that the lens and inner cornea do not produce inhibitors of lens regeneration. For instance, regeneration of a new lens will occur in the presence of the original lens provided an opening is created that allows for retinal factors to reach the corneal epithelium (Reeve and Wild, 1978; Filoni et al., 1978, 1980; Bosco et al., 1979, 1980). The process of lens regeneration can be recapitulated in culture (Bosco et al., 1993, 1994, 1997a,b). For example, transdifferentiation will occur in co-cultures of cornea epithelial and neural retina tissues (Figure 2D). In fact, the efficiency is nearly as good, or even better than that which occurs in vivo. This represents a very convenient system in which to study this process under a more controlled set of conditions. Direct contact between the cornea epithelium and the neural retina does not appear to be required for lens regeneration to take place, as lens cell differentiation will occur in cultures of corneal epithelium grown in retina-conditioned media (Bosco et al., 1997a). Together, these findings indicate that the retinal tissue provides diffusible factors sufficient to support lens regeneration.
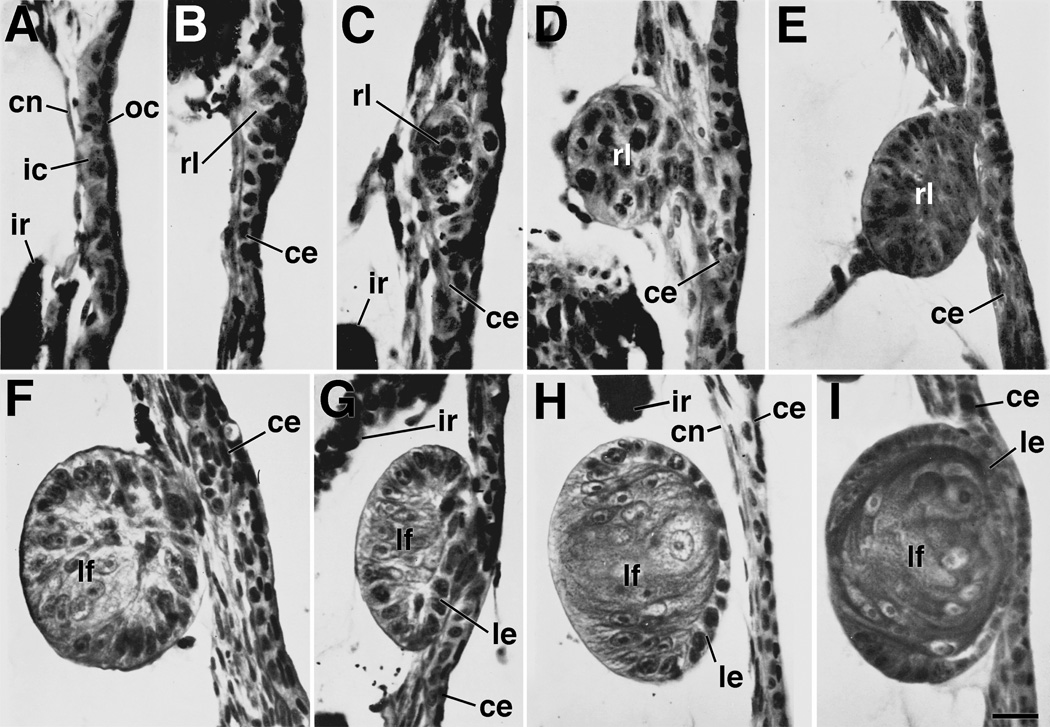
Figure 1.
Cornea-lens transdifferentiation in X. laevis. Sections show different stages of lens regeneration following removal of the lens (stages follow the convention of Freeman,1963). A. Stage 1. During this stage cells of the inner corneal epithelium assume a cuboidal shape within 24 hours following lens removal. B. Stage 2. During this stage, cells begin to assume a thickened placodal arrangement. Nuclei of these cells are found to contain one nucleolus (characteristic of lens epithelial cells), rather than two (characteristic of cornea epithelial cells). C. Early stage 3. D. Middle stage 3. E. Late stage 3. During stage 3, a loosely organized aggregate of cells begins to separate from the corneal epithelium. These cells begin to organize with distinct apical-basal polarity to form a lens vesicle. F. Early stage 4. G. Middle stage 4. H. Late stage 4. During stage 4 a definitive lens vesicle is present. Elongated primary lens fiber cells have formed on the side closest to the vitreous chamber. Typically the lens vesicle is separated from the overlying corneal epithelium at this stage. I. Early stage 5. During this stage, secondary lens fiber cells are being added from the lens epithelium at the equatorial zone. Nuclei of the primary fiber cells begin to disappear. This stage is followed by further addition of secondary fiber cells and growth of the lens. ce, corneal epithelium; cn, corneal endothelium; ic, inner layer of the corneal epithelium; ir, iris; oc, outer layer of the corneal epithelium; le, lens epithelium; lf, lens fibers; rl, regenerating lens vesicle. Refer to text for further details. Figure from Henry (2003, after Freeman, 1963). Scale bar equals 25µm.
Figure 2.
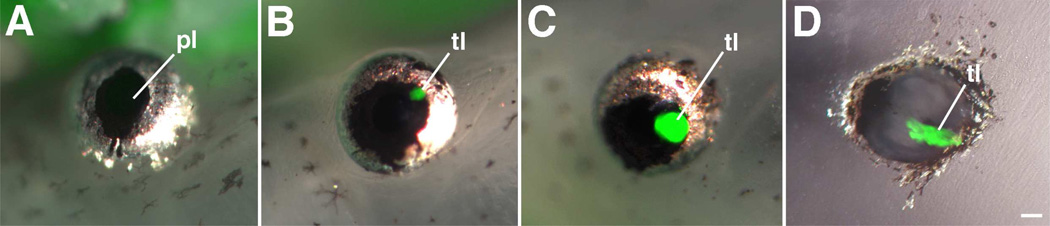
In vivo and in vitro lens regeneration in living transgenic X. tropicalis and X. laevis larval tissues carrying a transgene encoding the jellyfish green fluorescent protein (GFP) coupled to a γ-crystallin enhancer/promoter. Combined light and epifluorescence micrographs show expression of GFP (green) in the regenerating lenses of these examples. A. Eye of transgenic X. tropicalis following removal of the lens. Note absence of differentiated lens cells and GFP in the eye at one week following lens removal. B. Eye at 10 days following lens removal. Note the presence of a small regenerating lens containing GFP expressing cells. C. Eye at 21 days following lens removal. Note the growth of a larger regenerating lens containing GFP expressing cells. D. In vitro transdifferentiation in co-cultures of transgenic X, laevis corneal epithelium and non-transgenic larval eye cup. Note GFP expressing lens cells derived from the transgenic corneal epithelium. pl, pupil; tl, transdifferentiating lens cells. A–C from Henry et al. (2008, after Henry and Elkins, 2001). D, from L. Fukui and J. Henry (unpublished data, University of Illinois, Champaign-Urbana). Scale bar equals 200µm.
Through a variety of tissue transplantation experiments, it has been shown that only the larval cornea and the pericorneal epidermis, which includes a combined area twice the diameter of the eyecup, has the potential to form lens cells in X. laevis (Freeman 1963). The competence of this larval tissue to respond to these signals is established by developmental history, which includes the initial series of early and late embryonic lens inductive interactions and the presence of the eyecup (Bosco and Filoni 1992; Cannata et al., 2003; Arresta et al., 2005). Once competence is established, the continued presence of the eyecup does not appear to be required to maintain this condition (Cannata et al., 2003), since the eye can be removed late during embryogenesis or from the older larvae and the overlying tissues will still retain the ability to respond if transplanted into the vitreous chamber.
The success and extent of lens regeneration gradually decreases as the larvae approach metamorphosis (Freeman, 1963, Filoni et al., 1997). This appears to be due to the increasing rapidity with which the inner corneal endothelium heals back to cover the pupillary opening, and may also be related to the extent of differentiation of the cornea epithelium. Experimentally, one can demonstrate that the capacity to differentiate lens cells is still present in the corneal epithelium throughout this time interval and apparently even after metamorphosis, if one exposes this tissue to the key retinal factors via transplantation into the vitreous chamber (Freeman and Overton, 1962; Filoni et al., 1997). Likewise, the factors provided by the neural retina are still present in adult eyes (Bosco et al., 1992). Researchers have demonstrated that corneal epithelium of postmetamorphic frogs can undergo transdifferentiation, if implanted into the vitreous chamber of either pre- or post-metamorphic frogs (Freeman and Overton, 1961; Bosco and Willems, 1992; Bosco et al., 1992; Filoni et al., 1997). More recently, Yoshii et al. (2007) observed examples of lens regeneration in adult Xenopus and suggested that these may arise from small numbers of lens epithelial cells that remain in the eye following incomplete removal of the original lens (though no attempt to trace these cells was made in that study to prove this claim).
X. tropicalis is also capable of regenerating lenses though the success rate is much lower than that found in X. laevis (Figure 2A–C; Henry and Elkins, 2001). In the former species the inner corneal endothelium heals more rapidly compared to that in X. laevis, and this tends to cut off the critical signaling factors required to support lens regeneration. Another species, X. borealis does not normally regenerate a lens following lentectomy, due to the rapidity with which the inner cornea heals back to cover the pupillary opening (Filoni et al., 2006). On the other hand, the corneal epithelium of X. borealis is capable of forming a lens if implanted directly into the vitreous chamber where it can be continuously exposed to the key inducing factors. In contrast, the corneal epithelium of other frog species, (e.g., members of the genera Rana, Bufo, Hyla and Disscoglossus) do not appear to have the competence to respond to these inductive signals; however, it appears that the eyes of all frog species examined do produce the factors required to support regeneration, as established in xenoplastic (cross-species) transplantation experiments (reviewed by Henry and Elkins, 2001; Henry, 2003 and Filoni et al., 2006).
As mentioned above, the new lens normally arises from the tissue that lies directly over the pupillary opening. This suggests that the signals that trigger lens regeneration are either short ranged and/or the response is concentration dependant. The initial observations made by Freemen (1963) indicate that lens-forming transformations initially occur at multiple foci within this region (each reaching stages 1–2, refer to Figure 1 for description of these stages), however, only one of these foci eventually forms a lens. The other foci appear to regress. Rarely does one see the formation of multiple lenses. This suggests that some mechanism may operate to ensure that only one lens is ultimately formed, possibly involving some form of lateral inhibition (see further discussion below).
2.2. Molecular Aspects of Lens Regeneration in Xenopus
Patterns of crystallin expression have been characterized during lens regeneration in X. laevis and some interesting differences have been found compared to the situation encountered during embryonic lens formation. The use of antibodies reveals that α- and β-crystallin proteins are detected in both fiber cells and lens epithelial cells beginning at Freeman stage 4 of lens regeneration, while γ-crystallin proteins are apparently restricted only to lens fiber cells (Campbell, 1965, Brahma and McDevitt, 1974; Campbell and Truman, 1977, Reeve and Wild, 1978; Brahma, 1980; Henry and Mittleman, 1995). During embryonic lens development translation of various crystallins, however, is restricted to lens fiber cells, beginning around stage 29/30 of development (stages of Nieuwkoop and Faber, 1956). The transcription of certain crystallin genes has also been examined during lens regeneration (Schaefer et al., 1999; Mizuno et al., 1999a, Malloch et al., 2009). Again, some interesting differences have been observed compared to the patterns of expression detected during embryonic stages. For instance, αA-, and βB1-crystallin transcripts are first detected in presumptive lens fiber cells of the regenerated lens vesicle (middle to late Freeman stage 3), and subsequently only in differentiated lens fiber cells at later stages. γ-crystallin transcripts are not detected until early Freeman stage 4 of regeneration and only in lens fiber cells. However, during embryogenesis, αA-βB1- and γ-crystallin transcripts are detected simultaneously in the lens placode, and only in differentiated lens fiber cells at later stages of development. These finding indicate that there are some differences in the regulation of crystallin expression during regeneration vs. development of the lens. In contrast, Mizuno et al. (2005) demonstrated that the expression of βB1-crystallin during lens regeneration requires the same promotor elements as those required during embryonic lens development, suggesting that elements of a shared regulatory network appear to be operating in both of these lens-forming processes.
A large number of transcription factors play key roles in eye and lens development (Beebe, 1994; Wride, 1996; Cvekl an Piatigorsky, 1996; Graw, 1996; 2003; Oliver and Gruss, 1997; Tomarev, 1997; Fini et a., 1997; Jean et al., 1998; Kondoh, 1999; Ogino and Yasuda, 2000; Wawersik and Mass, 2000; Hanson, 2001; Chow and Lang, 2001; Zhang et al., 2002; Graw and Loster, 2003; Zuber et al., 2003; Zaghloul et al., 2005; Lupo et al., 2006; Adlar and Canto-Soler, 2007; Cvekl and Duncan, 2007), including Otx2, Pax6, Sox3 Pitx3, and Prox1 as well as others. For instance, Otx2 encodes an orthodenticle-related transcription factor that contains a bicoid class DNA-binding homeodomain. Otx2 acts as a “head-field selector” and is required for the development of the forebrain and midbrain (Chow and Lang, 2001, Ogino et al., 2008). Otx2 is expressed early in embryonic head ectoderm, including the presumptive lens ectoderm (Pannese et al., 1995; Kablar et al., 1996; Zygar et al., 1998), and is required for the formation of the lens and retinal pigmented epithelium in mice (Martinez-Morales et al., 2001). Otx2 has recently been shown to play an important role in regulating Notch induced FoxE3 expression (a forkhead transcription factor related to Xenopus lens1), which is essential for lens formation (Kenyon et al., 1999; Ogino et al., 2008). Pax6 represents a central transcriptional regulator of eye development, containing two separate DNA binding domains that include a homeodomain and a paired box (Ashery-Pedan and Gruss, 2001). Pax6 is expressed early during development in a relatively broad area of anterior embryonic ectoderm and plays specific roles in eye development and lens cell differentiation, including the regulation of crystallin gene expression (Pannese et al., 1995; Kablar et al., 1996; Cvekl and Piatigorsky, 1996; Zyger et al., 1998; Schaefer et al., 1999; Cui et al., 2004). As is the case in other organisms, ectopic expression of Pax6 leads to the formation of ectopic eyes and lenses in Xenopus (Altmann et al., 1997; Chow et a., 1999). Sox3 is a member of the SOX family of SRY testis determining factor family of HMG box transcription factors known to play important roles in eye development (Penzel et al., 1997; Furuta and Hogan, 1998; Zyger et al., 1998; Ashery-Pedan et al., 2000; Koster et al., 2000). Sox proteins play roles in regulating crystallin expression (Uwanogho et al., 1995; Kamachi et al., 1995, 1998, 2001; Nishiguchi et al., 1998; Chow an Lang, 2001; Ishibashi and Yasuda, 2001). Pitx3 is a paired-like (bicoid) homeodomain containing transcription factor expressed early in presumptive and placodal lens ectoderm (Chang et al., 2001; Pommereit et al., 2001). Pitx3 and a related gene Pitx1 are required for normal lens and retinal development (Semina et al., 2000; Khosrowshahian et al., 2005; Shi et al., 2005; Medina-Martinez et al., 2009). Another gene, Prox1 encodes a prospero-like transcription factor, which is initially expressed in the lens placode (Oliver et al., 1993, Tomarev et al., 1996, 1998; Schaefer et al., 1999; Chow and Lang, 2001; Reza et al., 2002). Prox1 is important for lens fiber cell differentiation (Wigle et al., 1999) and serves to activate crystallin expression (Cvekl and Piatigorsky, 1996; Ring et al., 2000).
