Background: Plasmodium falciparum invades red blood cells by the binding of specific parasite ligands to host cell receptors.
Results: We have solved the three-dimensional structure of the erythrocyte-binding Duffy binding-like domain of an important red blood cell-binding protein.
Conclusion: The architecture of this protein is distinct from other related proteins.
Significance: This has provided insights into the function of this protein and has enabled us to map a receptor-binding site.
Keywords: Infectious Diseases, Invasion, Ligand-binding Protein, Malaria, Structural Biology
Abstract
Invasion of human red blood cells by Plasmodium falciparum involves interaction of the merozoite form through proteins on the surface coat. The erythrocyte binding-like protein family functions after initial merozoite interaction by binding via the Duffy binding-like (DBL) domain to receptors on the host red blood cell. The merozoite surface proteins DBL1 and -2 (PfMSPDBL1 and PfMSPDBL2) (PF10_0348 and PF10_0355) are extrinsically associated with the merozoite, and both have a DBL domain in each protein. We expressed and refolded recombinant DBL domains for PfMSPDBL1 and -2 and show they are functional. The red cell binding characteristics of these domains were shown to be similar to full-length forms of these proteins isolated from parasite cultures. Futhermore, metal cofactors were found to enhance the binding of both the DBL domains and the parasite-derived full-length proteins to erythrocytes, which has implications for receptor binding of other DBL-containing proteins in Plasmodium spp. We solved the structure of the erythrocyte-binding DBL domain of PfMSPDBL2 to 2.09 Å resolution and modeled that of PfMSPDBL1, revealing a canonical DBL fold consisting of a boomerang shaped α-helical core formed from three subdomains. PfMSPDBL2 is highly polymorphic, and mapping of these mutations shows they are on the surface, predominantly in the first two domains. For both PfMSPDBL proteins, polymorphic variation spares the cleft separating domains 1 and 2 from domain 3, and the groove between the two major helices of domain 3 extends beyond the cleft, indicating these regions are functionally important and are likely to be associated with the binding of a receptor on the red blood cell.
Introduction
The recognition of host receptors by specific parasite ligands is a critical step in the pathogenic cycle of invasion and growth of Plasmodium parasites. Proteins belonging to the erythrocyte binding-like (EBL)4 superfamily are known to play key roles in the complex series of interactions required for merozoite invasion of erythrocytes (reviewed in Ref. 1). The defining feature that classifies members of this superfamily is the Duffy-binding-like (DBL) domain, so called from its identification in Plasmodium knowlesi and Plasmodium vivax as the ligand that binds the Duffy antigen receptor for chemokines (DARC) (2, 3). DBL domains are encoded in many genes within the Plasmodium falciparum genome and are found in both EBL and var genes (4). These domains are responsible for diverse pathogenic phenotypes, including sequestration of infected erythrocytes in various tissues (5) and rosetting (6), as well as erythrocyte invasion (1).
For invasion-related proteins, two types of EBLs have been identified in the genus, having either a single or double tandemly repeated DBL domain (7). In P. knowlesi and P. vivax, a single DBL-encoding gene exists (PkDBP and PvDBP), possessing a single DBL domain, which binds to the DARC receptor as a dimerized form (3, 8). The P. falciparum EBL ligands, which bind to sialic acid residues of distinct erythrocyte surface proteins, are erythrocyte-binding antigen-175 (EBA-175), which binds to glycophorin A (9), EBA-140 (also known as Baebl), which binds to glycophorin C (10–12), and EBA-181 (also known as Jsebl), whose receptor is unknown (13). In each of these P. falciparum proteins, two tandem DBL domains are present.
Two hypothetical proteins containing single DBL domains have been identified in P. falciparum (PF10_0348 and PF10_0355). Both proteins have been localized to the merozoite surface (14, 15), and functional studies have shown that the full-length form of the PF10_0348 protein has erythrocyte binding activity when expressed on the surface of COS cells (14). Furthermore, PF10_0355 was recently identified in a genome-wide association study designed to highlight putative genes involved in anti-malarial drug resistance and has been associated with resistance to halofantrine (16). Population genomic analysis has revealed a large number of polymorphisms in both genes, particularly within the dbl region, and that both genes are subject to strong balancing selection, an indicator that they are likely to be under intense immune pressure, consistent with their presence on the surface of the merozoite (17).
These DBL-containing merozoite surface proteins are of interest as potential malaria vaccine candidates, and as such, it will be useful to obtain a better understanding of their functional role during parasite invasion. Given the very polymorphic nature of these proteins and the functional importance of the DBL domain, a structural analysis would be informative to understand how they bind to their erythrocyte receptors. The crystal structures for both single and tandemly arranged DBL domains of PvDBP, PkDBP, and EBA-175, respectively, have been solved (3, 8, 18). In both cases, a dimer organization of the domain appears to be critical in receptor binding.
In this study, we characterize two closely related merozoite surface proteins, PfMSPDBL1 (PF10_0348) and -2 (PF10_0355), and show that erythrocyte binding occurs through the DBL domains of these proteins in a manner enhanced by the presence of specific metal ion cofactors. We have also derived the crystal structure of the erythrocyte-binding DBL domain of PfMSPDBL2 to 2.09 Å resolution.
EXPERIMENTAL PROCEDURES
Cloning, Protein Production, and Purification
Synthetic genes, codon-optimized for expression in Escherichia coli, were produced corresponding to residues Lys-161 to Leu-457 in PfMSPDBL2 and residues Lys-143 to Asp-443 in PfMSPDBL1 (Geneart). Each gene was cut from the supplier's vector using BamHI and XhoI and then ligated into the pProExHTb vector (Invitrogen), which added a cleavable N-terminal hexahistidine fusion tag. The vectors were then transformed into E. coli strain BL21 (DE3) for expression of the recombinant DBL domains. Bacteria were grown in super broth at 37 °C to an A600 nm of 0.5. Expression was induced with 1 mm isopropyl 1-thio-β-d-galactopyranoside, and bacteria were grown for a further 3 h at 37 °C. Both proteins were deposited as insoluble inclusion bodies. Cells were lysed by sonication, and the inclusion bodies solubilized by the addition of 6 m guanidine-HCl, pH 8.0. The solubilized proteins were purified by metal-chelating chromatography using a nickel-nitrilotriacetic acid column (Qiagen) and eluted in an 8 m urea, pH 8.0, solution containing 1 m imidazole. Each protein was refolded at room temperature for 2 days in 2 m urea, 100 mm NaCl, 20 mm Tris, pH 8.0, with 1 mm reduced and 1 mm oxidized glutathione to facilitate disulfide bond formation.
The refolded PfMSPDBL2 DBL domain was further purified using anion-exchange chromatography. Buffer A was composed of 20 mm Tris, pH 8.0, 250 mm urea, and buffer B was composed of 20 mm Tris, pH 8.0, 250 mm urea, 1 m NaCl. Bound proteins were eluted from a 1-ml Hitrap Q column (GE Healthcare) using a linear gradient from 1 to 50% B, and the relevant fractions were pooled and buffer-exchanged into 20 mm BisTris, pH 6.50, 20 mm NaCl and then concentrated to 3 mg/ml for crystallization. The PfMSPDBL1 DBL domain was further purified using cation-exchange chromatography. Buffer A was composed of 20 mm MES, pH 6.5, 250 mm urea, and buffer B was composed of 20 mm MES, pH 8.0, 250 mm urea, 1 m NaCl. Bound proteins were eluted from a 1-ml Hitrap S column (GE Healthcare) using a linear gradient from 1–50% B, and the relevant fractions were pooled and buffer-exchanged into 20 mm MES, pH 6.5, 20 mm NaCl and then concentrated to 3 mg/ml for crystallization trials. The majority of the N-terminal fusion tag was removed from the expressed proteins using TEV protease (Invitrogen). However, the five most C-terminal residues from the vector (GAMGS) on the P′ side of the TEV cleavage remained attached to the DBL domains at their N terminus.
Proteins were electrophoresed using 4–12% BisTris precast gels (Invitrogen) in the presence of DTT-reduced or nonreduced sample buffer. Proteins were electrophoretically transferred onto PVDF membrane using an iBlot apparatus (Invitrogen). Protein G-purified pooled hyperimmune sera, from individuals living in malaria endemic regions in PNG, were used to detect proteins on immunoblots.
Reverse Phase-HPLC analyses of the refolded or denatured forms of PfMSPDBL2 and PfMSPDBL1 DBL domains were performed using an Agilent 1100 LC system. Buffer A consisted of 0.05% (v/v) TFA (HPLC/Spectro grade, Pierce) in Milli-Q grade water (Millipore, Bedford, MA); buffer B c consisted of 0.05% (v/v) TFA in acetonitrile (ChromAR HPLC grade, Mallinckrodt, Paris, KY). Samples were loaded onto a C8 column (2.1 mm inner diameter × 100-mm (Brownlee columns, PerkinElmer Life Sciences)) in the presence of buffer A. Bound proteins were eluted using a linear gradient of 0–100% B over 12 min at a flow rate of 0.5 ml/min.
Reduced and alkylated forms of the DBL domain for PfMSPDBL1 and PfMSPDBL2 were produced by incubating with 50 mm DTT for 1 h at room temperature and then adding iodoacetic acid to a final concentration of 250 mm and leaving the solution for 1 h in the dark at room temperature. Proteins were then dialyzed into 2 m urea, 150 mm NaCl, 20 mm Tris, pH 8.0.
Generation of HA-tagged PfMSPDBL1 and PfMSPDBL2 Parasite Lines
To attach a triple HA tag (HA3) to the 3′ end of the Pfmspdbl1 (MSPDBL1) gene, an 834-bp fragment of Pfmspdbl1 was amplified from 3D7 genomic DNA using the primers 5′-ATATCCGCGGAATGTGATTGTAAATATAAAG-3′ and 5′-GAACCTCGAGTTTTTGAAATAAATCTGTC-3′ (SacII and XhoI restriction sites underlined). Similarly, to attach an HA3 tag to the 3′ end of the Pfmspdbl2 (Pfmspdbl2) gene, a 922-bp fragment of PfMSPDBL1 was amplified from 3D7 genomic DNA using the primers 5′-ATTCCGCGGAAAAGCTTTATTAGAAAAG-3′ and 5′-ATTACTCGAGATTTTTAAATAAATTTGTAATATC-3′. The DNA fragments were digested with SacII and XhoI and cloned into pHAST, a derivative of pGEM-3Z containing an HA3 tag and a Strep II tag in tandem. Parasites were transfected as described previously (19). Successful integration of the HA3 tag was determined by Western and Southern blotting.
Red Blood Cell Binding Assay Using HA-tagged PfMSPDBL1 and PfMSPDBL2 Derived from Post-invasion Culture Supernatants
For PfMSPDBL1-HA and PfMSPDBL2-HA RBC binding assays, 200 μl of red blood cells (type O+) from three donors were washed twice at room temperature (RT) with 10 ml of RPMI/Hepes containing 0.2% NaHCO3 (RHN buffer), followed by centrifugation at 1500 rpm for 5 min. Red blood cells were a gift from the Red Cross Blood Service (Melbourne, Australia). For enzyme-treated red blood cells, erythrocytes were incubated with trypsin (bovine pancreas l-1-tosylamido-2-phenylethyl chloromethyl ketone-treated, 1.5 mg/ml in RHN buffer) and neuraminidase (Vibrio cholera 0.06 units/ml (final) in RHN buffer) for 1 h at room temperature to digest red blood cell surface proteins or sialic acid residues, respectively. Cells were then washed for 15 min at room temperature with RHN + 0.5 mg/ml soybean trypsin inhibitor or RHN only buffers (for trypsin-treated or neuraminidase treated cells, respectively). The untreated and enzyme-treated red blood cells were then resuspended in 10 ml of post-invasion culture supernatant containing 1 mm Ca2+ and either PfMSPDBL1-HA, PfMSPDBL2-HA, or control supernatant lacking HA-tagged PfMSPDBL proteins, and incubated with gentle shaking for 2 h at room temperature. The culture supernatant was removed by centrifugation, and the red blood cells were layered onto 400 μl of oil (Dow Corning) and spun for 30 s at 10,000 rpm at room temperature in a microcentrifuge. The culture supernatant and oil were removed, and the red blood cells were washed with 1 ml of RHN buffer. The RHN buffer was discarded, and the red blood cells were resuspended in 200 μl of elution buffer (50 mm Tris-HCl, pH 7.6, 1.5 m NaCl) and incubated at room temperature for 10 min with gentle shaking before centrifuging at 10,000 rpm for 30 s. The eluate was subjected to an additional centrifugation step at a maximum speed for 3 min to pellet any red blood cells present. The supernatant was retained, and the protein was precipitated using a mixture of methanol/chloroform/water (20), resuspended in SDS-PAGE sample buffer, and subjected to Western blotting. RBC binding assays for PfMSPDBL1-HA and PfMSPDBL2-HA were carried out with or without 1 mm CaCl2 added to the culture supernatant.
