Abstract
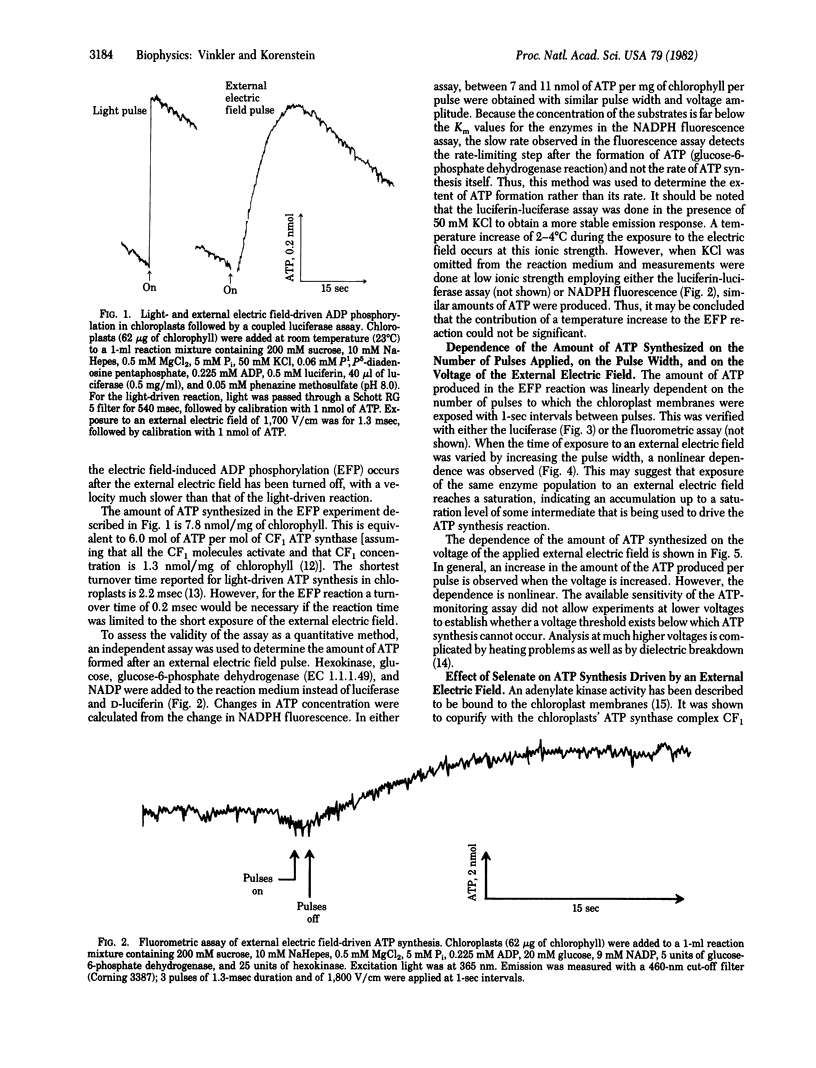
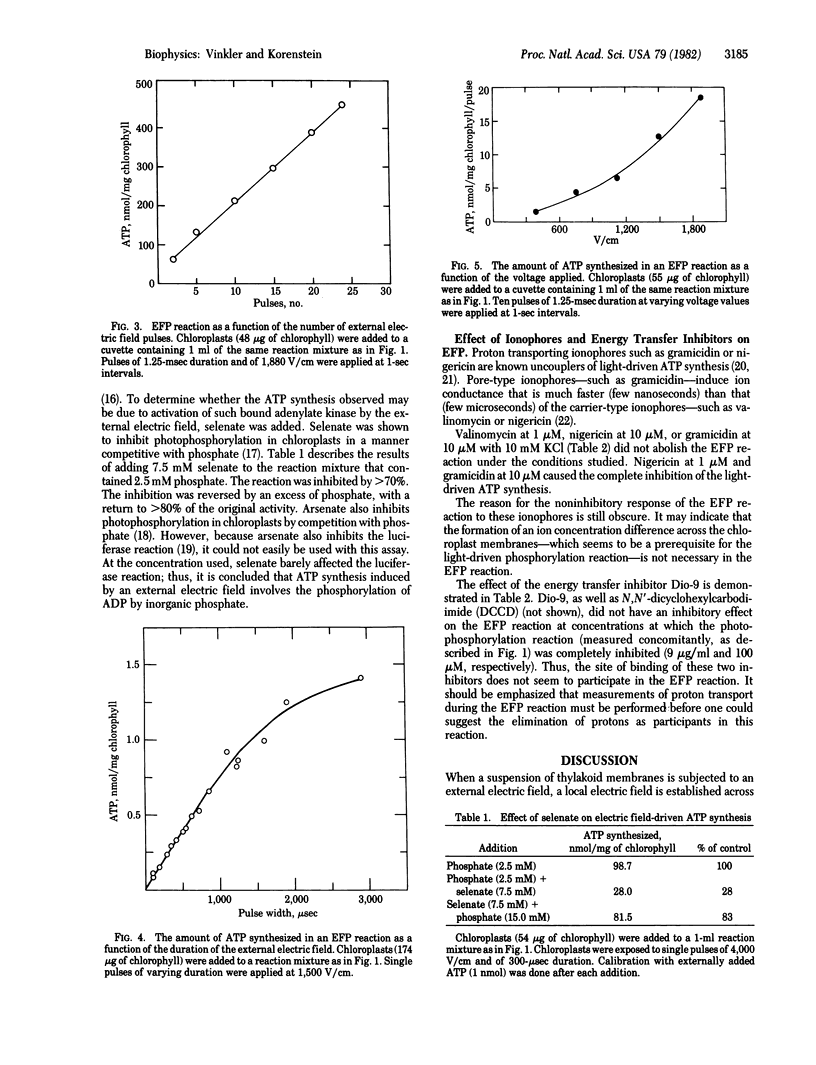
External electric field-induced ADP phosphorylation (EFP) in lettuce chloroplasts was monitored with a coupled luciferin-luciferase enzymatic assay. This assay made it possible to follow ATP synthesis in a kinetically competent manner. The EFP reaction was found to be a much slower process than the light-driven reaction in the same system. The amount of ATP synthesized after a single electric field pulse corresponds to many turnovers of the ATP synthase.
Keywords: electric field jump, cross-membrane potential, phosphorylation, luciferase, inhibitors
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVRON M., JAGENDORF A. T. Evidence concerning the mechanism of adenosine triphosphate formation by spinach chloroplasts. J Biol Chem. 1959 Apr;234(4):967–972. [PubMed] [Google Scholar]
- AVRON M. Photophosphorylation by swiss-chard chloroplasts. Biochim Biophys Acta. 1960 May 20;40:257–272. doi: 10.1016/0006-3002(60)91350-0. [DOI] [PubMed] [Google Scholar]
- Avron M., Shavit N. Inhibitors and uncouplers of photophosphorylation. Biochim Biophys Acta. 1965 Nov 29;109(2):317–331. doi: 10.1016/0926-6585(65)90160-3. [DOI] [PubMed] [Google Scholar]
- Bény M., Dolivo M. Separation of firefly luciferase using an anion exchanger. FEBS Lett. 1976 Nov;70(1):167–170. doi: 10.1016/0014-5793(76)80750-8. [DOI] [PubMed] [Google Scholar]
- DeLuca M., Wannlund J., McElroy W. D. Factors affecting the kinetics of light emission from crude and purified firefly luciferase. Anal Biochem. 1979 May;95(1):194–198. doi: 10.1016/0003-2697(79)90204-5. [DOI] [PubMed] [Google Scholar]
- Eisenman G., Enos B., Hägglund J., Sandblom J. Gramicidin as an example of a single-filing ionic channel. Ann N Y Acad Sci. 1980;339:8–20. doi: 10.1111/j.1749-6632.1980.tb15964.x. [DOI] [PubMed] [Google Scholar]