Significantly, Otx2, Pax6, Sox3, and prox1 are all expressed during lens regeneration in Xenopus (Schaeffer et al., 1999; Mizuno et al., 1999b; Cannata et al., 2003). Furthermore, Henry et al. (2002) showed that Pax6 is expressed in larval corneal epithelium prior to removal of the lens. Following lens removal, pax6 expression appears to be up-regulated in the cornea and regenerating lens (Schaefer et al., 1999; Mizuno et al., 1999b; Cannata et al., 2003). Gargioli et al. (2008) examined the expression of otx2, Pax6, Sox3, pitx3, prox1 and βB1-cry prior to lens removal in Xenopus. These investigators noted that only Pax6 is expressed in the larval ectoderm, and further, its expression is restricted to the lentogenic area that includes both the corneal and pericorneal ectoderm. They further demonstrated that injections of exogenous Pax6 in early embryonic cells (at the 2-cell stage) trigger ectopic, endogenous Pax6 expression. Presumably this occurred through auto-regulation of Pax6 expression, though they did not actually demonstrate that the injected, exogenous message was translated. This treatment, however, was apparently sufficient to impart lentogenic capacity in flank epidermis, as assayed by transplanting this tissue inside the vitreous chamber. These findings suggest that pax6 expression is linked with the acquisition of lens regeneration competence in larval ectoderm. Other researchers have described patterns of Pax6 expression as being linked with embryonic lens-forming competence in other vertebrate systems (e.g., chicken and rat, Li et al., 1994; Fujiwara et al., 1994).
In order to further characterize the molecular level events that underlie the process of lens regeneration in Xenopus laevis, a subtracted cDNA library enriched for genes expressed during the first four days of this process was prepared (Freeman stages 1–3; Henry et al., 2002; Malloch et al., 2009). Representative clones were sequenced and the information was submitted for BLAST analysis to characterize gene expression during the early stages of lens regeneration. A searchable database of information pertaining to each of these sequences is available on-line at www.life.illinois.edu/henry/EST_databases.html. A total of 734 unique genes were identified. The assemblage of genes is typical of those associated with metazoan organismal development and includes a high concentration of genes involved in regulating transcription and signal transduction. Groups with more prevalent representation include transcription factors, proteins involved in RNA synthesis and processing, integral membrane and transmembrane proteins, components of a number of conserved signaling pathways, proteins involved in protein processing, transport and degradation, proteins involved in matrix remodeling including a number of matrix metalloproteases (MMPs), regulators of the cell cycle, metabolism, apoptosis, inflammation and immune responses, proteins involved in chromatin remodeling in addition to known lens proteins (e.g., crystallins).
Of the transcription factors identified in this set, a number have not been previously implicated in the process of lens formation; though some, including eyes-absent homolog 2 and Prox1, are clearly important for eye/lens formation (Malloch et al., 2009). On the other hand, elements of several signal transduction cascades are also present, which have previously been shown to play important roles in lens development, regeneration and differentiation, including the Bone Morphogenetic Protein (BMP), Delta-Notch, Wnt, FGF, Hedgehog, TGF-beta, Rho-Ras and retinoic acid signaling pathways (Manns and Fritzsch, 1991; Hyuga et al., 1993; Kastner et al., 1994; Bosco et al., 1994, 1997b; Kodama and Eguchi, 1995; Graw 1996; Cvekl and Piatogorsky, 1996; Li et al., 1997; McDevitt et al., 1997; Del Rio Tsonis et al., 1997; 1998; Gopal-Srivastava et al., 1998; Enwright and Grainger, 2000; Wagner et al., 2000; Tsonis et al., 2000, 2002; 2004; de Inogh et al., 2001; Onuma et al., 2002; Kawamorita et al., 2002; Stump et al., 2003; Beebe et al., 2004; Ang, et al., 2004; Lang, 2004; Lupo et al., 2005; Lovicu and McAvoy, 2005; Grogg et al., 2005, 2006 ; Chen et al., 2008).
Expression patterns for most (703) of the genes identified by Malloch et al. (2009) were examined during embryonic development. 634 of these exhibited some embryonic expression, Significantly, 57.7% of the latter are expressed in developing eye tissues and nearly half (46.8%) included the developing lens. These findings indicate that there are significant similarities, as well as some differences, between the processes of lens development and lens regeneration. Some of the differences may be associated with the processes of wound healing, as well as cellular de-differentiation that may be associated with lens regeneration (Carinato et al., 2000; Henry et al., 2002; Malloch et al., 2009). It will be important to decipher the roles of those genes that only appear to be expressed in regenerating lenses.
Comparison of the genes expressed during lens regeneration in Xenopus with those expressed in other regenerating tissues/organisms, did not reveal a substantial conserved core of genes that appear to underlie different regenerative phenomena (Malloch et al., 2009). There are, however, some commonalities. For instance, MMPs appear to be expressed in a number of different regenerating tissues, including regenerating lenses (Yang an Bryant, 1994, Miyazaki et al., 1996; Yang et al., 1999; Kerif et al., 1999; Carinato, et al., 2000; Kato et al., 2003; Vinarsky et al., 2005; Odelberg, 2005; Malloch et al., 2009). In addition, elements of the stress, inflammation and immune response pathways also appear to be expressed in many regenerating systems, including Xenopus lens regeneration (Lu and Richardson, 1991; Meyer-Franke et al., 1998; Leon et al., 2000; Patruno et al., 2001; Imokawa and Brockes, 2003; Harty et al., 2003; Imokawa et al., 2004; Mescher and Neff, 2005; Makino et al., 2005; Kanao and Miyachi, 2006; Brockes and Kumar, 2006; Filbin 2006; Godwin and Brockes, 2006; Mescher et al., 2007; Pearl et al., 2008; Malloch, et al., 2009). These researchers propose conflicting hypotheses as to whether stress, inflammation and immune response pathways stimulate or inhibit regeneration.
2.3. Signaling Factors Involved in Xenopus Lens Regeneration
The inductive signals that trigger lens regeneration have not yet been identified. There is some evidence, however, that they may be similar to those that are responsible for embryonic lens induction (Henry and Mittleman, 1995; Cannata et al., 2003, 2008; Arresta et al., 2005). Studies indicate that members of the FGF family and specific BMPs (BMP4, and BMP7) play key roles in eye and lens development (Furuta and Hogen, 1998; Belecky-Adams et al., 2002; Chow and Lang, 2001; Yang, 2004; Esteve and Bovolenta, 2006; Cvekel and Duncan, 2006; Wordinger and Clark, 2007; Adler and Canto-Soler, 2007).
Using cultures of isolated larval corneal epithelium, Bosco et al., 1994, 1997b; demonstrated that addition of FGF1 appears to be sufficient to trigger lens cell differentiation. Furthermore, Arresta et al. (2005) found that a commercial polyclonal antibody that recognizes the FGFR2 receptor (bek variant) labels stage 53 larval cornea epithelium but not dorsal head or flank epidermis (stages of Nieuwkoop and Faber, 1956). In addition, this antibody was found to label dorsal head epidermis if this was previously subjected to eyes implanted at stage 46 of larval development. These observations link FGFR2 expression with the acquisition of lentogenic capacity, and provide further evidence that FGF signaling may be involved in lens regeneration in Xenopus. The Henry lab (University of Illinois, Urbana-Champaign) also has several lines of evidence that FGF signaling is functionally required in vivo for lens regeneration in Xenopus laevis (Fukui and Henry, unpublished data).
In another set of studies, Dr. Caroline Beck’s lab (University of Otago, New Zealand) has shown that over-expression of Noggin in transgenic Xenopus larvae inhibits lens regeneration, suggesting that elements of the TGF-β signaling pathways, specifically those involving the Bone Morphogenetic Proteins (BMPs) play an important role in this process (Caroline W. Beck, personal communication).
As mentioned above, the early observations of Freeman (1963) suggest that multiple, small populations of cells scattered over the pupillary opening begin to undergo transdifferentiation (reaching Freeman stages 1–2) but only one of these foci appears to form the news lens. An element of the Delta-Notch signaling pathway known to play roles in lateral inhibition and boundaries-inductive mechanisms (Artavanis-Tsakonas, et al., 1999; Schweisguth, 2004; Gazave et al., 2009) is expressed during lens regeneration (including Mind bomb homolog 1, which encodes a E3 ubiquitin protein ligase known to regulate Delta-mediated Notch signaling, Malloch et al., 2009). Perhaps Delta-Notch signaling plays a role in regulating the development of these foci. As argued above, some mechanism may operate to ensure that only a single lens is ultimately regenerated. A similar mechanism could also function in the context of embryonic lens development given that a larger field of competent tissue is established early during development than actually generates the developing lens. As mentioned above, direct contact between the cornea epithelium and the retina is not required for lens regeneration, and this might preclude the involvement of delta-notch signaling in that particular interaction.
3. Studies of Lens Regeneration and Development in Xenopus
As a model vertebrate system, Xenopus offers significant advantages for the study of regeneration, which have been extolled in a number of recent reviews (Slack et al., 2004, 2008; Callery, 2006; Tseng and Levin, 2008; Henry et al., 2008; Beck et al., 2009; Filoni, 2009). Tremendous molecular and genomic resources exist for this system (Klein et al., 2002, 2006; Carruthers and Stemple, 2006). Likewise, Xenopus has been used widely in the study of eye development (Henry et al., 2008). Maintenance of a colony of adult frogs is rather simple and inexpensive, and the adults provide an abundant supply of embryonic material, which is available year-round. Furthermore, the large eggs and embryos develop externally and rapidly. In addition, a vast array of molecular tools are available to facilitate studies of gene function in these animals. As previously mentioned, these include the availability of a sequenced genome (http://genome.jgipsf.org/Xentr4/Xentra4.home.html). The X. tropicalis genome has a high number of regions with long stretches of synteny with mammalian genomes (each typically a hundred or more genes). Hence, it is evolutionarily well positioned to identify conserved elements in vertebrate genomes (e.g. Ogino et al., 2008).
It is a relatively simple matter to carry out both gain- and loss-of-function analyses to examine the necessity and sufficiency of specific genes. For example morpholinos may be used to knockdown translation of specific messages (Ekker, 2000; Corey and Abrams, 2001; Nutt et al., 2001; Heasman, 2002; Gene Tools, LLC, Philomath, OR; www.gene-tools.com). Affymetrix produces micro-arrays to study gene expression in both X. laevis and X. tropicalis (www.affymetrix.com). A reverse genetics/TILLING resource (Stemple, 2004) at the Sanger Centre (Cambridge, UK) has recently led to the identification of the first null mutation in the Rx gene (a key homeobox regulator of eye formation, S. Carruthers, D. Stemple and R. Grainger. University of Virgina, Unpublished, see also Mathers et al., 1997) and other mutations are in the initial stages of characterization.
The relative ease with which one can prepare transgenic frogs is a tremendous benefit and facilitates molecular functional studies at more advanced stages of larval development, which is essential for studying regenerative phenomenon (Kroll and Amaya, 1996; Amaya and Kroll, 1999; Amaya et al. 1998; Huang et al., 1999; Marsh-Armstrong et al. 1999; Offield et al., 2000; Sparrow et al., 2000; Hirsch et al., 2002; Ogino et al., 2006; Pan et al., 2006; Slack et al., 2008: Beck et al., 2009). For instance, transgenic frogs carrying various genes under the control of the heat-shock (hsp70) promoter (see Wheeler et al., 2000) have been used to study tail and limb regeneration (Beck and Slack, 2001; Beck et al., 2003; 2006; Slack et al., 2004; Lin and Slack, 2008). Beck et al. (2003, 2006, see also Slack et al., 2004) investigated the activity of the BMP and Notch signaling pathways during spinal cord and muscle regeneration in the tail and limb, akin to the unpublished study by Beck’s lab that examined lens regeneration (as described above). Knocking down either BMP or Notch inhibited regeneration while activation of either promoted regeneration. These experiments showed further that BMP acts upstream of Notch in spinal cord regeneration, but these two pathways appeared to act independently in the case of muscle regeneration. Lin and Slack (2008) also used transgenic frogs to show that FGF and Wnt signaling are important in Xenopus tail regeneration by expressing the Wnt inhibitor dkk1 and a dominant negative (truncated) form of an FGF receptor under the control of the hsp70 heat-shock promoter.
Tissues isolated from transgenic frogs carrying a GFP reporter driven by the cytomegalovirus (CMV) promoter have been used in transplantation experiments to follow the fates of cells that contribute to regenerated tissues in the tail (Gargioli and Slack, 2004). This study demonstrated that notochord and spinal cord both regenerated from prexisting notochord and spinal cord stump tissues, respectively. However, muscles arose from a population of satellite “stem” cells, rather than from pre-existing myofibers. Chen et al. (2006) showed further that this population of satellite cells specifically express Pax7. Using a similar approach, Lin et al, (2007) showed that melanophores do not arise from a pre-existing population of unpigmented, melanophores precursors during tail regeneration. The cre/lox system has also been employed to generate transgenic frogs that express yellow fluorescent protein as a muscle specific lineage marker (under control of the cardiac actin promoter) to show that differentiated muscle cells do not undergo transdifferentiation to form other cell types during tail regeneration (Ryffel et al., 2003). Transgenic Xenopus tropicalis lines carrying a γ1-crystallin promoter driving lens GFP expression have proven to be very useful for the study of lens development and regeneration (Offield et al. 2000; Henry and Elkins, 2001, see Figure 2A–C). Labs, including our own, are now applying these transgenic approaches to study lens regeneration.