RBC Binding Assay Using Recombinant DBL Domains for PfMSPDBL1 and PfMSPDBL2
Assays using recombinant DBL domains for PfMSPDBL1 and PfMSPDBL2 were carried out as described above with several modifications. Recombinant proteins (10 μg/assay) were made up to 300 μl with the filtrate that was used for their concentration during purification and combined with 300 μl of RHN buffer to give a total volume of 600 μl. This volume was incubated with 200 μl of red blood cells to allow binding to occur. For some assays, 5 mm Ca2+ or other metal ions (5 mm, final concentration) were included in the assay. It was not necessary to concentrate the protein in the final high salt eluate for analysis of binding by Western blot.
FACS-based RBC Binding Assay Using the Recombinant DBL Domain for PfMSPDBL1
This assay was modified from a previously described protocol (21, 22). Trypsin- and neuraminidase-treated red blood cells were prepared as described above. In addition, chymotrypsin-treated red blood cells were produced in a similar manner to that of trypsin-treated red blood cells, using chymotrypsin (1-chloro-3-tosylamido-7-amino-2-heptanone-treated) at 1.5 mg/ml final concentration. Erythrocytes were washed three times with RHN and then diluted to a final volume of 1 × 107 cells/ml. For binding, the recombinant DBL domain of MSPDBL1 was incubated at 0.15 mg/ml for 1 h at room temperature in RHN buffer containing 1 mm Ca2+ (CaCl2). Post binding, erythrocytes were washed twice with 200 μl of PBS, 1% BSA and then incubated with rabbit serum containing α MSPDBL1-DBL domain antibodies diluted 1:100 in PBS, 1% BSA buffer. After an hour of incubation, the erythrocytes were washed two times with 200 μl of PBS, 1% BSA and incubated with goat α-rabbit Alexa Fluor 488-conjugated secondary antibodies (diluted 1:200 in PBS, 1% BSA, Molecular Probes) for 1 h. Samples were washed four times and resuspended in 400 μl of PBS, 0.1% BSA. 50,000 events were acquired per sample using CellQuest Software (BD Biosciences) on the FACs Calibur flow cytometer (BD Biosciences). The data were analyzed using Flowjo 8.8.7 (Treestar) where the Alexa Fluor 488 fluorescently labeled proteins bound to erythrocytes were calculated as a percentage over unbound erythrocytes. Recombinant PfRh4.9 protein was a generous gift from Dr. Wai-Hong Tham (Walter and Eliza Hall Institute). This protein was used as an additional control as its red blood cell binding behavior is sensitive to trypsin treatment but not neuraminidase treatment of erythrocytes. Recombinant PfRh4.9, in RHN buffer, was incubated with red blood cells at 0.1 mg/ml for 1 h at room temperature and then assayed as per the DBL domain of MSPDBL1. PfRh4.9 binding to erythrocytes was detected using an anti-PfRh4 IgG antibody, followed by a secondary anti-rabbit Alexa Fluor 488 antibody (21).
To demonstrate the specific effect of Ca2+ on the binding of the DBL domain of MSPDBL1 to red blood cells, RHN buffer containing protein and 1 mm CaCl2 was supplemented with increasing amounts of EGTA over a range from 0 to 60 mm.
Crystallization Diffraction Data and Structure Determination
Crystals obtained in initial sparse matrix screens with His-tagged protein diffracted relatively poorly, and diffraction was often not reproducible. Removal was effected by adding TEV protease (1–50 volume ratio) to the protein sample for 3 h prior to setting up drops, leaving five residues (GAMGS) from the vector attached to the N terminus of the DBL domain. The protein crystallized out of a variety of conditions containing medium weight polyethylene glycols and salt; the crystal used for data collection was crystallized at a protein concentration of 2 mg/ml from 20% PEG3350, 0.2 m sodium thiocyanate. In an attempt at derivatization, 0.8 μl of well solution and 0.2 μl of 10 mm KAuCl4 was added to the crystals 24 h prior to mounting and cryocooling; however, we found no evidence for derivatization in either the anomalous signal nor in the final maps. The crystal used for data collection was cryoprotected by quickly soaking in 25% PEG3350, 15% ethylene glycol, 0.2 m NaCl, and cryocooled by plunging into liquid nitrogen.
X-ray datasets were collected at the Australian Synchrotron on beam line MX2 at 100 K. Data were processed with HKL2000 (23), and the structure was solved by molecular replacement with PHASER (24) using a BALBES (25) model based on the structure of PfEMP1 VAR2CSA DBL 6e (PDB code 2WAU). Initial maps revealed little density beyond the molecular replacement model, and multiple attempts at building and refinement neither enhanced maps nor reduced Rwork/Rfree. Multicrystal averaging was subsequently performed using DMMULTI from the CCP4 suite (26) incorporating data from a second lower resolution crystal form (P21212, a = 61.6, b = 89.6, c = 60.8 Å; resolution 2.7 Å) grown in crystal drops lacking TEV protease and thus retaining vector-derived sequence. The subsequent maps were significantly improved permitting manual model building in COOT (27) and refinement in PHENIX (28). The final refinement statistics are shown in Table 1.
TABLE 1.
Crystallographic statistics
| Data collection | Nativea | Zn2+a |
|---|---|---|
| Space group | P21 | P21 |
| Cell dimensions | a = 36.6 Å, b = 101.5 Å, c = 38.4 Å, β = 101.6° | a = 36.7 Å, b = 102.1 Å, c = 38.2 Å, β = 101.8° |
| Wavelength | 1.0163 Å | 1.2718 Å |
| Resolution range | 50.0 to 2.09 Å (2.16 to 1.09 Å)b | 50.0 to 2.11 Å (2.24 to 2.11 Å) |
| Rmerge | 0.074 (0.489) | 0.085 (0.683) |
| I/σI | 19.23 (2.4) | 12.33 (2.15) |
| Completeness | 99.7% (97.5%) | 99.1% (95.7%) |
| Redundancy | 4.6 (4.0) | 3.7 (3.6) |
| Refinement | ||
| Resolution | 35.9 to 2.09 (2.25 to 2.09) | 37.4 to 2.11 (2.25 to 2.11) |
| No. of reflections Rwork | 15,167 (2552) | 14,918 (2434) |
| No. of reflections Rfree | 765 (133) | 791 (125) |
| Rwork | 0.18 (0.24) | 0.19 (0.27) |
| Rfree | 0.24 (0.31) | 0.24 (0.35) |
| No. of atoms | ||
| Protein | 2233 | 2287 |
| Water/ions | 55 | 74 |
| Root mean square deviations | ||
| Bond lengths | 0.008 Å | 0.018 Å |
| Bond angles | 1.0° | 1.0° |
| Ramachandran | ||
| Favored | 96.2% | 97.5% |
| Allowed | 3.8% | 2.5% |
| Rotamer outliers | 0.0% | 2.0% |
a The PDB codes for the native structure and with coordinated Zn2+ are 3VUU and 3VUV.
b Numbers in parentheses represent statistics for highest resolution shell.
Crystals of the structure determined for the DBL domain of PfMSPDBL2 in the presence of Zn2+ were obtained from a protein preparation of a larger refolded recombinant fragment of PfMSPDBL2.5 During crystallization trials, this protein was processed by an unknown protease leaving only the DBL domain of PfMSPDBL2. Crystals grew from this preparation in the same crystallization conditions described above for DBL domain and were of the same space group. Crystals were soaked for 5 min in mother liquor supplemented with 50 mm ZnCl2 and then washed in mother liquor prior to cryopreservation. X-ray datasets were collected at the Australian Synchrotron on beam line MX2 at 100 K and at a wavelength near the zinc absorption edge (1.2781 Å). The structure was solved by molecular replacement using PHASER (24) with the model of the DBL domain of PfMSPDBL2 as a search model. Model building was performed in COOT (27) with refinement performed, and anomalous difference maps were generated, with PHENIX (28).
Structural Comparison
Structural alignments of the DBL domains from PF10_0355, PfEBA175 (F1 and F2) (PDB code 1ZRO), PkDBP (PDB code 2C6J), PvDBP (PDB code 3RRC), PfEMP1-NTS-DBL1a-VarO (PDB code 2XU0), PfEMP1 VAR2CSA DBL3X (PDB codes 3BQI/3CML), PfEMP1 VAR2CSA DBL6 (PDB code 2WAU), and the modeled structure for Pf332 DBL domain (29) were performed using the Sequoia program (30).
Modeling of the PfMSPDBL1 Structure
The structure of the PfMSPDBL2 DBL domain was used as a template in comparative modeling of the corresponding DBL domain region in PfMSPDBL1 using the MODELLER (9, Version 7) program (31) based on the sequence alignment presented in supplemental Fig. S1. From 25 initial models, the model with the lowest modeler objective function was used for further loop modeling. Two loops missing the electron density of the structure of PfMSPDBL2 (residues 172–185, and 375–387) were refined using the loop modeling utility, a part of the MODELLER package; the final model yielded the lowest modeler objective function from 25 loop refinement models.
Molecular electrostatics were calculated using the MEAD program (32) using PARSE atomic charges and radii (33), an internal dielectric of 4.0, external dielectric of 78, and an ionic strength of 0.10. The molecular surface was calculated using the MSMS program (34). The molecular electrostatic surface of PfMSPDBL2 was calculated on a model in which the loops missing in the x-ray crystal structural were included using the MODELLER program.
RESULTS
PfMSPDBL2 Is a Red Cell-binding Protein on the Merozoite Surface That Contains DBL and SPAM Domains
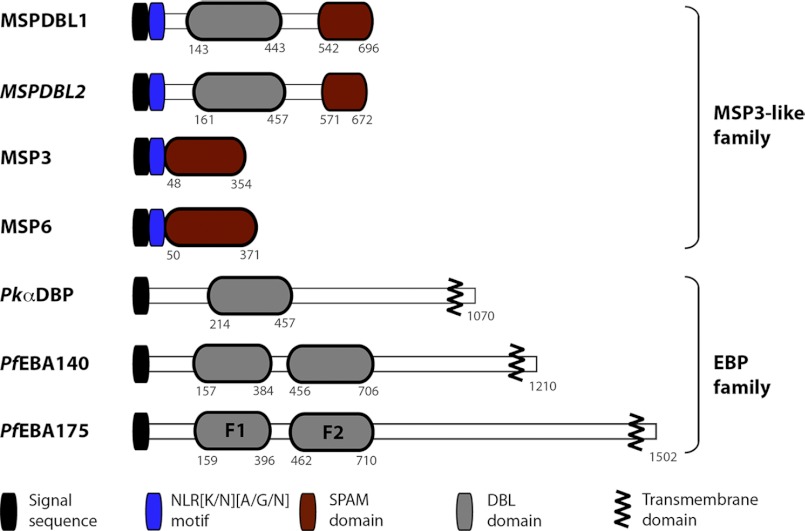
The merozoite surface protein PfMSPDBL1 (PF10_0348), which binds the erythrocyte surface, consists of DBL and SPAM domains (residues 542–696 in PfMSPDBL1 and residues 571–672 in PfMSPDBL2) (14), and the SPAM domain is characterized by an acid-rich region followed by a leucine zipper-like region (Fig. 1). In all members of the MSP3-like family and in both PfMSPDBLs, there is also a conserved sequence motif of NLR(K/N)(A/G/N) near the N terminus whose significance is not known. Because it lacks a transmembrane region or a glycosylphosphatidylinositol-anchoring motif, PfMSPDBL1 is extrinsically associated with the merozoite surface (Fig. 1). A second gene (PF10_0355) that is closely linked on the same chromosome encodes a protein of similar structure, also consisting of a single DBL and SPAM domain. We henceforth refer to this latter protein as PfMSPDBL2 and the former (PF10_0348) as PfMSPDBL1 (Fig. 1). The sequence identity and similarity between the DBL domains of PfMSPDBL2 and PfMSPDBL1 is 38 and 62%, respectively (supplemental Fig. S1), whereas for the SPAM domain it is 30 and 53%, respectively. The similar domain structure and significant sequence homology between PfMSPDBL1 and PfMSPDBL2 suggest that they are both involved in the interaction of the merozoite with the red blood cell (Fig. 1).