- Karlish S. J., Shavit N., Avron M. On the mechanism of uncoupling in chloroplasts by ion-permeability inducing agents. Eur J Biochem. 1969 Jun;9(2):291–298. doi: 10.1111/j.1432-1033.1969.tb00608.x. [DOI] [PubMed] [Google Scholar]
- Lemasters J. J., Hackenbrock C. R. Continuous measurement and rapid kinetics of ATP synthesis in rat liver mitochondria, mitoplasts and inner membrane vesicles determined by firefly-luciferase luminescence. Eur J Biochem. 1976 Aug 1;67(1):1–10. doi: 10.1111/j.1432-1033.1976.tb10625.x. [DOI] [PubMed] [Google Scholar]
- Lundin A., Thore A., Baltscheffsky M. Sensitive measurement of flash induced photophosphorylation in bacterial chromatophores by firefly luciferase. FEBS Lett. 1977 Jul 1;79(1):73–76. doi: 10.1016/0014-5793(77)80353-0. [DOI] [PubMed] [Google Scholar]
- McCarty R. E., Guillory R. J., Racker E. Dio-9, an inhibitor of coupled electron transport and phosphorylation in chloroplasts. J Biol Chem. 1965 Dec;240(12):4822–4823. [PubMed] [Google Scholar]
- Moudrianakis E. N., Tiefert M. A. Synthesis of bound adenosine triphosphate from bound adenosine diphosphate by the purified coupling factor 1 of chloroplasts. Evidence for direct involvement of the coupling factor in this "adenylate kinase-like" reaction. J Biol Chem. 1976 Dec 25;251(24):7796–7801. [PubMed] [Google Scholar]
- Nelson N., Eytan E., Notsani B. E., Sigrist H., Sigrist-Nelson K., Gitler C. Isolation of a chloroplast N,N'-dicyclohexylcarbodiimide-binding proteolipid, active in proton translocation. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2375–2378. doi: 10.1073/pnas.74.6.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick U., Avron M. A method for measuring the internal pH in illuminated chloroplasts based on the stimulation of proton uptake by amines. Eur J Biochem. 1976 Nov 15;70(2):569–576. doi: 10.1111/j.1432-1033.1976.tb11048.x. [DOI] [PubMed] [Google Scholar]
- Pick U., Avron M. Inorganic sulfate and selenate as energy transfer inhibitors of photophosphorylation. Biochim Biophys Acta. 1973 Nov 22;325(2):297–303. doi: 10.1016/0005-2728(73)90105-9. [DOI] [PubMed] [Google Scholar]