Finally, tremendous NIH-funded community web resources are available that provide valuable information on X. laevis and X. tropicalis biology and genomics (e.g, “Xenbase,” www.xenbase.org and see also “Xine,” http://blumberg.bio.uci.edu/xine/index.htm). A long-standing course taught at Cold Spring Harbor covers topics related to the cell and developmental biology of Xenopus, and the Embryology and Physiology Courses taught at the Marine Biological Laboratory also cover Xenopus biology, training future generations of scientists in the use of this system. These resources continue to position Xenopus as a key system for the study of regeneration and development for many years to come.
4. LENS REGENERATION IN NEWTS
4.1. Morphological events
In the case of the newt, lens regeneration involves transdifferentiation of the pigmented epithelial cells of the iris (PECs). Following removal of the lens, the PECs re-enter the cell cycle, dedifferentiate, and lose their characteristic pigmentation. In vivo, regeneration occurs from the dorsal iris population of PECs. Despite the apparent similarity between the dorsal and ventral PECs, the ability to regenerate through transdifferentiation under normal conditions, belongs exclusively to the dorsal PECs. Interestingly, however, culturing of both dorsal and ventral PECs results in lentoid body formation (Del Rio-Tsonis and Tsonis, 2003; Tsonis and Del Rio-Tsonis, 2004). This means that the ventral PECs have the potential for transdifferentiation, but this is not permited in vivo.
Early events of dedifferentiation occur through day 8 of the regeneration process. Cell proliferation is initiated by day 4 post-lentectomy. Visible dedifferentiation and loss of pigment from the PECs begins by day 8. By day 10 of the process, a lens vesicle has formed, which represents the precursor to what will become a fully differentiated lens. After the lens vesicle has formed, the posterior cells start to elongate, express crystallins and differentiate to lens fibers. The anterior cells become lens epithelium (Figure 3) (Eguchi, 1963; Eguchi, 1964).
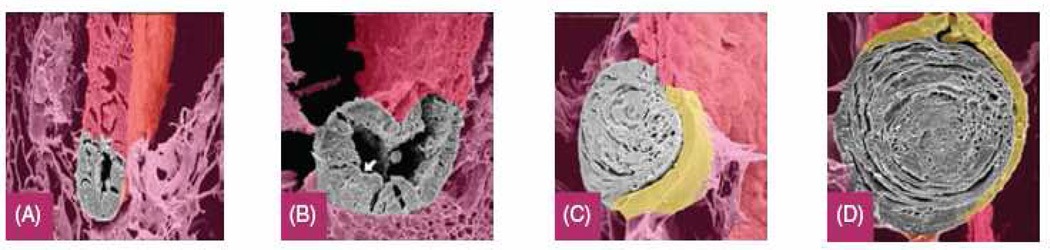
Figure 3.
The process of lens regeneration in newts. Lens formation from the dorsal iris during regeneration shown through scanning electron microscopy. (A) Day 10 after lentectomy, a lens vesicle forms at the dorsal margin of the iris. (B) Day 14, elongation and further lens development as differentiation of lens fibers occur at the posterior part (arrow) of the vesicle. (C) Day 20 of lens reformation. The anterior portion has become the lens epithelium (colored yellow). In contrast, lens fiber cells are forming in the posterior region. (D) Day 30, regeneration has concluded.
Once lens differentiation has started the process is remarkably similar to lens development (as far as the sequential appearance of the different crystallins is concerned). Indeed, studies using antibodies to crystallins or cDNA probes have conclusively shown that there is a parallel of their synthesis during developing and regenerating lens. For instance, αA, βB1 and γ-crystallins appeared all at the same time at the ventral-posterior portion of the lens vesicle (Sato stage IV–V; nearly 10–12 days postlentectomy (Yamada, 1977), with γ-crystallin (in contrast with the other two) being specific for the lens fibers and not the lens epithelial cells (Mizuno et al., 2002).
4.2. Gene Regulation
Focusing on the possible mechanisms of genetic regulation, it would only make sense to hypothesize that different genes are at play in the dorsal and ventral iris creating two very different populations of cells, one that regenerates and one that does not, respectively. There are of course, many genes and proteins to consider in the regeneration process. As previously mentioned, a master regulator gene in eye development, Pax-6 is found in both the dorsal and ventral iris during Wolffian lens regeneration (Del Rio-Tsonis et al., 1995; Madhavan et al., 2006). The same is true for Six-3, a partner of Pax-6 and by itself sufficient to induce lens when ectopically expressed in frogs and fish (Oliver et al, 1996; Altmann et al., 1997). Prox-1 is a transcriptional regulator of crystallin synthesis and is also expressed just before cells of the vesicle elongate and express crystallins (Del Rio-Tsonis et al., 1999). Interestingly these genes are also expressed in the ventral iris tissues. This finding was unexpected and it means that the ventral iris does initially undergo similar events as the dorsal iris In fact there may be a general inhibitory effect that prevents further regeneration from taking place.
To gain more insights about gene expression patterns, a microarray analysis with newt cDNA was utilized and it revealed that gene signatures in the dorsal and ventral iris are in fact very similar (Makarev et al., 2007). Even genes responsible for tissue remodeling, such as collagenase and cathepsin are present and up-regulated in both dorsal and ventral irises during regeneration. Surprisingly expression levels for some of these genes are even higher in the ventral iris. It is not unreasonable to suggest, given these unexpected results on gene expression, that we might encounter novel regulatory events during newt regenerative processes (Makarev et al., 2007). EST analysis from irises 8 days after lentectomy, when dedifferentiation of PECs is ongoing, has also revealed very important information. An initial set of 10449 cDNA sequence reads were obtained. The total length of these sequences was 9.85 Mbp that was reduced to 4.82 Mbp by assembling overlapping reads using CAP3 software (Huang and Madan, 1999). This assembly was composed of 4725 unique sequences (1368 contigs and 3357 singlets). Of 1219 contigs and 3357 singlets, 780 contigs and 1666 singlets were annotated by Blast2GO (Conesa et al., 2005). The 15 most frequently counted biological process terms, cellular component terms, and molecular functions were reported (Maki et al, 2010). It is interesting to note here that when compared with a same analysis performed during Xenopus lens regeneration the most frequent GO annotations were very similar (Table 1). GO (Gene Ontology) is used to describe the function of a gene as it pertains to processes and place in the cell.. There are 3 independent sets of ontologies, the molecular function (or molecular component, i.e. DNA binding), the biological process (i.e. transcription) and the cellular component (or compartment) where the gene product can be found (i.e. nucleus).
Table 1.
The 5 most frequent GO identified in Xenopus and Newt. In red are the common ones.
| Xenopus | Newt | |
|---|---|---|
| GO | Regulation of transcription, DNA dependent | Regulation of transcription, DNA dependent |
| Transcription | Signal transduction | |
| Biological Process | Transport | Ribosome biogenesis |
| Signal transduction | Translation | |
| Multicellular organismal development | Electron transport | |
| Nucleus | Cytoplasm | |
| Membrane | Nucleus | |
| Cellular Compartment | Cytoplasm | Integral to membrane |
| Integral to membrane | Transcription factor complex | |
| Intracellular | Extracellular space | |
| Protein binding | Protein binding | |
| Metal ion binding | ATP binding | |
| Molecular Component | DNA binding | Zinc ion binding |
| Nucleotide binding | RNA binding | |
| Zinc ion binding | DNA binding |
4.3. Signaling pathways in newt lens regeneration
A number of cell signaling pathways have been implicated in the process of lens regeneration in the newt. Members of the hedgehog signaling pathway are also expressed during the regeneration process; Sonic hedgehog (Shh) and Indian hedgehog (Ihh) only being expressed in the regenerating and developing lens, and no longer expressed once the lens is fully reformed. When one interferes with this signaling pathway, the overall regeneration process is inhibited. Also, cell proliferation rates are decreased and differentiation in the regenerating lens suffers (Tsonis et al., 2004). Retinoic acid receptors are also expressed in the lens during regeneration and their inhibition might account for aberrant lens formation (Tsonis et al., 2000; Tsonis et al., 2002).
Exogenous FGFs have been shown to elicit regeneration of a second lens from the dorsal iris (Del Rio-Tsonis et al., 1997; Hayashi et al, 2004). It has been suggested that this action of FGFs is mediated via an induction of cell proliferation. The FGF pathway seems to collaborate with the Wnt pathway. When explants of dorsal iris were treated with FGF2 in the presence of Wnt inhibitors, the action of FGF2 was inhibited. Combined addition of FGF2 and Wnt3a was in fact able to induce lens transdifferentiation of ventral explants as well (Hayashi et al., 2006).
Interestingly, research on another signaling pathway, the Bone Morphogenetic Protein pathway (BMP), revealed that its inhibition allows the ventral iris to transdifferentiate into lens. Importantly, such induction was also observed when ventral PECs were transfected with Six-3 and treated with retinoic acid (Grogg et al., 2005). These manipulations of signaling pathways and regulatory genes clearly indicates that induction of the ventral iris is possible and such observations might open new avenues in experimenting with higher animals that are unable to regenerate eye tissues.
4.4. MicroRNAs
MicroRNAs, or miRNAs are short RNAs, about 22 nucleotides long, which can bind to complementary sequences of RNA and subsequently prevent mRNA translation. miRNAs may have multiple binding sequences due to their short size and can block translation on a wide scale. As such they might be involved in the transition from one cell type to another as we see during regeneration. Cloning of newt miRNA from the eye has pinpointed differentiatial regulation in both dorsal and ventral iris. Some of the targets of the cloned miRNAs were predicted to be FGFR2, and SOX9 (for miR-124a), PAX3, chordin, and TGFβR1 for let7b (Makarev et al., 2006). Based on this, further study of the role of miRNA regulation in regeneration showed that members of the let7 family were found to be down-regulated in the regenerating dorsal iris. Examination of the ventral iris revealed that miR-148 is up-regulated in both intact and regenerating ventral iris when compared with dorsal counterparts (Tsonis et al., 2007). Thus, miRNAs might be very useful regulators of regenerative processes.
Our previous study on the expression of miRNAs during lens and hair cell regeneration in newts was the first to indicate a possible involvement and regulation. The present study shows that, indeed, miRNAs can have a role in regeneration. Other recent reports support this as well. During zebrafish fin regeneration it has been shown that miR-203 regulates the Wnt signaling pathway transcription factor Lef1. Down-regulation of Lef1 by miR-203 blocks regeneration, while loss of miR-203 results in up-regulation of Lef1 and fin overgrowth (Thatcher et al., 2008). In another study it was found that depletion of miR-133 promotes fin regeneration and that this is FGF-dependent (Yin et al., 2008). In a different study it was shown that miR-196 is involved in axolotl tail regeneration. Inhibition of miR-196 blocks regeneration by acting up-stream BMP4 and Pax-7 (Sehm et al., 2008).
4.5. Knock-down technology and gene expression in newts
Transgenesis and knockout technologies are not as developed in the newt when compared to Xenopus or even the axolotl (another salamander that can regenerate body parts such as tail and limb, but is unable to regenerate the lens). Nevertheless knock-down technology has been developed in the newt and it promises to shed more light to the mechanisms of regeneration. Application of such technology in the adult newt has been quite informative on the role of pax-6 in this process. When pax-6 expression is decreased in the eye, lens regeneration suffers dramatically due to the decreases in proliferation of the pigmented epithelial cells. When Pax-6 is knocked down during later regeneration events, crystallins are not made and lens fibers production is decreased (Figure 4) (Madhavan et al., 2006).
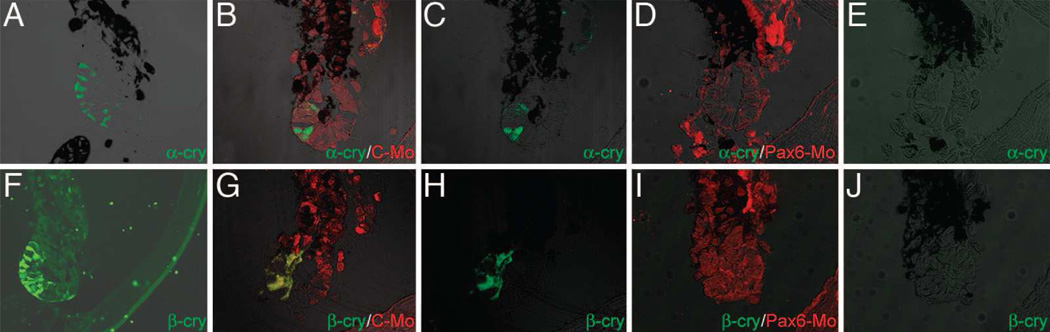
Figure 4.
Pax-6 morpholino treatment inhibits crystallin expression. Morpholinos were injected at day 10 after lentectomy, and animals were collected for assay at day 13 after lentectomy. (A and F) Lens vesicles from untreated control eyes express α- and β- crystallin (α-cry or β-cry). (B and G) Eyes treated with C-Mo also express α and β crystallin. (D and I) Lens vesicles from eyes treated with Pax6-Mo1 do not show any α- or β- crystallin expression. (C, E, H, and J) α- or β- crystallin staining only from B and D (C and E) and β- crystallin staining only from G and I (H and J).
4.6. Transdifferentiation in newts: A model for stem cell differentiation?
One could argue that during the process of lens regeneration the dedifferentiated cells become stem cell-like, going from a terminally differentiated state to an undifferentiated state and can then further differentiate into the cells needed to create a new lens. Such a hypothesis was first proposed in 2000, using the example of mesenchymal stem cell similarity to limb blastema cells (Tsonis, 2000; Tsonis, 2004). Similar processes may also occur during lens regeneration in the frog (Henry et al., 2003). Research in this area is being pursued, and some initial studies in the newt support this hypothesis. For example, the stem cell nuclear protein nucleostemin (also a cancer cell marker) is found highly expressed in the nucleus of undifferentiated cells, like pluripotent embryonic stem cells, as well as multipotent stem cells of the nervous system and primitive cells of the bone marrow (Maki et al., 2007). As the onset of differentiation of these cells occurs, accumulation of nucleostemin within the nucleus is shown to decrease. When dedifferentiated PECs were studied during regeneration, as compared to non-regenerating PECs, this stem cell nuclear protein was found to be highly expressed in both dorsal and ventral regenerating PECs. The amount of protein present decreased faster in the ventral PECs, and after lens differentiation the amount of nucleostemin also decreased in dorsal PECs (Maki et al., 2007).