FIGURE 1.
Structure of PfMSPDBL1 and -2, MSP3, and EBL proteins. Schematic shows the important structural characteristics for PfMSPDBL1 and -2 in relation to the MSP3 and EBL protein families.
To further analyze PfMSPDBL2 and PfMSPDBL1, we transfected the 3D7 strain of P. falciparum with a plasmid that, when inserted by homologous recombination, would place a sequence encoding a hemagglutinin (HA) tag at the 3′ end of the pfmspdbl1 and pfmspdbl2 genes (3D7MSPDBL1-HA and 3D7MSPDBL2-HA). Immunoblots with anti-HA antibodies confirmed that both 3D7MSPDBL1-HA- and 3D7MSPDBL2-HA-modified parasite lines expressed full-length forms of HA-tagged PfMSPDBL1 and PfMSPDBL2, respectively (Fig. 2A). Interestingly, parasite-derived PfMSPDBL1 and -2 harvested from invasion supernatants was largely observed as a processed form that was ∼10–15 kDa smaller than the larger forms. This processing must occur at the N terminus as the HA tag used for recognition remained attached to the C terminus of these proteins that were expressed in transfected parasites. Similar processing of parasite-derived PfMSPDBL1 has also been observed previously; however, the orientation of the cleavage event could not be determined by the antibodies used for detection of this protein (14). PfSUB1 cleavage sites have been predicted near the N terminus of PfMSPDBL1 and -2, and cleavage at these sites would result in processed forms of similar size to those observed in Fig. 2A (35). Similar processing was also observed in wild type parasites (supplemental Fig. S2). Saponin lysis of late-stage schizonts revealed that the majority of the PfMSPDBL proteins were found in a soluble and unprocessed form. However, the processed forms of these proteins were found only in the insoluble pellet fraction, suggesting that processing is required before they can be incorporated onto the merozoite surface (supplemental Fig. S2).
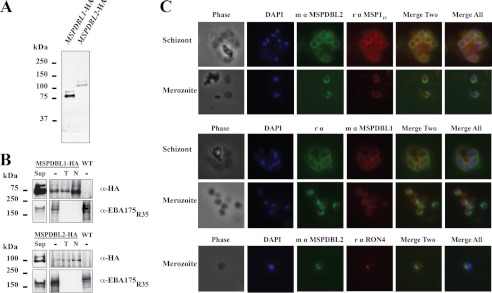
FIGURE 2.
Expression, red cell binding, and surface localization of parasite-derived PfMSPDBL1 and -2. A, HA tagging of endogenous PfMSPDBL1 and PfMSPDBL2 proteins. Triple HA tags (HA3) were attached to the 3′ end of the PfMSPDBL1 and PfMSPDBL2 genes as described under “Experimental Procedures.” Full-length forms of the HA-tagged proteins were detected on immunoblots using a mouse anti-HA antibody. B, parasite-derived HA-tagged PfMSPDBL1 and PfMSPDBL2 proteins bind to red blood cells in an enzyme-independent manner. Post-invasion culture supernatants (Sup) from parasites expressing endogenously HA-tagged PfMSPDBL proteins or from wild type (WT) parasites, were incubated with untreated red blood cells (−) or cells treated with either trypsin (T) or neuraminidase (N) as described under “Experimental Procedures.” Bound proteins were eluted and detected on immunoblots using mouse anti-HA antibodies. Rabbit anti-EBA175 antibodies were used to confirm that the enzyme treatments had effectively removed selective red blood cell surface receptors. C, PfMSPDBL1 and -2 localize to the merozoite surface. Immunofluorescence assays of late stage schizonts and merozoites were prepared from P. falciparum (3D7 line) parasites probed with either rabbit or mouse polyclonal antibodies made to PfMSPDBL2 and marker proteins MSP119 (1st and 2nd rows), PfMSPDBL1 (3rd and 4th rows), or RON4 (5th row). 1st column, phase view; 2nd column, DAPI stained parasites; 3rd column, localization of PfMSPDBL2 (green); 4th column, localization of marker protein (red); 5th column, the merge of 3rd and 4th columns, and 6th column, the merge of columns 2–4.
To confirm erythrocyte binding activity of native parasite protein PfMSPDBL1 and to determine whether PfMSPDBL2 was also able to bind human erythrocytes, C-terminal HA-tagged protein was harvested from culture supernatants and tested in red blood cell binding assays (Fig. 2A). Both PfMSPDBL1 and PfMSPDBL2 parasite-derived proteins bound red blood cells. Binding of both ligands was consistently resistant to trypsin and neuraminidase treatment of the red blood cells (Fig. 2B). This was in contrast to binding of EBA-175 on the same enzyme-treated erythrocytes, which was sensitive to both enzyme treatments as described previously (36). The binding phenotype observed for parasite-derived PfMSPDBL1 differs from that previously reported for this protein expressed on the surface of COS-7 cells (14). However, it was clear that PfMSPDBL2 is also a P. falciparum invasion ligand that binds to human erythrocytes suggesting that it plays a direct role in merozoite invasion. PfMSPDBL1 and -2 were also found to have identical red blood cell binding phenotypes under the conditions used.
The location of PfMSPDBL2 on the merozoite surface was determined using immunofluorescence assays with anti-PfMSPDBL2 antibodies. It showed strong colocalization with the known merozoite surface protein merozoite surface protein 1 (MSP1) in both schizont and free merozoite stages (Fig. 2C, 1st and 2nd rows). PfMSPDBL2 colocalized with PfMSPDBL1 in mature schizont stages, both giving a “bunch of grapes” pattern, consistent with their presence on the merozoite surface (Fig. 2C, 1st and 3rd rows). They were also colocalized on the surface of free merozoites, although PfMSPDBL2 appeared to be more concentrated at the apical end of the cell as indicated by its close apposition to RON4, a protein located in the neck of the rhoptries (Fig. 2C, bottom row) (37). This confirmed previous work showing that PfMSPDBL1 was located on the merozoite surface and also demonstrated that PfMSPDBL2 had a similar localization consistent with them having similar functions (14).
Recombinant DBL Domains of PfMSPDBL1 and PfMSPDBL2 Bind Human Red Blood Cells
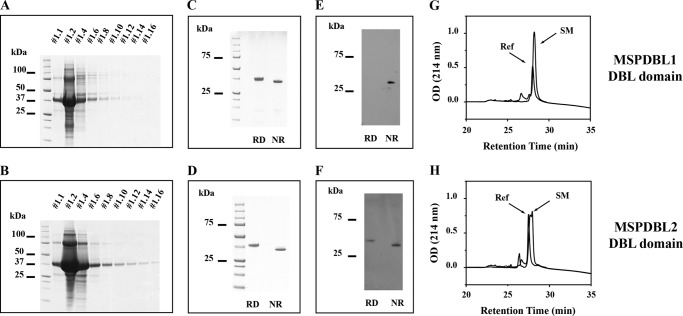
To further analyze the binding of PfMSPDBL1 and PfMSPDBL2 to red blood cells, we produced recombinant forms of the DBL domains for both proteins in E. coli. These expressed proteins were found in inclusion bodies that were separated and dissolved in 6 m guanidinium hydrochloride, purified under denaturing conditions by nickel-nitrilotriacetic acid immobilized metal affinity chromatography, oxidatively refolded, and further purified by ion-exchange chromatography (Fig. 3, A–D). Differential migration was observed for both refolded proteins on SDS-PAGE under reducing and nonreducing conditions indicating an influence of disulfide bond architecture on the shape and/or SDS-binding ability of these domain fragments (Fig. 3, C and D). Attainment of native conformational epitopes in the in vitro refolded DBL domains was demonstrated using immunoblots. The DBL domains of MSPDBL1 and -2 were electrophoresed in sample buffer with or without DTT and transferred onto PVDF membranes, and the immunoblots were probed with antibodies from pooled hyperimmune sera obtained from adult individuals living in malaria endemic regions of Papua, New Guinea (Fig. 3, E and F). The ability of these antibodies to recognize the DBL domains was largely dependent on the existence of epitopes with a conformation stabilized by disulfide bonds. Antibodies targeting the PfMSPDBL1 DBL domain reacted exclusively with disulfide bond-dependent epitopes (Fig. 3E), whereas those targeting the PfMSPDBL2 DBL domain largely recognized conformational epitopes, but additionally linear epitopes were also recognized in the DTT-reduced sample (Fig. 3F). Reverse phase-HPLC was used to demonstrate a decrease in hydrophobicity because each of the DBL domains had occurred as a result of the oxidative in vitro refolding process (Fig. 3, G and H). The refolded material eluted significantly earlier than the denatured starting material consistent with internalization of hydrophobic residues upon refolding (Fig. 3, G and H). The monomeric forms of these DBL domains were found to be stable for extended periods at 4 °C, further indicating no reactive surface-accessible Cys residues were present in the final products.
FIGURE 3.
Expression and purification of the DBL domains from PfMSPDBL2 and PfMSPDBL1. A and B show the elution profiles for PfMSPDBL1 and PfMSPDBL2, respectively, from nickel-nitrilotriacetic acid resin under denaturing conditions. C and D show SDS-PAGE analyses for the refolded DBL domains of PfMSPDBL1 and PfMSPDBL2, respectively, after ion-exchange purification. Samples were electrophoresed in sample buffer with (RD) or without (NR) reducing agent. E and F show immunoblots for PfMSPDBL1 and PfMSPDBL2 DBL domains, respectively, probed with IgG obtained from pooled hyperimmune sera from malaria endemic regions of PNG. Proteins were electrophoresed with (RD) or without (NR) reducing agent in the sample buffer prior to transfer onto PVDF membrane. G and H show reverse phase-HPLC profiles for the denatured (SM) and refolded (50) DBL domains for PfMSPDBL1 and PfMSPDBL2. See “Experimental Procedures” for additional information.
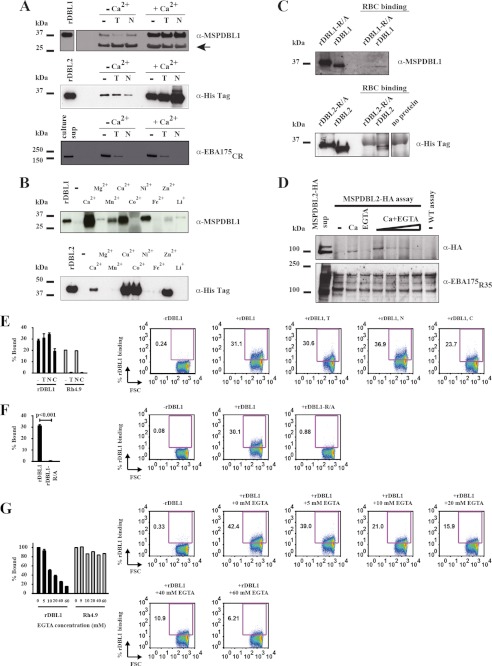
Recombinant PfMSPDBL1 and PfMSPDBL2 DBL domains were functional in traditional red blood cell binding assays in which bound proteins are eluted from the cells, after passage through oil, and detected on immunoblots. Both DBL domains demonstrated a binding phenotype that was insensitive to treatment of erythrocytes with either trypsin or neuraminidase (Fig. 4A). Futhermore, the addition of Ca2+ to these assays was found to enhance the level of binding for both the parasite-derived, full-length (Figs. 2B and 4D), and recombinant DBL domains of PfMSPDBL1 and PfMSPDBL2 (Fig. 4A), although the effect could also be reversed in the presence of EGTA (Fig. 4D). However, this increased level of binding was only induced by the presence of specific metal ions, with Ca2+, Cu2+, and Ni2+ having the greatest effect for PfMSPDBL1, and Cu2+, Co2+, Zn2+, and Ca2+ having the greatest effect for PfMSPDBL2 (Fig. 4B). In addition, binding was found to be dependent on a disulfide bond-stabilized conformation within the DBL domains of MSPDBL1 and -2, as reduced and alkylated forms of these proteins could not bind red blood cells (Fig. 4C).
FIGURE 4.