- Roy H., Moudrianakis E. N. Interactions between ADP and the coupling factor of photophosphorylation. Proc Natl Acad Sci U S A. 1971 Feb;68(2):464–468. doi: 10.1073/pnas.68.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rögner M., Ohno K., Hamamoto T., Sone N., Kagawa Y. Net ATP synthesis in H+ -atpase macroliposomes by an external electric field. Biochem Biophys Res Commun. 1979 Nov 14;91(1):362–367. doi: 10.1016/0006-291x(79)90627-2. [DOI] [PubMed] [Google Scholar]
- Schlodder E., Witt H. T. Relation between the initial kinetics of ATP synthesis and of conformational changes in the chloroplast ATPase studied by external field pulses. Biochim Biophys Acta. 1981 May 13;635(3):571–584. doi: 10.1016/0005-2728(81)90115-8. [DOI] [PubMed] [Google Scholar]
- Strehler B. L. Bioluminescence assay: principles and practice. Methods Biochem Anal. 1968;16:99–181. doi: 10.1002/9780470110348.ch2. [DOI] [PubMed] [Google Scholar]
- Strotmann H., Hesse H., Edelmann K. Quantitative determination of coupling factor CF1 of chloroplasts. Biochim Biophys Acta. 1973 Aug 31;314(2):202–210. doi: 10.1016/0005-2728(73)90135-7. [DOI] [PubMed] [Google Scholar]
- Witt H. T. Energy conversion in the functional membrane of photosynthesis. Analysis by light pulse and electric pulse methods. The central role of the electric field. Biochim Biophys Acta. 1979 Mar 14;505(3-4):355–427. doi: 10.1016/0304-4173(79)90008-9. [DOI] [PubMed] [Google Scholar]
- Witt H. T., Schlodder E., Gräber P. Membrane-bound ATP synthesis generated by an external electrical field. FEBS Lett. 1976 Oct 15;69(1):272–276. doi: 10.1016/0014-5793(76)80702-8. [DOI] [PubMed] [Google Scholar]
- de Grooth B. G., van Gorkom H. J., Meiburg R. F. Electrochromic absorbance changes in spinach chloroplasts induced by an external electrical field. Biochim Biophys Acta. 1980 Feb 8;589(2):299–314. doi: 10.1016/0005-2728(80)90046-8. [DOI] [PubMed] [Google Scholar]