Further studies were undertaken to characterize gene expression during lens regeneration in the newt. A list of the lens-regenerating iris ESTs, possible candidate genes for participating in nuclear regulation during newt dedifferentiation have been identified (Maki et al, 2010). This is similar to the types of genes expressed during lens regenerationin the frog (Malloch et al., 2009). Epigenetic regulation, a range of heritable chromatin modifications including histone modifications, DNA methylation and chromatin remodeling, play a pivotal role in the control of differentiation and maintenance of cellular identity. So it is expected that epigenetic regulation plays an important role during newt PEC dedifferentiation. Indeed, many factors involved in reprogramming were identified in the ESTs and interestingly were also found in newt ovarian tissues as well.
The expression patterns of these genes may be significant in the field of cellular reprogramming, stem cells and regeneration in general. While the newt does not reprogram its adult differentiated cells to form pluripotent progenitors, it does reprogram them to specific progenitor stem cell state according to their origin and to their contribution, as evidenced by their ability to regenerate with precision only those parts lost to injury (Kragl et al., 2009). Indeed, aggregates from dorsal iris PECs will transdifferentiate only to lens even if transplanted to regenerating limb (Ito et al, 1999; Tsonis, 2000). These data might suggest an underlying mechanism for this property in the newt.
Recently, it has been shown that differentiated mammalian cells, including human, can also be reprogrammed to become induced pluripotent stem cells (iPSCs) that can subsequently differentiate to different tissues. This reprogramming is mediated by inducing the expression of four factors, Oct4, Sox-2, c-myc and Klf4. Another factor, nanog, seems to be also important (Takahashi et al., 2006, 2007; Okita et al., 2007; Wernig et al., 2007). All these are transcriptional factors expressed in embryonic stem cells. The critical question that arises from these studies is: Does reprogramming that mediates the formation iPSCs share any similarities to reprogramming during regeneration in newts? A recent study examined expression of these stem cell pluripotency-maintaining factors during newt lens regeneration (Maki et al., 2009). Interestingly, there was significant regulation of three of the factors that we examined, Sox-2, Klf4 and c-myc. Oct4 and nanog were not detected in these tissues beyond the levels of negative control (-RT), however, they were expressed in control ovarian tissues (meaning that the newt does have these genes).
During lens regeneration, Sox2 and Klf4 were upregulated during the very early stages of regeneration (day 2), while c-myc showed a peak of expression at day 8. Day 2 marks an early response to lens removal and is expected to be characterized by events that may prepare pre-existing tissues for reprogramming and cell cycle re-entry. In fact, cell proliferation is not detected until day 4. Those rapid responses to lens removal prior to cell cycle re-entry are similar to those observed for nucleostemin (Maki et al., 2007). c-myc showed quite opposite patterns to Sox2 and Klf4. It was highly expressed at day 8, which correlated with the establishment of the lens vesicle, but without major differences between dorsal and ventral iris. It should be noted here that the regeneration-incompetent ventral iris does show activity such as cell cycle re-entry and gene expression, which must be suppressed later (Grogg et al., 2005; Madhavan et al., 2006). Therefore, expression differences between dorsal and ventral iris might be correlated with these events.
The specific stage-related regulation of Sox2, Klf4 and c-myc, and the absence of Oct4 and nanog expression might indicate why the newt cells do not become pluripotent. Manipulating these factors in the newt will open new avenues in the field and should be of enormous importance to compare or contrast the biology of regeneration in classical models and in stem cells.
5. Xenopus and Newt Lens Regeneration: Some Additional Questions
While there have been many advances in our understanding of the cellular and molecular mechanisms that control lens regeneration in Xenopus, and newts, a number of key questions still need to be addressed in these systems, in addition to those already raised above. The process of “transdifferentiation” is described as one that takes a population of differentiated cells though an initial process of cellular de-differentiation, followed by re-differentiation along an altered trajectory (e.g., Eguchi and Kodama, 1993; Burke and Tosh, 2005). While it is clear this does take place during Wolffian lens regeneration in the newt, investigators have questioned whether the process of lens regeneration in Xenopus actually involves de-differentiation. In fact, the cornea is not fully differentiated until after the process of metamorphosis is completed (Yamada, 1982, McDevitt and Brahma, 1982, Bosco, 1988, Henry 2003). The larval cornea and lens are originally derived from the same embryonic tissue, and the phenomenon of lens regeneration in Xenopus may simply represent a condition in which there is persistent competence of embryonic and larval ectoderm to respond to key lens inducing signals. Likewise, the precise relationships between embryonic lens development and lens regeneration, though apparently similar processes, is not entirely clear (see Schaefer et al., 1999; Mizuno et al., 1999a, 2005; Henry et al., 2002; Henry, 2003; Cannata et al., 2003; Malloch et al., 2009; Filoni, 2009; Mizuno et al., 2002). Answers to these questions will require careful molecular characterization and comparison of the genes expressed in these tissues and the changes that occur during the processes of lens regeneration and development.
It is unclear whether the larval cornea or the adult iris contains populations of specialized somatic stem cells. For instance, it is known in other vertebrates that there exists a population of limbal stem cells that supports repair of the cornea epithelium (Buck 1985; Cotseralis et al., 1989; Pellegrini et al., 1999; Lavker, et al., 1998; Auran et al., 1995; Huang and Tseng, 1995; Tseng and Sun 1999). We have looked for a population of slowly dividing stem cells (BrdU-retaining-cells) in Xenopus and have not found any evidence that these exist in the limbal region. Rather, these preliminary investigations revealed that dividing cells are seen scattered throughout the cornea epithelium (see also Freeman, 1963). Hence, it is not clear if there is a specialized subpopulation of stem cells within the cornea that support regeneration. Likewise it is not believed at the present time that the newt iris contains a stem cell population. Rather, all the evidence testifies for a clear case of transdifferentiation. However, more studies on cell populations using advanced techniques will eventually clear this issue.
Though the regenerated lenses appear to be normal on the basis of morphological and histological criteria, no attempt has been made to examine lens function or visual acuity following regeneration in either Xenopus or newts. In addition, it is unclear whether other associated structures are also fully regenerated, such as the muscles that reposition the lens, etc.
Finally, we do not understand what factors may prevent lens regeneration from occurring in other vertebrates, including humans. Do all vertebrates possess a similar conserved suite of genes that could be active in these processes? Some evidence suggests that there might be species specific differences that underlie some regenerative phenomena (e.g., limb regeneration in newts, Kumar et al., 2007; Brockes and Kumar, 2008; Garza-Garcia et al., 2010). In the case of lens regeneration, barriers may be present to ensure that abnormal, supernumerary lenses do not form within the eye that would prevent regeneration from occurring. This could involve the production of specific inhibitors of lens regeneration, or it is possible that key inductive signals are either not present or the receptors or downstream elements of the signal cascade are missing in eye tissues. Once we understand the factors required to support lens regeneration, and the reasons why some vertebrates are unable to regenerate these structures, it may be possible to “restore” this capacity, even in humans, as a therapeutic approach to treat injured or diseased lenses.
Acknowledgements
The authors’ research is supported by NIH/NEI grant EY09844 (JJH) and EY10540 (PAT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler R, Canto-Soler MV. Molecular mechanisms of optic vesicle development: Complexities, ambiguities and controversies. Developmental Biology. 2007;305:1–13. doi: 10.1016/j.ydbio.2007.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann CR, Chow RL, Lang RA, HemmatiBrivanlou A. Lens induction by Pax-6 in Xenopus laevis. Developmental Biology. 1997;185:119–123. doi: 10.1006/dbio.1997.8573. [DOI] [PubMed] [Google Scholar]
- Amaya E, Kroll KL. A method for generating transgenic frog embryos. Molecular Embryology. 1999;97:393–414. doi: 10.1385/1-59259-270-8:393. [DOI] [PubMed] [Google Scholar]
- Amaya E, Offield MF, Grainger RM. Frog genetics: Xenopus tropicalis jumps into the future. Trends in Genetics. 1998;14:253–255. doi: 10.1016/s0168-9525(98)01506-6. [DOI] [PubMed] [Google Scholar]
- Ang SJ, Stump RJW, Lovicu FJ, McAvoy JW. Spatial and temporal expression of Wnt and Dickkopf genes during murine lens development. Gene Expression Patterns. 2004;4:289–295. doi: 10.1016/j.modgep.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Arresta E, Bernardini S, Gargioli C, Filoni S, Cannata SM. Lens-forming competence in the epidermis of Xenopus laevis during development. Journal of Experimental Zoology Part a-Comparative Experimental Biology. 2005;303A:1–12. doi: 10.1002/jez.a.138. [DOI] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: Cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- Ashery-Padan R, Gruss P. Pax6 lights-up the way for eye development. Current Opinion in Cell Biology. 2001;13:706–714. doi: 10.1016/s0955-0674(00)00274-x. [DOI] [PubMed] [Google Scholar]
- Ashery-Padan R, Marquardt T, Zhou XL, Gruss P. Pax6 activity in the lens primordium is required for lens formation and for correct placement of a single retina in the eye. Genes & Development. 2000;14:2701–2711. doi: 10.1101/gad.184000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auran JD, Koester CJ, Kleiman NJ, Rapaport R, Bomann JS, Wirotsko BM, Florakis GJ, Koniarek JP. Scanning Slit Confocal Microscopic Observation of Cell Morphology and Movement within the Normal Human Anterior Cornea. Ophthalmology. 1995;102:33–41. doi: 10.1016/s0161-6420(95)31057-3. [DOI] [PubMed] [Google Scholar]
- Beck CW, Belmonte JCI, Christen B. Beyond Early Development: Xenopus as an Emerging Model for the Study of Regenerative Mechanisms. Developmental Dynamics. 2009;238:1226–1248. doi: 10.1002/dvdy.21890. [DOI] [PubMed] [Google Scholar]
- Beck CW, Christen B, Barker D, Slack JMW. Temporal requirement for bone morphogenetic proteins in regeneration of the tail and limb of Xenopus tadpoles. Mech Dev. 2006;123:674–688. doi: 10.1016/j.mod.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Beck CW, Christen B, Slack JMW. Molecular pathways needed for regeneration of spinal cord and muscle in a vertebrate. Developmental Cell. 2003;5:429–439. doi: 10.1016/s1534-5807(03)00233-8. [DOI] [PubMed] [Google Scholar]
- Beck CW, Slack JM. An amphibian with ambition: a new role for Xenopus in the 21st century. Genome Biol. 2001;2 doi: 10.1186/gb-2001-2-10-reviews1029. REVIEWS1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe D, Garcia C, Wang XH, Rajagopal R, Feldmeier M, Kim JY, Chytil A, Moses H, Ashery-Padan R, Rauchman M. Contributions by members of the TGFbeta superfamily to lens development. International Journal of Developmental Biology. 2004;48:845–856. doi: 10.1387/ijdb.041869db. [DOI] [PubMed] [Google Scholar]
- Beebe DC. Homeobox Genes and Vertebrate Eye Development. Investigative Ophthalmology & Visual Science. 1994;35:2897–2900. [PubMed] [Google Scholar]
- Belecky-Adams TL, Adler R, Beebe DC. Bone morphogenetic protein signaling and the initiation of lens fiber cell differentiation. Development. 2002;129:3795–3802. doi: 10.1242/dev.129.16.3795. [DOI] [PubMed] [Google Scholar]
- Bosco L. Transdifferentiation of Ocular-Tissues in Larval Xenopus-Laevis. Differentiation. 1988;39:4–15. doi: 10.1111/j.1432-0436.1988.tb00074.x. [DOI] [PubMed] [Google Scholar]
- Bosco L, Filoni S. Relationships between Presence of the Eye Cup and Maintenance of Lens-Forming Capacity in Larval Xenopus-Laevis. Development Growth & Differentiation. 1992;34:619–625. doi: 10.1111/j.1440-169X.1992.tb00030.x. [DOI] [PubMed] [Google Scholar]
- Bosco L, Filoni S, Cannata S. Relationships between Eye Factors and Lens-Forming Transformations in the Cornea and Peri-Corneal Epidermis of Larval Xenopus-Laevis. Journal of Experimental Zoology. 1979;209 doi: 10.1002/jez.1402090208. 261-&. [DOI] [PubMed] [Google Scholar]