Red blood cell binding of recombinant DBL domains from PfMSPDBL1 and -2. A, recombinant DBL domains from PfMSPDBL1 and -2 proteins (rDBL1 and rDBL2, respectively) bind to red blood cells in an enzyme-independent manner with binding enhanced in the presence of Ca2+. Recombinant DBL domains were incubated with untreated red blood cells (−) or cells treated with either trypsin (T) or neuraminidase (N) in the presence or absence of 5 mm Ca2+, as described under “Experimental Procedures.” After passage through oil, the bound proteins were eluted with high salt, subjected to Western transfer, and then detected on immunoblots with either rabbit anti-PfMSPDBL1 or a mouse monoclonal antibody directed against the hexaHis tag of the recombinant PfMSPDBL2 DBL domain. For rDBL1 (top blot), lower bands were additionally shown (arrow) in the immunoblot as an indication of sample loading. These bands were of red blood cell protein origin and found to react only with the secondary sheep anti-rabbit IgG HRP-conjugated antibody and not the primary anti-PfMSPDBL1 antibody. Sheep anti-mouse IgG HRP antibodies used to assist in visualizing the rDBL2 (middle blot) did not cross-react with red blood cell proteins. EBA-175, obtained from parasite culture supernatants (Sup), was used to demonstrate effective trypsin and neuraminidase treatment of red blood cells. The EBA-175-binding phenotype is sensitive to treatment with either of these enzymes. An antibody targeting the C-terminal cysteine-rich region (CR) or region 6 of EBA175 was used for detection (lower blot) (51). B, binding of the recombinant DBL domains of MSPDBL1 and -2 to erythrocytes is enhanced in the presence of specific metal ions. Red blood cells were incubated with recombinant DBL domains in the presence of a selection of di- and monovalent cations (5 mm final concentration) as described under “Experimental Procedures.” Only metal ions that enhanced the binding of rDBL1 or rDBL2 to erythrocytes resulted in detectable levels of these proteins on immunoblots. Assays were performed using untreated erythrocytes and carried out as described in A and under “Experimental Procedures.” Lanes labeled rDBL1 or rDBL2 show recombinant protein not used in the binding assay. Other lanes show outcome of binding assays performed with different metal ions in conjunction with rDBL1 (upper blot) or rDBL2 (lower blot). C, binding of recombinant DBL domains of PfMSPDBL1 and -2 to red blood cells is dependent upon their disulfide bond-stabilized conformation. Red blood cells were incubated with refolded rDBL domain (rDBL1 or rDBL2) or reduced and alkylated rDBL domains (rDBL1-R/A or rDBL2-R/A) in the presence of 5 mm Ca2+ as described in A and under “Experimental Procedures.” 1st and 2nd lanes of each immunoblot contain recombinant rDBL1 or rDBL2 and rDBL1-R/A or rDBL2-R/A not used in binding assays as an identification control. 4th and 5th lanes show the recovery of the same proteins when used in binding assays. Note that the rDBL1-R/A or rDBL2-R/A proteins could not be recovered from these assays. D, effect of Ca2+ on the binding of parasite-derived, full-length PfMSPDBL2 to red blood cells can be reversed in the presence of EGTA. HA-tagged MSPDBL2 (i.e. full-length MSPDBL2-HA) derived from parasite cultures (see Fig. 2A) was used in these binding assays. 1st lane, parasite culture supernatant only, not used in the binding assay. 2nd lane, no Ca2+ added to the culture supernatant used in the binding assay. 3rd lane, 1 mm Ca2+ added to the culture supernatant and used in the binding assay. 4th lane, 10 mm EGTA in culture supernatant and binding assay. 5th lane, 1 mm Ca2+ + 1 mm EGTA in culture supernatant and binding assay. 6th lane, 1 mm Ca2+ + 2 mm EGTA in culture supernatant and binding assay. 7th lane, 1 mm Ca2+ + 5 mm EGTA in culture supernatant and binding assay. 8th lane, 1 mm Ca2+ + 10 mm EGTA in culture supernatant and binding assay; 9th lane, a wild type parasite culture supernatant control, in which MSPDBL2 does not have the C-terminal HA tag + 1 mm Ca2+ used in the binding assay. EBA-175, obtained from the same parasite culture supernatants used as a source of PfMSPDBL2, was used as a binding control under identical assay conditions. The rabbit polyclonal antibody used to detect EBA-175 targeted regions 3–5 of the molecule (R35) (51). E, histograms and dot blots obtained from FACS analyses of the binding of rDBL1 to untreated and enzyme-treated erythrocytes. Enzyme treatments and assay conditions are discussed further under “Experimental Procedures.” Error bars on histograms indicate means ± S.E. obtained from three independent assays. Recombinant PfRh4.9 was used as a binding control for enzyme-treated erythrocytes. This protein can bind to neuraminidase-treated erythrocytes but not erythrocytes treated with either trypsin or chymotrypsin. Representative dot blots are shown for each of the rDBL1 assay conditions. Numbers outside of the purple boxes refer to the percentage of erythrocytes with bound rDBL1 relative to the erythrocyte population. The various enzyme-treated erythrocytes used in the assays are indicated by the following symbols: −, no treatment; T, trypsin treatment; N, neuraminidase treatment; C, chymotrypsin treatment. F, histograms and dot blots obtained from FACS analyses of the binding of refolded and reduced and alkylated forms of rDBL1 to erythrocytes. Binding was performed on untreated erythrocytes in the presence of 1 mm Ca2+. See E and under “Experimental Procedures” for further details. A Student's t test was performed on the mean values obtained for the % binding of the refolded (rDBL1) and reduced and alkylated (rDBL-R/A) forms of rDBL1. A p < 0.001 is considered significant. G, histograms and dot blots obtained from FACS analyses of the binding of rDBL1 in the presence of increasing concentrations of EGTA. Binding was performed using untreated erythrocytes with 1 mm Ca2+ present in the binding buffer. The EGTA concentration within the binding assays ranged from 0 to 60 mm as indicated. Recombinant PfRh4.9 was used as a binding control over the EGTA concentration range used in the binding assays. See A and under “Experimental Procedures” for further details.
To further validate the red blood cell binding behavior of the DBL domains of MSPDBL1 and -2, we used FACS-based assays to assess their binding to a suspension of cells. The antibody used for the detection of rDBL1 on immunoblots (Fig. 4, A–C) was suitable for use in the FACS-based assay. However, several different antibodies, including the mouse anti-Hexa-His monoclonal antibody could not detect rDBL2, recombinant or parasite-derived forms of full-length MSPDBL1 and -2, or parasite-derived EBA175. We assume this is due to steric hindrance arising from the interaction between the red blood cell receptor(s) and these proteins. However, the FACS-based erythrocyte binding data obtained for rDBL1 (Fig. 4, E–G) was found to complement the observations determined for this protein in binding assays where rDBL1 was eluted from erythrocytes and detected on immunoblots (Fig. 4, A–C). The binding of rDBL1 was found to be insensitive to treatment of erythrocytes with either trypsin, neuraminidase, or chymotrypsin (Fig. 4E). Furthermore, the binding of rDBL1 to erythrocytes was dependent on the disulfide bond-stabilized conformation of this domain (Fig. 4F). The effect of Ca2+ on binding was demonstrated by titrating EGTA (a metal ion-chelating agent) over a concentration range from 0 to 60 mm. This effect was specific for rDBL1 as the binding of recombinant PfRh4.9 to erythrocytes was unaffected by the level of EGTA under similar assay conditions (Fig. 4G).
Combined, these results show that the recombinant DBL domains of PfMSPDBL1 and PfMSPDBL2 have the same functional properties as the full-length mature forms of the PfMSPDBL proteins derived from parasite culture supernatants (Fig. 2A), and the results suggest that the affinity for receptor binding of both PfMSPDBL1 and PfMSPDBL2 is influenced by the presence of specific metal ions.
Structure of the PfMSPDBL2 DBL Domain
We determined the crystal structure of the PfMSPDBL2 DBL domain (residues 161–457) to 2.09 Å resolution revealing a canonical DBL fold consisting of a boomerang-shaped α-helical core formed from three subdomains (Table 1 and Fig. 5). Electron density was apparent for residues 161–171, 186–374, and 388–457. Patches of poorly defined density connected residues 171–186 but was of inadequate quality for confident model building. However, this region contains Cys-177, and it is likely that density associated with Cys-212 represents a disulfide bond to this residue. Comparison with other DBL structures reveals the closest previously solved structures as PfEMP1 VAR2CSA DBL 6e (PDB code 2WAU), DBL3X (PDB code 3BQI), and PfEBA-175 F2 DBL (PDB code 1ZRL) with root-mean-square deviation of 2.0, 3.1, and 3.8 Å, and Dali Z scores of 24.2, 19.6, and 19.6, respectively (38).
FIGURE 5.
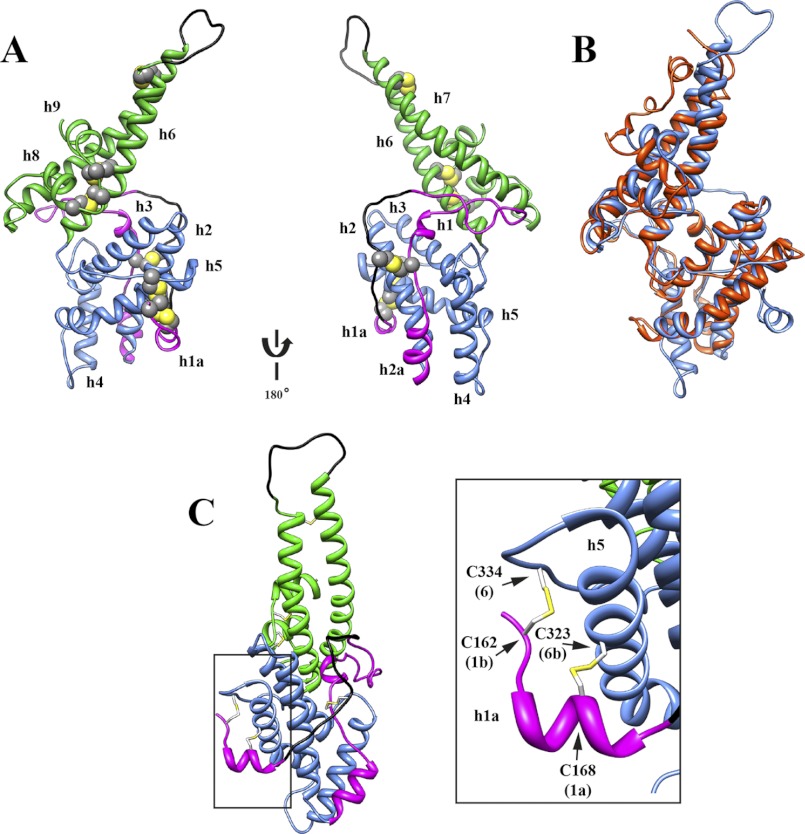
Structure of the DBL domain from PfMSPDBL2. A, ribbon diagram displaying the structure of the PfMSPDBL2 DBL domain. Disulfide bridges are highlighted in yellow. A 180° rotation of the structure is shown, and helices are labeled as depicted in Fig. 6. Regions corresponding to subdomains 1–3 are colored magenta, blue, and green, respectively. Only electron density corresponding to Cys-177 was observed in the region between residues Ser-172 and Asn-185, and no electron density was observed for the loop (Gln-375–Val-387) between helices 5 and 6. These regions were modeled using the LoopModel utility, part of the MODELLER package (31), and are colored in black. B, overlay of PfMSPDBL2-DBL (blue) with the DBL 6e domain of PfEMP1 VAR2CSA (red). C, schematic showing the disulfide linkages (yellow) between subdomains 1 (magenta) and 2 (blue) in the DBL domain of PfMSPDBL2. The depicted view is obtained by a 90° anticlockwise rotation of the first schematic in A. The PDB code for the native structure is 3VUU.
The DBL domain structure is conventionally divided into three subdomains based on the P. knowlesi DBL domain structure (3). In PfMSPDBL2, subdomain 1 consists of residues 161–225 and, like most other DBL domains, lacks abundant secondary structure but for a single 5-residue helix (helix 1) (Fig. 6) found near the junction of subdomains 2 and 3, which is stabilized by a hydrogen bond network involving Arg-207 from subdomain 1, Asp-266 from subdomain 2, and Glu-352 from subdomain 3. Subdomain 2 incorporates residues 226–341 and is composed of four structurally conserved helices (helices 2–5) (Fig. 6). Subdomains 1 and 2 contain a number of disulfide linkages, including the aforementioned disulfide connectivity between Cys-177 and Cys-212, which was conserved in the canonical structure (Fig. 7) (18). However, the disulfide bond that was found nested within the Cys-177 to Cys-212 linkage (C2-C3 discussed below) was not present in subdomain 1. Additionally, unlike the canonical structure, disulfide linkages occur between Cys-162 and Cys-334 and between Cys-168 and Cys-323 in PfMSPDBL2 (Fig. 7). These linkages tether a small helical region in subdomain 1 (helix 1a), between residues 161 and 171, to the C-terminal end of helix 5 and the loop region between helix 5 and helix 6 in subdomain 2 (Figs. 5C and 6).