- Bosco L, Filoni S, Cioni C. Lens Formation from Cornea in the Presence of the Old Lens in Larval Xenopus-Laevis. Journal of Experimental Zoology. 1980;213:9–14. doi: 10.1002/jez.1402130103. [DOI] [PubMed] [Google Scholar]
- Bosco L, Testa O, Venturini G, Willems D. Lens fibre transdifferentiation in cultured larval Xenopus laevis outer cornea under the influence of neural retina-conditioned medium. Cellular and Molecular Life Sciences. 1997;53:921–928. doi: 10.1007/PL00013198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco L, Testa O, Venturini G, Willems D. Lens fibre transdifferentiation in cultured larval Xenopus laevis outer cornea under the influence of neural retina-conditioned medium. Cellular and Molecular Life Sciences. 1997;53:921–928. doi: 10.1007/PL00013198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco L, Valle C, Willems D. In-Vivo and in-Vitro Experimental-Analysis of Lens Regeneration in Larval Xenopus-Laevis. Development Growth & Differentiation. 1993;35:257–270. doi: 10.1111/j.1440-169X.1993.00257.x. [DOI] [PubMed] [Google Scholar]
- Bosco L, Venturini G, Willems D. First evidence of lens-transdifferentiation of larval Xenopus laevis induced by brain-derived acidic FGF. Rendiconti Lincei. 1994;5:261–268. [Google Scholar]
- Bosco L, Venturini G, Willems D. In vitro lens transdifferentiation of Xenopus laevis outer cornea induced by Fibroblast Growth Factor (FGF) Development. 1997;124:421–428. doi: 10.1242/dev.124.2.421. [DOI] [PubMed] [Google Scholar]
- Bosco L, Venturini G, Willems D. In vitro lens transdifferentiation of Xenopus laevis outer cornea induced by Fibroblast Growth Factor (FGF) Development. 1997;124:421–428. doi: 10.1242/dev.124.2.421. [DOI] [PubMed] [Google Scholar]
- Bosco L, Willems D. Persistence of the lens-inducing capacity of the neural retina in adult Anura. Rendiconti Lincei. 1992;3:345–351. [Google Scholar]
- Brahma SK. Isofocusing and Immunoelectrophoretic Studies of Soluble Eye Lens Proteins from Regenerated and Normally Developed Xenopus-Laevis. Experimental Eye Research. 1980;30:269–275. doi: 10.1016/0014-4835(80)90007-x. [DOI] [PubMed] [Google Scholar]
- Brahma SK, McDevitt DS. Ontogeny and Localization of Lens Crystallins in Xenopus-Laevis Lens Regeneration. Journal of Embryology and Experimental Morphology. 1974;32:783–794. [PubMed] [Google Scholar]
- Brahma SK, McDevitt DS. Ontogeny and Localization of Lens Crystallins in Xenopus-Laevis Lens Regeneration. Journal of Embryology and Experimental Morphology. 1974;32:783–794. [PubMed] [Google Scholar]
- Brockes JR, Kumar A. Comparative Aspects of Animal Regeneration. Annual Review of Cell and Developmental Biology. 2008;24:525–549. doi: 10.1146/annurev.cellbio.24.110707.175336. [DOI] [PubMed] [Google Scholar]
- Buck RC. Measurement of Centripetal Migration of Normal Corneal Epithelial-Cells in the Mouse. Investigative Ophthalmology & Visual Science. 1985;26:1296–1299. [PubMed] [Google Scholar]
- Burke ZD, Tosh D. Therapeutic potential of transdifferentiated cells. Clinical Science. 2005;108:309–321. doi: 10.1042/CS20040335. [DOI] [PubMed] [Google Scholar]
- Callery EM. There's more than one frog in the pond: A survey of the Amphibia and their contributions to developmental biology. Seminars in Cell & Developmental Biology. 2006;17:80–92. doi: 10.1016/j.semcdb.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Campbell JC. An Immuno-Fluorescent Study of Lens Regeneration in Larval Xenopus Laevis. Journal of Embryology and Experimental Morphology. 1965;13 171-&. [PubMed] [Google Scholar]
- Campbell JC, Truman DES. Variations in Differentiation in Regenerating Lens of Xenopus-Laevis. Experimental Eye Research. 1977;25:99–100. doi: 10.1016/0014-4835(77)90250-0. [DOI] [PubMed] [Google Scholar]
- Cannata SM, Arresta E, Bernardini S, Gargioli C, Filoni S. Tissue interactions and lens-forming competence in the outer cornea of larval Xenopus laevis. Journal of Experimental Zoology Part a-Comparative Experimental Biology. 2003;299A:161–171. doi: 10.1002/jez.a.10275. [DOI] [PubMed] [Google Scholar]
- Cannata SM, Bernardini S, Filoni S, Gargioli C. The optic vesicle promotes cornea to lens transdifferentiation in larval Xenopus laevis. Journal of Anatomy. 2008;212:621–626. doi: 10.1111/j.1469-7580.2008.00886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carinato ME, Walter BE, Henry JJ. Xenopus laevis gelatinase B (Xmmp-9): Development, regeneration, and wound healing. Developmental Dynamics. 2000;217:377–387. doi: 10.1002/(SICI)1097-0177(200004)217:4<377::AID-DVDY5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Carruthers S, Stemple DL. Genetic and genomic prospects for Xenopus tropicalis research. Seminars in Cell & Developmental Biology. 2006;17:146–153. doi: 10.1016/j.semcdb.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Chang WY, KhosrowShahian F, Chang R, Crawford MJ. xPitx1 plays a role in specifying cement gland and head during early Xenopus development. Genesis. 2001;29:78–90. [PubMed] [Google Scholar]
- Chen Y, Lin GF, Slack JMW. Control of muscle regeneration in the Xenopus tadpole tail by Pax7. Development. 2006;133:2303–2313. doi: 10.1242/dev.02397. [DOI] [PubMed] [Google Scholar]
- Chen YJ, Stump RJW, Lovicu FJ, Shimono A, McAvoy JW. Wnt signaling is required for organization of the lens fiber cell cytoskeleton and development of lens three-dimensional architecture. Developmental Biology. 2008;324:161–176. doi: 10.1016/j.ydbio.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow RL, Altmann CR, Lang RA, Hemmati-Brivanlou A. Pax6 induces ectopic eyes in a vertebrate. Development. 1999;126:4213–4222. doi: 10.1242/dev.126.19.4213. [DOI] [PubMed] [Google Scholar]
- Chow RL, Lang RA. Early eye development in vertebrates. Annual Review of Cell and Developmental Biology. 2001;17:255–296. doi: 10.1146/annurev.cellbio.17.1.255. [DOI] [PubMed] [Google Scholar]
- Cioni C, Filoni S, Bosco L. Inhibition of Lens Regeneration in Larval Xenopus-Laevis. Journal of Experimental Zoology. 1982;220:103–108. doi: 10.1002/jez.1402200113. [DOI] [PubMed] [Google Scholar]
- Collucci V. Sulla rigenerazione parziale deell’occhio nei tritoni: Isogenesi esvilluppo-Studio seprimentale. Mem. Accad. Sci. Istt. Bologna, Ser 5. 1891;1:593–629. [Google Scholar]
- Conesa A, Gotz S, Garcia-Gomez JM, Terol J, Talon M, Robles M. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21:3674–3676. doi: 10.1093/bioinformatics/bti610. [DOI] [PubMed] [Google Scholar]
- Corey DR, Abrams JM. Morpholino antisense oligonucleotides: tools for investigating vertebrate development. Genome Biol. 2001;2 doi: 10.1186/gb-2001-2-5-reviews1015. REVIEWS1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotsarelis G, Cheng SZ, Dong G, Sun TT, Lavker RM. Existence of Slow-Cycling Limbal Epithelial Basal Cells That Can Be Preferentially Stimulated to Proliferate - Implications on Epithelial Stem-Cells. Cell. 1989;57:201–209. doi: 10.1016/0092-8674(89)90958-6. [DOI] [PubMed] [Google Scholar]
- Cui WW, Tomarev SI, Piatigorsky J, Chepelinsky AB, Duncan MK. Mafs, Prox1, and Pax6 can regulate chicken beta B1-crystallin gene expression. Journal of Biological Chemistry. 2004;279:11088–11095. doi: 10.1074/jbc.M312414200. [DOI] [PubMed] [Google Scholar]
- Cvekl A, Duncan MK. Genetic and epigenetic mechanisms of gene regulation during lens development. Progress in Retinal and Eye Research. 2007;26:555–597. doi: 10.1016/j.preteyeres.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvekl A, Piatigorsky J. Lens development and crystallin gene expression: Many roles for Pax-6. Bioessays. 1996;18:621–630. doi: 10.1002/bies.950180805. [DOI] [PubMed] [Google Scholar]
- de Iongh RU, Lovicu FJ, Overbeek PA, Schneider MD, Joya J, Hardeman ED, McAvoy JW. Requirement for TGFbeta receptor signaling during terminal lens fiber differentiation. Development (Cambridge) 2001;128:3995–4010. doi: 10.1242/dev.128.20.3995. [DOI] [PubMed] [Google Scholar]
- Del Rio-Tsonis K, Trombley MT, McMahon G, Tsonis PA. Regulation of lens regeneration by fibroblast growth factor receptor 1. Developmental Dynamics. 1998;213:140–146. doi: 10.1002/(SICI)1097-0177(199809)213:1<140::AID-AJA14>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Del Rio-Tsonis K, Tsonis PA. Eye regeneration at the molecular age. Developmental Dynamics. 2003;226:211–224. doi: 10.1002/dvdy.10224. [DOI] [PubMed] [Google Scholar]
- DelRioTsonis K, Jung JC, Chiu IM, Tsonis PA. Conservation of fibroblast growth factor function in lens regeneration. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:13701–13706. doi: 10.1073/pnas.94.25.13701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delriotsonis K, Washabaugh CH, Tsonis PA. Expression of Pax-6 During Urodele Eye Development and Lens Regeneration. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:5092–5096. doi: 10.1073/pnas.92.11.5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi G. Electron microscopic studies on lens regeneration I. Mechanism of depigmentation of the iris. Embryologia. 1963;8:45–62. [Google Scholar]
- Eguchi G. Electron microscopic studies on lens regeneration II. Formation and growth of lens vesicle and differentiation of lens fibers. Embryologia. 1964;8:247–287. [Google Scholar]
- Eguchi G, Kodama R. Transdifferentiation. Current Opinion in Cell Biology. 1993;5:1023–1028. doi: 10.1016/0955-0674(93)90087-7. [DOI] [PubMed] [Google Scholar]
- Ekker SC. Morphants: a new systematic vertebrate functional genomics approach. Yeast. 2000;17:302–306. doi: 10.1002/1097-0061(200012)17:4<302::AID-YEA53>3.0.CO;2-#. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enwright JF, Grainger RM. Altered retinoid signaling in the heads of small eye mouse embryos. Developmental Biology. 2000;221:10–22. doi: 10.1006/dbio.2000.9652. [DOI] [PubMed] [Google Scholar]
- Esteve P, Bovolenta P. Secreted inducers in vertebrate eye development: more functions for old morphogens. Current Opinion in Neurobiology. 2006;16:13–19. doi: 10.1016/j.conb.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Filbin MT. How inflammation promotes regeneration. Nature Neuroscience. 2006;9:715–717. doi: 10.1038/nn0606-715. [DOI] [PubMed] [Google Scholar]
- Filoni S. Retina and lens regeneration in anuran amphibians. Seminars in Cell & Developmental Biology. 2009;20:528–534. doi: 10.1016/j.semcdb.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Filoni S, Bernardini S, Cannata SM. Experimental analysis of lens-forming capacity in Xenopus borealis larvae. Journal of Experimental Zoology Part a-Comparative Experimental Biology. 2006;305A:538–550. doi: 10.1002/jez.a.297. [DOI] [PubMed] [Google Scholar]
- Filoni S, Bernardini S, Cannata SM, Dalessio A. Lens regeneration in larval Xenopus laevis: Experimental analysis of the decline in the regenerative capacity during development. Developmental Biology. 1997;187:13–24. doi: 10.1006/dbio.1997.8598. [DOI] [PubMed] [Google Scholar]
- Filoni S, Bosco L, Cannata S. Primi dati sperimentali sui fattori necessari per la transformazione lentogena della cornea esterna di larve di Xenopus laevis. Acta Embryol. Exp. 1978;3:344. [Google Scholar]
- Filoni S, Bosco L, Cioni C. Experimental analysis of lens regeneration in larval Xenopus laevis, the role of the retina and lens. Acta Embryol. Morphol. Exp. 1981;2:XVI–XXVII. [PubMed] [Google Scholar]
- Filoni S, Bosco L, Cioni C. The role of neural retina in lens regeneration from cornea in larval Xenopus laevis. Acta Embryol Morphol Exp. 1982;3:15–28. [PubMed] [Google Scholar]
- Filoni S, Bosco L, Cioni C, Venturini G. Lens Forming Transformations in Larval Xenopus-Laevis Induced by Denatured Eye-Cup or Its Whole Protein Complement. Experientia. 1983;39:315–317. doi: 10.1007/BF01955324. [DOI] [PubMed] [Google Scholar]
- Filoni S, Bosco L, Paglioni N, Cioni C. Lens Formation from Pericorneal Epidermis in the Presence of the Old Lens in Larval Xenopus-Laevis. Journal of Experimental Zoology. 1980;211:303–309. doi: 10.1002/jez.1402130103. [DOI] [PubMed] [Google Scholar]
- Fini ME, Strissel KJ, WestMays JA. Perspectives on eye development. Developmental Genetics. 1997;20:175–185. doi: 10.1002/(SICI)1520-6408(1997)20:3<175::AID-DVG1>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Freeman G. Lens Regeneration from Cornea in Xenopus Laevis. Journal of Experimental Zoology. 1963;154 doi: 10.1002/jez.1401540105. 39-&. [DOI] [PubMed] [Google Scholar]
- Freeman G, Overton J. Lens regeneration from the cornea in Xenopus laevis. American Zoologist. 1961;1:448–449. [Google Scholar]
- Freeman G, Overton J. The effects of thyroxin on the competence of lens regeneration in Xenopus. Anatomical Record. 1962;142:305. [Google Scholar]
- Fujiwara M, Uchida T, Osumiyamashita N, Eto K. Uchida Rat (Rsey) - a New Mutant Rat with Craniofacial Abnormalities Resembling Those of the Mouse Sey Mutant. Differentiation. 1994;57:31–38. doi: 10.1046/j.1432-0436.1994.5710031.x. [DOI] [PubMed] [Google Scholar]
- Furuta Y, Hogan BLN. BMP4 is essential for lens induction in the mouse embryo. Genes & Development. 1998;12:3764–3775. doi: 10.1101/gad.12.23.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargioli C, Giambra V, Santoni S, Bernardini S, Frezza D, Filoni S, Cannata SM. The lens-regenerating competence in the outer cornea and epidermis of larval Xenopus laevis is related to pax6 expression. Journal of Anatomy. 2008;212:612–620. doi: 10.1111/j.1469-7580.2008.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargioli C, Slack JMW. Cell lineage tracing during Xenopus tail regeneration. Development. 2004;131:2669–2679. doi: 10.1242/dev.01155. [DOI] [PubMed] [Google Scholar]