FIGURE 6.
Structure-based sequence alignment for various DBL domains found in Plasmodium spp. Residues participating in helices are colored red, and those in β-strands are colored blue. Two cysteine residues not engaged in disulfide bond formation are underlined. Residues that were either engineered mutations or that are anomalous are presented in italics. Cysteine residues engaged in disulfide bonds and strictly conserved residues are highlighted. The location of the canonical helices is shown. Residues in lowercase for PfMSPDBL2 are not observed in the x-ray structure. Residues presented in boldface and highlighted have been implicated in the binding of substrate. Magenta, blue, and green underlines represent regions defined as subdomains 1–3, respectively. Protein identification is based on the PDB identifier code with 1ZRO = PfEBA175 DBL; 3RRC = Pv DBP DBL; 2C6J = Pk DBP DBL; 2XUO = PfEMP1-NTS-DBL1a1–VarO; 3BQI/3CML = PfEMP1 VAR2CSA DBL 3X; and 2WAU = PfEMP1 VARCSA DBL 6ϵ.
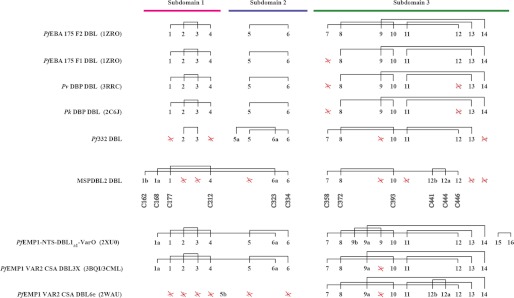
FIGURE 7.
Comparison of the disulfide bond architectures found in various DBL domains of Plasmodium spp. Numbers 1–14 represent the relative position of cysteine residues conserved in the structure of the EBA-175 F2 DBL domain. Additional numbers and letters represent a shift in the position of cysteine residues found in other DBL domains relative to those in the EBA-175 F2 DBL domain. A red cross through numbers 1–14 represents the loss of a cysteine from a position found in EBA-175 F2 DBL domain. The identity of individual Cys residues involved in disulfide bonds within the DBL domain of PfMSPDBL2 is shown. Magenta, blue, and green lines indicate the location of Cys residues in subdomain 1–3, respectively. PDB identifier codes for each structure are shown in parentheses where known. The disulfide architecture for Pf332 is shown as described (29).
PfMSPDBL2 subdomain 3 (344–460) is a helical bundle composed of two long α-helices (helix 6 and 7) and two smaller α-helices (helix 8 and 9) (Figs. 6 and 5D). The two larger helices are tethered to each other via a single disulfide bond (Cys-372 to Cys-393) at the distal end of the bundle. A Cys-358 to Cys-446 disulfide linkage anchors the small loop region between helices 8 and 9 to the mid-region of helix 6. A disulfide linkage between Cys-441 and Cys-444 constrains this same loop region and brings helices 8 and 9 in close proximity to each other in an anti-parallel orientation (Fig. 7). The loop between helices 6 and 7 (residues 374–388) was disordered (Fig. 6), as has been observed for other DBL structures (e.g. PfEMP1 VAR2CSA DBL 6ϵ, PDB code 2WAU) (38). Structures of DBL domains in which this region is visible, for example the PfEBA175 F1 DBL (PDB code 1ZRO F1) (18), contain C-terminal extensions that interact with this loop and thus stabilize the region. Residues corresponding to this C-terminal extension were not included in the construct for PfMSPDBL2 used in these studies.
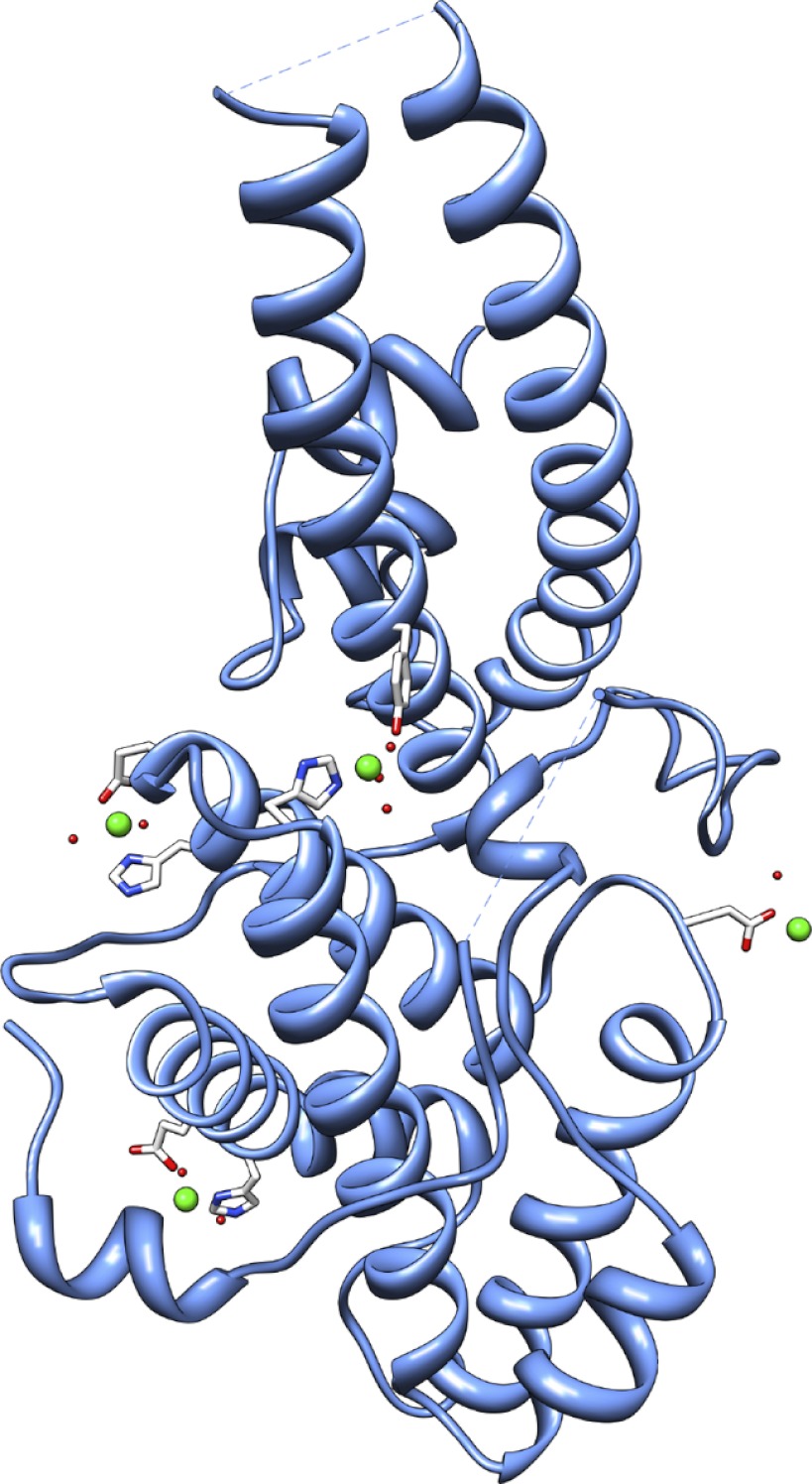
For a second structure of PfMSPDBL2, crystals were soaked with ZnCl2, and data were collected near the zinc absorption edge. Anomalous difference maps revealed the location of four sites of zinc coordination, none of which conform to the classical zinc functional types (catalytic, structural, cocatalytic, or interfacial) (Fig. 8) (39). At three sites the zinc ion coordinates a single histidine and either a glutamate or tyrosine. Water molecules filled the remaining coordination valency at all three sites. At the fourth site a single aspartate coordinates the metal ion. Although the data for this structure were nominally at lower resolution than that collected for the native, electron density unambiguously extended beyond residue 171, and in the native dataset only patchy density had been observed. This permitted additional model building from residue Ser-172 to Lys-179, including the cysteine residue at position 177; this additional density also confirmed the disulfide connectivity between Cys-177 and Cys-212.
FIGURE 8.
Structure of PfMSPDBL2 and the zinc-binding sites. The four zinc-binding sites are shown S1, His-249 and Tyr-356; S2, Glu-254 and His-257; S3, His-316 and Glu-319; S4, Asp-278, with the zinc cation represented as a green sphere. The PDB code for the structure with coordinated Zn2+ is 3VUV.
Model of the DBL Domain for PfMSPDBL1
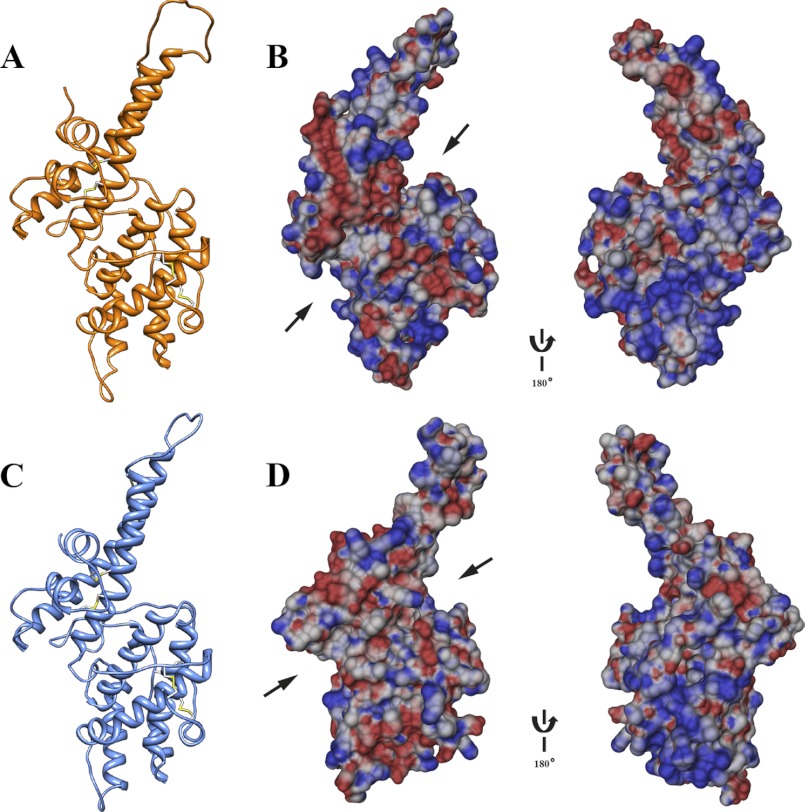
The DBL domain of PfMSPDBL1 would not crystallize under the myriad of conditions used. The high sequence similarity between PfMSPDBL1 and PfMSPDBL2 permitted the comparative modeling of the DBL domain of PfMSPDBL1 using PfMSPDBL2 as a template; in particular, the disulfide bond connectivity pattern was predicted to be similar in both domains (supplemental Fig. S1). Notably, however, an additional Cys residue (Cys-398) remains unpaired in helix 7 of subdomain 3 of PfMSPDBL1. This Cys residue differs in location to other unpaired Cys residues found in PDB codes 2WAU (Cys-2385 in a small helix found in subdomain 1) and 1ZRO(F1) (Cys-273 in helix 9 of subdomain 3) (Fig. 6). Ligand interaction sites in DBL domains other than PfMSPDBL1 and PfMSPDBL2 are associated with clusters of positively charged basic residues. The electrostatic potential surface of PfMSPDBL1 and PfMSPDBL2 (Fig. 9, B and D, respectively) show large patches of positive potential, particularly at the C terminus of helix 2a. The cleft between subdomains 2 and 3 of PfMSPDBL1 shows a large negatively charged region, which was not observed on the surface of PfMSPDBL2.
FIGURE 9.
Modeled structures of the PfMSPDBL1 and -2 DBL domains. A, ribbon schematic of the modeled PfMSPDBL1 DBL domain. Disulfide bridges are displayed in yellow. B, electrostatic surface potential diagrams, with 180° rotation, for the DBL domain of PfMSPDBL1. C, ribbon schematic of the modeled DBL domain for PfMSPDBL2. Disulfide bridges are displayed in yellow. D, electrostatic surface potential diagrams, with 180° rotation, for the DBL domain of PfMSPDBL2. Electrostatic surface diagrams in B and D are based on the modeled structures for the DBL domains of PfMSPDBL1 and -2, including loops missing in the x-ray structure of PfMSPDBL2. Arrows indicate the location of the cleft that occurs between subdomains 2 and 3 in these DBL domains.