- Garza-Garcia AA, Driscoll PC, Brockes JP. Evidence for the local evolution of mechanisms underlying limb regeneration in salamanders. 2010 doi: 10.1093/icb/icq022. [DOI] [PubMed] [Google Scholar]
- Gazave E, Lapebie P, Richards GS, Brunet F, Ereskovsky AV, Degnan BM, Borchiellini C, Vervoort M, Renard E. Origin and evolution of the Notch signalling pathway: an overview from eukaryotic genomes. BMC Evolutionary Biology. 2009;9 doi: 10.1186/1471-2148-9-249. Article No. 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godwin JW, Brockes JP. Regeneration, tissue injury and the immune response. Journal of Anatomy. 2006;209:423–432. doi: 10.1111/j.1469-7580.2006.00626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal-Srivastava R, Cvekl A, Piatigorsky J. Involvement of retinoic acid retinoid receptors in the regulation of murine alpha B-crystallin small heat shock protein gene expression in the lens. Journal of Biological Chemistry. 1998;273:17954–17961. doi: 10.1074/jbc.273.28.17954. [DOI] [PubMed] [Google Scholar]
- Graw J. Genetic aspects of embryonic eye development in vertebrates. Developmental Genetics. 1996;18:181–197. doi: 10.1002/(SICI)1520-6408(1996)18:3<181::AID-DVG1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Graw J. The genetic and molecular basis of congenital eye defects. Nature Reviews Genetics. 2003;4:876–888. doi: 10.1038/nrg1202. [DOI] [PubMed] [Google Scholar]
- Graw J, Loster J. Developmental genetics in ophthalmology. Ophthalmic Genet. 2003;24:1–33. doi: 10.1076/opge.24.1.1.13888. [DOI] [PubMed] [Google Scholar]
- Grogg MW, Call MK, Okamoto M, Vergara MN, Del Rio-Tsonis K, Tsonis PA. BMP inhibition-driven regulation of six-3 underlies induction of newt lens regeneration. Nature. 2005;438:858–862. doi: 10.1038/nature04175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grogg MW, Call MK, Tsonis PA. Signaling during lens regeneration. Seminars in Cell & Developmental Biology. 2006;17:753–758. doi: 10.1016/j.semcdb.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdon JB, Hopwood N. The introduction of Xenopus laevis into developmental biology: of empire, pregnancy testing and ribosomal genes. International Journal of Developmental Biology. 2000;44:43–50. [PubMed] [Google Scholar]
- Hanson IM. Mammalian homologues of the Drosophila eye specification genes. Seminars in Cell & Developmental Biology. 2001;12:475–484. doi: 10.1006/scdb.2001.0271. [DOI] [PubMed] [Google Scholar]
- Harty M, Neff AW, King MW, Mescher AL. Regeneration or scarring: An immunologic perspective. Developmental Dynamics. 2003;226:268–279. doi: 10.1002/dvdy.10239. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Mizuno N, Takada R, Takada S, Kondoh H. Determinative role of Wnt signals in dorsal iris-derived lens regeneration in newt eye. Mechanisms of Development. 2006;123:793–800. doi: 10.1016/j.mod.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Mizuno N, Ueda Y, Okamoto M, Kondoh H. FGF2 triggers iris-derived lens regeneration in newt eye. Mechanisms of Development. 2004;121:519–526. doi: 10.1016/j.mod.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Heasman J. Morpholino oligos: Making sense of antisense? Developmental Biology. 2002;243:209–214. doi: 10.1006/dbio.2001.0565. [DOI] [PubMed] [Google Scholar]
- Henry JJ. The cellular and molecular bases of vertebrate lens regeneration. International Review of Cytology - a Survey of Cell Biology, Vol 228. 2003;228:195–265. doi: 10.1016/s0074-7696(03)28005-0. [DOI] [PubMed] [Google Scholar]
- Henry JJ, Carinato ME, Schaefer JJ, Wolfe AD, Walter BE, Perry KJ, Elbl TN. Characterizing gene expression during lens formation in Xenopus laevis: Evaluating the model for embryonic lens induction. Developmental Dynamics. 2002;224:168–185. doi: 10.1002/dvdy.10097. [DOI] [PubMed] [Google Scholar]
- Henry JJ, Elkins MB. Cornea-lens transdifferentiation in the anuran, Xenopus tropicalis. Development Genes and Evolution. 2001;211:377–387. doi: 10.1007/s004270100163. [DOI] [PubMed] [Google Scholar]
- Henry JJ, Mittleman JM. The Matured Eye of Xenopus-Laevis Tadpoles Produces Factors That Elicit a Lens-Forming Response in Embryonic Ectoderm. Developmental Biology. 1995;171:39–50. doi: 10.1006/dbio.1995.1258. [DOI] [PubMed] [Google Scholar]
- Henry JJ, Wever JA, Veragara MN, Fukui L. An Ideal Vertebrate System for Studies of Eye Development and Regeneration. In: Tsonis PA, editor. Animal Models for Eye Research. City. San Diego: Academic Press; 2008. pp. 57–92. [Google Scholar]
- Hirsch N, Zimmerman LB, Gray J, Chae J, Curran KL, Fisher M, Ogino H, Grainger RM. Xenopus tropicalis transgenic lines and their use in the study of embryonic induction. Developmental Dynamics. 2002;225:522–535. doi: 10.1002/dvdy.10188. [DOI] [PubMed] [Google Scholar]
- Huang AJW, Tseng SCG. Corneal Epithelial Wound-Healing in the Absence of Limbal Epithelium. Investigative Ophthalmology & Visual Science. 1991;32:96–105. [PubMed] [Google Scholar]
- Huang HC, Marsh-Armstrong N, Brown DD. Metamorphosis is inhibited in transgenic Xenopus laevis tadpoles that overexpress type III deiodinase. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:962–967. doi: 10.1073/pnas.96.3.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XQ, Madan A. CAP3: A DNA sequence assembly program. Genome Research. 1999;9:868–877. doi: 10.1101/gr.9.9.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyuga M, Kodama R, Eguchi G. Basic Fibroblast Growth-Factor as One of the Essential Factors Regulating Lens Transdifferentiation of Pigmented Epithelial-Cells. International Journal of Developmental Biology. 1993;37:319–326. [PubMed] [Google Scholar]
- Imokawa Y, Brockes JP. Selective activation of thrombin is a critical determinant for vertebrate lens regeneration. Current Biology. 2003;13:877–881. doi: 10.1016/s0960-9822(03)00294-x. [DOI] [PubMed] [Google Scholar]
- Imokawa Y, Simon A, Brockes JP. A critical role for thrombin in vertebrate lens regeneration. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 2004;359:765–776. doi: 10.1098/rstb.2004.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi S, Yasuda K. Distinct roles of maf genes during Xenopus lens development. Mechanisms of Development. 2001;101:155–166. doi: 10.1016/s0925-4773(00)00585-2. [DOI] [PubMed] [Google Scholar]
- Ito M, Hayashi T, Kuroiwa A, Okamoto M. Lens formation by pigmented epithelial cell reaggregate from dorsal iris implanted into limb blastema in the adult newt. Development Growth & Differentiation. 1999;41:429–440. doi: 10.1046/j.1440-169x.1999.00447.x. [DOI] [PubMed] [Google Scholar]
- Jean D, Ewan K, Gruss P. Molecular regulators involved in vertebrate eye development. Mechanisms of Development. 1998;76:3–18. doi: 10.1016/s0925-4773(98)00117-8. [DOI] [PubMed] [Google Scholar]
- Kablar B, Vignali R, Menotti L, Pannese M, Andreazzoli M, Polo C, Giribaldi MG, Boncinelli E, Barsacchi G. Xotx genes in the developing brain of Xenopus laevis. Mechanisms of Development. 1996;55:145–158. doi: 10.1016/0925-4773(96)00497-2. [DOI] [PubMed] [Google Scholar]
- Kamachi Y, Sockanathan S, Liu QR, Breitman M, Lovellbadge R, Kondoh H. Involvement of Sox Proteins in Lens-Specific Activation of Crystallin Genes. Embo Journal. 1995;14:3510–3519. doi: 10.1002/j.1460-2075.1995.tb07357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamachi Y, Uchikawa M, Collignon J, Lovell-Badge R, Kondoh H. Involvement of Sox1, 2 and 3 in the early and subsequent molecular events of lens induction. Development. 1998;125:2521–2532. doi: 10.1242/dev.125.13.2521. [DOI] [PubMed] [Google Scholar]
- Kamachi Y, Uchikawa M, Tanouchi A, Sekido R, Kondoh H. Pax6 and SOX2 form a co-DNA-binding partner complex that regulates initiation of lens development. Genes & Development. 2001;15:1272–1286. doi: 10.1101/gad.887101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanao T, Miyachi Y. Lymphangiogenesis promotes lens destruction and subsequent lens regeneration in the newt eyeball, and both processes can be accelerated by transplantation of dendritic cells. Developmental Biology. 2006;290:118–124. doi: 10.1016/j.ydbio.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Kastner P, Grondona JM, Mark M, Gansmuller A, Lemeur M, Decimo D, Vonesch JL, Dolle P, Chambon P. Genetic-Analysis of Rxr-Alpha, Developmental Function - Convergence of Rxr and Rar Signaling Pathways in Heart and Eye Morphogenesis. Cell. 1994;78:987–1003. doi: 10.1016/0092-8674(94)90274-7. [DOI] [PubMed] [Google Scholar]
- Kato T, Miyazaki K, Shimizu-Nishikawa K, Koshiba K, Obara M, Mishima HK, Yoshizato K. Unique expression patterns of matrix metalloproteinases in regenerating newt limbs. Developmental Dynamics. 2003;226:366–376. doi: 10.1002/dvdy.10247. [DOI] [PubMed] [Google Scholar]
- Kawamorita M, Suzuki C, Saito G, Sato T, Sato K. In vitro differentiation of mouse embryonic stem cells after activation by retinoic acid. Hum Cell. 2002;15:178–182. doi: 10.1111/j.1749-0774.2002.tb00112.x. [DOI] [PubMed] [Google Scholar]
- Kay BK, Peng HB. Xenopus-Laevis - Practical Uses in Cell and Molecular-Biology - Preface. Methods in Cell Biology. 1991;36:R19–R22. [PubMed] [Google Scholar]
- Kenyon KL, Moody SA, Jamrich M. A novel fork head gene mediates early steps during Xenopus lens formation. Development. 1999;126:5107–5116. doi: 10.1242/dev.126.22.5107. [DOI] [PubMed] [Google Scholar]
- Kherif S, Lafuma C, Dehaupas M, Lachkar S, Fournier JG, Verdiere-Sahuque M, Fardeau M, Alameddine HS. Expression of matrix metalloproteinases 2 and 9 in regenerating skeletal muscle: A study in experimentally injured and mdx muscles. Developmental Biology. 1999;205:158–170. doi: 10.1006/dbio.1998.9107. [DOI] [PubMed] [Google Scholar]
- Khosrowshahian F, Wolanski M, Chang WY, Fujiki K, Jacobs L, Crawford MJ. Lens and retina formation require expression of Pitx3 in Xenopus pre-lens Ectoderm. Developmental Dynamics. 2005;234:577–589. doi: 10.1002/dvdy.20540. [DOI] [PubMed] [Google Scholar]
- Klein SL, Gerhard DS, Wagner L, Richardson P, Schriml LM, Sater AK, Warren WC, McPherson JD. Resources for genetic and genomic studies of Xenopus. Methods in Molecular Biology. 2006;1:16. doi: 10.1007/978-1-59745-000-3_1. [DOI] [PubMed] [Google Scholar]
- Klein SL, Strausberg RL, Wagner L, Pontius J, Clifton SW, Richardson P Washington Univ Est Sequencing, G., and Natl Inst Hlth Xenopus Working, G. Genetic and genomic tools for Xenopus research: The NIH Xenopus initiative. Developmental Dynamics. 2002;225:384–391. doi: 10.1002/dvdy.10174. [DOI] [PubMed] [Google Scholar]
- Kodama R, Eguchi G. From Lens Regeneration in the Newt to in-Vitro Transdifferentiation of Vertebrate Pigmented Epithelial-Cells. Seminars in Cell Biology. 1995;6:143–149. doi: 10.1006/scel.1995.0020. [DOI] [PubMed] [Google Scholar]
- Kondoh H. Transcription factors for lens development assessed in vivo. Current Opinion in Genetics & Development. 1999;9:301–308. doi: 10.1016/s0959-437x(99)80045-8. [DOI] [PubMed] [Google Scholar]
- Koster RW, Kuhnlein RP, Wittbrodt J. Ectopic Sox3 activity elicits sensory placode formation. Mechanisms of Development. 2000;95:175–187. doi: 10.1016/s0925-4773(00)00356-7. [DOI] [PubMed] [Google Scholar]
- Kragl M, Knapp D, Nacu E, Khattak S, Maden M, Epperlein HH, Tanaka EM. Cells keep a memory of their tissue origin during axolotl limb regeneration. Nature. 2009;460:U60–U69. doi: 10.1038/nature08152. [DOI] [PubMed] [Google Scholar]
- Kroll KL, Amaya E. Transgenic Xenopus embryos from sperm nuclear transplantations reveal FGF signaling requirements during gastrulation. Development. 1996;122:3173–3183. doi: 10.1242/dev.122.10.3173. [DOI] [PubMed] [Google Scholar]
- Kumar A, Godwin JW, Gates PB, Garza-Garcia AA, Brockes JP. Molecular basis for the nerve dependence of limb regeneration in an adult vertebrate. Science. 2007;318:772–777. doi: 10.1126/science.1147710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang RA. Pathways regulating lens induction in the mouse. International Journal of Developmental Biology. 2004;48:783–791. doi: 10.1387/ijdb.041903rl. [DOI] [PubMed] [Google Scholar]
- Lavker RM, Wei ZG, Sun TT. Phorbol ester preferentially stimulates mouse fornical conjunctival and limbal epithelial cells to proliferate in vivo. Investigative Ophthalmology & Visual Science. 1998;39:301–307. [PubMed] [Google Scholar]
- Leon S, Yin YQ, Nguyen J, Irwin N, Benowitz LI. Lens injury stimulates axon regeneration in the mature rat optic nerve. Journal of Neuroscience. 2000;20:4615–4626. doi: 10.1523/JNEUROSCI.20-12-04615.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HS, Yang JM, Jacobson RD, Pasko D, Sundin O. Pax-6 Is First Expressed in a Region of Ectoderm Anterior to the Early Neural Plate - Implications for Stepwise Determination of the Lens. Developmental Biology. 1994;162:181–194. doi: 10.1006/dbio.1994.1077. [DOI] [PubMed] [Google Scholar]