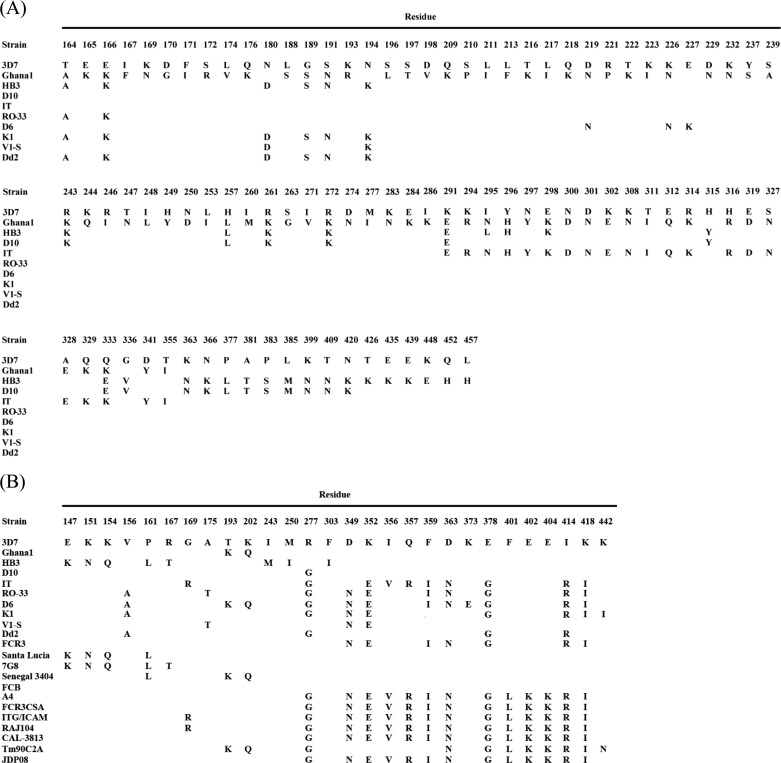
DBL Domains of PfMSPDBL2 and PfMSPDBL1 Are Highly Polymorphic
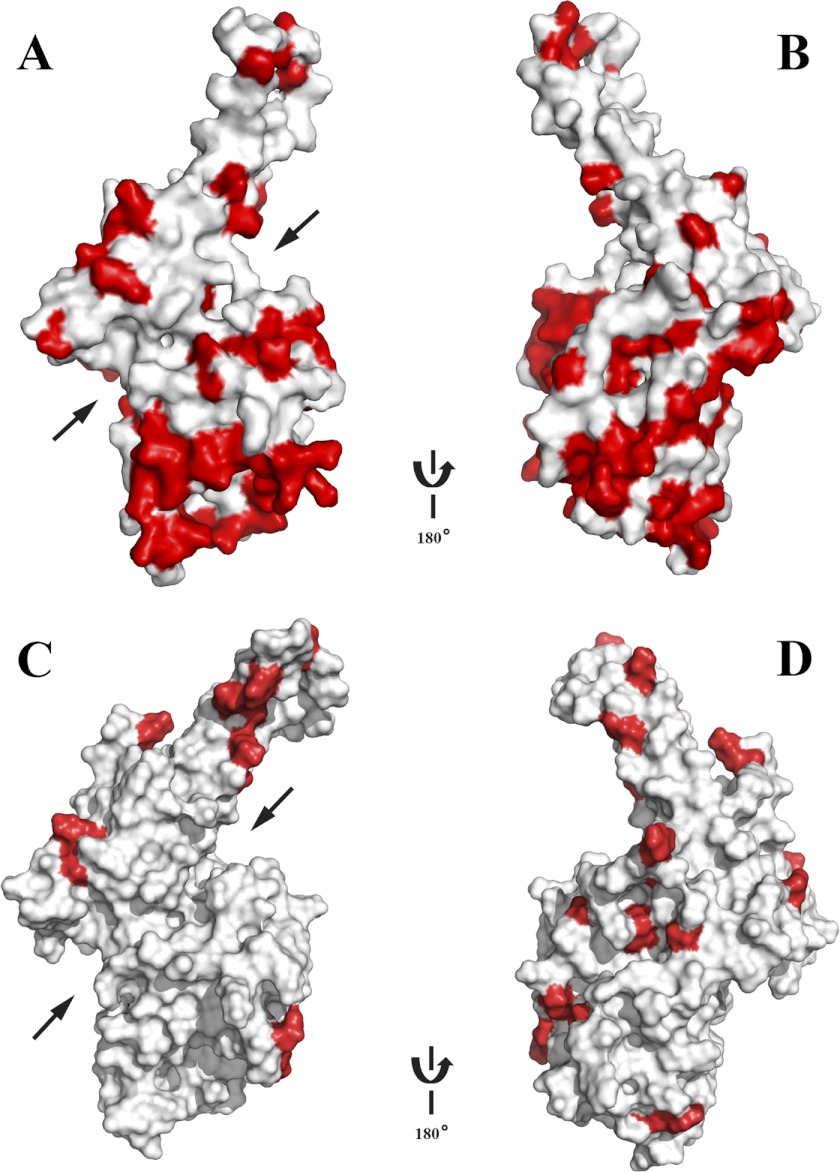
Both PfMSPDBL2 and PfMSPDBL1 proteins display some of the highest levels of sequence diversity so far found within the P. falciparum genome, with the majority of the polymorphisms located in the DBL domains of these proteins (17). Many of the mutations are dimorphic in nature, with ∼10–20% of the mutated amino acids involving a switch in polarity (Table 2). Placement of the naturally occurring polymorphisms onto a surface structure for the DBL domain of PfMSPDBL2 showed good coverage around the surface of the molecule (Fig. 10, A and B), except for a cleft region that lies between helices 1 and 6 and the groove between helices 6 and 7 that extends beyond the cleft (Fig. 10A). The composition of the residues associated with this region between subdomains 2 and 3 is conserved and restricted from mutational change, indicating that this region of the DBL domain is functionally important and may be associated with the interaction of a receptor on the surface of the red blood cell. Significantly fewer polymorphisms are known for PfMSPDBL1 than for PfMSPDBL2. Mapping of the known polymorphisms of PfMSPDBL1 on the surface of the modeled structure reveals that most are distributed equally across the surface (Fig. 10D), and like PfMSPDBL2, the region between subdomains 2 and 3 remains free of polymorphisms.
TABLE 2.
Location of polymorphisms within the DBL domains of PfMSPDBL2 (A) and PfMSPDBL1 (B)
The location of amino acid polymorphisms within the DBL domains of PfMSPDBL1 and −2 in various strains of P. falciparum was obtained on line. Additional polymorphisms for the DBL domain of PfMSPDBL1 were obtained from Ref. 14.
FIGURE 10.
Naturally occurring polymorphisms of the PfMSPDBL2 and PfMSPDBL1 DBL domains. The structures are depicted in surface format with polymorphisms colored red. The orientation in A is as that displayed in Fig. 5A. The orientation in B is rotated 180° to that displayed in A. The orientation in C is as that displayed in Fig. 9A. The orientation in D is rotated 180° to that displayed in C. Arrows shown in A and C indicate the location of the cleft between subdomains 2 and 3 in the DBL domains of PfMSPDBL2 and -1, respectively. Coloring of the electrostatic potential is from red (−15 kJ mol−1), through white (0 kJ mol−1) to blue (+15 kJ mol−1).
DISCUSSION
Key host/parasite molecular interactions that occur during the invasion process remain poorly understood. Here, we have characterized two unusual members of the PfMSP3 family that interact with red blood cells via a single DBL domain located near each N terminus. We have determined the three-dimensional structure of the DBL domain from PfMSPDBL2 and used this information modeled PfMSPDBL1 to show that they both have a canonical DBL fold consisting of a boomerang-shaped α-helical core formed from three subdomains. We have used this to map the sequence polymorphisms associated with these proteins in the P. falciparum population, and this has highlighted a region most likely corresponding to the receptor-binding domain. The similar domain structures for PfMSPDBL1 and -2 and their shared subcellular localization on the merozoite surface suggest that they both play a similar role in merozoite invasion, most likely in the initial interaction of the parasite cell with the host.
PfMSPDBL1 and -2 have no apparent transmembrane spanning domains or GPI-anchoring motifs, and they associate peripherally with other parasite proteins to form complexes at the merozoite surface. We have found that processing of these proteins was linked with their association on the merozoite surface (supplemental Fig. S2). Both PfMSPDBL1 and -2 have potential PfSUB1 cleavage sites located near to their N terminus, and processing by this protease would produce fragments of similar size to those observed for the processed forms of these proteins. A similar PfSUB1 processing event is required for incorporation of MSP6, another member of the MSP3 family, into the MSP1 complex (40, 41). It is possible that unprocessed forms of PfMSPDBL1 and -2, which are not incorporated into the merozoite surface (supplemental Fig. S2), may act as immuno-decoys as these proteins will be presented to the host immune system in a different form to the PfMSPDBL proteins, which form part of a complex on the merozoite surface.
We believe the DBL domain from each PfMSPDBL protein is the region of the molecule that interacts with the receptor(s) on the surface of the red blood cell for the following reasons: (i) the red blood cell binding properties of the recombinant DBL domains and the mature full-length forms of the PfMSPDBL proteins, obtained from parasite culture supernatants, are similar and greatly enhanced in the presence of specific metal ions, such as Ca2+; (ii) other members of the MSP3 family, which do not contain DBL domains yet retain the C-terminal SPAM domain, as found in the PfMSPDBL proteins, do not bind red blood cells in identical binding assays6; and (iii) the interaction between the DBL domains of other merozoite surface proteins involved in invasion, such as members of the EBL family, is well documented (18).
The structure of the DBL domain for PfMSPDBL2 was also determined in the presence of Zn2+, which was one of the metal ions identified to enhance the binding of this DBL domain to red blood cells. Four distinct Zn2+-binding sites were identified within the structure. Functional zinc-binding sites generally employ at least three side-chain ligands to coordinate the metal ion (42). In three of the binding sites identified, two side-chain ligands are observed, and in the fourth only a single side-chain ligand is observed. Enhanced binding of PfMSPDBL1 and PfMSPDBL2 to red blood cells is also observed for calcium ions and these do not generally coordinate histidine. Therefore, it is unlikely that calcium could function in at least three of these sites in the same manner as zinc, indicating other metal ion-binding sites may exist within these DBL domains. However, the enhanced binding of these parasite ligands to the red blood cell was found to be specific to only a few metal ions, as not all cations tested in the red blood cell binding assay resulted in elevated levels of binding for each DBL domain. Some members of the lectin family rely on the presence of metal ions, such as Ca2+, to enable the formation of high affinity interactions with carbohydrate moieties in their binding partners (43). The Ca2+ concentration in the parasitophorous vacuole, which contains the dividing merozoites, is ∼100 nm and 1 mm in serum, and therefore it is likely that some of the Ca2+-binding sites in PfMSPDBL1 and PfMSPDBL2 are occupied thus enabling enhanced affinities of these surface proteins for their specific receptors (44). It will be interesting to determine whether DBL domains in other EBL family members are able to bind Ca2+ or metal ions and if this plays a role in affinity of binding to their receptor.
The binding of the DBL domains, from the PfMSPDBL proteins, to their receptors on the red blood cell surface was dependent on a conformation stabilized by disulfide bonds, as the reduced and alkylated forms of these proteins were unable to bind the host cell. Furthermore, both proteins had a similar red blood cell binding phenotype that was both trypsin- and neuraminidase-resistant. This binding phenotype was also observed for the parasite-derived proteins but differed from that previously reported for PfMSPDBL1 expressed on the surface of COS-7 cells (14). It is not clear why the pattern of binding differs between these two studies, but it may possibly be due to misfolding of the protein expressed in COS-7 cells. It will be of interest to identify the specific receptor(s) for these merozoite surface proteins.
The SPAM domain, which is present in most other members of the MSP3 family, consists of a glutamic acid-rich region and a leucine zipper-like region; the alanine-rich heptad repeat region of the parent SPAM protein (45) is absent from all other members of the MSP3 family in P. falciparum (15). The leucine zipper-like region has been shown to be responsible for oligomerization of MSP3. Analysis of the physical properties of MSP3 has suggested that it forms a highly extended and oligomeric structure on the merozoite surface that would allow it to interact with the red blood cell at relatively long distances (46), most notably, dimerization of MPS3 is solely through the leucine zipper-like domain. As PfMSPDBL1 and -2 bind red blood cells through the DBL domains and share with MSP3 a SPAM domain at the C terminus, they are also likely to be long extended molecules able to bind to their specific receptors. The function of these proteins is likely to be associated with the initial interaction of the merozoite with the red blood cell and as such important for the invasion process.
Although the overall structures for the various DBL domains found in Plasmodium spp. are relatively conserved, there is great variation in the host receptors with which they interact (1, 47). The specificity of the DBL domain structure is poorly understood in relation to function/receptor binding. An alignment of the sequences of DBL domains shows that the size and location of the major helices (i.e. helices 1–9) are highly conserved in the structure of known DBL domains (Fig. 6). However, the extent of the connecting loops between these helices varies with each structure. The DBL fold is classically divided into three subdomains. Subdomain 1 contains little conserved secondary structure except for a short helical segment (helix 1) between Cys-3 and Cys-4 conserved in all structures except 2C6J. However, this region of 2C6J includes a sequence anomaly (Val-39 rather than Asp) in the solved structure. Subdomain 2 is composed of four structurally conserved helices (helices 2–5), with helix 5 bent mid-way in all structures. Subdomain 3 is formed by two long anti-parallel helices (6 and 7) and several smaller helices across one face of this helix bundle; in the canonical DBL structure, this region includes two helices, 8 and 9; however, there are five helices found in the structure PDB 2WAU.
Despite a high degree of sequence similarity, few residues are strictly conserved across the seven DBL structures determined to date. An arginine (Arg-207) in the short helix between Cys-3 and Cys-4 forms salt bridges with a conserved aspartate (Asp-266) on helix 2 and glutamate (Glu-352) on helix 6. A conserved glutamate in helix 2 forms a hydrogen bond with the backbone amide nitrogen immediately prior to Cys-4. The indole nitrogen of a conserved tryptophan in helix 6 (Trp-349 in PfMSPDBL2) forms a hydrogen bond with the carboxylate side chain of a conserved aspartate in helix 3 (Asp-266 in PfMSPDBL2) and packs against a conserved proline at the beginning of the short helix between Cys-3 and Cys-4 (Pro-205 in PfMSPDBL2; notably, in PDB code 2C6J, this residue is a serine, and the short helix 1 is not correctly folded). The N terminus of helix 1 is capped by another conserved tryptophan two residues C-terminal of Cys-2 (Trp-187 in PfMSPDBL2). The conserved aspartate in helix 3 (Asp-266 in PfMSPDBL2) also forms hydrogen bonds with a conserved glutamine (Gln-345 in PfMSPDBL2) at the beginning of helix 6. Thus, residue conservation in these DBL domains is spatially focused about the junction between helices 1 and 6.
Although the DBL domains from different protein families share similar overall folds, different DBL subgroups demonstrate distinct disulfide linkage patterns (Fig. 7). The canonical disulfide linkage pattern is based on that observed in the F2 DBL domain of PfEBA175, which is a member of the EBL family that binds glycophorin A (18). Members of the EBL family generally do not have disulfide connectivity between any of the subdomains, conserve the C1-C4 and C2-C3 linkages in subdomain 1, conserve the C5-C6 linkage in subdomain 2, and conserve the C8-C10, C9-C14, and C11-C13 linkages in subdomain 3. In contrast, PfEMP1 family members have a modified linking pattern across subdomain 3. In particular, C14 links to a cysteine not observed in the EBL family (C9a) located in an insertion between helices 6 and 7, and C9 is either absent or links with an additional cysteine (C9b) also within this insertion. Additionally, PfEMP1-NTS-DBL1α1 and PfEMP1 VAR2CSA DBL3X contain an extra disulfide link connecting subdomains 1 and 2 (C1a–C6a). Although disulfide connectivity for PfEMP1 VAR2CSA DBL 6ϵ is similar to PfEMP1-NTS-DBL1α1 and PfEMP1 VAR2CSA DBL3X within subdomain 3, it is unique in that it has lost all disulfide links within subdomains 1 and 2. Additionally, it has acquired an extra disulfide linkage (C12b–C12a) arising from cysteine residues present in a small insertion at the C terminus of α8. Among all the structures determined to date, there occur only two instances where a cysteine residue is not found in a disulfide bond, Cys-273 in PDB code 1ZRO(F1) and Cys-2385 in PDB code 2WAU. Both are surface-exposed and could potentially form a disulfide bond with partner cysteine residues.
The disulfide bond architecture observed in the DBL domain of PfMSPDBL2 is similar to that of PfEMP1-NTS-DBL1α1 and PfEMP1 VAR2CSA DBL3X in that it contains disulfide links connecting subdomains 1 and 2 (C1a–C6a). However, unlike other DBL domains, C6 of PfMSPDBL2 links to a novel N-terminal cysteine (C1b) providing an extra covalent tether between these domains. Additionally, PfMSPDBL2 has lost the C2-C3 linkage in subdomain 1, and the C9–C14 and C11–C13 linkages in subdomain 3. Subdomain 3 contains the small insertion at the end of α8 containing the short hairpin disulfide bond between C12b and C12a observed in PfEMP1 VAR2CSA DBL 6e. These unique characteristics place the PfMSPDBL proteins in their own subgroup in regard to their disulfide architecture.
Previous structures of DBL domains show almost no similarity in the location of proposed substrate-binding sites (supplemental Fig. S3). Additionally, the majority of residues implicated in ligand interaction are not conserved across DBL domains (Fig. 6). A heparin-binding site on PfEMP1-NTS-DBL1α1 (PDB code 2XU0) maps to the N-terminal extension to subdomain 1 and the flanking C-terminal region of subdomain 3 (48). In contrast, the primary binding site for chondroitin sulfate proteoglycans on VAR2CSA DBL6e (PDB code 2WAU) lies at the N terminus of helix 2, on the “distal side” of subdomain 2 (49). The ligand-binding site on PkαDBL (PDB code 2C6J), which binds to Duffy antigen receptor for chemokines (DARC), comprises residues from the N terminus of helix 3 and the loop region between subdomains 2 and 3 (3). The proposed binding site for chondroitin sulfate A on VAR2CSA DBL3x (PDB codes 3BQI and 3CML) is located in the region formed at the junction of all three domains. Although disparate in location, most sites identified contain lysine residues. Likewise, PfMSPDBL2 contains lysine residues in similar regions indicating that it has the capacity to form similar types of interaction.
It has also been proposed for both EBA-175 (PDB code 1ZRO) and PvDBP (PDB code 3RCC) that dimerization is required for receptor binding and that residues at the dimer interfaces are involved in these interactions. Again, however, two disparate dimerization models have been identified with different ligand-binding sites. EBA-175 contains two tandem DBL domains joined through a three-turn helical linker (18). Dimerization of these domains produces a “handshake” orientation between two RII regions and results in the formation of a channel. The glycan (sialic acid)-binding sites occur at dimer interfaces, on loops between strands in subdomain 1 and the base of subdomain 3. However, as PfMSPDBL2 contains only one DBL domain, and the ligand is not neuraminidase-sensitive, it is unlikely that it utilizes the same binding mechanism. Unlike EBA-175, the DARC-binding site of PvDBP DBL (PDB code 3RCC) occurs at the interface of a pair of dimers involving subdomain 2 (supplemental Fig. S3). The PfMSPDBL2 structure is incompatible with this dimer due to steric clashes at the interface involving helix 1c and helix 3. Thus, it is also unlikely that PfMSPDBL2 utilizes this dimerization surface for ligand interaction.
Although PfMSPDBL2 is unlikely to adopt the dimerization interfaces described for either EBA-175 (18) or PvDBP DBL (3), it remains possible that dimerization is still required for ligand recognition. In the full-length protein, dimerization is probably facilitated by the C-terminal SPAM domain present in both PfMSPDBL1 and -2, as is the case for MSP3 (46). Dimerization via this motif would likely orient two DBL domains in a parallel fashion; however, flexibility within the linker region between the DBL and SPAM domains may allow for a variety of DBL dimer interfaces. The structure of full-length PfMSPDBL1 or -2, or of the DBL domains in complex with receptor, will likely inform the nature of the receptor recognition complex.
A large positively charged region on the surface of PfMSPDBL2 is reminiscent of similarly charged regions on the surfaces of other DBL domains, regions that are associated with binding of receptors or protein partners. However, this region on the surface of PfMSPDBL2 is highly polymorphic, and thus it is doubtful that the positive potential has any significance for receptor binding. The significance of the acidic region at the interface of subdomains 2 and 3 of PfMSPDBL1 is less clear because the characterization of polymorphisms is less complete, although this region is resistant to substitution in PfMSPDBL2 also. This region coincides with the few residues conserved across all DBL domains (which tether helix 1), raising the prospect that this region also plays a role in receptor binding.
In summary, we have characterized both PfMSPDBL1 and PfMSPDBL2 and have shown that the DBL domains of these proteins are responsible for red blood cell binding. This binding, although not sensitive to trypsin, chymotrypsin, or neuraminidase treatment, is enhanced in the presence of divalent cations, specifically Ca2+, Co2+, Cu2+, or Zn2+. We have determined the structure of PfMSPDBL2 and find that it displays a typical DBL fold. We propose that the disulfide bonding architecture of PfMSPDBL1 and PfMSPDBL2 is distinct from both the PfEMP1 family and the EBL family differentiating DBL proteins into three subgroups. We also find that the structure, and domain architecture, of PfMSPDBL2 is inconsistent with dimerization models proposed for other DBL domains of erythrocyte-binding ligands.
Supplementary Material
Acknowledgments
We thank the Red Cross Blood Service (Melbourne, Australia) for the supply of red blood cells and serum. Crystallization trials were performed at the Bio21 Collaborative Crystallization Centre, Victoria, Australia. Diffraction data were collected at the Australian Synchrotron, and we thank staff from the macromolecular beam lines for their assistance.
This work was supported by Victorian State Government Operational Infrastructure Support and Australian Government National Health and Medical Research Council Independent Research Institutes Infrastructure Support Scheme.

This article contains supplemental Figs. 1–3.
The atomic coordinates and structure factors (codes 3VUV and 3VUU) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
C. S. Lin, A. N. Hodder, and A. F. Cowman, unpublished data.
A. D. Uboldi, A. N. Hodder, and A. F. Cowman, unpublished data.
- EBL
- erythrocyte binding-like
- DBL
- Duffy binding-like
- PDB
- Protein Data Bank
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- DARC
- Duffy antigen receptor for chemokine
- TEV
- tobacco etch virus.