- Li X, Cvekl A, Bassnett S, Piatigorsky J. Lens-preferred activity of chicken delta 1- and delta 2-crystallin enhancers in transgenic mice and evidence for retinoic acid-responsive regulation of the delta 1-crystallin gene. Developmental Genetics. 1997;20:258–266. doi: 10.1002/(SICI)1520-6408(1997)20:3<258::AID-DVG8>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Lin G, Slack JMW. Requirement for Wnt and FGF signaling in Xenopus tadpole tail regeneration. Developmental Biology. 2008;316:323–335. doi: 10.1016/j.ydbio.2008.01.032. [DOI] [PubMed] [Google Scholar]
- Lin GF, Chen Y, Slack JMW. Regeneration of neural crest derivatives in the Xenopus tadpole tail. Bmc Developmental Biology. 2007;7 doi: 10.1186/1471-213X-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovicu FJ, McAvoy JW. Growth factor regulation of lens development. Developmental Biology. 2005;280:1–14. doi: 10.1016/j.ydbio.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Lu X, Richardson PM. Inflammation near the Nerve-Cell Body Enhances Axonal Regeneration. Journal of Neuroscience. 1991;11:972–978. doi: 10.1523/JNEUROSCI.11-04-00972.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luigi B, Filoni S. Relationships between Presence of the Eye Cup and Maintenance of Lens-Forming Capacity in Larval Xenopus laevis. Development, Growth and Differentiation. 1992;34:619–625. doi: 10.1111/j.1440-169X.1992.tb00030.x. [DOI] [PubMed] [Google Scholar]
- Lupo G, Andreazzoli M, Gestri G, Liu Y, He RQ, Barsacchi G. Homeobox genes in the genetic control of eye development. International Journal of Developmental Biology. 2000;44:627–636. [PubMed] [Google Scholar]
- Lupo G, Harris WA, Lewis KE. Mechanisms of ventral patterning in the vertebrate nervous system. Nature Reviews Neuroscience. 2006;7:103–114. doi: 10.1038/nrn1843. [DOI] [PubMed] [Google Scholar]
- Lupo G, Liu Y, Qiu R, Chandraratna RAS, Barsacchi G, He RQ, Harris WA. Dorsoventral patterning of the Xenopus eye: a collaboration of Retinoid, Hedgehog and FGF receptor signaling. Development. 2005;132:1737–1748. doi: 10.1242/dev.01726. [DOI] [PubMed] [Google Scholar]
- Madhavan M, Haynes TL, Frisch NC, Call MK, Minich CM, Tsonis PA, Del Rio-Tsonis K. The role of Pax-6 in lens regeneration. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:14848–14853. doi: 10.1073/pnas.0601949103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarev E, Call MK, Grogg MW, Atkinson DL, Milash B, Odelberg SJ, Tsonis PA. Gene expression signatures in the newt irises during lens regeneration. Febs Letters. 2007;581:1865–1870. doi: 10.1016/j.febslet.2007.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarev E, Spence J, Del Rio-Tsonis K, Tsonis P. Identification of microRNAs and other small RNAs from the adult newt eye. Molecular Vision. 2006;12:1386–1391. [PubMed] [Google Scholar]
- Maki N, Martinson J, Nishimura O, Tarui H, Meller J, Tsonis PA, Agata K. Expression profiles during dedifferentiation in newt lens regeneration revealed by expressed sequence tags. Molecular Vision. 16:72–78. [PMC free article] [PubMed] [Google Scholar]
- Maki N, Suetsugu-Maki R, Tarui H, Agata K, Del Rio-Tsonis K, Tsonis PA. Expression of Stem Cell Pluripotency Factors During Regeneration in Newts. Developmental Dynamics. 2009;238:1613–1616. doi: 10.1002/dvdy.21959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki N, Takechi K, Sano S, Tarui H, Sasai Y, Agata K. Rapid accumulation of nucleostemin in nucleolus during newt regeneration. Developmental Dynamics. 2007;236:941–950. doi: 10.1002/dvdy.21027. [DOI] [PubMed] [Google Scholar]
- Makino S, Whitehead GG, Lien CL, Kim S, Jhawar P, Kono A, Kawata Y, Keating MT. Heat-shock protein 60 is required for blastema formation and maintenance during regeneration. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:14599–14604. doi: 10.1073/pnas.0507408102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malloch EL, Perry KJ, Fukui L, Johnson VR, Wever J, Beck CW, King MW, Henry JJ. Gene Expression Profiles of Lens Regeneration and Development in Xenopus laevis. Developmental Dynamics. 2009;238:2340–2356. doi: 10.1002/dvdy.21998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns M, Fritzsch B. The Eye in the Brain - Retinoic Acid Effects Morphogenesis of the Eye and Pathway Selection of Axons but Not the Differentiation of the Retina in Xenopus-Laevis. Neuroscience Letters. 1991;127:150–154. doi: 10.1016/0304-3940(91)90782-o. [DOI] [PubMed] [Google Scholar]
- Marsh-Armstrong N, Huang HC, Berry DL, Brown DD. Germ-line transmission of transgenes in Xenopus laevis. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:14389–14393. doi: 10.1073/pnas.96.25.14389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Morales JR, Signore M, Acampora D, Simeone A, Bovolenta P. Otx genes are required for tissue specification in the developing eye. Development. 2001;128:2019–2030. doi: 10.1242/dev.128.11.2019. [DOI] [PubMed] [Google Scholar]
- Mathers PH, Grinberg A, Mahon KA, Jamrich M. The Rx homeobox gene is essential for vertebrate eye development. Nature. 1997;387:603–607. doi: 10.1038/42475. [DOI] [PubMed] [Google Scholar]
- McDevitt DS, Brahma SK, Courtois Y, Jeanny JC. Fibroblast growth factor receptors and regeneration of the eye lens. Developmental Dynamics. 1997;208:220–226. doi: 10.1002/(SICI)1097-0177(199702)208:2<220::AID-AJA9>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Medina-Martinez O, Shah R, Jamrich M. Pitx3 Controls Multiple Aspects of Lens Development. Developmental Dynamics. 2009;238:2193–2201. doi: 10.1002/dvdy.21924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher AL, Neff AW. Regenerative capacity and the developing immune system. Regenerative Medicine I: Theories, Models and Methods. 2005;93:39–66. doi: 10.1007/b99966. [DOI] [PubMed] [Google Scholar]
- Mescher AL, Wolf WL, Moseman A, Hartman B, Harrison C, Nguyen E, Neff AW. Cells of cutaneous immunity in Xenopus: Studies during larval development and limb regeneration. Developmental and Comparative Immunology. 2007;31:383–393. doi: 10.1016/j.dci.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Meyer-Franke A, Wilkinson GA, Kruttgen A, Hu M, Munro E, Hanson MG, Reichardt LF, Barres BA. Depolarization and cAMP elevation rapidly recruit TrkB to the plasma membrane of CNS neurons. Neuron. 1998;21:681–693. doi: 10.1016/s0896-6273(00)80586-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitashov VI. Comparitive studies of lens regeneration in Cobitid fish (Misgurnus fossilis, Nemachilus barbatulus, Nemachhilus dorsalis) Dokl. Akad. Nauk. SSSR. 1966;170:1439–1442. [PubMed] [Google Scholar]
- Miyazaki K, Uchiyama K, Imokawa Y, Yoshizato K. Cloning and characterization of cDNAs for matrix metalloproteinases of regenerating newt limbs. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:6819–6824. doi: 10.1073/pnas.93.13.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno N, Agata K, Sawada K, Mochii M, Eguchi G. Expression of crystallin genes in embryonic and regenerating newt lenses. Development Growth & Differentiation. 2002;44:251–256. doi: 10.1046/j.1440-169x.2002.00639.x. [DOI] [PubMed] [Google Scholar]
- Mizuno N, Mochii M, Takahashi TC, Eguchi G, Okada TS. Lens regeneration in Xenopus is not a mere repeat of lens development, with respect to crystallin gene expression. Differentiation. 1999;64:143–149. doi: 10.1046/j.1432-0436.1999.6430143.x. [DOI] [PubMed] [Google Scholar]
- Mizuno N, Mochii M, Yamamoto TS, Takahashi TC, Eguchi G, Okada TS. Pax-6 and Prox 1 expression during lens regeneration from Cynops iris and Xenopus cornea: evidence for a genetic program common to embryonic lens development. Differentiation. 1999;65:141–149. doi: 10.1046/j.1432-0436.1999.6530141.x. [DOI] [PubMed] [Google Scholar]
- Mizuno N, Ueda Y, Kondoh H. Requirement for beta B1-crystallin promoter of Xenopus laevis in embryonic lens development and lens regeneration. Development Growth & Differentiation. 2005;47:131–140. doi: 10.1111/j.1440-169X.2005.00789.x. [DOI] [PubMed] [Google Scholar]
- Nishiguchi S, Wood H, Kondoh H, Lovell-Badge R, Episkopou V. Sox1 directly regulates the gamma-crystallin genes and is essential for lens development in mice. Genes & Development. 1998;12:776–781. doi: 10.1101/gad.12.6.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt SL, Bronchain OJ, Hartley KO, Amaya E. Comparison of morpholino based translational inhibition during the development of Xenopus laevis and Xenopus tropicalis. Genesis. 2001;30:110–113. doi: 10.1002/gene.1042. [DOI] [PubMed] [Google Scholar]
- Odelberg SJ. Cellular plasticity in vertebrate regeneration. Anatomical Record Part B:The New Anatomist. 2005;287 doi: 10.1002/ar.b.20080. ISSN 1552–4906(print)|1552–4914(electronic). [DOI] [PubMed] [Google Scholar]
- Offield MF, Hirsch N, Grainger RM. The development of Xenopus tropicalis transgenic lines and their use in studying lens developmental timing in living embryos. Development. 2000;127:1789–1797. doi: 10.1242/dev.127.9.1789. [DOI] [PubMed] [Google Scholar]
- Ogino H, Fisher M, Grainger RM. Convergence of a head-field selector Otx2 and Notch signaling: a mechanism for lens specification. Development. 2008;135:249–258. doi: 10.1242/dev.009548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogino H, McConnell WB, Grainger RM. Highly efficient transgenesis in Xenopus tropicalis using I-SceI meganuclease. Mechanisms of Development. 2006;123:103–113. doi: 10.1016/j.mod.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Ogino H, Yasuda K. Sequential activation of transcription factors in lens induction. Development Growth & Differentiation. 2000;42:437–448. doi: 10.1046/j.1440-169x.2000.00532.x. [DOI] [PubMed] [Google Scholar]
- Ogino H, Yasuda K. Sequential activation of transcription factors in lens induction. Development Growth & Differentiation. 2000;42:437–448. doi: 10.1046/j.1440-169x.2000.00532.x. [DOI] [PubMed] [Google Scholar]
- Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–U1. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–U1. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- Oliver G, Gruss P. Current views on eye development. Trends in Neurosciences. 1997;20:415–421. doi: 10.1016/s0166-2236(97)01082-5. [DOI] [PubMed] [Google Scholar]
- Oliver G, Gruss P. Current views on eye development. Trends in Neurosciences. 1997;20:415–421. doi: 10.1016/s0166-2236(97)01082-5. [DOI] [PubMed] [Google Scholar]
- Oliver G, Loosli F, Koster R, Wittbrodt J, Gruss P. Ectopic lens induction in fish in response to the murine homeobox gene Six3. Mechanisms of Development. 1996;60:233–239. doi: 10.1016/s0925-4773(96)00632-6. [DOI] [PubMed] [Google Scholar]
- Oliver G, Loosli F, Koster R, Wittbrodt J, Gruss P. Ectopic lens induction in fish in response to the murine homeobox gene Six3. Mechanisms of Development. 1996;60:233–239. doi: 10.1016/s0925-4773(96)00632-6. [DOI] [PubMed] [Google Scholar]
- Oliver G, Sosapineda B, Geisendorf S, Spana EP, Doe CQ, Gruss P. Prox-1, a Prospero-Related Homeobox Gene Expressed During Mouse Development. Mechanisms of Development. 1993;44:3–16. doi: 10.1016/0925-4773(93)90012-m. [DOI] [PubMed] [Google Scholar]
- Onuma Y, Takahashi S, Asashima M, Kurata S, Gehring WJ. Conservation of Pax 6 function and upstream activation by Notch signaling in eye development of frogs and flies. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:2020–2025. doi: 10.1073/pnas.022626999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onuma Y, Takahashi S, Asashima M, Kurata S, Gehring WJ. Conservation of Pax 6 function and upstream activation by Notch signaling in eye development of frogs and flies. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:2020–2025. doi: 10.1073/pnas.022626999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal table of Xenopus laevis Daudin. Amsterdam: North Holland Publishing; 1956. [Google Scholar]
- Pan FC, Chen YL, Loeber J, Henningfeld K, Pieler T. I-SceI meganuclease-mediated transgenesis in Xenopus. Developmental Dynamics. 2006;235:247–252. doi: 10.1002/dvdy.20608. [DOI] [PubMed] [Google Scholar]
- Pan FC, Chen YL, Loeber J, Henningfeld K, Pieler T. I-SceI meganuclease-mediated transgenesis in Xenopus. Developmental Dynamics. 2006;235:247–252. doi: 10.1002/dvdy.20608. [DOI] [PubMed] [Google Scholar]
- Pannese M, Polo C, Andreazzoli M, Vignali R, Kablar B, Barsacchi G, Boncinelli E. The Xenopus Homolog of Otx2 Is a Maternal Homeobox Gene That Demarcates and Specifies Anterior Body Regions. Development. 1995;121:707–720. doi: 10.1242/dev.121.3.707. [DOI] [PubMed] [Google Scholar]
- Pannese M, Polo C, Andreazzoli M, Vignali R, Kablar B, Barsacchi G, Boncinelli E. The Xenopus Homolog of Otx2 Is a Maternal Homeobox Gene That Demarcates and Specifies Anterior Body Regions. Development. 1995;121:707–720. doi: 10.1242/dev.121.3.707. [DOI] [PubMed] [Google Scholar]
- Patruno M, Thorndyke MC, Carnevali MDC, Bonasoro F, Beesley PW. Growth factors, heat-shock proteins and regeneration in echinoderms. Journal of Experimental Biology. 2001;204:843–848. doi: 10.1242/jeb.204.5.843. [DOI] [PubMed] [Google Scholar]
- Patruno M, Thorndyke MC, Carnevali MDC, Bonasoro F, Beesley PW. Growth factors, heat-shock proteins and regeneration in echinoderms. Journal of Experimental Biology. 2001;204:843–848. doi: 10.1242/jeb.204.5.843. [DOI] [PubMed] [Google Scholar]