REFERENCES
- 1. Cowman A. F., Crabb B. S. (2006) Invasion of red blood cells by malaria parasites. Cell 124, 755–766 [DOI] [PubMed] [Google Scholar]
- 2. Chitnis C. E., Miller L. H. (1994) Identification of the erythrocyte binding domains of Plasmodium vivax and Plasmodium knowlesi proteins involved in erythrocyte invasion. J. Exp. Med. 180, 497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Singh S. K., Hora R., Belrhali H., Chitnis C. E., Sharma A. (2006) Structural basis for Duffy recognition by the malaria parasite Duffy-binding-like domain. Nature 439, 741–744 [DOI] [PubMed] [Google Scholar]
- 4. Gardner M. J., Hall N., Fung E., White O., Berriman M., Hyman R. W., Carlton J. M., Pain A., Nelson K. E., Bowman S., Paulsen I. T., James K., Eisen J. A., Rutherford K., Salzberg S. L., Craig A., Kyes S., Chan M. S., Nene V., Shallom S. J., Suh B., Peterson J., Angiuoli S., Pertea M., Allen J., Selengut J., Haft D., Mather M. W., Vaidya A. B., Martin D. M., Fairlamb A. H., Fraunholz M. J., Roos D. S., Ralph S. A., McFadden G. I., Cummings L. M., Subramanian G. M., Mungall C., Venter J. C., Carucci D. J., Hoffman S. L., Newbold C., Davis R. W., Fraser C. M., Barrell B. (2002) Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 419, 498–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Howell D. P., Levin E. A., Springer A. L., Kraemer S. M., Phippard D. J., Schief W. R., Smith J. D. (2008) Mapping a common interaction site used by Plasmodium falciparum Duffy binding-like domains to bind diverse host receptors. Mol. Microbiol. 67, 78–87 [DOI] [PubMed] [Google Scholar]
- 6. Rowe J. A., Moulds J. M., Newbold C. I., Miller L. H. (1997) P. falciparum rosetting mediated by a parasite-variant erythrocyte membrane protein and complement-receptor 1. Nature 388, 292–295 [DOI] [PubMed] [Google Scholar]
- 7. Adams J. H., Blair P. L., Kaneko O., Peterson D. S. (2001) An expanding ebl family of Plasmodium falciparum. Trends Parasitol. 17, 297–299 [DOI] [PubMed] [Google Scholar]
- 8. Batchelor J. D., Zahm J. A., Tolia N. H. (2011) Dimerization of Plasmodium vivax DBP is induced upon receptor binding and drives recognition of DARC. Nat. Struct. Mol. Biol. 18, 908–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sim B. K., Chitnis C. E., Wasniowska K., Hadley T. J., Miller L. H. (1994) Receptor and ligand domains for invasion of erythrocytes by Plasmodium falciparum. Science 264, 1941–1944 [DOI] [PubMed] [Google Scholar]
- 10. Mayer D. C., Kaneko O., Hudson-Taylor D. E., Reid M. E., Miller L. H. (2001) Characterization of a Plasmodium falciparum erythrocyte-binding protein paralogous to EBA-175. Proc. Natl. Acad. Sci. U.S.A. 98, 5222–5227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maier A. G., Duraisingh M. T., Reeder J. C., Patel S. S., Kazura J. W., Zimmerman P. A., Cowman A. F. (2003) Plasmodium falciparum erythrocyte invasion through glycophorin C and selection for Gerbich negativity in human populations. Nat. Med. 9, 87–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lobo C. A., Rodriguez M., Reid M., Lustigman S. (2003) Glycophorin C is the receptor for the Plasmodium falciparum erythrocyte-binding ligand PfEBP-2 (baebl). Blood 101, 4628–4631 [DOI] [PubMed] [Google Scholar]
- 13. Gilberger T. W., Thompson J. K., Triglia T., Good R. T., Duraisingh M. T., Cowman A. F. (2003) A novel erythrocyte binding antigen-175 paralogue from Plasmodium falciparum defines a new trypsin-resistant receptor on human erythrocytes. J. Biol. Chem. 278, 14480–14486 [DOI] [PubMed] [Google Scholar]
- 14. Wickramarachchi T., Cabrera A. L., Sinha D., Dhawan S., Chandran T., Devi Y. S., Kono M., Spielmann T., Gilberger T. W., Chauhan V. S., Mohmmed A. (2009) A novel Plasmodium falciparum erythrocyte-binding protein associated with the merozoite surface, PfDBLMSP. Int. J. Parasitol. 39, 763–773 [DOI] [PubMed] [Google Scholar]
- 15. Singh S., Soe S., Weisman S., Barnwell J. W., Pérignon J. L., Druilhe P. (2009) A conserved multigene family induces cross-reactive antibodies effective in defense against Plasmodium falciparum. PLoS One 4, e5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Van Tyne D., Park D. J., Schaffner S. F., Neafsey D. E., Angelino E., Cortese J. F., Barnes K. G., Rosen D. M., Lukens A. K., Daniels R. F., Milner D. A., Jr., Johnson C. A., Shlyakhter I., Grossman S. R., Becker J. S., Yamins D., Karlsson E. K., Ndiaye D., Sarr O., Mboup S., Happi C., Furlotte N. A., Eskin E., Kang H. M., Hartl D. L., Birren B. W., Wiegand R. C., Lander E. S., Wirth D. F., Volkman S. K., Sabeti P. C. (2011) Identification and functional validation of the novel antimalarial resistance locus PF10_0355 in Plasmodium falciparum. PLoS Genet 7, e1001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ochola L. I., Tetteh K. K., Stewart L. B., Riitho V., Marsh K., Conway D. J. (2010) Allele frequency-based and polymorphism versus divergence indices of balancing selection in a new filtered set of polymorphic genes in Plasmodium falciparum. Mol. Biol. Evol. 27, 2344–2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tolia N. H., Enemark E. J., Sim B. K., Joshua-Tor L. (2005) Structural basis for the EBA-175 erythrocyte invasion pathway of the malaria parasite Plasmodium falciparum. Cell 122, 183–193 [DOI] [PubMed] [Google Scholar]
- 19. Crabb B. S., Cooke B. M., Reeder J. C., Waller R. F., Caruana S. R., Davern K. M., Wickham M. E., Brown G. V., Coppel R. L., Cowman A. F. (1997) Targeted gene disruption shows that knobs enable malaria-infected red cells to cytoadhere under physiological shear stress. Cell 89, 287–296 [DOI] [PubMed] [Google Scholar]
- 20. Wessel D., Flügge U. I. (1984) A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal. Biochem. 138, 141–143 [DOI] [PubMed] [Google Scholar]
- 21. Tham W. H., Wilson D. W., Lopaticki S., Schmidt C. Q., Tetteh-Quarcoo P. B., Barlow P. N., Richard D., Corbin J. E., Beeson J. G., Cowman A. F. (2010) Complement receptor 1 is the host erythrocyte receptor for Plasmodium falciparum PfRh4 invasion ligand. Proc. Natl. Acad. Sci. U.S.A. 107, 17327–17332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tham W. H., Schmidt C. Q., Hauhart R. E., Guariento M., Tetteh-Quarcoo P. B., Lopaticki S., Atkinson J. P., Barlow P. N., Cowman A. F. (2011) Plasmodium falciparum uses a key functional site in complement receptor type-1 for invasion of human erythrocytes. Blood 118, 1923–1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Otwinowski Z., Minor W. (1997) Processing of x-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 [DOI] [PubMed] [Google Scholar]
- 24. McCoy A. J., Grosse-Kunstleve R. W., Adams P. D., Winn M. D., Storoni L. C., Read R. J. (2007) Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Long F., Vagin A. A., Young P., Murshudov G. N. (2008) BALBES. A molecular replacement pipeline. Acta Crystallogr. D Biol. Crystallogr. 64, 125–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Collaborative Computational Project No. 4 (1994) The CCP4 suite. Programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 50, 760–763 [DOI] [PubMed] [Google Scholar]
- 27. Emsley P., Cowtan K. (2004) Coot. Model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 28. Adams P. D., Afonine P. V., Bunkóczi G., Chen V. B., Davis I. W., Echols N., Headd J. J., Hung L. W., Kapral G. J., Grosse-Kunstleve R. W., McCoy A. J., Moriarty N. W., Oeffner R., Read R. J., Richardson D. C., Richardson J. S., Terwilliger T. C., Zwart P. H. (2010) PHENIX. A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hodder A. N., Maier A. G., Rug M., Brown M., Hommel M., Pantic I., Puig-de-Morales-Marinkovic M., Smith B., Triglia T., Beeson J., Cowman A. F. (2009) Analysis of structure and function of the giant protein Pf332 in Plasmodium falciparum. Mol. Microbiol. 71, 48–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bruns C. M., Hubatsch I., Ridderström M., Mannervik B., Tainer J. A. (1999) Human glutathione transferase A4-4 crystal structures and mutagenesis reveal the basis of high catalytic efficiency with toxic lipid peroxidation products. J. Mol. Biol. 288, 427–439 [DOI] [PubMed] [Google Scholar]
- 31. Fiser A., Sali A. (2003) Modeller. Generation and refinement of homology-based protein structure models. Methods Enzymol. 374, 461–491 [DOI] [PubMed] [Google Scholar]
- 32. Bashford D. (1997) An object-oriented programming suite for electrostatic effects in biological molecules. Lecture Notes in Computer Science 1343, 233–240 [Google Scholar]
- 33. Sitkoff D., Sharp K. A., Honig B. (1994) Accurate calculation of hydration free energies using macroscopic solvent models. J. Phys. Chem. 98, 1978–1988 [Google Scholar]
- 34. Sanner M. F., Olson A. J., Spehner J. C. (1996) Reduced surface. An efficient way to compute molecular surfaces. Biopolymers 38, 305–320 [DOI] [PubMed] [Google Scholar]
- 35. Silmon de Monerri N. C., Flynn H. R., Campos M. G., Hackett F., Koussis K., Withers-Martinez C., Skehel J. M., Blackman M. J. (2011) Global identification of multiple substrates for Plasmodium falciparum SUB1, an essential malarial processing protease. Infect. Immun. 79, 1086–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Camus D., Hadley T. J. (1985) A Plasmodium falciparum antigen that binds to host erythrocytes and merozoites. Science 230, 553–556 [DOI] [PubMed] [Google Scholar]
- 37. Richard D., MacRaild C. A., Riglar D. T., Chan J. A., Foley M., Baum J., Ralph S. A., Norton R. S., Cowman A. F. (2010) Interaction between Plasmodium falciparum apical membrane antigen 1 and the rhoptry neck protein complex defines a key step in the erythrocyte invasion process of malaria parasites. J. Biol. Chem. 285, 14815–14822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Holm L., Rosenström P. (2010) Dali server. Conservation mapping in 3D. Nucleic Acids Res. 38, W545–W549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Auld D. S. (2009) The ins and outs of biological zinc sites. Biometals 22, 141–148 [DOI] [PubMed] [Google Scholar]
- 40. Kauth C. W., Epp C., Bujard H., Lutz R. (2003) The merozoite surface protein 1 complex of human malaria parasite Plasmodium falciparum. Interactions and arrangements of subunits. J. Biol. Chem. 278, 22257–22264 [DOI] [PubMed] [Google Scholar]
- 41. Kauth C. W., Woehlbier U., Kern M., Mekonnen Z., Lutz R., Mücke N., Langowski J., Bujard H. (2006) Interactions between merozoite surface proteins 1, 6, and 7 of the malaria parasite Plasmodium falciparum. J. Biol. Chem. 281, 31517–31527 [DOI] [PubMed] [Google Scholar]
- 42. Auld D. S. (2001) Zinc coordination sphere in biochemical zinc sites. Biometals 14, 271–313 [DOI] [PubMed] [Google Scholar]
- 43. Zelensky A. N., Gready J. E. (2005) The C-type lectin-like domain superfamily. FEBS J. 272, 6179–6217 [DOI] [PubMed] [Google Scholar]
- 44. Gazarini M. L., Thomas A. P., Pozzan T., Garcia C. R. (2003) Calcium signaling in a low calcium environment. How the intracellular malaria parasite solves the problem. J. Cell Biol. 161, 103–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McColl D. J., Silva A., Foley M., Kun J. F., Favaloro J. M., Thompson J. K., Marshall V. M., Coppel R. L., Kemp D. J., Anders R. F. (1994) Molecular variation in a novel polymorphic antigen associated with Plasmodium falciparum merozoites. Mol. Biochem. Parasitol. 68, 53–67 [DOI] [PubMed] [Google Scholar]
- 46. Burgess B. R., Schuck P., Garboczi D. N. (2005) Dissection of merozoite surface protein 3, a representative of a family of Plasmodium falciparum surface proteins, reveals an oligomeric and highly elongated molecule. J. Biol. Chem. 280, 37236–37245 [DOI] [PubMed] [Google Scholar]
- 47. Tham W. H., Healer J., Cowman A. F. (2012) Erythrocyte and reticulocyte binding-like proteins of Plasmodium falciparum. Trends Parasitol. 28, 23–30 [DOI] [PubMed] [Google Scholar]
- 48. Juillerat A., Lewit-Bentley A., Guillotte M., Gangnard S., Hessel A., Baron B., Vigan-Womas I., England P., Mercereau-Puijalon O., Bentley G. A. (2011) Structure of a Plasmodium falciparum PfEMP1 rosetting domain reveals a role for the N-terminal segment in heparin-mediated rosette inhibition. Proc. Natl. Acad. Sci. U.S.A. 108, 5243–5248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Khunrae P., Philip J. M., Bull D. R., Higgins M. K. (2009) Structural comparison of two CSPG-binding DBL domains from the VAR2CSA protein important in malaria during pregnancy. J. Mol. Biol. 393, 202–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hodder A. N., Crewther P. E., Matthew M. L., Reid G. E., Moritz R. L., Simpson R. J., Anders R. F. (1996) The disulfide bond structure of Plasmodium apical membrane antigen-1. J. Biol. Chem. 271, 29446–29452 [DOI] [PubMed] [Google Scholar]
- 51. Reed M. B., Caruana S. R., Batchelor A. H., Thompson J. K., Crabb B. S., Cowman A. F. (2000) Targeted disruption of an erythrocyte binding antigen in Plasmodium falciparum is associated with a switch toward a sialic acid-independent pathway of invasion. Proc. Natl. Acad. Sci. U.S.A. 97, 7509–7514 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.