- Pearl EJ, Barker D, Day RC, Beck CW. Identification of genes associated with regenerative success of Xenopus laevis hindlimbs. Bmc Developmental Biology. 2008;8 doi: 10.1186/1471-213X-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl EJ, Barker D, Day RC, Beck CW. Identification of genes associated with regenerative success of Xenopus laevis hindlimbs. Bmc Developmental Biology. 2008;8 doi: 10.1186/1471-213X-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini G, Golisano O, Paterna P, Lambiase A, Bonini S, Rama P, De Luca M. Location and clonal analysis of stem cells and their differentiated progeny in the human ocular surface. Journal of Cell Biology. 1999;145:769–782. doi: 10.1083/jcb.145.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini G, Golisano O, Paterna P, Lambiase A, Bonini S, Rama P, De Luca M. Location and clonal analysis of stem cells and their differentiated progeny in the human ocular surface. Journal of Cell Biology. 1999;145:769–782. doi: 10.1083/jcb.145.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzel R, Oschwald R, Chen YL, Tacke L, Grunz H. Characterization and early embryonic expression of a neural specific transcription factor xSOX3 in Xenopus laevis. International Journal of Developmental Biology. 1997;41:667–677. [PubMed] [Google Scholar]
- Penzel R, Oschwald R, Chen YL, Tacke L, Grunz H. Characterization and early embryonic expression of a neural specific transcription factor xSOX3 in Xenopus laevis. International Journal of Developmental Biology. 1997;41:667–677. [PubMed] [Google Scholar]
- Pommereit D, Pieler T, Hollemann T. Xpitx3: a member of the Rieg/Pitx gene family expressed during pituitary and lens formation in Xenopus laevis. Mechanisms of Development. 2001;102:255–257. doi: 10.1016/s0925-4773(01)00305-7. [DOI] [PubMed] [Google Scholar]
- Pommereit D, Pieler T, Hollemann T. Xpitx3: a member of the Rieg/Pitx gene family expressed during pituitary and lens formation in Xenopus laevis. Mechanisms of Development. 2001;102:255–257. doi: 10.1016/s0925-4773(01)00305-7. [DOI] [PubMed] [Google Scholar]
- Reeve JG, Wild AE. Lens Regeneration from Cornea of Larval Xenopus-Laevis in the Presence of the Lens. Journal of Embryology and Experimental Morphology. 1978;48:205–214. [PubMed] [Google Scholar]
- Reza HM, Ogino H, Yasuda K. L-Maf, a downstream target of Pax6, is essential for chick lens development. Mechanisms of Development. 2002;116:61–73. doi: 10.1016/s0925-4773(02)00137-5. [DOI] [PubMed] [Google Scholar]
- Ring BZ, Cordes SP, Overbeek PA, Barsh GS. Regulation of mouse lens fiber cell development and differentiation by the Maf gene. Development. 2000;127:307–317. doi: 10.1242/dev.127.2.307. [DOI] [PubMed] [Google Scholar]
- Ryffel GU, Werdien D, Turan G, Gerhards A, Goosses S, Senkel S. Tagging muscle cell lineages in development and tail regeneration using Cre recombinase in transgenic Xenopus. Nucleic Acids Research. 2003;31:e44. doi: 10.1093/nar/gng044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T. Über bei der Cobitiden-Fischen. I. Misgurnus anguillicandatus (Cantor) Embryologia. 1961;6:251–290. [Google Scholar]
- Schaefer JJ, Oliver G, Henry JJ. Conservation of gene expression during embryonic lens formation and cornea-lens transdifferentiation in Xenopus laevis. Developmental Dynamics. 1999;215:308–318. doi: 10.1002/(SICI)1097-0177(199908)215:4<308::AID-AJA3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Schweisguth F. Regulation of notch signaling activity. Current Biology. 2004;14:R129–R138. [PubMed] [Google Scholar]
- Sehm T, Sachse C, Frenzel C, Echeverri K. miR-196 is an essential early-stage regulator of tail regeneration, upstream of key spinal cord patterning events. Developmental Biology. 2009;334:468–480. doi: 10.1016/j.ydbio.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Semina EV, Murray JC, Reiter R, Hrstka RF, Graw J. Deletion in the promoter region and altered expression of Pitx3 homeobox gene in aphakia mice. Human Molecular Genetics. 2000;9:1575–1585. doi: 10.1093/hmg/9.11.1575. [DOI] [PubMed] [Google Scholar]
- Shi XH, Bosenko DV, Zinkevich NS, Foley S, Hyde DR, Semina EV, Vihtelic TS. Zebrafish pitx3 is necessary for normal lens and retinal development. Mechanisms of Development. 2005;122:513–527. doi: 10.1016/j.mod.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Slack JMW, Beck CW, Gargioli C, Christen B. Cellular and molecular mechanisms of regeneration in Xenopus. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 2004;359:745–751. doi: 10.1098/rstb.2004.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack JMW, Lin G, Chen Y. The Xenopus tadpole: a new model for regeneration research. Cellular and Molecular Life Sciences. 2008;65:54–63. doi: 10.1007/s00018-007-7431-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow DB, Latinkic B, Mohun TJ. A simplified method of generating transgenic Xenopus. Nucleic Acids Res. 2000;28:E12. doi: 10.1093/nar/28.4.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemple DL. TILLING - a high-throughput harvest for functional genomics. Nature Reviews Genetics. 2004;5:145–U19. doi: 10.1038/nrg1273. [DOI] [PubMed] [Google Scholar]
- Stump RJW, Ang S, Chen YJ, von Bahr T, Lovicu FJ, Pinson K, de Iongh RU, Yamaguchi TP, Sassoon DA, McAvoy JW. A role for Wnt/beta-catenin signaling in lens epithelial differentiation. Developmental Biology. 2003;259:48–61. doi: 10.1016/s0012-1606(03)00179-9. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Thatcher EJ, Paydar I, Anderson KK, Patton JG. Regulation of zebrafish fin regeneration by microRNAs. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:18384–18389. doi: 10.1073/pnas.0803713105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinsley RC, Kobel HR. The Biology of Xenopus. Oxford, Great Briton: Clarendon Press; 1996. [Google Scholar]
- Tomarev SI. Pax-6, Eyes absent, and Prox 1 in eye development. International Journal of Developmental Biology. 1997;41:835–842. [PubMed] [Google Scholar]
- Tomarev SI, Sundin O, BanerjeeBasu S, Duncan MK, Yang JM, Piatigorsky J. Chicken homeobox gene Prox 1 related to Drosophila prospero is expressed in the developing lens and retina. Developmental Dynamics. 1996;206:354–367. doi: 10.1002/(SICI)1097-0177(199608)206:4<354::AID-AJA2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Tomarev SI, Zinovieva RD, Chang B, Hawes NL. Characterization of the mouse Prox1 gene. Biochemical and Biophysical Research Communications. 1998;248:684–689. doi: 10.1006/bbrc.1998.8989. [DOI] [PubMed] [Google Scholar]
- Tseng AS, Levin M. Tail regeneration in Xenopus laevis as a model for understanding tissue repair. Journal of Dental Research. 2008;87:806–816. doi: 10.1177/154405910808700909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng S, Sun T-T. Stem Cells: Occular surface maintenance. In: Brigthbill F, editor. Corneal Surgery: Theory, Technique Tisue. Mosby: St Louis; 1999. pp. 9–18. [Google Scholar]
- Tsonis PA. Regeneration in vertebrates. Developmental Biology. 2000;221:273–284. doi: 10.1006/dbio.2000.9667. [DOI] [PubMed] [Google Scholar]
- Tsonis PA. Stem cells from differentiated cells. Molecular Interventions. 2004;4 doi: 10.1124/mi.4.2.4. 81-+ [DOI] [PubMed] [Google Scholar]
- Tsonis PA, Call MK, Grogg MW, Sartor MA, Taylor RR, Forge A, Fyffe R, Goldenberg R, Cowper-Sallari R, Tomlinson CR. MicroRNAs and regeneration: Let-7 members as potential regulators of dedifferentiation in lens and inner ear hair cell regeneration of the adult newt. Biochemical and Biophysical Research Communications. 2007;362:940–945. doi: 10.1016/j.bbrc.2007.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsonis PA, Del Rio-Tsonis K. Lens and retina regeneration: transdifferentiation, stem cells and clinical applications. Experimental Eye Research. 2004;78:161–172. doi: 10.1016/j.exer.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Tsonis PA, Trombley MT, Rowland T, Chandraratna RAS, Del Rio-Tsonis K. Role of retinoic acid in lens regeneration. Developmental Dynamics. 2000;219:588–593. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1082>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Tsonis PA, Tsavaris M, Call MK, Chandraratna RAS, Del Rio-Tsonis K. Expression and role of retinoic acid receptor alpha in lens regeneration. Development Growth & Differentiation. 2002;44:391–394. doi: 10.1046/j.1440-169x.2002.00652.x. [DOI] [PubMed] [Google Scholar]
- Tsonis PA, Vergara MN, Spence JR, Madhavan M, Kramer EL, Call MK, Santiago WG, Vallance JE, Robbins DJ, Del Rio-Tsonis K. A novel role of the hedgehog pathway in lens regeneration. Developmental Biology. 2004;267:450–461. doi: 10.1016/j.ydbio.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Uwanogho D, Rex M, Cartwright EJ, Pearl G, Healy C, Scotting PJ, Sharpe PT. Embryonic Expression of the Chicken Sox2, Sox3 and Sox11 Genes Suggests an Interactive Role in Neuronal Development. Mechanisms of Development. 1995;49:23–36. doi: 10.1016/0925-4773(94)00299-3. [DOI] [PubMed] [Google Scholar]
- Vinarsky V, Atkinson DL, Stevenson TJ, Keating MT, Odelberg SJ. Normal newt limb regeneration requires matrix metalloproteinase function. Developmental Biology. 2005;279:86–98. doi: 10.1016/j.ydbio.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Wagner E, McCaffery P, Drager UC. Retinoic acid in the formation of the dorsoventral retina and its central projections. Developmental Biology. 2000;222:460–470. doi: 10.1006/dbio.2000.9719. [DOI] [PubMed] [Google Scholar]
- Wawersik S, Maas RL. Vertebrate eye development as modeled in Drosophila. Human Molecular Genetics. 2000;9:917–925. doi: 10.1093/hmg/9.6.917. [DOI] [PubMed] [Google Scholar]
- Wernig M, Meissner A, Foreman R, Brambrink T, Ku MC, Hochedlinger K, Bernstein BE, Jaenisch R. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318-U2. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- Wheeler GN, Hamilton FS, Hoppler S. Inducible gene expression in transgenic Xenopus embryos. Current Biology. 2000;10:849–852. doi: 10.1016/s0960-9822(00)00596-0. [DOI] [PubMed] [Google Scholar]
- Wigle JT, Chowdhury K, Gruss P, Oliver G. Prox1 function is crucial for mouse lens-fibre elongation. Nature Genetics. 1999;21:318–322. doi: 10.1038/6844. [DOI] [PubMed] [Google Scholar]
- Wolff G. Entwicklungsphyiologische Studien. I. Die Regeneration der Urodelenlinse. Wilhelm Roux’ Arch. Entwickl. Mech. Org. 1895;1:380–390. [Google Scholar]
- Wordinger RJ, Clark AF. Bone morphogenetic proteins and their receptors in the eye. Experimental Biology and Medicine. 2007;232:979–992. doi: 10.3181/0510-MR-345. [DOI] [PubMed] [Google Scholar]
- Wride MA. Cellular and molecular features of lens differentiation: A review of recent advances. Differentiation. 1996;61:77–93. doi: 10.1046/j.1432-0436.1996.6120077.x. [DOI] [PubMed] [Google Scholar]
- Yamada T. Control mechanisms in cell-type conversion in newt lens regeneration. Monogr Dev Biol. 1977;13:1–126. [PubMed] [Google Scholar]
- Yamada T. Transdifferentation of lens cells and its regulation. In: M DS, editor. Cell Biology of the Eye. New York: Academc Press; 1982. pp. 193–242. [Google Scholar]
- Yang EV, Bryant SV. Developmental Regulation of a Matrix Metalloproteinase During Regeneration of Axolotl Appendages. Developmental Biology. 1994;166:696–703. doi: 10.1006/dbio.1994.1348. [DOI] [PubMed] [Google Scholar]
- Yang EV, Gardiner DM, Carlson MRJ, Nugas CA, Bryant SV. Expression of Mmp-9 and related matrix metalloproteinase genes during axolotl limb regeneration. Developmental Dynamics. 1999;216:2–9. doi: 10.1002/(SICI)1097-0177(199909)216:1<2::AID-DVDY2>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Yang XH. Roles of cell-extrinsic growth factors in vertebrate eye pattern formation and retinogenesis. Seminars in Cell & Developmental Biology. 2004;15:91–103. doi: 10.1016/j.semcdb.2003.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin VP, Thomson JM, Thummel R, Hyde DR, Hammond SM, Poss KD. Fgf-dependent depletion of microRNA-133 promotes appendage regeneration in zebrafish. Genes & Development. 2008;22:728–733. doi: 10.1101/gad.1641808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshii C, Ueda Y, Okamoto M, Araki M. Neural retinal regeneration in the anuran amphibian Xenopus laevis post-metamorphosis: Transdifferentiation of retinal pigmented epithelium regenerates the neural retina. Developmental Biology. 2007;303:45–56. doi: 10.1016/j.ydbio.2006.11.024. [DOI] [PubMed] [Google Scholar]
- Zaghloul NA, Yan B, Moody SA. Step-wise specification of retinal stem cells during normal embryogenesis. Biology of the Cell. 2005;97:321–337. doi: 10.1042/BC20040521. [DOI] [PubMed] [Google Scholar]
- Zhang SSM, Fu XY, Barnstable CJ. Molecular aspects of vertebrate retinal development. Molecular Neurobiology. 2002;26:137–152. doi: 10.1385/MN:26:2-3:137. [DOI] [PubMed] [Google Scholar]
- Zuber ME, Gestri G, Viczian AS, Barsacchi G, Harris WA. Specification of the vertebrate eye by a network of eye field transcription factors. Development. 2003;130:5155–5167. doi: 10.1242/dev.00723. [DOI] [PubMed] [Google Scholar]
- Zygar CA, Cook TL, Grainger RM. Gene activation during early stages of lens induction in Xenopus. Develo. 1998 doi: 10.1242/dev.125.17.3509. [DOI] [PubMed] [Google Scholar]